Abstract
Since the classic experiments by Tigerstedt and Bergman that established the role of renin in hypertension a century ago, aggressive efforts have been launched to effectively block the renin-angiotensin system (RAS). Blockade of RAS is advocated at multiple levels by direct renin inhibitor, angiotensin-converting enzyme inhibitor and/or angiotensin II type 1 receptor blocker, or aldosterone inhibitor (spironolactone), and has now become part of the standard of care to control hypertension and related metabolic diseases including diabetes. However, recent lessons learned from randomized clinical trials question the wisdom of blocking RAS at multiple levels. In this context, it is highly pertinent that components of RAS are evolutionarily conserved, and novel physiological/adaptive/protective roles for renin and angiotensin-converting enzyme are currently emerging. Angiotensin II, the classical RAS effector peptide responsible for hypertension, hypertrophy, fluid retention and fibrosis, manifests its cardiovascular protective effect when it activates the angiotensin II type 2 receptor. Additionally, angiotensin-converting enzyme 2 and the angiotensin II metabolite Ang-(1–7) that acts through the Mas proto-oncogene constitute the cardiovascular and renal protective branch of RAS. It is conceivable that modulating this vasodilative/anti-inflammatory branch of RAS by activation of the RAS components that constitute this branch may offer a safer long-term treatment strategy to balance RAS activity and achieve homeostasis compared to chronic multilevel RAS inhibition.
Key Words: Renin-angiotensin system, Angiotensin II type 1 receptor, Angiotensin II type 2 receptor, Angiotensin-converting enzyme 2, Chymase
Introduction
In our society ravaged by the chronic ill health resulting from overnutrition and metabolic syndrome (MetS), the renin-angiotensin system (RAS) indeed is infamous since it serves as a link between obesity and low-grade systemic inflammation [1,2,3,4,5]. Chronic activation of RAS underlies a plethora of metabolic diseases such as hypertension, insulin resistance, cardiac and renal diseases, and polycystic ovarian syndrome. The simple view of RAS is that the aspartyl protease renin serves as the rate-limiting step in the RAS biochemical pathway where the serpin family member angiotensinogen (AGT), an α2-globulin, is cleaved by renin to split off the biologically inactive N-terminal decapeptide angiotensin I (Ang I), which is then cleaved by the dipeptidase angiotensin-converting enzyme (ACE) to produce the biologically active octapeptide angiotensin II (Ang II). Ang II would then bind to the Ang II type 1 receptor (AT1R) and induce signaling pathways that promote muscle constriction, salt and water retention, fibrosis, hypertrophy, and hyperplasia that underlie many metabolic diseases and poor cardiovascular and renal prognosis. Blockade of RAS can be exerted at multiple levels, via inhibition of renin, ACE, or AT1R signaling [6,7,8,9,10,11,12]. Efficient RAS blockers at all these levels have been developed and are currently in use to block overactivation of RAS and to offer protection from RAS-related metabolic diseases including diabetes [8,9,10,11,12,13]. However, evidence of increased adverse effects from randomized clinical trials such as Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) using double RAS blockade strongly advises against blocking RAS at multiple levels [14,15,16,17]. Furthermore, the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) also demonstrated that dual RAS blockade was not beneficial compared to monotherapy with an ACE inhibitor (ACEi) or an AT1R blocker (ARB) in preventing serious outcomes in patients with known vascular disease or diabetes with end-organ damage [18,19,20]. Clinical evidence supporting the association of RAS inactivation to renal diseases and basic research on RAS have begun to unveil the intricate self-regulatory signaling loops that fine-tune RAS activation and the adaptive/protective role of RAS in many tissues [21,22]. Moreover, given the fact that the AT1R is a G protein-coupled receptor (GPCR) that has pleotropic effects and a multitude of cellular protein partners, the quest is now underway to develop ARBs with functional selectivity such as β-arrestin-biased agonists [23,24]. This review focuses on the protective physiological roles of RAS and novel strategies to regulate the vasoconstrictive/pro-inflammatory effects of RAS in cardiovascular and renal tissues.
The Changing Image of RAS
The first clinical evidence for a correlation between cardiac hypertrophy and renal disease was reported in 1836 by Richard Bright [[25], and references therein]. However, the first insight into the regulation of blood pressure came 61 years later from the discovery of a pressor principle termed ‘renin’ by Tigerstedt and Bergman. In 1940, Page and Helmer identified a ‘renin activator’ that later proved to be AGT. Subsequently, the pressor substance was isolated and characterized as the octapeptide Ang II. Following this major discovery, all RAS components that lead to an increase in blood pressure, including AGT, ACE, Ang II, AT1R, and degraded products of Ang II such as Ang-(2–8) (Ang III) and Ang-(3–8) (Ang IV), were isolated and characterized [4,5,25]. Meanwhile, the complexity of the RAS signal cascade gradually expanded due to the uncovering of additional biologically active truncated angiotensin peptides such as Ang-(1–9) and Ang-(1–7), receptors such as AT2R, AT3R, AT4R (insulin regulated aminopeptidase), and Mas receptor, and additional enzymes that contribute to the generation of RAS-related peptides such as the ACE-related carboxypeptidase ACE2, cathepsin D, cathepsin G, kallikrein, pepsin, tissue plasminogen activator, tonin, trypsin, and chymase [4,5,25,26,27,28,29] (fig. 1). The fragmented peptides Ang III and IV are produced from Ang II by the action of aminopeptidases A and N at the N-terminus, while Ang-(1–7) is produced by the action of the carboxypeptidase ACE2, a close relative of ACE, at the C-terminus. Characterization of the signaling pathways activated by ACE2, AT2R, and Ang-(1–7) further revealed the cardiovascular and renal protective branch of RAS (fig. 2).
Fig. 1.
An integrated model of RAS and resulting physiological and pathophysiological effects. The precursor AGT is cleaved by renin to form the decapeptide Ang I, which is subsequently cleaved by the dipeptidase ACE to form the octapeptide Ang II. AGT may also be cleaved to Ang-(1–12), which is further cleaved to Ang II by the action of chymase. Chymases can also act on Ang I and cleave it to form Ang II. Ang II is the central active component of RAS. Activation of the AT1R by Ang II stimulates vasoconstrictive/pro-inflammatory pathways, whereas that of the AT2R activates the vasodilative/anti-inflammatory branch of RAS. Ang II is further cleaved by ACE2 to Ang-(1–7). ACE2 can also directly cleave Ang I to form Ang-(1–9). Ang-(1–9) is cleaved by ACE to Ang-(1–7). Ang-(1–7) activates the vasodilative/anti-inflammatory branch of RAS via the Mas receptor, a GPCR that activates NO synthase/cGMP signaling. Ang II can also be cleaved to form Ang III and Ang IV by the action of aminopeptidases (aminopeptidases A and N; AP-A and AP-N). Ang III is a preferred ligand for the AT2R. Ang IV binds to the insulin-regulated aminopeptidase receptors (IRAP). Direct renin inhibitors (DRIs) that block renin can increase AGT levels, and ACEi that block ACE can increase Ang I levels. In such conditions, local RAS gets activated by the action of chymase and other proteases that generate Ang II.
Fig. 2.
ARBs redirect Ang II to bind and activate more AT2R. Cleavage of Ang II by ACE2 to generate Ang-(1–7) that binds to the Mas receptor increases vasodilatation via activation of the NO/cGMP system. Novel molecular scaffolds that directly bind to the intracellular tail of the AT2R such as AT2 receptor-interacting proteins (ATIPs) contribute to vascular remodeling and growth inhibition. The AT2R suppresses serine/threonine and tyrosine phosphorylation of proteins via activation of phosphatases such as mitogen-activated protein kinase phosphatase-1 (MPK1), protein phosphatase 2A (PP2A), and Src homology region 2 domain-containing phosphatase-1 (SHP1), or via direct interaction as in the case of ErbB3.
Mutations in the genes encoding components of RAS in rodents or humans cause congenital abnormalities of the kidney and urinary tract [22,30,31]. Importantly, recent reports that show autosomal recessive renal tubular dysgenesis (RTD), a severe disorder of renal tubular development that is characterized by persistent fetal anuria and perinatal death, is linked to a spectrum of mutations in the genes encoding renin (REN), AGT, ACE, and AT1R (AGTR1) highlight the essential role of RAS in normal kidney development [22,32]. Infants with RTD surviving on dialysis and respiratory assistance show profound life-threatening and refractory hypotension and renal RAS suppression. Studies on 48 unrelated families have uncovered 54 mutations: 11 different mutations in the REN gene were identified in 10 families, 6 different mutations in the AGT gene were identified in 4 unrelated families, 33 different ACE mutations were identified in 31 families (some consanguineous), and 4 different mutations in the AGTR1 gene were identified in 3 families. Thus, ACE mutations were more frequent and observed in two-thirds of the families (64.6%). The severity of the clinical course of RTD in these patients was similar irrespective of the function of the mutated RAS component. These observations underscore the importance of a functional RAS in the maintenance of blood pressure and renal blood flow during the life of a human fetus since nephrogenesis in humans is completed before birth. Moreover, prolonged fetal exposure to RAS blockers is shown to cause a phenotype quite similar to that in autosomal recessive RTD [22]. Conversely, in rats and mice whose nephrogenesis is completed only after birth, inactivation of different components of RAS does not lead to RTD, and the animals show normal embryo-fetal development [22]. However, lack of functional REN, AGT, ACE, or AGTR1 genes in these animals causes polyuria, and most of them die due to dehydration before weaning [22,30,31,32,33,34].
Recent studies also highlight the requirement for functional RAS to achieve the full therapeutic potential of transplantation of autologous bone marrow cells (BMCs) to treat ischemic diseases [35]. Rapid revascularization is crucial to restore organ functions in conditions of ischemia and injury; however, inducing efficient revascularization remains a major issue in the medical field. Novel treatment strategies that utilize BMC transplantation to induce revascularization are highly promising, and animal studies with endothelial progenitor cells (EPCs) have demonstrated that these cells augment reparative neovascularization either via differentiation into mature endothelial cells or by indirect paracrine stimulation of resident endothelial cell proliferation [36]. Though EPC therapy is shown to successfully restore vascularization after ischemic events in the myocardium, retina, brain, and limbs in experimental studies on healthy animals, clinical studies in patients with cardiovascular disease risk factors and endothelial dysfunction did not show such success. de Resende et al. [35] have shown that, in Dahl salt-sensitive rats (SS/Mcwi), skeletal muscle angiogenesis induced by electrical stimulation is significantly impaired. This effect was independent of their salt intake. Consomic SS-13BN/Mcwi rats in which RAS dysregulation is corrected by the replacement of chromosome 13 derived from the Brown Norway (BN) rat exhibited normal angiogenesis under similar conditions. Thus, reduced angiogenesis seemed to be a renin-dependent mechanism. Moreover, BMCs isolated from SS-13BN/Mcwi and SS/Mcwi rats infused with Ang II effectively restored the skeletal muscle angiogenesis in response to electrical stimulus in the SS/Mcwi recipients, whereas BMCs isolated from SS/Mcwi or SS/Mcwi rats infused with saline failed to restore the angiogenic response. These observations underpin the need for a normal functional RAS for achieving a positive response to EPC therapy.
It is well established that RAS activation is an evolutionarily conserved adaptive response in conditions of hypovolemia that helps in maintaining fluid homeostasis and sodium appetite. An Ang II-activated adaptive dipsogenic effect is seen in mammals, birds, reptiles, and bony fish [37]. Signaling by different RAS components is tightly regulated via an inherent balancing act by its vasoconstrictive/pro-inflammatory branch and vasodilative/anti-inflammatory branches, as discussed below.
The Vasoconstrictive/Pro-Inflammatory Branch
The vasoconstrictive/pro-inflammatory branch of RAS involves the RAS components that contribute to the formation of Ang II and activation of the AT1R that leads to vasoconstriction and inflammatory pathways. However, evidence gathered from clinical trials and basic research strongly suggests that the chronic inhibition of different RAS components (renin, ACE, AT1R) involved in this pathway does not stop new-onset cardiac and kidney diseases and may activate RAS back-up systems such as local chymases or other proteases that contribute to the formation of local Ang II in tissues such as heart and kidney.
Renin
The preprohormone renin synthesized by juxtaglomerular cells of the kidney contains a signal sequence necessary for the translocation of the protein to the endoplasmic reticulum where it is processed into prorenin, an inactive precursor. Enzymatically active renin is formed by the proteolytic removal of a 43-amino-acid prosegment peptide from the N-terminus of prorenin. Release of renin to circulation upon demand by renin-expressing cells of the kidney is controlled by cyclic adenosine monophosphate and by calcium-signaling pathways that are activated by a variety of systemic and local factors. Renin is also locally expressed in many tissues. In mice, the adrenal gland is the primary source of renin during fetal development [38].
Direct renin inhibitors (for example aliskiren) have been shown to have beneficial effects in the management of hypertension and diabetes, and novel 5- or 7-azaindole derivatives with remarkable potency for renin inhibition have been identified recently [9,10,11,39]. However, according to European Medicines Agency recommendations, aliskiren should not be prescribed to diabetic patients in combination with ACEi or ARBs [40]. The long-term, randomized, placebo-controlled morbidity/mortality trial ALTITUDE, which included 8,600 patients with type 2 diabetes, proteinuria, and a high cardiovascular risk already treated with an ACEi or ARB, was terminated in December 2011 due to an increased incidence of serious adverse events in the aliskiren 300 mg arm [15,16,17,40]. In this context, it is pertinent that chronic suppression of renin does not protect against the profibrotic influence of a chronically elevated urine flow. For example, mice with deletion of Gsα in renin-producing cells (RC/FF mice) have greatly reduced renin production and lack of responsiveness of renin secretion to acute stimuli [41]. Urine osmolality and glomerular filtration rate (GFR) declined progressively in these mice, and expression of collagen I and IV, fibronectin, and α-smooth muscle actin in kidneys increased with age. Moreover, RC/FF mice showed a progressive reduction of body weight, an increase in urine albumin excretion, and an increase of blood pressure with aging. This was accompanied by focal segmental glomerulosclerosis and periglomerular interstitial fibrosis [41]. Thus, a balanced renin function is required for the protection of the renal function. It is conceivable that the increased events of non-fatal stroke, renal complications, hyperkalemia, and hypotension in the aliskiren group of the ALTITUDE study simply reflects the additive effects of hypotension and eGFR reduction due to the addition of potent RTD in patients with already well-controlled blood pressure.
AGT
AGT is a 485-amino-acid protein (482 amino acids in mice) synthesized and secreted by hepatocytes, which serves as the parental peptide for all angiotensin peptides in the RAS pathway [42]. Renin cleaves AGT to generate Ang I and des(AngI)-AGT that accounts for more than 95% of the AGT protein sequence and maintains a typical serpin folding. Both AGT and des(AngI)-AGT are suggested to have anti-angiogenic properties. The crystal structure of human AGT suggests that the formation of a Cys18-Cys138 disulphide bridge confers a conformational change that allows access of renin to its cleavage site of AGT, and the oxidative status of AGT influences the rate of AGT-renin reaction [42]. Mice deficient in AGT develop hydronephrosis and dilated cardiomyopathy and have a low neonatal survival rate, highlighting the need of AGT for normal cardiac and renal functions [33].
ACE
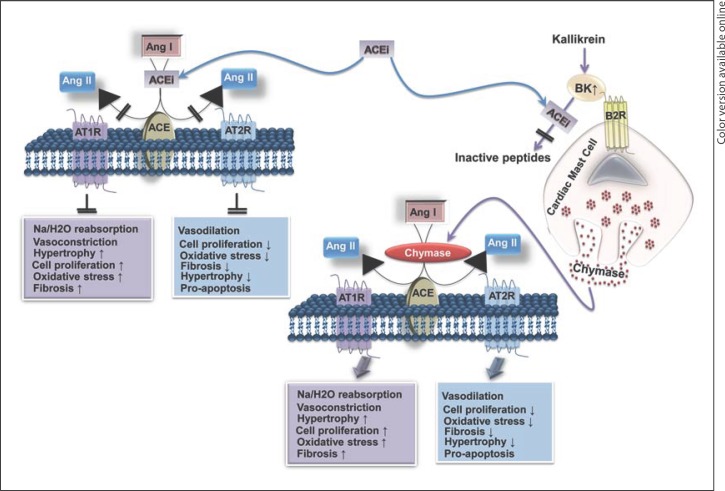
ACEi are highly effective in controlling hypertension and reducing the risk of developing heart failure, left ventricular hypertrophy, insulin resistance, and microalbuminuria in diabetes [12,13,18,19,20]. Since ACE generates the vasoconstrictor peptide Ang II from Ang I and degrades the vasodilator bradykinin (BK), ACEi treatment improves vasodilatation by reducing Ang II and increasing BK. However, recent evidence shows development of new heart failure in patients chronically treated with ACEi and beta-blockers and new-onset albuminuria in patients receiving an ACEi for years. Xiao et al. [34] demonstrated that ACE null mice show abnormal anatomic findings with thickened arterioles and underdeveloped renal medulla and papilla. These anatomic abnormalities are attributed to the lack of Ang II [34]. Moreover, mutations in the ACE gene were predominant among the mutations in RAS components associated with RTD [22]. Though ACE is the main dipeptidase that cleaves circulating Ang I to generate Ang II, other local dipeptidases such as mast cell chymase can contribute significantly to increase Ang II production locally [28] (fig. 3). Studies using microdialysis probes tethered to the heart of conscious mice have shown that Ang II levels remained high in the left ventricular (LV) interstitial fluid (ISF) during chronic ACEi treatment [28]. This was due to an ACEi-induced and BK/B2 receptor-mediated increase in LV ISF chymase activity, and this effect was absent in mast cell-deficient KitW/KitW-v mice [28]. Similarly, in the diabetic kidney of db/db mice, ACEi was not effective in inhibiting the intrarenally formed Ang II from the substrate Ang I and resultant potent afferent arteriole vasoconstriction [43]. However, in the normal kidney, afferent arteriolar vasoconstriction to intrarenally formed Ang II was attenuated by ACEi treatment. Interestingly, the serine protease inhibitor could suppress intrarenal Ang II production in db/db mice, emphasizing the importance of ACE-independent pathways of Ang II production in the kidney [43]. While these observations underscore the need for novel drugs such as chymase inhibitors to treat cardiorenal disease progression, they also unveil the complex back-up signaling pathways of RAS to maintain local Ang II production in cardiorenal tissues in conditions of ACE inhibition (for example, the ACEi-induced and BK/B2 receptor-mediated increase in LV ISF chymase activity).
Fig. 3.
ACEi inhibit cleavage of Ang I to Ang II and of BK to inactive peptides. Blockade of ACE by ACEi increases the levels of Ang I and BK. BK binds to the BK 2 receptor (B2R) on cardiac mast cells and increases the release of chymase that cleaves Ang I to Ang II and bypass ACEi effects in heart tissue.
AT1R
The AT1R is an evolutionarily conserved GPCR composed of 359 amino acids that can activate several signaling networks that are either G protein dependent or independent [23,44]. The human AT1R gene (AGTR1) is present on chromosome 3. The receptor is the product of a single gene that contains five exons. ARB treatment is highly efficient in regulating blood pressure and ameliorating cardiovascular and renal diseases and insulin resistance [4,8,9,11]. However, a recent analysis of data from 85 clinical trials (21,708 patients) indicated that there is no significant reduction in the risk of all-cause mortality or fatal cardiac-cerebrovascular outcomes with ARB treatment alone or in combination with ACEi [45]. Moreover, the existing dogma that the AT1R is a major contributor to the RAS-induced vascular pathology has been partially challenged recently by the results of studies on mice with cell-specific deletion of AT1R in vascular smooth muscle cells (VSMCs). The SM22α-Cre+Agtr1a flox/flox (SMKO) mice used in this study had their AT1aR deleted from VSMCs in larger vessels, but not from resistance vessels [46]. Therefore, Ang II infusion for 4 weeks could induce hypertension in SMKO mice and control mice to a similar extent. However, lack of the AT1aR in SMKO mice protected them from vascular oxidative stress. Nevertheless, the SMKO mice developed Ang II-induced vascular remodeling (aortic medial expansion) similar to control mice, indicating that the AT1aR-induced oxidative stress is not central to Ang II-induced vascular remodeling [46].
It is also conceivable that chronic AT1R inhibition would dysregulate the mechanism of urine concentration and thus contributes to progression of renal diseases. In mice lacking AT1aR in the collecting duct (CD-KO mice), kidneys were structurally normal, and urine osmolalities and urine volumes were similar to those in control mice [47]. However, in conditions of water deprivation or vasopressin administration, normal response of an increase in urine osmolality was attenuated in CD-KO mice, indicating that AT1aR regulates this renal function via an aquaporin-mediated mechanism [47]. These studies have begun to provide an explanation for why cardiorenal diseases progress in some populations in spite of RAS suppression by ACEi or ARB treatments.
The aforementioned observations highlight the need for developing efficient, functionally selective ARBs that can precisely target desired AT1R effects and inhibit AT1R-mediated inflammatory signaling. Recent studies have identified a novel, potent β-arrestin-biased agonist of the AT1R, TRV120027 [48]. This new ARB is shown to block AT1R-mediated G protein signaling and inhibit Ang II-mediated vasoconstriction and, simultaneously, activate β-arrestin and improve cardiomyocyte contractility. Studies on heart failure in canines have shown that acute treatment with TRV120027 could decrease mean arterial pressure, pulmonary capillary wedge pressure, right atrial pressure as well as systemic and renal vascular resistances and improve cardiac output and renal blood flow [48]. These unique properties of TRV120027 indicate that, in in vivo conditions, it serves as an antagonist, analogous to ARBs, when tested for G protein coupling, p38 mitogen-activated protein kinase (MAPK) activation, and effects on blood pressure. However, it serves as an agonist when assessed for efficacy in recruiting β-arrestins to the AT1R, receptor internalization, β-arrestin-mediated signals, and cardiomyocyte contractility. Thus, a β-arrestin-biased AT1R agonist holds new hope for a better treatment of acute heart failure patients.
Ang II and Its Derivatives: The Paradigm Shift and the Vasodilative/Anti-Inflammatory Branch
The concept that Ang II is the principal effector peptide of RAS and its primary role is to serve as a pressor molecule that regulates vascular tone is currently undergoing a re-evaluation with the unveiling of new roles for its degraded products. The octapeptide Ang II is converted to des-aspartyl1-Ang II (Ang III) by aminopeptidase A and further metabolized to angiotensin IV by aminopeptidase N. Ang III has now emerged as the predominant activator of the AT2R in the proximal tubule and significant contributor to AT2R-mediated natriuresis [49]. Ang III infusion in the presence of the aminopeptidase N inhibitor is shown to increase urine sodium excretion, fractional sodium, and lithium excretion, whereas Ang II infusion did not exhibit this effect. Our studies indicate that Ang III also activates endogenous chloride channels and induces germinal vesicle breakdown in oocytes [50]. Thus, Ang III has a crucial biological role in RAS-mediated physiological end points.
Though Ang II exerts vasoconstriction when it activates the AT1R, it also has a depressor role when it acts through the AT2R and induces vasodilation via kinin-/nitric oxide (NO)-dependent mechanisms. Ang-(1–7) generated from Ang II by the action of the ACE homologue ACE2 is another metabolite of Ang II that contributed significantly to the paradigm shift regarding the cardiorenal protective role of RAS. Indeed, unveiling of the AT2R/ACE2/Ang-(1–7)/Mas receptor axis of RAS [5] that exerts the vasoprotective and anti-inflammatory actions of RAS highlights the dual role of RAS in maintaining blood pressure and homeostasis (fig. 2).
AT2R
The AT2R is also a GPCR that shares only 34% sequence identity with the AT1R and forms heterodimers with the AT1R [51]. The human AGTR2 gene that codes for the AT2R is located on the X-chromosome and consists of three exons with an uninterrupted coding region that codes a 363-amino-acid receptor in the third exon. Sequence comparisons reveal a high level of homology with a 99% sequence identity between rat and mouse and a 79% sequence identity between rat and human. The divergence between the rodent and the human sequence lies in the amino terminus. Even though the AT2R belongs to the GPCR family of proteins, its signaling mechanisms are atypical and remain elusive. Activated AT2R induces a vasodilator cascade of BK/NO/cyclic guanosine monophosphate (cGMP), stimulates various protein phosphatases (SHP1, MKP1, and PP2A), and inhibits cell growth. We have shown that the AT2R exerts antagonistic effects on AT1R signaling, and this effect is mediated via the third intracellular loop of the AT2R [52]. We have also shown that the AT2R interacts with the ErbB family receptors and Na+/H+ exchanger NHE6 via its third intracellular loop and C-terminal cytoplasmic domain [52,53]. The AT2R also interacts with a family of AT2R-interacting proteins involved in neuronal differentiation, vascular remodeling, and tumor suppression via its C-terminal domain [54]. Chronic ARB treatment can result in redirecting Ang II to the AT2R that is usually co-expressed with the AT1R in cardiovascular tissues, increased AT2R activation, and enhanced AT1R-AT2R cross talk (fig. 2). Indeed, in AT2R knock-out mice, valsartan failed to attenuate acute-phase post-infarction remodeling, suggesting that the AT2R is required for the cardioprotective effects of ARBs. Cardiovascular protective effects of the AT2R are highlighted by the fact that moderate cardiac-specific AT2R overexpression protects the heart from ischemic injury [55]. The AT2R agonist compound 21 is shown to attenuate vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats [56]. Since pro-inflammatory signaling by the AT1R is usually activated in these conditions, it is conceivable that cross talk between the AT1R and the AT2R that can lead to inhibition of pro-inflammatory signaling by the AT1R in cardiovascular and renal tissues is part of the intricate self-regulatory signaling network activated by RAS.
The ACE2/Ang-(1–7)/Mas Axis
The monocarboxypeptidase ACE2 that cleaves the Pro7-Phe8 bond of Ang II to form Ang-(1–7) emerged as an important component of the vasodilative/anti-inflammatory arm of RAS for the following reasons: (a) ACE2 gene deletion is accompanied by severe cardiac contractility defects; (b) ACE2 overexpression reverses cardiac hypertrophy and progression of atherosclerosis, and (c) ACE2 inhibitors worsen renovascular hypertension [4,5,57]. The heptapeptide Ang-(1–7) was originally discovered as a vasopressin-releasing molecule. Now it has emerged as a natural antagonist of Ang II and a first-in-class anti-angiogenic drug in clinical trials for cancer treatment. Ang-(1–7) acts through the Mas receptor and promotes vasodilatation as well as anti-hypertrophic, anti-fibrotic, and anti-proliferative effects. Indeed, lentivirus-mediated overexpression of Ang-(1–7) and ACE2 could improve cardiac functions, attenuate ischemia-induced cardiac pathology, and ameliorate lung fibrosis and pulmonary hypertension [57]. Ang-(1–7) binds to the Mas proto-oncogene which is a GPCR and activates the NO/cGMP pathway via the phosphatidylinositol 3′-kinase-protein kinase B (Akt) pathway leading to endothelial NO synthase activation and NO generation. Indeed, the NO-releasing properties of Ang-(1–7) were abolished in cardiomyocytes from Mas-deficient mice. Moreover, Ang-(1–7) inhibits agonist-stimulated activation of kinases involved in cardiac remodeling like MAPK activation and ERK1/2 in heart [4,5].
RAS Activation in MetS
RAS activation also occurs in conditions of overnutrition/obesity and is associated with hyperinsulinemia. In the Zucker obese (fa/fa) rat, an animal model that exhibits the traits of MetS and cardiac contractile dysfunction, RAS is activated. Interestingly, these hyperinsulinemic rats have about 10 times higher plasma insulin concentrations compared to their lean controls. It is known that the presence of insulin or IGF-1 stimulates the expression of AT2R in VSMCs in serum-free conditions, and an insulin response sequence is located at −126 to −117 of the 5′-flanking region of the rat AT2R gene [58,59]. Studies on Zucker obese rat aortas showed that these tissues exhibit increased expression of AT2R mRNA compared to those from age-matched lean rats [58]. Since AT2R activation promotes vasodilation, such upregulation of the vascular AT2R in hyperinsulinemic conditions may serve as an adaptive mechanism that counteracts AT1R-induced vasoconstriction.
Recent reports suggest that Ang II-induced AT1R activation results in suppression of food intake, and mice lacking AT1R exhibit hyperphagia and obesity [60]. One of the mechanisms induced by AT1R activation in the hypothalamus is the upregulation of anorexigenic corticotropin-releasing hormone (Crh) gene expression that results in suppression of food intake. It is also reported that Ang II downregulates expression of orexigenic neuropeptide Y and orexin in the hypothalamus [60]. In conditions of chronic RAS activation, Ang II-induced suppression of food intake can contribute to significant loss of appetite that can underlie cachexia, muscle atrophy, fatigue, and weakness. Conversely, it is conceivable that in conditions of overnutrition and overweight, adaptive RAS activation may be a natural response to regulate food intake.
Resistant Hypertension
Data from the National Health and Nutrition Examination Survey from 2003 through 2008 indicate that, among US adults with hypertension, 8.9% deal with a condition termed resistant hypertension. Resistant hypertension is defined as hypertension that cannot be controlled by antihypertensive medications from three different drug classes. The majority of the individuals who suffer from resistant hypertension include elderly, non-Hispanic blacks, and those with higher body mass index. The comorbidities include albuminuria, renal dysfunction, coronary heart disease, heart failure, stroke, and diabetes mellitus. The recent Addition of Spironolactone in Patients With Resistant Arterial Hypertension (ASPIRANT) clinical trial administrated 25 mg spironolactone, an anti-mineralocorticoid agent that inhibits aldosterone, to patients whose blood pressure was not controlled by three antihypertensive drugs, including a diuretic. Analysis of 111 patients indicated that spironolactone significantly decreased systolic blood pressure, though its effect was not prominent on respective diastolic blood pressure [61]. These studies suggest that spironolactone may be an effective alternative to treat elevated systolic blood pressure in patients with resistant hypertension.
Conclusion
The standard of care to treat the vasoconstrictive/pro-inflammatory effects of RAS activation involves chronic RAS inhibition using direct renin inhibitors, ACEi, or ARBs, either alone or in combination. However, data merging from randomized clinical trials and basic research indicate that, although these treatments are very effective in controlling the progression of existing disease, they are not as effective in preventing new-onset cardiac and renal diseases. Moreover, inhibiting RAS at multiple levels increases unwanted detrimental effects since novel physiological roles for renin, ACE, and AT1R are currently emerging. In this scenario, selectively inhibiting AT1R-mediated inflammatory pathways with appropriate, biased agonists such as TRV120027 that would regulate blood pressure and improve cardiomyocyte contractility offers new hope for RAS treatment. Furthermore, since RAS activation also leads to activation of the vasodilative/anti-inflammatory branch of RAS, an alternative approach to modulate RAS has begun to emerge that utilizes novel agonists of the AT2R and formulations of recombinant Ang-(1–7).
Acknowledgements
This work is supported in part by NIHLB-HL060241-02 and University of Missouri Mission Enhancement Fund (to L.P.), and NIH R01-HL073101 and R01-HL107910 and VA Merit Award (to J.R.S.).
References
- 1.Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136–149. doi: 10.1111/j.1467-789X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 2.Pulakat L, Demarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell AT, Sowers JR. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011;301:R885–R895. doi: 10.1152/ajpregu.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertens Res. 2009;32:533–536. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell KN, Raij L, Mundel P. Role of angiotensin II in the development of nephropathy and podocytopathy of diabetes. Curr Diabetes Rev. 2011;7:3–7. doi: 10.2174/157339911794273973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Miyashita Y, Shirai K. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Balakrishnan A, Howatt DA, Wu C, Charnigo R, Liau G, Cassis LA, Daugherty A. Comparative effects of different modes of renin angiotensin system inhibition on hypercholesterolaemia-induced atherosclerosis. Br J Pharmacol. 2012;165:2000–2008. doi: 10.1111/j.1476-5381.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Düsing R, Brunel P, Baek I, Baschiera F. Sustained decrease in blood pressure following missed doses of aliskiren or telmisartan: the ASSERTIVE double-blind, randomized study. J Hypertens. 2012;30:1029–1040. doi: 10.1097/HJH.0b013e328351c263. [DOI] [PubMed] [Google Scholar]
- 10.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117:3199–3205. doi: 10.1161/CIRCULATIONAHA.108.767202. [DOI] [PubMed] [Google Scholar]
- 11.Lam S. Azilsartan: a newly approved angiotensin II receptor blocker. Cardiol Rev. 2011;19:300–304. doi: 10.1097/CRD.0b013e31822e9ba3. [DOI] [PubMed] [Google Scholar]
- 12.Hale TM, Robertson SJ, Burns KD, Deblois D. Short-term ACE inhibition confers long-term protection against target organ damage. Hypertens Res. 2012;35:604–610. doi: 10.1038/hr.2012.2. [DOI] [PubMed] [Google Scholar]
- 13.Barodka V, Silvestry S, Zhao N, Jiao X, Whellan DJ, Diehl J, Sun JZ. Preoperative renin-angiotensin system inhibitors protect renal function in aging patients undergoing cardiac surgery. J Surg Res. 2011;167:e63–e69. doi: 10.1016/j.jss.2009.11.702. [DOI] [PubMed] [Google Scholar]
- 14.Elijovich F, Laffer CL. Detrimental effects of dual ACEI-ARB therapy: is the (pro)renin receptor the culprit? Kidney Int. 2011;80:911–914. doi: 10.1038/ki.2011.264. [DOI] [PubMed] [Google Scholar]
- 15.Azizi M, Ménard J.Renin inhibitors and cardiovascular and renal protection: an endless quest? Cardiovasc Drugs Ther 2012, E-pub ahead of print [DOI] [PubMed]
- 16.Sarafidis PA, Ruilope LM. Cardiorenal disease development under chronic renin-angiotensin-aldosterone system suppression. J Renin Angiotensin Aldosterone Syst. 2012;13:217–219. doi: 10.1177/1470320312439140. [DOI] [PubMed] [Google Scholar]
- 17.Powers BJ, Coeytaux RR, Dolor RJ, Hasselblad V, Patel UD, Yancy WS, Jr, Gray RN, Irvine RJ, Kendrick AS, Sanders GD. Updated report on comparative effectiveness of ACE inhibitors, ARBs, and direct renin inhibitors for patients with essential hypertension: much more data, little new information. J Gen Intern Med. 2011;27:716–729. doi: 10.1007/s11606-011-1938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, ONTARGET investigators Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomized, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C, ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 20.Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JF, ONTARGET and TRANSCEND Investigators Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation. 2011;123:1098–1107. doi: 10.1161/CIRCULATIONAHA.110.964171. [DOI] [PubMed] [Google Scholar]
- 21.O'Hare AM, Kaufman JS, Covinsky KE, Landefeld CS, McFarland LV, Larson EB. Current guidelines for using angiotensin-converting enzyme inhibitors and angiotensin II-receptor antagonists in chronic kidney disease: is the evidence base relevant to older adults? Ann Intern Med. 2009;150:717–724. doi: 10.7326/0003-4819-150-10-200905190-00010. [DOI] [PubMed] [Google Scholar]
- 22.Gribouval O, Morinière V, Pawtowski A, Arrondel C, Sallinen SL, Saloranta C, Clericuzio C, Viot G, Tantau J, Blesson S, Cloarec S, Machet MC, Chitayat D, Thauvin C, Laurent N, Sampson JR, Bernstein JA, Clemenson A, Prieur F, Daniel L, Levy-Mozziconacci A, Lachlan K, Alessandri JL, Cartault F, Rivière JP, Picard N, Baumann C, Delezoide AL, Belar Ortega M, Chassaing N, Labrune P, Yu S, Firth H, Wellesley D, Bitzan M, Alfares A, Braverman N, Krogh L, Tolmie J, Gaspar H, Doray B, Majore S, Bonneau D, Triau S, Loirat C, David A, Bartholdi D, Peleg A, Brackman D, Stone R, DeBerardinis R, Corvol P, Michaud A, Antignac C, Gubler MC. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326. doi: 10.1002/humu.21661. [DOI] [PubMed] [Google Scholar]
- 23.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 24.Kendall RT, Strungs EG, Rachidi SM, Lee MH, El-Shewy HM, Luttrell DK, Janech MG, Luttrell LM. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1, Ile4, Ile8] angiotensin II regulates a robust G protein-independent signaling network. J Biol Chem. 2011;286:19880–19891. doi: 10.1074/jbc.M111.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basso N, Terragno NA. History about the discovery of the renin-angiotensin system. Hypertension. 2001;38:1246–1249. doi: 10.1161/hy1201.101214. [DOI] [PubMed] [Google Scholar]
- 26.Haulica I, Bild W, Serban DN. Angiotensin peptides and their pleiotropic actions. J Renin Angiotensin Aldosterone Syst. 2005;6:121–131. doi: 10.3317/jraas.2005.018. [DOI] [PubMed] [Google Scholar]
- 27.Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA. Angiotensin-(1–9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension. 2012;59:300–307. doi: 10.1161/HYPERTENSIONAHA.111.177485. [DOI] [PubMed] [Google Scholar]
- 28.Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, Saku K, Urata H, Dell'italia LJ, Husain A. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120:1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension. 2011;57:614–619. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 32.Michaud A, Bur D, Gribouval O, Muller L, Iturrioz X, Clemessy M, Gasc JM, Gubler MC, Corvol P. Loss-of-function point mutations associated with renal tubular dysgenesis provide insights about renin function and cellular trafficking. Hum Mol Genet. 2011;20:301–311. doi: 10.1093/hmg/ddq465. [DOI] [PubMed] [Google Scholar]
- 33.Walther T, Steendijk P, Westermann D, Hohmann C, Schulze K, Heringer-Walther S, Schultheiss HP, Tschöpe C. Angiotensin deficiency in mice leads to dilated cardiomyopathy. Eur J Pharmacol. 2004;493:161–165. doi: 10.1016/j.ejphar.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Xiao HD, Fuchs S, Frenzel K, Teng L, Li P, Shen XZ, Adams J, Zhao H, Keshelava GT, Bernstein KE, Cole JM. The use of knockout mouse technology to achieve tissue selective expression of angiotensin converting enzyme. J Mol Cell Cardiol. 2004;36:781–789. doi: 10.1016/j.yjmcc.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 35.de Resende MM, Stodola TJ, Greene AS. Role of the renin angiotensin system on bone marrow-derived stem cell function and its impact on skeletal muscle angiogenesis. Physiol Genomics. 2010;42:437–444. doi: 10.1152/physiolgenomics.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 37.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz A. Control of renin synthesis and secretion. Am J Hypertens. 2012;24:839–847. doi: 10.1038/ajh.2011.246. [DOI] [PubMed] [Google Scholar]
- 39.Matter H, Scheiper B, Steinhagen H, Böcskei Z, Fleury V, McCort G. Structure-based design and optimization of potent renin inhibitors on 5- or 7-azaindole-scaffolds. Bioorg Med Chem Lett. 2011;21:5487–5492. doi: 10.1016/j.bmcl.2011.06.112. [DOI] [PubMed] [Google Scholar]
- 40.McMurray JJ, Abraham WT, Dickstein K, Køber L, Massie BM, Krum H. Aliskiren, ALTITUDE, and the implications for ATMOSPHERE. Eur J Heart Fail. 2012;14:341–343. doi: 10.1093/eurjhf/hfs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal failure in mice with Gsalpha deletion in juxtaglomerular cells. Am J Nephrol. 2010;32:83–94. doi: 10.1159/000314635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujino M, Miura S, Kiya Y, Tominaga Y, Matsuo Y, Karnik SS, Saku K. A small difference in the molecular structure of angiotensin II receptor blockers induces AT1 receptor-dependent and -independent beneficial effects. Hypertens Res. 2010;33:1044–1052. doi: 10.1038/hr.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maione A, Navaneethan SD, Graziano G, Mitchell R, Johnson D, Mann JF, Gao P, Craig JC, Tognoni G, Perkovic V, Nicolucci A, De Cosmo S, Sasso A, Lamacchia O, Cignarelli M, Manfreda VM, Gentile G, Strippoli GF. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2011;26:2827–2847. doi: 10.1093/ndt/gfq792. [DOI] [PubMed] [Google Scholar]
- 46.Sparks MA, Parsons KK, Stegbauer J, Gurley SB, Vivekanandan-Giri A, Fortner CN, Snouwaert J, Raasch EW, Griffiths RC, Haystead TA, Le TH, Pennathur S, Koller B, Coffman TM. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57:577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stegbauer J, Gurley SB, Sparks MA, Woznowski M, Kohan DE, Yan M, Lehrich RW, Coffman TM. AT1 receptors in the collecting duct directly modulate the concentration of urine. J Am Soc Nephrol. 2011;22:2237–2246. doi: 10.1681/ASN.2010101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC., Jr Cardiorenal actions of TRV120027, a novel β-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- 49.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60:387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes R, Pulakat L, Miledi R, Martínez-Torres A. Mammalian AT2 receptors expressed in Xenopus laevis oocytes couple to endogenous chloride channels and stimulate germinal vesicle break down. Cell Physiol Biochem. 2009;24:45–52. doi: 10.1159/000227812. [DOI] [PubMed] [Google Scholar]
- 51.Funke-Kaiser H, Reinemund J, Steckelings UM, Unger T. Adapter proteins and promoter regulation of the angiotensin AT2 receptor – implications for cardiac pathophysiology. J Renin Angiotensin Aldosterone Syst. 2010;11:7–17. doi: 10.1177/1470320309343652. [DOI] [PubMed] [Google Scholar]
- 52.Kumar V, Knowle D, Gavini N, Pulakat L. Identification of the region of AT2 receptor needed for inhibition of the AT1 receptor-mediated inositol 1,4,5-triphosphate generation. FEBS Lett. 2002;532:379–386. doi: 10.1016/s0014-5793(02)03713-4. [DOI] [PubMed] [Google Scholar]
- 53.Pulakat L, Rahman S, Gray A, Knowle D, Gavini N. Roles of the intracellular regions of angiotensin II receptor AT2 in mediating reduction of intracellular cGMP levels. Cell Signal. 2005;17:395–404. doi: 10.1016/j.cellsig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues-Ferreira S, Nahmias C. An ATIPical family of angiotensin II AT2 receptor-interacting proteins. Trends Endocrinol Metab. 2010;21:684–690. doi: 10.1016/j.tem.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Qi Y, Li H, Shenoy V, Li Q, Wong F, Zhang L, Raizada MK, Sumners C, Katovich MJ. Moderate cardiac-selective overexpression of angiotensin II type 2 receptor protects cardiac functions from ischaemic injury. Exp Physiol. 2012;97:89–101. doi: 10.1113/expphysiol.2011.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steckelings UM, Paulis L, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens. 2012;21:142–146. doi: 10.1097/MNH.0b013e328350261b. [DOI] [PubMed] [Google Scholar]
- 57.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Díez-Freire C, Dooies A, Jun JY, Sriramula S, Mariappan N, Pourang D, Venugopal CS, Francis J, Reudelhuber T, Santos RA, Patel JM, Raizada MK, Katovich MJ. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010;182:1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kambayashi Y, Nagata K, Ichiki T, Inagami T. Insulin and insulin-like growth factors induce expression of angiotensin type-2 receptor in vascular-smooth-muscle cells. Eur J Biochem. 1996;239:558–565. doi: 10.1111/j.1432-1033.1996.0558u.x. [DOI] [PubMed] [Google Scholar]
- 59.Ichiki T, Kambayashi Y, Inagami T. Multiple growth factors modulate mRNA expression of angiotensin II type-2 receptor in R3T3 cells. Circ Res. 1995;77:1070–1076. doi: 10.1161/01.res.77.6.1070. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012;153:1411–1420. doi: 10.1210/en.2011-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Václavík J, Sedlák R, Plachy M, Navrátil K, Plásek J, Jarkovsky J, Václavík T, Husár R, Kociánová E, Táborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]