Abstract
Ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, is frequently used in pediatric general anesthesia. Accumulating evidence from animal experiments has demonstrated that ketamine causes neuronal cell death during the brain growth spurt. To elucidate the underlying mechanisms associated with ketamine-induced neuronal toxicity and search for approaches or agents to prevent ketamine’s adverse effects on the developing brain, a primary nerve cell culture system was utilized. Neurons harvested from the forebrain of newborn rats were maintained under normal control conditions or exposed to either ketamine (10µM) or ketamine plus l-carnitine (an antioxidant; 1–100µM) for 24h, followed by a 24-h withdrawal period. Ketamine exposure resulted in elevated NMDA receptor (NR1) expression, increased generation of reactive oxygen species (ROS) as indicated by higher levels of 8-oxoguanine production, and enhanced neuronal damage. Coadministration of l-carnitine significantly diminished ROS generation and provided near complete protection of neurons from ketamine-induced cell death. NMDA receptors regulate channels that are highly permeable to calcium, and calcium imaging data demonstrated that neurons exposed to ketamine had a significantly elevated amplitude of calcium influx and higher intracellular free calcium concentrations ([Ca2+]i) evoked by NMDA (50µM), compared with control neurons. These findings suggest that prolonged ketamine exposure produces an increase in NMDA receptor expression (compensatory upregulation), which allows for a higher/toxic influx of calcium into neurons once ketamine is removed from the system, leading to elevated ROS generation and neuronal cell death. l-Carnitine appears to be a promising agent in preventing or reversing ketamine’s toxic effects on neurons at an early developmental stage.
Key Words: ketamine, NMDA receptor, reactive oxygen species, calcium influx, l-carnitine
Ketamine, an FDA-approved anesthetic agent, is commonly used in general pediatric anesthesia because of its ability to produce a stable state of unconsciousness with minimal respiratory depression in comparison to other available agents. At clinically relevant concentrations, ketamine acts to block the N-methyl-d-aspartate (NMDA) receptor, a subtype of the glutamate receptor which is involved in a variety of processes including development and differentiation of the nervous system; learning and memory; and synaptic plasticity (Collingridge et al., 1983; D’Souza et al., 1993; Meldrum and Garthwaite, 1990; Muller et al., 1996). NMDA receptors are excitatory in neurons that play key roles in many physiological and pathological processes. These receptors are well-known mediators of neuronal cell death in numerous neuropathological conditions (Bubenikova-Valesova et al., 2008; Kari et al., 1978; Medeiros et al., 2011; Wakschlag et al., 2010).
NMDA receptors are heteromeric complexes composed of obligatory NR1 subunits as well as subunits from the NR2 subfamily (NR2A, NR2B, NR2C, and NR2D) or the NR3 subfamily (NR3A or NR3B) (Furukawa et al., 2005; Laube et al., 1998; Premkumar and Auerbach, 1997; Ulbrich and Isacoff, 2007). Various combinations of subunits generate a large number of different NMDA receptors with differing pharmacological and biological properties. NMDA receptors are a class of ion channel-forming receptors that are highly permeable to calcium. Cytosolic calcium is an important mediator of neuronal signal transduction, participating in diverse biochemical reactions that elicit changes in synaptic function, metabolic rate, and gene transcription. Upregulation of NMDA receptors can result in an excessive entry of calcium, triggering a series of cytoplasmic and nuclear processes such as loss of mitochondrial membrane potential, which ultimately results in neuronal cell death.
Several lines of evidence have demonstrated that ketamine causes neuronal cell death in important brain areas of experimental animals at an early developmental stage, e.g. during the brain growth spurt (Ikonomidou et al., 1999; Scallet et al., 2004; Slikker et al., 2007; Zou et al., 2009a). Apoptosis is a common mechanism of ketamine-induced neuronal cell death in rodents (Yon et al., 2005; Zou et al., 2009b). Previous work based on mRNA levels showed that NMDA receptor NR1 expression in ketamine-treated rat pup brains was significantly higher than in controls (Shi et al., 2010; Slikker et al., 2007; Zou et al., 2009b). This evidence suggests that upon removal of ketamine from the extracellular milieu, the now upregulated NMDA receptor population (compensatory regulation as a consequence of continued or prolonged NMDA receptor blockade) will “over” respond to normal levels of extracellular glutamate, resulting in glutamatergic excitotoxicity. However, several important questions remain unanswered.
First, direct evidence of ketamine-induced upregulation of NMDA receptors at the protein level is necessary for testing our working hypothesis. Previously published in situ hybridization and microarray data do provide partial support for this phenomenon (Shi et al., 2010; Slikker et al., 2007; Wang et al., 2005a, 2006).
Second, the interactions between altered ionotropic receptors and intracellular signaling, as evidenced by increases in intracellular calcium ([Ca2+]i) need to be demonstrated. Will enhanced Ca2+ flux associated with upregulated NMDA receptors (as a consequence of ketamine exposure) increase reactive oxygen species (ROS) generation and subsequent neuronal apoptosis?
Third, will antioxidants, e.g., l-carnitine mitigate or prevent the adverse effects of ketamine that occur at critical neurodevelopmental stages and provide a potential pathway toward the development of protective strategies for this anesthetic-induced neuronal damage that may have clinical relevance? The goal of the current project was to address these questions using a primary mixed neuronal-glial cell culture system.
MATERIALS AND METHODS
Drugs and other materials.
Ketamine hydrochloride (Ketaset) was purchased from Fort Dodge Animal Health (Fort Dodge, IA, USA) and diluted in Dulbecco’s Modified Eagle’s Medium (DMEM). Ketamine was identified and its purity confirmed using high-performance liquid chromatography and mass spectrometry (Slikker et al., 2007). DMEM and fetal bovine serum were obtained from Invitrogen (Grand Island, NY). l-Carnitine was purchased from Sigma (St Louis, MO).
Primary cell culture.
All animal procedures were approved by the National Center for Toxicological Research Institutional Animal Care and Use Committee and conducted in full accordance with the PHS Policy on Humane Care and Use of Laboratory Animals. Forebrain tissues were obtained from newborn rat pups (Wang et al., 2000, 2005b). Briefly, the brains were quickly removed and the forebrain portion was dissected and placed in ice-cold (4°C) Hank’s solution (without Mg2+ and Ca2+). The cells were then dissociated by mechanical trituration through a fire-polished Pasteur pipette. After centrifugation (200 × g for 10min), cells were washed with DMEM, distributed and grown on polylysine-coated coverslips in DMEM supplemented with 10% (vol/vol) fetal bovine serum. Glial proliferation was inhibited by a mitotic inhibitor, cytosine β-d-arabinofuranoside, beginning on day 3 of culture.
On day 5, cultures were exposed to ketamine (10µM) for 24h in the presence and absence of l-carnitine (1, 30, or 100µM). Cells were maintained in normal culture media for 7 days in the control cultures. Immunocytochemical staining, immunoblotting, calcium imaging, and potential neuronal toxic effects were evaluated 24h after ketamine washout using the various assays as described below.
Immunocytochemistry and nuclear staining.
A mouse monoclonal antibody to NR1 (1:100, BD Biosciences Pharmingen, San Diego, CA) was used to identify the NMDA receptor NR1 subunit on the cultured cells. A mouse monoclonal antibody to polysialic acid neural cell adhesion molecule (PSA-NCAM; 1:500; Miltenyi Biotec Inc., Auburn, CA) was used to identify neurons and a rabbit polyclonal antibody to glial fibrillary acidic protein (GFAP; 1:200, Dakopatts, Copenhagen, Denmark) was used to label glial cells (astrocytes). Also, a mouse monoclonal antibody to 8-oxoguanine (1:150, USBiological, Swampscott, MA) was used as a surrogate marker of oxidative DNA damage.
Briefly, cells were rinsed with PBS, fixed with ice-cold (4°C) 4% paraformaldehyde in 0.1M phosphate buffer and permeabilized with a solution of PBS/0.5% bovine serum albumin (BSA)/Triton X-100 for 1h. For double labeling, all primary antibodies were diluted in PBS/0.5% BSA solution. The cells were incubated with primary antibodies at 4°C overnight. Bound antibodies were revealed with fluorescein isothiocyanate-conjugated sheep anti-mouse IgG second antibody (1:80 diluted in PBS/0.5% BSA solution) and/or rhodamine-conjugated sheep anti-rabbit IgG secondary antibody (1:40 diluted in PBS/0.5% BSA solution). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), sealed with mounting medium and viewed using an Olympus FV1000 confocal microscope (Olympus, Center Valley, PA).
Western blotting.
NR1 expression at the protein level was evaluated using Western blot. The cells were lysed using a lysis buffer (1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS in normal PBS; pH 6.8) containing a protease inhibitor cocktail (10 µl/ml; Sigma-Aldrich). The homogenate was centrifuged at 14,000 × g for 10min at 4°C, and the supernatant was collected and stored at −80°C until assayed. The protein concentration was measured using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Equal amounts of protein (10 µg per lane) were loaded and run on SDS-polyacrylamide gels with a Tris-glycine running buffer system and then transferred to a polyvinylidene difluoride membrane (0.2 µm) in a Mini Electrotransfer Unit (Bio-Rad, Hercules, CA). The blots were subsequently probed with anti-NR1 (1:300, monoclonal, BD Biosciences Pharmingen), and antiactin (1:3000, monoclonal, Chemicon, Temecula, CA). Immunoblot analysis was performed with horseradish peroxidase (HRP) conjugated anti-mouse IgG using enhanced chemiluminescence Western blotting detection reagents (Amersham Bioscience, Piscataway, NJ). The bands corresponding to NR1 and β-actin were scanned and densitometrically analyzed using an automatic image analysis system (Alpha Innotech Corporation, San Leandro, CA). The ratios of densitometric value of NR1 to β-actin are presented as means ± SEM.
Single-cell gel electrophoresis (Comet assay).
To determine the level of DNA breakage produced by ketamine exposure, single-cell gel electrophoresis (Comet assay) was performed. Isolated neurons were suspended in 200 µl ice-cold PBS, then 50 µl of the cell suspension was mixed with 450 µl low melting point agarose (LMAgarose; Trevigen, Gaithersburg, MD) and warmed to 42°C. A 50-µl aliquot of the mixture was loaded onto a CometSlide to cool. The slide was subsequently incubated in ice-cold lysis solution (Trevigen) for 30min at 4°C and alkali buffer solution (0.6g NaOH and 100 µl 0.5M EDTA in 50ml purified water) for 30min at room temperature. After washing, the slide was placed in the electrophoresis chamber, electrophoresed, then removed from the apparatus, fixed, and air dried in the dark. The slide was examined using an Olympus FV1000 confocal microscope (Olympus) after the sample area was covered with 50 µl of 0.01% SYBR Green (Trevigen) in Tris-EDTA buffer. For quantitative purposes, 50 neurons per slide were imaged from three independent experiments.
HT 8-hydroxy-2′-deoxyguanosine ELISA.
8-Hydroxy-2′-deoxyguanosine (8-oxo-dG) and its analogs have been used as biomarkers of oxidative DNA damage and oxidative stress. To detect oxidative DNA damage and to test the effect of ketamine on neural cell survival, an 8-oxo-dG ELISA was utilized allowing for a determination of the levels of 8-oxo-dG and its analogs in the culture media, according to the manufacturer’s instructions (Trevigen). In brief, samples (culture media) were diluted 1:5 (vol/vol) in sample diluent. Fifty microliters of each sample were transferred to a 96-well precoated plate; then 50 µl immunoreagent mix was added and the mixture incubated for 1h at room temperature. After washing with wash buffer, anti-mouse IgG-HRP conjugate was placed into each well and incubated at room temperature for 1h. The incubation was stopped by washing, followed by incubation with 100 µl of the substrate for 15min. The reaction was terminated by the addition of 100 µl stop solution (phosphate buffer). A microplate reader was set at a wavelength of 450nm, and the absorbance was determined.
Cell death detection by ELISA.
A quantitative determination of cytosolic histone-associated DNA fragments (mono- and oligonucleosomes) was performed using a photometric enzyme-immunoassay to evaluate the rate of neuronal apoptosis (cell death detection ELISA, Roche Diagnostics Corporation, Indianapolis, IN). The cells were first lysed in 3ml of lysis buffer. After centrifugation to remove nuclei and other cellular debris, the supernatant, which contained the cytosolic low molecular mass and fragmented DNA, was diluted in the ratio 1:2 (vol/vol) with lysis buffer. Twenty microliters of each sample were transferred to a 96-well plate precoated with antihistone antibody, and 80 µl of immunoreagent mix was added. After incubation and washing, the chromogen 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) was placed in each well as a substrate. The intensity of the color that developed was measured using a plate reader (Bio-Tek Instruments, Winooski, VT) at 405nm, whereas a measurement at 490nm was used as a blank (reference wavelength).
Intracellular free calcium measurements.
Determination of cytosolic calcium concentrations was performed using a fluorescent calcium indicator (fura-2-acetoxymethyl [Fura-2 AM] [Life, Grand Island, NY]) which diffuses across the cell membrane and is deesterified by cellular esterases to yield Fura-2 free acid (Barreto-Chang and Dolmetsch, 2009). Cultures were loaded with Fura-2 AM (1µM) at 37°C for 30min in a dark incubator. Coverslips (polylysine coated) on which cultured neurons were mounted were placed in the imaging chamber for calcium measurements. Fura-2-loaded neurons were imaged using an inverted microscope (Olympus IX 81) with a 40× objective. Fluorescence excitations (340/380nm) were provided by a 75-W xenon arc lamp. Ratio images were obtained by acquiring pairs of images at alternate excitation wavelengths (340/380nm) and filtering the emission at 510nm. Image acquisition and processing were controlled by a computer connected to the camera and filter wheel (LAMBDA 10-3, Sutter Instrument Company, Novato, CA). The preparations were continuously superfused (1ml/min) at 37°C with the following modified Krebs-Ringer bicarbonate salt solution buffered with hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) and gassed with air (mM): 158 NaCl, 5.6 KCl, 2.5 CaCl2, and 5 NaHCO3; when not indicated, MgCl2 was 0.5; HEPES, 10.0; pH 7.4.
Statistical analyses.
The expression of NR1 NMDA receptor subunits and the changes in intracellular calcium concentrations in control and ketamine-treated neurons were analyzed using the Student’s t-test. A parametric one-way ANOVA with Tukey’s post hoc analysis for individual differences was used to compare the differences among multiple groups. The null hypothesis was rejected at a probability level of p < 0.05. All experiments were independently repeated at least three times.
RESULTS
Presence and Regulation of NMDA Receptor NR1 Subunit Protein on Cultured Neurons
The NMDA receptor is a pentameric transmembrane protein that is composed of different subunits (Moriyoshi et al., 1991; Paoletti and Neyton, 2007; Seeburg et al., 1995). The NR1 subunit is essential for function, whereas members of the NR2 subunit class, produced by four different genes (NR2A-D), can be considered modulatory (Moriyoshi et al., 1991; Seeburg et al., 1995). We first assayed for the presence and regulation of NR1 protein in cultured neurons using immunocytochemical analysis with a monoclonal NR1-specific antibody. Cells from a control culture (Fig. 1A) and a ketamine-exposed culture (Fig. 1B) were double-immunostained with antibodies to the NMDA receptor NR1 subunit protein and GFAP. NMDA receptor NR1 subunit protein is specifically expressed by neurons, whereas GFAP is an astrocyte-specific marker. NR1 immunoreactivity (green color) was localized specifically on the neurons (Figs. 1A and 1B). There was no NR1 signal on astrocytes which were counterstained using an antibody to GFAP (red color; Figs. 1A and 1B). Importantly, it was demonstrated that administration of 10µM ketamine (for 24h) caused a marked increase in immunoreactivity to NMDA receptor NR1 subunits on the neurons (green) (Fig. 1B) compared with control (Fig. 1A).
FIG. 1.
Immunostained micrographs from primary forebrain cultures. Cells from a control culture (A) and a ketamine-exposed culture (B) were double-immunostained with a mouse monoclonal antibody to NMDA receptor NR1 subunit protein (green) and a rabbit polyclonal antibody to GFAP (red; astrocytes). NR1 immunoreactivity (green) was localized specifically on neurons and the fluorescent density was remarkably upregulated in ketamine-exposed cultures. Scale bar = 50 µm. The NMDA receptor NR1 protein levels were also evaluated by Western blot analysis. A major protein band at about 130kDa was observed in both control and ketamine-exposed cultures (D). Ketamine administration produced a marked upregulation of the NR1 protein compared with controls. No significant difference was detected in GFAP expression levels (astrocytes) between control and ketamine-treated cultures. Densitometry measurements from three independent experiments were used to calculate a ratio of NMDA receptor NR1 protein to β-actin (C). The data are shown as means ± S.D. *p < 0.05 was considered significant compared with control.
The presence and regulation of NR1 protein was further assessed using Western blot analysis. A major protein band at about 130kDa was observed in both control and ketamine-exposed cultures (Fig. 1D). However, ketamine administration produced a remarkable upregulation of the NR1 protein levels compared with controls (Fig. 1D). Quantitative densitometry revealed that ketamine administration produced a significant increase (about twofold) in protein expression ratio of NR1 protein to β-actin. Here, β-actin was used as a loading or internal control (Fig. 1C).
Intracellular Calcium Concentration Measurements
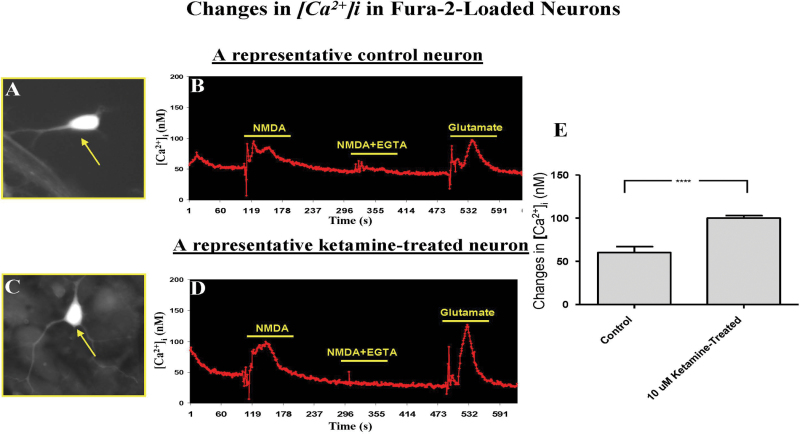
Because NMDA receptor-regulated ion channels are known to be highly permeable to calcium, we determined whether intracellular calcium concentrations could be altered by NMDA receptor subunit dysregulation that results from prolonged ketamine exposure. The application of NMDA to typical neurons (Figs. 2A and 2C) with glycine (100µM) in Mg2+-free medium produced an immediate elevation of intracellular free Ca2+ in both control and ketamine-exposed neurons. Ethylene glycol tetraacetic acid (EGTA) is a chelating agent with a high affinity for calcium and was used here to sequester extracellular calcium. To test whether NMDA produced its elevation of [Ca2+]i through the mobilization of Ca2+ from extracellular Ca2+ stores, NMDA was applied in the absence of extracellular Ca2+ (i.e., after chelation with EGTA; Figs. 2B and 2D). Under these conditions, no NMDA-evoked [Ca2+]i increase was observed. As a control, 25µM glutamate, a concentration known to preferentially stimulate NMDA receptors, was tested on the same cells without EGTA, and it produced a similar Ca2+ increase as observed previously with NMDA (Figs. 2B and 2D).
FIG. 2.
Dynamic changes in intracellular calcium concentrations [Ca2+]i of a control neuron (A) and a ketamine-exposed neuron (C). Application of NMDA (50µM) or glutamate (25µM) caused an immediate elevation in intracellular free Ca2+ for both control (B) and ketamine-exposed (D) neurons. No NMDA-evoked [Ca2+]i rise was observed when the extracellular Ca2+ was chelated and, thus, unavailable for intracellular transport (50µM NMDA + 200µM EGTA in the perfusion buffer). A significant increase in intracellular free calcium [Ca2+]i was detected in ketamine-exposed neurons (D and E) compared with control neurons (B and E) after NMDA (50µM) stimulation. Each condition was assessed at least in triplicate and experiments were repeated independently three times. Data are presented as means ± SD.
In addition, the ketamine-exposed neurons showed a significantly greater increase in intracellular free calcium [Ca2+]i than that seen in the neurons in control cultures (Fig. 2E). The change in intracellular calcium resulting from NMDA stimulation was approximately 40% higher in the neurons from ketamine-exposed cultures. Taken together, these observations are consistent with the view that prolonged/continuous exposure of developing neurons to NMDA antagonists such as ketamine causes compensatory upregulation of NMDA receptors, and activation of these altered NMDA receptors generates [Ca2+]i elevations that arise primarily from Ca2+ influx, not from release of intracellular stores.
Ketamine-Induced Oxidative Stress and Apoptotic Cell Death
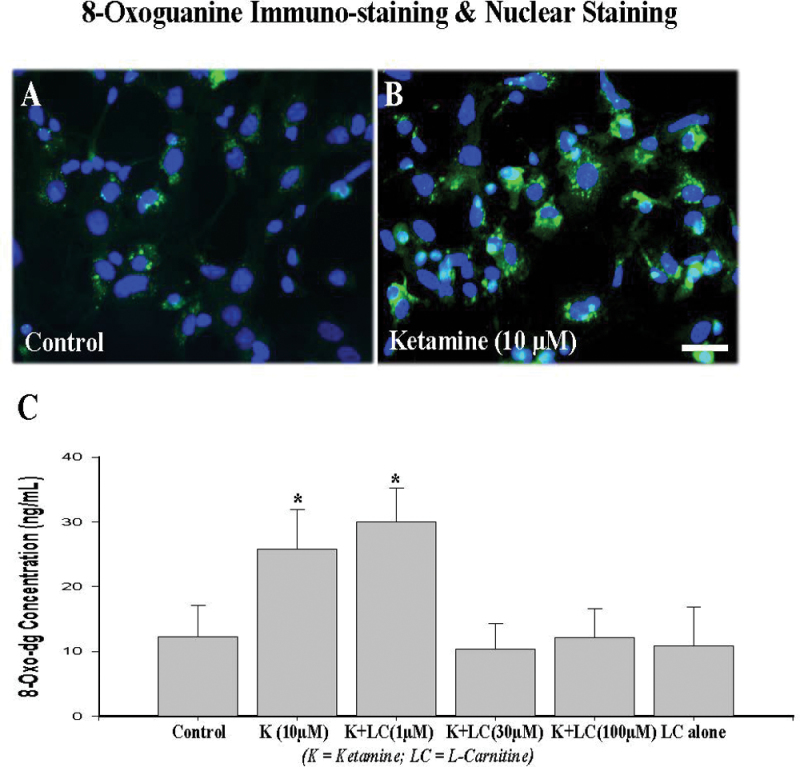
To examine whether the upregulation of NMDA receptor NR1 protein is associated with increased generation of ROS, 8-oxoguanine levels were evaluated immunocytochemically and using ELISA. 8-Oxoguanine is a mutagenic oxidative damage product of guanine. Oxidatively damaged 8-oxo guanine is generated in DNA due to the presence of ROS. To detect and localize oxidative DNA damage associated with ketamine administration, a monoclonal anti-8-oxoguanine antibody was utilized to detect 8-oxoguanine levels. Meanwhile, DAPI nuclear stain was used to reveal the total number of cells (in blue). Figure 3 demonstrates that ketamine administration at a concentration of 10µM increases 8-oxoguanine levels in both nuclei and mitochondria (Fig. 3B) compared with controls (Fig. 3A).
FIG. 3.
Immunohistochemical staining of oxidized DNA with 8-oxoguanine (green) and nuclear staining with DAPI (blue). A 24-h exposure to ketamine (10µM) markedly increased oxidative DNA damage as evidenced by increased 8-oxoguanine formation in ketamine-exposed cultures (B) compared with controls (A). Scale bar = 50 µm. An 8-oxo-dG ELISA (C) showed that coadministration of l-carnitine (30 or 100µM) effectively blocked the 8-oxo-dG increase induced by ketamine. No significant effects were observed when l-carnitine was administered alone. Each condition was assessed at least in triplicate and experiments were repeated independently three times. Data are presented as means ± SD. *p < 0.05 was considered significant compared with control.
l-Carnitine plays an integral role in attenuating the brain injury associated with mitochondrial neurodegenerative disorders (Zou et al., 2008). To investigate whether coadministration of l-carnitine can protect against ketamine-induced cell damage, cell cultures were incubated with ketamine alone, or ketamine (10µM) plus 1, 30, or 100µM l-carnitine.
An 8-oxo-dG ELISA is a fast and sensitive competitive immunoassay for the detection and quantitation of 8-oxoguanine. Figure 3C shows that enhanced oxidative stress was observed in the cultures exposed to 10µM ketamine as revealed by elevated levels of 8-oxoguanine, indicating more ROS generation, compared with controls. Coadministration of l-carnitine (30 or 100µM) significantly attenuated the elevation in 8-oxo-dG levels induced by ketamine. No significant protective effect was detected at the l-carnitine concentration of 1µM, and no significant effect was observed when l-carnitine was administered alone.
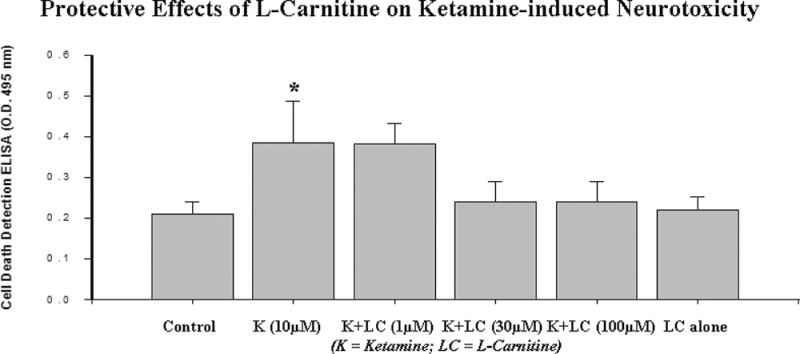
In concordance, the data shown in Figure 4 indicate that ketamine administration results in an increase in neuronal death as determined using an ELISA for histone-associated DNA fragmentation. The data from both the 8-oxo-dG ELISA (Fig. 3C) and the cell death ELISA (Fig. 4) indicate that ketamine-induced DNA damage and neurotoxicity were effectively prevented by the coadministration of 30 or 100µM l-carnitine. No significant effect was observed when l-carnitine was administered alone.
FIG. 4.
Effect of l-carnitine on ketamine-induced apoptotic cell death. Ketamine (10µM) exposure for 24h resulted in a significant increase in cell death as indicated by an ELISA for histone-associated DNA fragmentation. Coadministration of l-carnitine (30 or 100µM) effectively blocked the cell death induced by ketamine. No significant protective effect of l-carnitine was observed at a concentration of 1µM, and no significant neurotoxic effects were observed when l-carnitine was administered alone. Each condition was assessed at least in triplicate and the experiments were repeated independently three times. Data are presented as means ± SD. *p < 0.05 was considered significant compared with control.
Oxidative DNA Damage and Apoptotic Cell Death Evaluated by Comet Assay
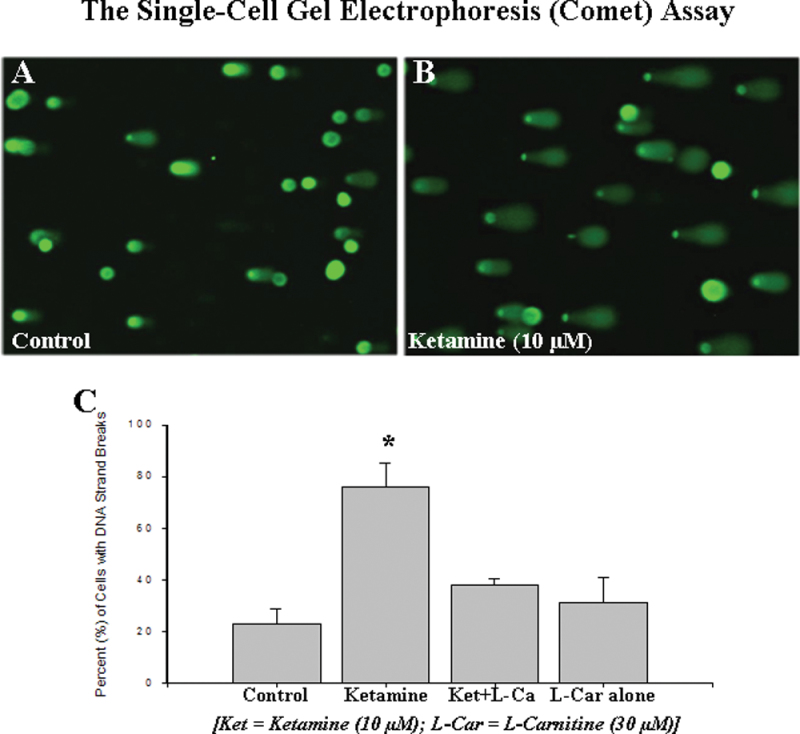
The single-cell gel electrophoresis (Comet) assay is a simple, rapid, and sensitive technique for analyzing DNA damage at the individual cell level and specifically for detecting DNA strand breaks induced by oxidative stimuli. To further investigate the severity of ketamine-induced neuronal damage and the potential protective properties of l-carnitine in ketamine-induced neuronal apoptosis, cells from forebrain cultures were exposed to either ketamine alone or ketamine plus l-carnitine. Twenty-four hours after the removal of ketamine, cultures were assessed using the Comet assay. A clear increase in the number of cells with DNA strand breaks (those having “tails”) was apparent in the cultures exposed to ketamine (Fig. 5B) compared with controls (Fig. 5A). However, coadministration of l-carnitine (30µM) effectively prevented the DNA damage/neurotoxicity induced by ketamine (Fig. 5C). The data from the 8-oxo-dG ELISA and Comet assay suggest that ketamine-induced DNA damage and neuronal apoptosis could result from elevated oxidative stress.
FIG. 5.
Single-cell gel electrophoresis (Comet) assay for rat primary neuronal cells. A marked increase in the number of cells with DNA strand breaks (tails) was apparent in the cultures exposed to ketamine (B) compared with control cultures (A). Quantitative analysis of the percent of cells exhibiting DNA strand breaks indicated a significant increase in DNA damage in ketamine-exposed neurons, but not in ketamine-exposed cells that were also treated with l-carnitine or in cells treated with l-carnitine alone. Experiments were repeated independently three times. Data are presented as means ± SD. *p < 0.05 was considered significant.
To better understand the nature of ketamine-induced neurotoxicity, PSA-NCAM (a neuron-specific marker) immunostained images were acquired from control and ketamine-treated (10µM) cultures. In the control cultures, PSA-NCAM immunoreactivity (Fig. 6A) was intense on the surface of cell bodies and processes of neurons. Figure 6B shows that ketamine exposure (24h) dramatically diminished PSA-NCAM immunoreactivity: PSA-NCAM expression was weak and characterized by typical cellular residue and fragmentation and neuronal shrinking.
FIG. 6.
Effect of ketamine on levels of PSA-NCAM (a neuron-specific marker). PSA-NCAM immunoreactivity was intense in control cultures (A) and diminished in ketamine-treated cultures (B). Scale bar = 50 µm.
DISCUSSION
The safety of anesthetic use in infants and children is an issue of major concern, and over the past 10 years, the use of ketamine, a noncompetitive NMDA receptor antagonist, in pediatric anesthesia has been extensively examined. Preclinical studies have shown that exposure of developing animals to ketamine can trigger neurodegeneration in several brain regions and cause persistent learning/memory deficits later in life (Paule et al., 2011; Scallet et al., 2004; Shi et al., 2010; Slikker et al., 2007; Yon et al., 2005; Zou et al., 2009b). Clinical studies also indicate that some learning disabilities and behavioral disturbances in children correlate with anesthetic exposure during surgery before 4 years of age, even in children experiencing only single exposures to anesthesia (Flick et al., 2011; Kalkman et al., 2009; Sprung et al., 2012; Wilder et al., 2009). To understand the relevant mechanisms underlying the etiology of the neurotoxicity associated with developmental exposure to ketamine, and presumably other NMDA receptor antagonists, a primary culture system was utilized in this study. This system provides a reliable, simple in vitro model for evaluating potential neurotoxic effects and allows for the investigation of cellular mechanisms, such as disturbed calcium homeostasis, which may be associated with ketamine-induced cell death. Although it is difficult to equate in vitro concentrations in rat cell cultures to human in vivo doses, the in vitro experimental conditions, approaches, and results are consistent with those from in vivo nonhuman primates (Slikker et al., 2007; Zou et al., 2009a) and represent a suitable platform for studying the mechanisms of anesthetic action on neuronal receptor channels. Thus, this study was designed to address how specific receptor subunits and intracellular signaling events may be involved in ketamine-induced neurotoxicity. We explored, at the cellular and functional receptor ion channel levels, the biochemical and molecular mechanisms associated with ketamine-induced neurotoxicity, particularly during sensitive developmental stages.
NMDA receptors constitute a subfamily of glutamate receptors identified by specific molecular composition and pharmacological and functional properties. NMDA receptors are densely localized on neurons of most major brain areas and are physically connected to proteins involved in cell-signaling cascades (Arundine et al., 2003; Arundine and Tymianski, 2003). Glutamate is the primary excitatory neurotransmitter in the central nervous system (CNS) of mammals. In addition to ionotropic receptors responsible for fast excitatory neurotransmission in the CNS, glutamate also activates a number of metabotropic glutamate (mGlu) receptors, which belong to the G-protein-coupled receptor family of receptors. Metabotropic glutamate receptor activation may lead to calcium release from internal store, and the roles of these receptors in neurodegeneration remain controversial (Baudry et al., 2012; Pellegrini-Giampietro, 2003; Vincent and Maiese, 2000; Zhu et al., 2004). Glutamate stimulates the opening of the channels that the ionotropic receptors regulate to enable the influx of various ions and excessive activation of NMDA-type glutamate receptors is implicated in the pathophysiology of several neurological conditions including hypoxia-ischemia and seizure-mediated excitotoxic damage, neuropathic pain, and opiate dependence. Exploring the mechanisms by which anesthetic agents might disturb NMDA receptor expression patterns should help identify avenues for protection or prevention of potential anesthetic-induced neuronal damage.
The experiments described here provide several lines of evidence demonstrating how prolonged ketamine exposure affects specific NMDA receptor subunit expression, altered receptor function, and elevated ROS generation, as well as subsequent enhanced neuronal damage. First, we provide direct evidence at the protein level that prolonged ketamine exposure causes a compensatory upregulation of the NMDA receptor NR1 subunit. The administration of noncompetitive NMDA receptor antagonists such as ketamine, phencyclidine (PCP), and MK-801 to rats during a critical period of development results in neurotoxicity/neurodegeneration in several major brain areas (Ikonomidou et al., 1999; Scallet et al., 2004). Previous studies have also shown that repeated administration of PCP to young rats results in a sensitized locomotor response when these animals are subjected to a later PCP challenge (Johnson et al., 1998). This sensitization is associated with apoptotic cell death and an increase in NMDA receptor NR1 subunit mRNA (Wang et al., 1999). Similarly, our previous in vivo data demonstrated that the expression of the NMDA receptor subunit gene, Grin1 (NR1), was significantly upregulated in ketamine-exposed rat pups as detected using in situ hybridization and microarray techniques (Shi et al., 2010). In addition, our previous work also showed altered expression levels of the NMDA receptor NR2 family, such as NR2A and NR2C (Shi et al., 2010), after repeated ketamine exposure. Taken together, previous and current mRNA and protein data clearly demonstrate that extended exposure of the developing mammal to NMDA receptor antagonists (e.g., ketamine) perturbs the endogenous NMDA receptor system and upregulates the expression of NMDA receptors, especially the essential NR1 subtype.
Second, we examined whether the upregulated NMDA receptor expression in ketamine exposed neurons is of functional significance. Here, it was demonstrated that ketamine exposure has a significant impact on intracellular Ca2+ homoeostasis: the amplitudes of calcium influx caused by activating concentrations of NMDA were significantly increased in neurons from ketamine-exposed cultures. It has been reported that local and global elevations in neuronal cytosolic calcium are important for a variety of physiological and pathological processes (Berridge, 1998). Here, we used calcium imaging techniques to investigate the potential interactions between NMDA-evoked calcium influx and/or NMDA receptor activation of mGlu receptors in the mediation of calcium signals in cultured neurons. In this study, NMDA-elicited increases in intracellular Ca2+ were blocked by perfusing cultures with Ca2+-free buffer (e.g., in the presence of EGTA), clearly demonstrating that the NMDA-evoked increases in intracellular calcium originated from an extracellular source, rather than from a depletion or release of calcium from the endoplasmic reticulum (intracellular calcium store).
It should be mentioned that a magnesium-free buffer (perfusion) was used in the calcium imaging study in order to minimize magnesium-blockade of NMDA receptor activation. Because neurons in frontal cortical cultures are known to contain other Ca2+ channels such as voltage-dependent Ca2+ channels and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors that are Ca2+ permeable (Nakanishi, 1992), it was not a surprise to see an even higher intracellular calcium concentration (Fig. 2) when the neurons were stimulated by glutamate. Together, these observations provide further support for the hypothesis that enhanced NMDA-type glutamate receptor expression (compensatory upregulation after prolonged NMDA receptor blockade) promotes the specific signal transduction (e.g., enhanced Ca2+ influx) that plays a critical role in ketamine-induced neurotoxicity.
Although calcium is necessary for cell growth, survival, and normal functioning, in excess it can be neurotoxic (Lynch and Guttmann, 2002). Ketamine, a PCP derivative, is an anesthetic agent with a well-defined effect on the NMDA receptor at clinical concentrations (Irifune et al., 1992). Racemic ketamine blocks the open channel by reducing mean channel open time and decreasing the frequency of channel opening by an allosteric mechanism while in the open state (Orser et al., 1997). In addition to the NMDA receptor, ketamine also affects other receptors including the AMPA- and kainate-type glutamate receptors and nicotinic and muscarinic acetylcholine receptors at concentrations necessary for anesthesia (Friederich et al., 2000; Sasaki et al., 2000). Although there seems to be a lack of stereoselectivity of ketamine on the above-mentioned receptors in hippocampal neurons (Brau et al., 1997; Durieux and Nietgen, 1997), the non–NMDA-type receptor systems are unlikely to account for the stereoselective effects. Although the exact mechanisms underlying its anesthetic action and associated neuronal damage are unknown, ketamine is considered to act primarily by a noncompetitive blockade of the NMDA receptor. Furthermore, our previous pharmacological data demonstrate that application of the AMPA/kainate receptor antagonist, CNQX, or nifedipine (an antagonist of the L-type voltage-sensitive calcium channel) does not produce a significant protective effect against ketamine-induced neuronal apoptosis, compared with the strong protective effect obtained with the selective NMDA antagonist, d-APV (Wang et al., 2005b, 2006). Also, coadministration of antisense oligonucleotides that specifically target NMDA receptor NR1 and NR2A subunit mRNAs is able to block the neuronal damage induced by ketamine or PCP (Wang et al., 2005a,b, 2006). These data clearly indicate a specific involvement of NMDA receptor-mediated excitation.
Third, associated with the increased Ca2+ influx seen in ketamine-exposed cultures was an increase in the generation of ROS that appear to originate in mitochondria. Recent evidence suggests that general anesthetics, administered at the peak of synaptogenesis, causes protracted injury to mitochondria including significant enlargement of mitochondria, impairment of their structural integrity and a decrease in their regional distribution (Sanchez et al., 2012). Along with morphological changes, the general anesthetic exposure also causes functional impairment of the immature neuronal mitochondria (Boscolo et al., 2012). Injured mitochondria could be a significant source of ROS (Boscolo et al., 2012). In this study, ketamine administration markedly elevated both nuclear and mitochondrial levels of 8-oxoguanine expression. The concordance between elevated 8-oxo-dG levels, enhanced DNA fragmentation and increases in the number of cells with DNA strand breaks following ketamine exposure, suggests key roles for calcium homeostasis and mitochondrial ROS production in inducing neuronal DNA damage and ketamine-induced cell death via apoptotic pathways.
It is becoming increasingly apparent that mitochondria lie at the center of the cell death regulation process (Hongpaisan et al., 2003, 2004; Waxman and Lynch, 2005). ROS generated by mitochondria are not just damaging by-products of respiration, but they are also important for cell signaling (Brookes and Darley-Usmar, 2002; Brookes et al., 2002). Calcium is one determinant of ultimate cell survival. However, excessive calcium sequestration by mitochondria can produce injury, leading to respiratory inhibition, uncoupling of oxidative phosphorylation, and ultimately to the release of cytochrome C from the mitochondrial membrane into the cytosol (Blaylock et al., 2010; Bosnjak et al., 2012; Zhang et al., 2010). Most of the mitochondrial effects of Ca2+ require its entry across the double membrane into the matrix. Although the mitochondrial outer membrane was thought to be permeable to Ca2+, recent studies suggest that the outer membrane voltage-dependent anion channel is a ruthenium red (RuRed) sensitive Ca2+ channel and serves to regulate Ca2+ entry into the mitochondrial intermembrane space (Gincel et al., 2001). In this study, to test whether mitochondrial function is regulated by disturbed Ca2+ influx, l-carnitine, an antioxidant dietary supplement, was utilized. l-Carnitine plays an integral role in attenuating brain injury associated with mitochondria-related oxidative stress (Zou et al., 2008). In this study, cells from forebrain cultures were exposed to ketamine or ketamine plus l-carnitine. After removal of ketamine, cultures were assayed using 8-oxo-dG and cell death detection ELISAs as well as the Comet assay. The data from all three of these assays indicated that aspects of ketamine-induced DNA damage and neurotoxicity can be effectively blocked by l-carnitine. The neuronal protective mechanism of l-carnitine is very complicated and still unclear. It may involve cell membrane stabilization, increased heat shock protein and superoxide dismutase production, and decreased expression of iNOS. Here, it is proposed that l-carnitine’s protective effects are likely due to its membrane modulatory effects, presumably by reducing ROS generation or increasing ROS scavenging, to preserve mitochondrial membrane integrity, a process thought to be downstream of ketamine-induced receptor alterations and disturbed Ca2+ homeostasis.
At the heart of understanding how Ca2+ can be both a physiological and a pathological regulator of mitochondrial function is the issue of how Ca2+ can modulate mitochondrial ROS generation. Several ROS, including superoxide anion (O2 −) and nitric oxide (NO), have been implicated in glutamate-induced neuronal death. However, little is known about the signaling pathway(s) that mediates the postulated roles of altered Ca2+ influx. Ca2+ could enhance ROS output by making the whole mitochondrion work faster and consume more O2. Indeed, mitochondrial ROS generation correlates well with metabolic rate (Perez-Campo et al., 1998; Sohal and Allen, 1985) suggesting that faster metabolism simply results in more leakage from the respiratory chain electron. In addition, Ca2+ stimulation of nitric oxide synthase (Alderton et al., 2001) generates NO, which inhibits complex IV (Cleeter et al., 1994), thus enhancing ROS generation. Importantly, several recent studies using blockers of oxidative stress such as melatonin (Yon et al., 2006), the superoxide dismutase mimetic, M40403 (Wang et al., 2003), the NOS inhibitor, 7-nitroindazole (Wang et al., 2008), hypothermia (Friedman, 2009), and EUK-134, a synthetic ROS scavenger, or R(+) pramipexole (PPX), a synthetic aminobenzothiazol derivative that restores mitochondrial integrity (Boscolo et al., 2012), have indicated that reduction of oxidative stress may protect the developing animal from anesthetic-induced brain cell death. In addition, Trolox, a ROS scavenger, has also been demonstrated to significantly attenuate ketamine-induced increases in ROS formation, caspase-3 activity, and cell damage (Bosnjak et al., 2012).
In summary, our experiments provide direct evidence that ketamine-induced neuronal cell death involves compensatory upregulation of the NMDA receptor, disturbed calcium influx, elevated ROS generation, and subsequent neuronal apoptosis. In addition, the antioxidant l-carnitine effectively attenuates ketamine’s toxic effects and serves as a potential protective agent.
Overall, these experimental results are consistent with our working hypothesis (Slikker et al., 2007; Wang et al., 2006) that continuous blockade of NMDA receptors by ketamine causes a compensatory upregulation of NMDA receptors and this upregulation makes neurons bearing these receptors more vulnerable, after ketamine withdrawal, to the excitotoxic effects of endogenous glutamate. Activation of upregulated NMDA receptors results in a calcium overload that exceeds the buffering capacity of the mitochondria and interferes with electron transport in a manner that results in an elevated production of ROS and subsequent neuronal damage including apoptosis.
FUNDING
The National Center for Toxicological Research (NCTR) /US Food and Drug Administration (FDA) Protocol E-7405; the Center for Drug Evaluation and Research (CDER)/FDA; the National Toxicology Program (NTP) Protocol E-7255/NIEHS; the National Institute of Child Health and Human Development (NICHD).
REFERENCES
- Alderton W. K., Cooper C. E., Knowles R. G. (2001). Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 357(Pt 3), 593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M., Sanelli T., Ping He B., Strong M. J. (2003). NMDA induces NOS 1 translocation to the cell membrane in NGF-differentiated PC 12 cells. Brain Res. 976, 149–158 [DOI] [PubMed] [Google Scholar]
- Arundine M., Tymianski M. (2003). Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34, 325–337 [DOI] [PubMed] [Google Scholar]
- Barreto-Chang O. L., Dolmetsch R. E. (2009). Calcium imaging of cortical neurons using Fura-2 AM. J. Vis. Exp, doi:10.3791/1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Greget R., Pernot F., Bouteiller J. M., Bi X. (2012). Roles of group I metabotropic glutamate receptors under physiological conditions and in neurodegeneration. WIREs Membr. Transp. Signal 1, 523–532 [Google Scholar]
- Berridge M. J. (1998). Neuronal calcium signaling. Neuron 21, 13–26 [DOI] [PubMed] [Google Scholar]
- Blaylock M., Engelhardt T., Bissonnette B. (2010). Fundamentals of neuronal apoptosis relevant to pediatric anesthesia. Paediatr. Anaesth. 20, 383–395 [DOI] [PubMed] [Google Scholar]
- Boscolo A., Starr J. A., Sanchez V., Lunardi N., DiGruccio M. R., Ori C., Erisir A., Trimmer P., Bennett J., Jevtovic-Todorovic V. (2012). The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: The importance of free oxygen radicals and mitochondrial integrity. Neurobiol. Dis. 45, 1031–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak Z. J., Yan Y., Canfield S., Muravyeva M. Y., Kikuchi C., Wells C. W., Corbett J. A., Bai X. (2012). Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr. Drug Saf. 7, 106–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brau M. E., Sander F., Vogel W., Hempelmann G. (1997). Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology 86, 394–404 [DOI] [PubMed] [Google Scholar]
- Brookes P., Darley-Usmar V. M. (2002). Hypothesis: The mitochondrial NO(*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: An alternative function for cytochrome C oxidase. Free Radic. Biol. Med. 32, 370–374 [DOI] [PubMed] [Google Scholar]
- Brookes P. S., Levonen A. L., Shiva S., Sarti P., Darley-Usmar V. M. (2002). Mitochondria: Regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 33, 755–764 [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V., Horácek J., Vrajová M., Höschl C. (2008). Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 32, 1014–1023 [DOI] [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. (1994). Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54 [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. (1983). Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. (Lond.) 334, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza S. W., McConnell S. E., Slater P., Barson A. J. (1993). Glycine site of the excitatory amino acid N-methyl-D-aspartate receptor in neonatal and adult brain. Arch. Dis. Child. 69, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux M. E., Nietgen G. W. (1997). Synergistic inhibition of muscarinic signaling by ketamine stereoisomers and the preservative benzethonium chloride. Anesthesiology 86, 1326–1333 [DOI] [PubMed] [Google Scholar]
- Flick R. P., Katusic S. K., Colligan R. C., Wilder R. T., Voigt R. G., Olson M. D., Sprung J., Weaver A. L., Schroeder D. R., Warner D. O. (2011). Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128, e1053–e1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich P., Dybek A., Urban B. W. (2000). Stereospecific interaction of ketamine with nicotinic acetylcholine receptors in human sympathetic ganglion-like SH-SY5Y cells. Anesthesiology 93, 818–824 [DOI] [PubMed] [Google Scholar]
- Friedman R. (2009). Hypothermia approach may avoid apoptosis from anesthesia in children. Neurol. Today 9, 19–20 [Google Scholar]
- Furukawa H., Singh S. K., Mancusso R., Gouaux E. (2005). Subunit arrangement and function in NMDA receptors. Nature 438, 185–192 [DOI] [PubMed] [Google Scholar]
- Gincel D., Zaid H., Shoshan-Barmatz V. (2001). Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mitochondrial function. Biochem. J. 358(Pt 1), 147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongpaisan J., Winters C. A., Andrews S. B. (2003). Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol. Cell. Neurosci. 24, 1103–1115 [DOI] [PubMed] [Google Scholar]
- Hongpaisan J., Winters C. A., Andrews S. B. (2004). Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J. Neurosci. 24, 10878–10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., Tenkova T. I., Stefovska V., Turski L., Olney J. W. (1999). Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74 [DOI] [PubMed] [Google Scholar]
- Irifune M., Shimizu T., Nomoto M., Fukuda T. (1992). Ketamine-induced anesthesia involves the N-methyl-D-aspartate receptor-channel complex in mice. Brain Res. 596, 1–9 [DOI] [PubMed] [Google Scholar]
- Johnson K. M., Phillips M., Wang C., Kevetter G. A. (1998). Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J. Neurosci. Res. 52, 709–722 [DOI] [PubMed] [Google Scholar]
- Kalkman C. J., Peelen L., Moons K. G., Veenhuizen M., Bruens M., Sinnema G., de Jong T. P. (2009). Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110, 805–812 [DOI] [PubMed] [Google Scholar]
- Kari H. P., Davidson P. P., Kohl H. H., Kochhar M. M. (1978). Effects of ketamine on brain monoamine levels in rats. Res. Commun. Chem. Pathol. Pharmacol. 20, 475–488 [PubMed] [Google Scholar]
- Laube B., Kuhse J., Betz H. (1998). Evidence for a tetrameric structure of recombinant NMDA receptors. J. Neurosci. 18, 2954–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. R., Guttmann R. P. (2002). Excitotoxicity: Perspectives based on N-methyl-D-aspartate receptor subtypes. J. Pharmacol. Exp. Ther. 300, 717–723 [DOI] [PubMed] [Google Scholar]
- Medeiros L. F., Rozisky J. R., de Souza A., Hidalgo M. P., Netto C. A., Caumo W., Battastini A. M., Torres I. L. (2011). Lifetime behavioural changes after exposure to anaesthetics in infant rats. Behav. Brain Res. 218, 51–56 [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. (1990). Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol. Sci. 11, 379–387 [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. (1991). Molecular cloning and characterization of the rat NMDA receptor. Nature 354, 31–37 [DOI] [PubMed] [Google Scholar]
- Muller D., Wang C., Skibo G., Toni N., Cremer H., Calaora V., Rougon G., Kiss J. Z. (1996). PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17, 413–422 [DOI] [PubMed] [Google Scholar]
- Nakanishi S. (1992). Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597–603 [DOI] [PubMed] [Google Scholar]
- Orser B. A., Pennefather P. S., MacDonald J. F. (1997). Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 86, 903–917 [DOI] [PubMed] [Google Scholar]
- Paoletti P., Neyton J. (2007). NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 7, 39–47 [DOI] [PubMed] [Google Scholar]
- Paule M. G., Li M., Allen R. R., Liu F., Zou X., Hotchkiss C., Hanig J. P., Patterson T. A., Slikker W., Jr, Wang C. (2011). Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol. Teratol. 33, 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E. (2003). The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol. Sci. 24, 461–470 [DOI] [PubMed] [Google Scholar]
- Perez-Campo R., López-Torres M., Cadenas S., Rojas C., Barja G. (1998). The rate of free radical production as a determinant of the rate of aging: Evidence from the comparative approach. J. Comp. Physiol. B 168, 149–158 [DOI] [PubMed] [Google Scholar]
- Premkumar L. S., Auerbach A. (1997). Stoichiometry of recombinant N-methyl-D-aspartate receptor channels inferred from single-channel current patterns. J. Gen. Physiol. 110, 485–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V., Feinstein S. D., Lunardi N., Joksovic P. M., Boscolo A., Todorovic S. M., Jevtovic-Todorovic V. (2012). General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology 115, 992–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Andoh T., Watanabe I., Kamiya Y., Itoh H., Higashi T., Matsuura T. (2000). Nonstereoselective inhibition of neuronal nicotinic acetylcholine receptors by ketamine isomers. Anesth. Analg. 91, 741–748 [DOI] [PubMed] [Google Scholar]
- Scallet A. C., Schmued L. C., Slikker W., Jr, Grunberg N., Faustino P. J., Davis H., Lester D., Pine P. S., Sistare F., Hanig J. P. (2004). Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol. Sci. 81, 364–370 [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Burnashev N., Köhr G., Kuner T., Sprengel R., Monyer H. (1995). The NMDA receptor channel: Molecular design of a coincidence detector. Recent Prog. Horm. Res. 50, 19–34 [DOI] [PubMed] [Google Scholar]
- Shi Q., Guo L., Patterson T. A., Dial S., Li Q., Sadovova N., Zhang X., Hanig J. P., Paule M. G., Slikker W., Jr, et al. (2010). Gene expression profiling in the developing rat brain exposed to ketamine. Neuroscience 166, 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W., Jr, Zou X., Hotchkiss C. E., Divine R. L., Sadovova N., Twaddle N. C., Doerge D. R., Scallet A. C., Patterson T. A., Hanig J. P., et al. (2007). Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol. Sci. 98, 145–158 [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Allen R. G. (1985). Relationship between metabolic rate, free radicals, differentiation and aging: A unified theory. Basic Life Sci. 35, 75–104 [DOI] [PubMed] [Google Scholar]
- Sprung J., Flick R. P., Katusic S. K., Colligan R. C., Barbaresi W. J., Bojanić K., Welch T. L., Olson M. D., Hanson A. C., Schroeder D. R., et al. (2012). Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin. Proc. 87, 120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich M. H., Isacoff E. Y. (2007). Subunit counting in membrane-bound proteins. Nat. Methods 4, 319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. M., Maiese K. (2000). The metabotropic glutamate system promotes neuronal survival through distinct pathways of programmed cell death. Exp. Neurol. 166, 65–82 [DOI] [PubMed] [Google Scholar]
- Wakschlag L. S., Kistner E. O., Pine D. S., Biesecker G., Pickett K. E., Skol A. D., Dukic V., Blair R. J., Leventhal B. L., Cox N. J., et al. (2010). Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol. Psychiatry 15, 928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Fridley J., Johnson K. M. (2005a). The role of NMDA receptor upregulation in phencyclidine-induced cortical apoptosis in organotypic culture. Biochem. Pharmacol. 69, 1373–1383 [DOI] [PubMed] [Google Scholar]
- Wang C., Kaufmann J. A., Sanchez-Ross M. G., Johnson K. M. (2000). Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J. Pharmacol. Exp. Ther. 294, 287–295 [PubMed] [Google Scholar]
- Wang C., McInnis J., West J. B., Bao J., Anastasio N., Guidry J. A., Ye Y., Salvemini D., Johnson K. M. (2003). Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J. Pharmacol. Exp. Ther. 304, 266–271 [DOI] [PubMed] [Google Scholar]
- Wang C., Sadovova N., Fu X., Schmued L., Scallet A., Hanig J., Slikker W. Jr. (2005b). The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience 132, 967–977 [DOI] [PubMed] [Google Scholar]
- Wang C., Sadovova N., Hotchkiss C., Fu X., Scallet A. C., Patterson T. A., Hanig J., Paule M. G., Slikker W., Jr (2006). Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol. Sci. 91, 192–201 [DOI] [PubMed] [Google Scholar]
- Wang C., Sadovova N., Patterson T. A., Zou X., Fu X., Hanig J. P., Paule M. G., Ali S. F., Zhang X., Slikker W., Jr (2008). Protective effects of 7-nitroindazole on ketamine-induced neurotoxicity in rat forebrain culture. Neurotoxicology 29, 613–620 [DOI] [PubMed] [Google Scholar]
- Wang C., Showalter V. M., Hillman G. R., Johnson K. M. (1999). Chronic phencyclidine increases NMDA receptor NR1 subunit mRNA in rat forebrain. J. Neurosci. Res. 55, 762–769 [DOI] [PubMed] [Google Scholar]
- Waxman E. A., Lynch D. R. (2005). N-methyl-D-aspartate receptor subtypes: Multiple roles in excitotoxicity and neurological disease. Neuroscientist 11, 37–49 [DOI] [PubMed] [Google Scholar]
- Wilder R. T., Flick R. P., Sprung J., Katusic S. K., Barbaresi W. J., Mickelson C., Gleich S. J., Schroeder D. R., Weaver A. L., Warner D. O. (2009). Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110, 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon J. H., Carter L. B., Reiter R. J., Jevtovic-Todorovic V. (2006). Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol. Dis. 21, 522–530 [DOI] [PubMed] [Google Scholar]
- Yon J. H., Daniel-Johnson J., Carter L. B., Jevtovic-Todorovic V. (2005). Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135, 815–827 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Dong Y., Wu X., Lu Y., Xu Z., Knapp A., Yue Y., Xu T., Xie Z. (2010). The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J. Biol. Chem. 285, 4025–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., DeCoster M. A., Bazan N. G. (2004). Interplay among platelet-activating factor, oxidative stress, and group I metabotropic glutamate receptors modulates neuronal survival. J. Neurosci. Res. 77, 525–531 [DOI] [PubMed] [Google Scholar]
- Zou X., Patterson T. A., Divine R. L., Sadovova N., Zhang X., Hanig J. P., Paule M. G., Slikker W., Jr, Wang C. (2009a). Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int. J. Dev. Neurosci. 27, 727–731 [DOI] [PubMed] [Google Scholar]
- Zou X., Patterson T. A., Sadovova N., Twaddle N. C., Doerge D. R., Zhang X., Fu X., Hanig J. P., Paule M. G., Slikker W., Jr, et al. (2009b). Potential neurotoxicity of ketamine in the developing rat brain. Toxicol. Sci. 108, 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Sadovova N., Patterson T. A., Divine R. L., Hotchkiss C. E., Ali S. F., Hanig J. P., Paule M. G., Slikker W., Jr, Wang C. (2008). The effects of L-carnitine on the combination of, inhalation anesthetic-induced developmental, neuronal apoptosis in the rat frontal cortex. Neuroscience 151, 1053–1065 [DOI] [PubMed] [Google Scholar]