Abstract
Each year ~1 billion kg of herbicides are used worldwide to control the unwanted growth of plants. In the United States, over a quarter of a billion kg of herbicides are used, representing 28% of worldwide use. (Kiely, T., Donaldson, D., and Grube, A. [2004]. Pesticide Industry Sales and Usage. 2000 and 2001 Market Estimates. Available at: http://www.epa.gov/pesticides/pestsales/01pestsales/market_estimates2001.pdf. Accessed October 25, 2012.) Propanil (3,4-dichloropropionanilide [DCPA]) is a commonly used herbicide in the United States, with 2–4 million kg applied annually to 2 million acres of crop land. The immunomodulatory effects of DCPA have been well documented, but limited data are available on the effects of its metabolites. (Salazar, K. D., Ustyugova, I. V., Brundage, K. M., Barnett, J. B., and Schafer, R. [2008]. A review of the immunotoxicity of the pesticide 3,4-dichloropropionanalide. J. Toxicol. Environ. Health B Crit. Rev. 11, 630–645.) In mammals, hepatic enzymes metabolize DCPA, resulting in the production of 3,4-dichloroaniline (DCA). Further biotransformation of DCA leads to the production of 6-hydroxy-3,4-dichloroaniline (6OH-DCA) and N-hydroxy-3,4-dichloroaniline (NOH-DCA). We report, for the first time, the immunotoxic effects of DCPA metabolites on T-cell function. Human Jurkat T cells were exposed to varying concentrations of DCPA or its metabolites and assayed for effects on T-cell function. In addition, fluorine analogs of DCPA and DCA were investigated to determine the relative role of chlorine substituents on T-cell immunotoxicity. Here we report that exposure of Jurkat T cells to DCPA and DCA alters IL-2 secretion, nuclear factor of activated T cells (NFAT) activity, and calcium influx. However, exposure to 6OH-DCA and NOH-DCA reduces IL-2 secretion and NFAT activity but has no effect on calcium flux. When both chlorines in DCPA and DCA were substituted with fluorines all effects were abrogated. Our data indicate that metabolites of DCPA have differential effects on T-cell function and the presence of chlorines plays an important role in eliciting these effects.
Key Words: T cells; 3,4-dichloropropionanilide; propanil; metabolites; 3,4-dichloroaniline; N-hydroxy-3,4-dichloroaniline; 6-hydroxy-3,4-dichloroaniline; fluorine substitution; DCA; DCPA
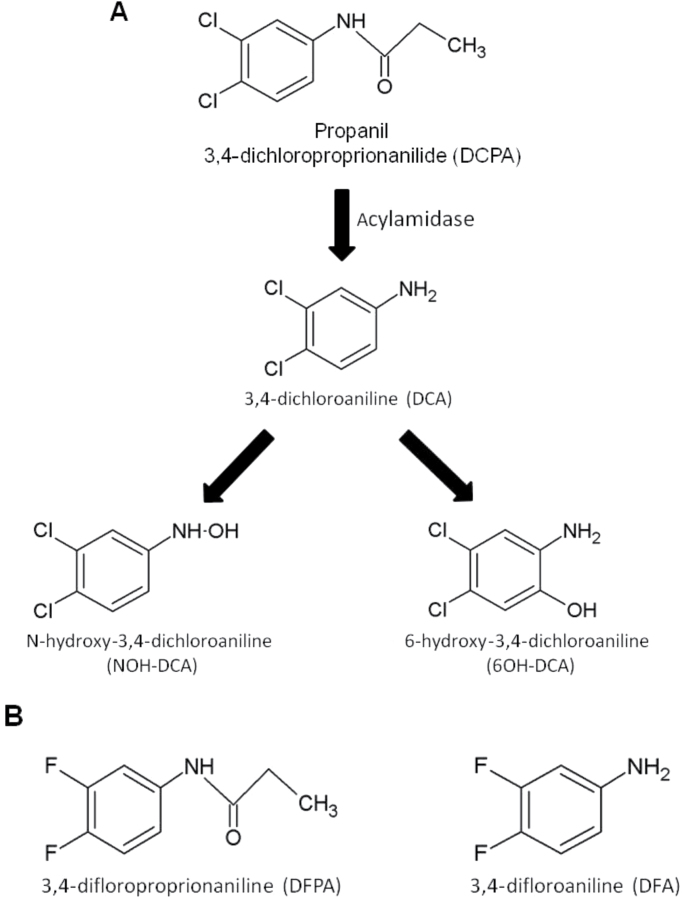
Approximately half a billion kg of pesticides are applied annually across the United States, with 15 of the top 25 most used pesticides falling under the class of herbicides (Kiely et al., 2004). Propanil (3,4-dichloropropionanilide [DCPA]) is a postemergent contact herbicide that is applied several times per crop season for control of broadleaf and grassy weeds. Annual use of DCPA is estimated to be 2–4 million kg per year and is distributed over 2 million acres of crop land, primarily rice fields. Rice plants are able to avoid the herbicidal effects of DCPA because they produce acylamidase, an enzyme that cleaves the DCPA amide bond, resulting in the production of two metabolites, 3,4-dichloroaniline (DCA) and propionic acid (Gaynor and Still, 1983). In mammals, DCPA can also be metabolized to DCA and propionic acid via hepatic acylamidases (McMillan et al., 1990a). Although propionic acid is nontoxic and quickly converts into carbon dioxide and water, limited data are available on the effects of DCA and its oxidative metabolites. DCA is a stable compound that persists in the environment with a predicted half-life of 1000 days (European Union Risk Assessment Report, 2006). DCA can undergo further transformation in the liver to produce the oxidative metabolites, N-hydroxy-3,4-dichloroaniline (NOH-DCA) and 6-hydroxy-3,4-dichloroaniline (6OH-DCA) (McMillan et al., 1990a) (Fig. 1A).
Fig. 1.
(A) Metabolic pathway and structures of DCPA and its metabolites in mammals and (B) structures of DFPA and DFA, fluorine analogs of DCPA and DCA.
Workers involved in manufacturing or handling (applicators) of DCPA are at greatest risk of exposure, but there have also been reports of non-applicator exposure (Richards et al., 2001). Numerous in vitro and in vivo studies have reported the immunomodulatory effects of exposure to DCPA and have been reviewed in Salazar et al. (2008). Exposure to DCPA alters cytokine production and the phagocytic ability of macrophages as well as the lytic function of CD8+ T cells after secondary stimulation. Other immunotoxic effects include decreased natural killer (NK) cell function and an increase in antibody secreting cells. Recent studies have also demonstrated that exposure of human Jurkat T cells to DCPA results in a concentration-dependent decrease in IL-2 production that is mediated by alterations in nuclear factor of activated T cells (NFAT) translocation and calcium (Ca2+) homeostasis (Lewis et al., 2008). Limited data are available on the toxic effects of DCA, NOH-DCA, and 6OH-DCA. It has been reported that DCA and NOH-DCA are toxic to the bladder, liver, and kidney of rats (Valentovic et al., 1995). DCA can also alter the male reproductive system and binds weakly to the androgen receptor, possibly acting as an endocrine disruptor (Bauer et al., 1998; Zhang and Lin, 2009). In a study examining occupational exposure to DCPA, several alterations in immune parameters were reported (Corsini et al., 2007). Agricultural workers exposed to DCPA had increased plasma IgG1 and IL-6 production in whole blood assays and decreased IL-10 and interferon-γ (IFN-γ), when compared with control subjects. In vitro assays demonstrated similar results and at molar equivalent concentrations, both DCPA and DCA inhibited IL-10 and IFN-γ production in enriched human CD4+CD8+ cells. In addition, alterations in calcium homeostasis and cytokine production were observed in anti-CD3 and phytohemagglutinin-stimulated peripheral blood mononuclear cells, suggesting that T cells may be sensitive to the effects of DCPA.
Using Jurkat T cells, we investigated the effects of DCA, NOH-DCA, 6OH-DCA, and the role of halogen substitution on T-cell function (Fig. 1). Pesticides containing chlorine substituents are widely used, with 45% of all pesticides introduced into the market after 1989 containing a chlorine-carbon bond (Crinnion, 2009). The addition of chlorine to many chemical compounds can increase its activity with negative biological consequences (Crinnion, 2009). DDT (dichlorodiphenyltrichloroethane) is a well-known organochlorine pesticide whose effects are mediated through the presence and positioning of two key chlorines. Although alterations in calcium-dependent signaling events have been reported in human Jurkat T cells exposed to DCPA, the effects of its metabolites in humans are unknown. We report that exposure of Jurkat T cells to DCA altered IL-2 secretion in a calcium-dependent manner but required higher concentrations than that observed with DCPA. In addition, NOH-DCA and 6OH-DCA are cytotoxic to T cells at lower concentrations than that of DCPA and DCA and inhibited IL-2 secretion in a calcium-independent manner. Finally, fluorine substitution of chlorines in DCPA and DCA (Fig. 1B) resulted in an abrogation of all effects indicating that the location of the chlorines is critical in eliciting immunotoxic effects. We report, for the first time, the adverse effects of DCA metabolites on T-cell function and advance our knowledge on the metabolic and structural effects of DCPA.
Materials and Methods
Cell lines and reagents.
Experiments were performed using the human T-cell leukemia cell line, Jurkat clone E6-1, obtained from the American Type Culture Collection (Manassas, VA). Jurkat cells were maintained in complete Roswell Park Memorial Institute media (RPMI 1640; Mediatech Inc., Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; vol/vol; Hyclone Inc., Logan, UT), 10 units/ml penicillin (BioWhittaker, Walkersville, MD), 100 µg/ml streptomycin (Bio Whittaker), 20mM glutamine (BioWhittaker), and 50 µM 2-mercaptoethanol (Sigma, St Louis, MO). The cultures were kept at 37°C in 5% CO2.
Stock solutions of DCPA (ChemServices, West Chester, PA), DCA (ChemServices), 3,4-difluoropropionanilide (DFPA), and 3,4-difluoroaniline (DFA; Sigma) were prepared by dissolving the compounds in absolute ethanol (AAPER Alcohol and Chemical Company, Shelbyville, KY). Vehicle control samples were treated with an equivalent concentration (0.1% vol/vol) of ethanol. The NOH-DCA and 6OH-DCA (a generous gift from G. Rankin) were dissolved in dimethyl sulphoxide (DMSO; Sigma) and vehicle control samples were treated with an equivalent concentration (0.1% vol/vol) of DMSO.
Synthesis of DFPA and NOH-DCA.
DFPA was synthesized from DFA (Sigma-Aldrich, Milwaukee, WI). Two grams of propanoic acid (Sigma-Aldrich) was added to 1g of DFA and heated to 100°C for 1h followed by addition of 5ml of water and continued heating at 100°C for an additional hour. The precipitate was cooled to room temperature, filtered through a sintered glass funnel, washed with water, and dried in vacuo resulting in 1.26g of DFPA. The crude DFPA was then recrystallized from a 1:1 water and ethanol solution.
NOH-DCA was synthesized by methods described by Lerman et al. (2005). In the presence of nitric acid, dichloromethane, and tetra-n-butylammonium bromide, 3,4-dichlorophenol was converted to 4,5-dichloro-2-nitrophenol, which was analyzed using nuclear magnetic resonance (NMR) and mass spectroscopy and was found to be in agreement with others (Lerman et al., 2005). Ethanol (100%) was added to 0.32g 4,5-dichloro-2-nitrophenol and 20mg platinum dioxide and hydrogenated (30–50 psi) on a Parr shaker for 1h. The mixture was filtered through celite and concentrated in vacuo to yield NOH-DCA.
Production and purity of DFPA, NOH-DCA, and the intermediate, 4,5-dichloro-2-nitrophenol were verified using NMR spectra from a Varian Unity-300 NMR spectrometer (Palo Alto, CA) and exact mass data were obtained using a Thermo-Fisher LTQ-FTICR and were in agreement with previously reported analytical data (Lerman et al., 2005; Lok et al., 1996).
The following concentrations of compounds were used for various assays: DCPA and DCA: 25, 50, 100, and 200µM; 6OH-DCA and NOH-DCA: 5, 25, 50, and 100µM; DFPA and DFA: 100, 200, and 400µM.
Viability assays.
Viability assays were performed for DCPA, DCA, NOH-DCA, 6OH-DCA, DFPA, DFA using 7-aminoactinomycin (7-AAD; BD Pharmingen, San Diego, CA), and following the manufacturer’s protocol. Briefly, 1.0 × 106 Jurkat cells were treated with or without varying concentrations of DCPA, DCA, NOH-DCA, 6OH-DCA, DFPA, DFA, and including ethanol and DMSO vehicle controls and incubated at 37°C in 5% CO2 for 24h. Cells were then incubated in PBS with 5 µl (0.25 µg) 7-AAD and incubated on ice for 20min in the dark. Cells were then washed and resuspended in 0.4% paraformaldehyde and analyzed by flow cytometry. Emission was detected in the FL-3 channel (> 650nm) using a FACSCalibur flow cytometer (Becton Dickson, Franklin Lakes, NJ).
Calcium fluorescence measurements.
Jurkat T cells were loaded with the calcium-indicator dye fluo-3 AM (Invitrogen, Carlsbad, CA) as previously described (Grynkiewicz et al., 1985). Briefly, cells were harvested and resuspended at a concentration of 5 × 106 cells/ml and incubated for 30min (37°C in 5% CO2) in complete RPMI media (1.5% FBS, vol/vol) containing 0.1µM fluo-3 AM in the presence of 0.02% pluronic F-127 (Invitrogen) and 2.5mM probenecid (Invitrogen). Cells were washed twice in Ca2+- and Mg2+-free Hanks Balanced Salt Solution (Mediatech Inc.) containing 10mM N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid, pH 7.4, 2% FBS and 2.5mM probenecid, resuspended to a concentration of 1 × 106 cells/ml and incubated 30min at room temperature. Because the addition of 2% FBS is essential for cell viability, the media contains a nominal concentration of Ca2+ (2.5µM). Samples were kept at room temperature and protected from light until ready for analysis. For each sample, 2 × 106 cells were placed in a quartz cuvette and the fluorescence was measured using a PTI QM-2000-4 spectrofluorometer (Photon Technology International [PTI], Birmingham, NJ) with constant stirring. The fluorescence of the fluo-3 dye was measured with excitation at 490nm and emission at 525nm. The fluorescence was measured and digitized at 1 Hz using the software program FeliX 1.42b (PTI). Data points were collected every second and cells were either treated with vehicle control, DCPA, its metabolites or analogs and 2µM thapsigargin (Sigma) or 2µM thapsigargin alone. Reagents were added after baseline data were collected for 45 s. Following the return of fluorescence to background levels, 2.5mM CaCl2 (Sigma) was added to the media. Addition of 200µM ionomycin (Sigma) ensured even loading of the cells. Cell membranes were lysed with 0.1% (vol/vol) Triton X-100 (Fisher Scientific, Hampton, NH) to measure the maximum fluorescence (F max) parameter for calculation of [Ca2+]i and to monitor compartmentalization of the dye. To chelate the free Ca2+ to a nominally Ca2+-free level (F min), 50mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt (EGTA), pH 7.5 (Sigma) was added to the cuvette.
Fluorescence values were converted to [Ca2+]i using the following equation:
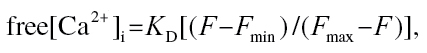 |
where K D (360nM) is the dissociation constant of the fluo-3/Ca2+ complex, F is the measured fluorescence intensity, F min is the minimum fluorescence at very low [Ca2+]i (fluorescence after the addition of 50mM EGTA), and F max is the fluorescence measured at high [Ca2+]i (fluorescence after the addition of Triton X-100) (Grynkiewicz et al., 1985). The background fluorescence obtained from unloaded cells over a 3-min time period was subtracted from all data points before [Ca2+]i was calculated.
Measurement of IL-2 production.
Jurkat cells were cultured in complete RPMI media at 5 × 105 cells/well in 48-well plates (Costar, Corning, NY) coated with mouse antihuman CD3 antibody (10 µg/ml) (BD Biosciences, San Diego, CA). Cells were treated with varying concentrations of DCPA, DCA, DFPA, DFA, NOH-DCA, 6OH-DCA, ethanol, or DMSO vehicle control. Cells were also simultaneously stimulated with anti-CD28 antibody (2 µg/ml) (BD Biosciences). Cells were incubated at 37°C in 5% CO2 for 24h, after which supernatants were collected and placed at −20°C. IL-2 production was determined using the sandwich ELISA method and following the manufacturer’s protocol (BD Pharmingen). All cultures and ELISA analyses were performed in triplicate and the experiment was repeated at minimum of three times. IC50 for the metabolites based on IL-2 production was determined from linear regression analysis employing JMP v 8.0.1 software (SAS Institute Inc., Cary, NC).
Transfections and measurement of NFAT activity by luciferase assay.
Jurkat cells were plated in RPMI media with 1.5% FBS at 6 × 105 cells/well in a 6-well plate (Costar). For each well, 750ng of pNFAT-luc (firefly luciferase plasmid; Stratagene, Wilmington, DE) and 10ng pRL-TK (Renilla luciferase plasmid; Promega, Madison, WI) were transfected with 1 µl Lipofectamine 2000 (Invitrogen). Cells were incubated for 5h at 37°C in 5% CO2 and media was then replaced with complete RPMI + 10% FBS and incubated over night at 37°C in 5% CO2. Following transfection, cells were treated with or without varying concentrations of DCPA, DCA, DFPA, DFA, NOH-DCA, 6OH-DCA, ethanol, or DMSO and stimulated with antihuman CD3 (10 µg/ml; BD Biosciences) and antihuman CD28 (2 µg/ml; BD Biosciences). In addition, robust experimental T-cell stimulation was achieved using phorbol 12-myristate 13-acetate (10ng/ml PMA; Sigma) and calcium ionophore A23187 (1 µg/ml). Cells were incubated for 4h at 37°C in 5% CO2, centrifuged, lysed, and stored at −70°C until ready for analysis. NFAT activity was determined using the Dual Luciferase Assay Kit and following the manufacturer’s protocol (Promega). Briefly, 25 µl of lysate was added to 100 µl of Luciferase Assay Reagent II and the firefly luciferase activity measured. Addition of Stop & Glo quenched the firefly luminescence and provided a substrate for the Renilla luciferase activity. Luminescence was detected using a Berthold Lumat LB 9507 (Berthold Technologies, Oak Ridge, TN). NFAT-firefly luciferase transfection efficiency was normalized to the Renilla luciferase activity and the percent change compared with the vehicle control was reported. All luciferase assays were performed in triplicate and the experiment was repeated at least twice.
Statistical analyses.
Cell viability, IL-2 secretion, and NFAT expression data were analyzed using MS Excel 2007 (Redmond, WA) and Sigma Stat 3.1 (Port Richmond, CA). ANOVA with a Student-Newman-Keuls post hoc test was used to determine statistical significance with a p value of < 0.05 considered significant. Calcium fluorescence was quantified using the area under the curve (AUC) from the time point when external calcium was added, until unified length of time, on at least three experiments completed at three independent replicates/times. The statistical analysis of the effect of DCPA, DCA, 6OH-DCA, NOH-DCA, DFPA, DFA at their treatment levels (independent variable) on Ca2+ fluorescence curves—AUC (dependent variable) was done using polynomial orthogonal contrasts applied in PROC GLM of SAS (Statistical Analysis System vs. 9.2, Cary, NC). This allowed us to indirectly test if there is a linear, quadratic, or cubic relationship of the dose of the herbicide or its metabolites on the Ca2+ influx following intracellular Ca2+ depletion. For instance, if linear effect is significant (p < 0.05) with negative slope of the line, we can conclude that the Ca2+ influx in Jurkat cells is linearly decreasing with respect to increasing concentration of the particular herbicide. In addition, ANOVA followed by a Student-Newman-Keuls multiple comparison test was used on AUC calcium influx data to depict the differences between the treatment levels, with alpha of 0.05.
Results
Hydroxylated Metabolites of DCPA Are More Cytotoxic Than the Parent Compound
Metabolism of DCPA is reported to occur in the liver through the action of acylamidases, which cleave the amide side chain, resulting in the production of DCA (McMillan et al., 1990a). Several studies have also reported detectable levels of DCA in the blood and urine of DCPA-exposed individuals (Roberts et al., 2009). In mammals, hydroxylation of DCA to 6OH-DCA and NOH-DCA also occurs in the liver and both oxidative metabolites are capable of converting oxyhemoglobin (Hb) to metHb. In order to assess the cytotoxicity of DCPA and its metabolites on Jurkat T cells, viability and proliferation assays were conducted over a range of concentrations. Cells exposed to DCPA, DCA, DFPA, and DFA required the use of ethanol (0.1% vol/vol) as a vehicle control and DMSO (0.1% vol/vol) was used as a vehicle control for 6OH-DCA and NOH-DCA. Jurkat T cells were treated or left untreated, loaded with 7-AAD and viability was assessed after 24h (Supplementary data). The viability of cells, treated with increasing concentrations of DCPA, did not decrease up to 100µM DCPA (Supplementary fig. 1). Treatment with 200µM DCPA decreased viability by ~14.6% (Supplementary fig. 1A) and subsequent studies were conducted using a maximum concentration of 100µM of DCPA. DCA appeared to be less toxic and concentrations up to 200µM were not cytotoxic (Supplementary fig. 1B). Jurkat T cells were more sensitive to the hydroxylated DCA metabolites and cytoxicity was observed at 100µM for both 6OH-DCA (Supplementary fig. 1C) and NOH-DCA (Supplementary fig. 1D). Proliferation assays were also conducted on Jurkat T cells and inhibition was observed only at those concentrations that were also cytotoxic (data not shown).
DCPA and Its Metabolites Decrease IL-2 Secretion
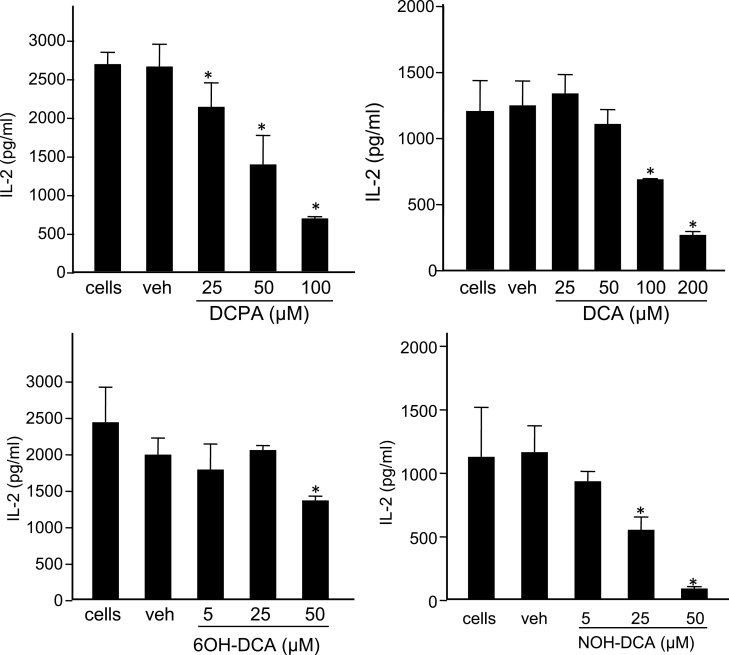
We have previously reported that exposure to DCPA decreased IL-2 production in Jurkat T cells in a calcium-dependent manner (Lewis et al., 2008). IL-2 is an important early cytokine produced by T cells and is critical for the activation, differentiation, and proliferation of several immune cells, including NK cells, T cells, and B cells. The effects of DCPA metabolites on T-cell function are unknown. To determine if exposure to DCPA metabolites alter IL-2 production, Jurkat T cells were treated with increasing concentrations, with or without vehicle controls (DMSO for the hydroxylated metabolites and ethanol for all others), and stimulated with anti-CD3 and anti-CD28 antibodies. After 24h in culture IL-2 secretion levels in the supernatant were assessed using an ELISA. DCPA decreased IL-2 secretion in a concentration-dependent manner with significant decreases compared with vehicle control observed at 25, 50, and 100µM DCPA and representing a 20, 48, and 74% decrease in IL-2 production, respectively (Fig. 2A). These data are in agreement with previously reported decreases of IL-2 in response to DCPA treatment (Lewis et al., 2008).
Fig. 2.
DCPA and its metabolites inhibit IL-2 secretion. Jurkat T cells were treated with (A) DCPA, (B) DCA, (C) 6OH-DCA, (D) NOH-DCA in the concentrations indicated on the figure. Additional treatment groups included a vehicle (veh) control and a no treatment (labeled “cells”). All experimental groups were stimulated with anti-CD3 and anti-CD28 for 24h as described in the Materials and Methods section. Supernatants were analyzed by standard ELISA methods to quantitate IL-2 secretion. Graphs are representative of three separate experiments, each performed in triplicate wells. Error bars reflect ± SEM and asterisks (*) indicate statistically significant results for all experimental units, p < 0.05 using ANOVA with a Student-Newman-Keuls post hoc test.
When Jurkat T cells were exposed to increasing concentrations of DCA (25–200µM), concentration-dependent decreases in IL-2 secretion were also observed (Fig. 2B). A trend of decreasing IL-2 production is observed at a concentration of 50µM with statistically significant decreases at 100 and 200µM DCA (Fig. 2B). Exposure to 50, 100, and 200µM DCA results in a 10, 45, and 78% decrease in IL-2, respectively. In addition, IC50 values for DCPA and DCA were calculated as 70.3 and 123µM, respectively. These results suggest that, in T cells, DCPA is more potent than its metabolite DCA.
Because NOH-DCA and 6OH-DCA are cytotoxic at 100µM in Jurkat T cells, lower concentrations were evaluated to assess the effects on IL-2 secretion. Both 6OH-DCA and NOH-DCA decreased IL-2 secretion; however, NOH-DCA is a more potent inhibitor of IL-2 (Figs. 2C and 2D). IL-2 production was decreased by 30% at the highest concentration of 6OH-DCA (50µM), whereas exposure to 25 and 50µM NOH-DCA significantly decreased IL-2 secretion by 51 and 90%, respectively. No change in IL-2 secretion was observed in cells treated with 5µM NOH-DCA. The IC50 value for NOH-DCA was 24.7µM but the IC50 for 6OH-DCA was not determined, as it would be greater than 50µM (133.2µM by extrapolation) and toxic to the cells. These data indicate that Jurkat T cells are highly sensitive when exposed to NOH-DCA and greatly reduced IL-2 production.
DCPA and Its Metabolites Alter NFAT Expression
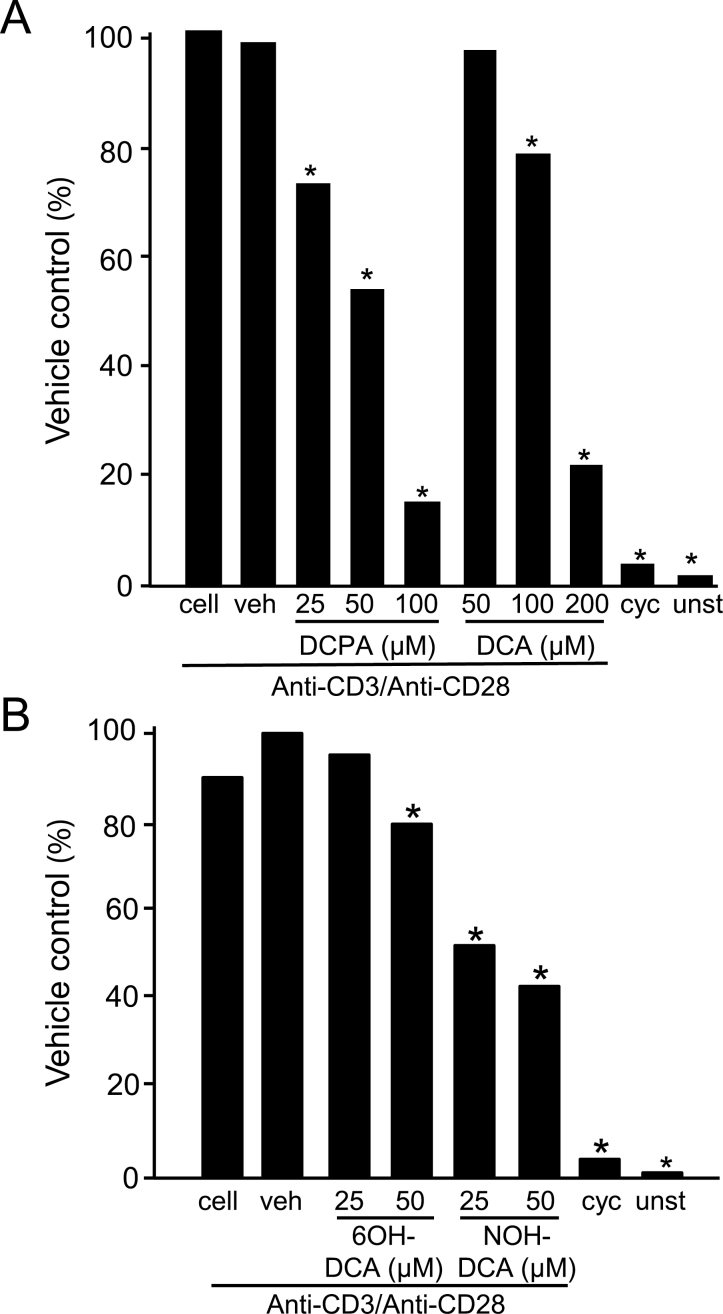
In T cells, optimal transcription of the IL-2 gene requires the coordinated binding of three transcription factors; NFAT, NF-κB, and activator protein-1 (AP-1; Jain et al., 1995). AP-1 is a heterodimer that includes c-fos and c-jun members and has been previously shown that exposure of Jurkat cells to DCPA decreases AP-1 binding ability and c-jun protein and phosphorylation levels (Brundage et al., 2004). To further understand the effects of DCPA and its metabolites on IL-2 secretion and to advance our previous studies (Lewis et al., 2008), we investigated the effects of DCPA and its metabolites on NFAT due to its direct dependence on intracellular calcium. Jurkat T cells were cotransfected with a NFAT luciferase plasmid and a Renilla luciferase plasmid that was used as a control for transfection efficiency. In cells exposed to 25, 50, and 100µM DCPA, and stimulated with antihuman CD3 and CD28 antibodies, we found a significant decrease in NFAT expression compared with vehicle control (Fig. 3A). A decrease of 26, 45, and 84% was observed in cells exposed to 25, 50, and 100µM DCPA, respectively. Cells treated with DCA and stimulated with antihuman CD3 and CD28 antibodies also decreased NFAT expression although significant decreases of 20 and 87% were only observed at the 100 and 200µM, respectively (Fig. 3A).
Fig. 3.
DCPA and its metabolite alter NFAT expression. Jurkat T cells were cotransfected with a firefly luciferase NFAT reporter plasmid and a Renilla luciferase reporter plasmid to control for transfection efficiency. Cells were treated with varying concentrations of (A) DCPA and DCA; (B) 6OH-DCA and NOH-DCA or vehicle (veh) as indicated on the figure. Unstimulated (unst) and cyclosporine A (cyc)-treated cells were included as controls. All groups were stimulated with anti-CD3 and anti-CD28 for 4h as described in the Materials and Methods section. Cell lysates were analyzed for luciferase activity and normalized to Renilla activity. Results are representative of at least two experiments each treatment performed in triplicate wells. Asterisks (*) indicate statistically significant results, p < 0.05 using ANOVA with a Student-Newman-Keuls post hoc test.
When we treated Jurkat cells with varying concentrations NOH-DCA and 6OH-DCA and stimulated with antihuman CD3 and CD28 antibodies there was a significant decrease in NFAT expression only at 50µM of 6OH-DCA (30% decrease) but at 25 and 50µM of NOH-DCA NFAT expression was reduced by 48 and 54%, respectively (Fig. 3B).
In addition to stimulation of Jurkat T cells with antihuman CD3 and CD28 antibodies, which mimics the in vivo T-cell activation, cells were also robustly stimulated with PMA/A23187, finding similar significant reduction of NFAT expression (Supplementary fig. 2). Specifically, there was a 37, 63, and 87% decrease in NFAT expression in cells treated with 25, 50, and 100µM DCPA, respectively. Similarly, cells treated with DCA and stimulated with PMA/A23187 also had decreases in NFAT expression (Supplementary fig. 2A) at the same concentrations that caused decreased IL-2 secretion (Fig. 2B). PMA/A23187-stimulated cells treated with 6OH-DCA had a 30% decrease in NFAT expression at 50µM with no change at 25µM (Supplementary fig. 2B). NOH-DCA decreased NFAT expression by 33 and 47% at 25 and 50µM, respectively (Supplementary fig. 2B). All experiments, under both stimulation conditions, included cells treated with cyclosporine A, an inhibitor of NFAT that prevents dephosphorylation, and produced similar levels of NFAT as those in unstimulated cells. These data indicate that DCPA and its metabolites inhibit NFAT expression.
DCPA and Its Metabolites Have Differential Effects on Ca2+ Homeostasis
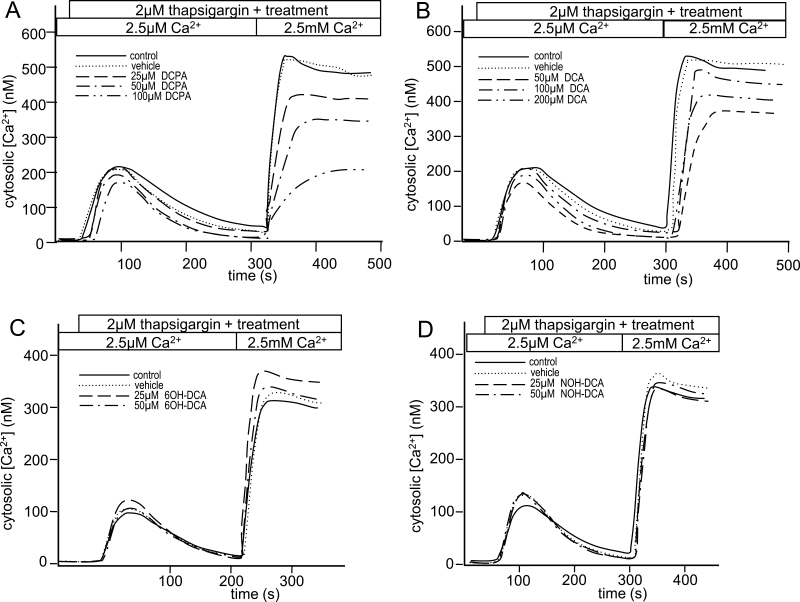
It has been previously reported that exposure of Jurkat T cells to DCPA results in decreased intracellular calcium influx following endoplasmic reticulum (ER) Ca2+ store depletion (Lewis et al., 2008). To examine the effect of DCPA metabolites on calcium homeostasis, Jurkat T cells were loaded with a calcium-sensitive dye and changes in intracellular calcium were monitored over time. At the start of these experiments, depletion of ER Ca2+ stores with thapsigargin, an inhibitor of the Sarco/ER Ca2+ ATPase pump, results in a small, transient increase in intracellular Ca2+ (Fig. 4). Addition of 2mM Ca2+ to the media results in a large and sustained increase in intracellular Ca2+ and reflects store-operated calcium influx (Fig. 4). In agreement with previous reports (Lewis et al., 2008), Jurkat T cells exposed to DCPA showed decreased calcium influx following store depletion in a concentration and linear manner (Fig. 4A). Specifically, calcium influx was significantly lower at 100µM of DCPA than at vehicle control and 25µM DCPA. Similarly, a linear decrease in calcium influx occurred with increased doses of DCA, with a significant decrease at 200µM when compared with vehicle control and 50µM (Fig. 4B). It should be noted that DCPA and DCA-treated cells do not alter the transient increase in intracellular calcium observed when ER calcium stores are depleted. Interestingly, cells treated with 6OH-DCA and NOH-DCA did not inhibit ER calcium store depletion or calcium influx (Figs. 4C and 4D), as demonstrated by lack of linear, quadratic, or cubic relationship between the dose and Ca2+ influx for both 6OH-DCA and NOH-DCA. These data support the conclusion that the hydroxylated metabolites alter IL-2 secretion through a different mechanism than DCPA and DCA.
Fig. 4.
Differential effects of DCPA and its metabolites on calcium influx. Jurkat T cells were loaded with the calcium-sensitive dye, fluo-3, and treated with (A) DCPA, (B) DCA, (C) 6OH-DCA, (D) NOH-DCA at concentrations indicated on the figure. Additional treatment groups included a vehicle (veh) control and a no treatment control (labeled “control”). Following addition of the indicated treatment, 2µM thapsigargin was immediately added to deplete ER Ca2+ stores. When fluorescence returned to baseline, 2mM CaCl2 was added and the effect on Ca2+ influx was recorded. Graphs are representative of at least three experiments, and statistical analysis was done collectively on all experimental units (n = 3 for each of DCPA, DCA, and NOH-DCA).
Chlorine Substituents Play a Role in the Immunotoxic Effects of DCPA
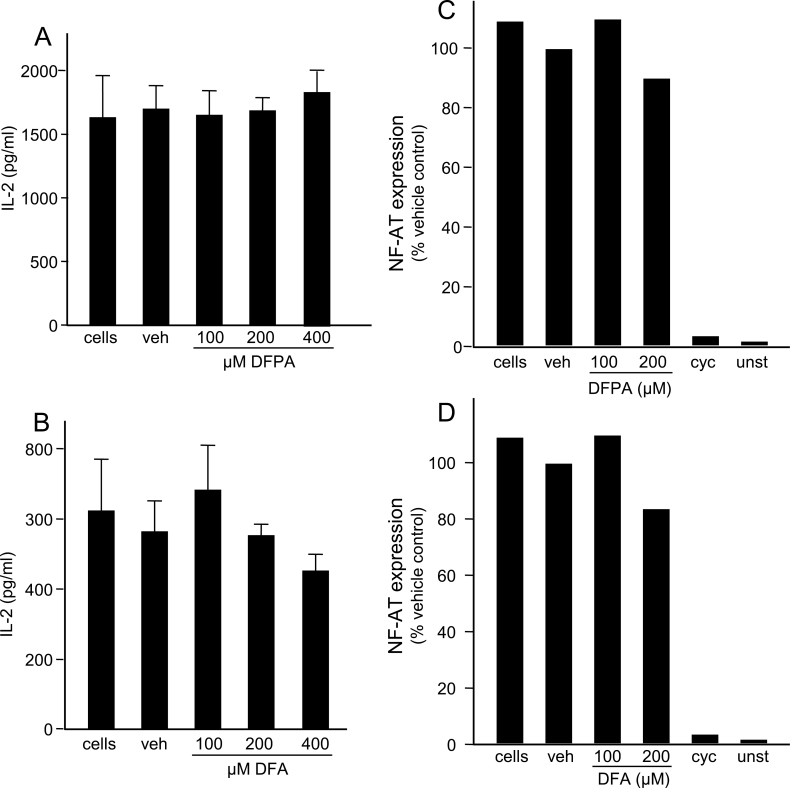
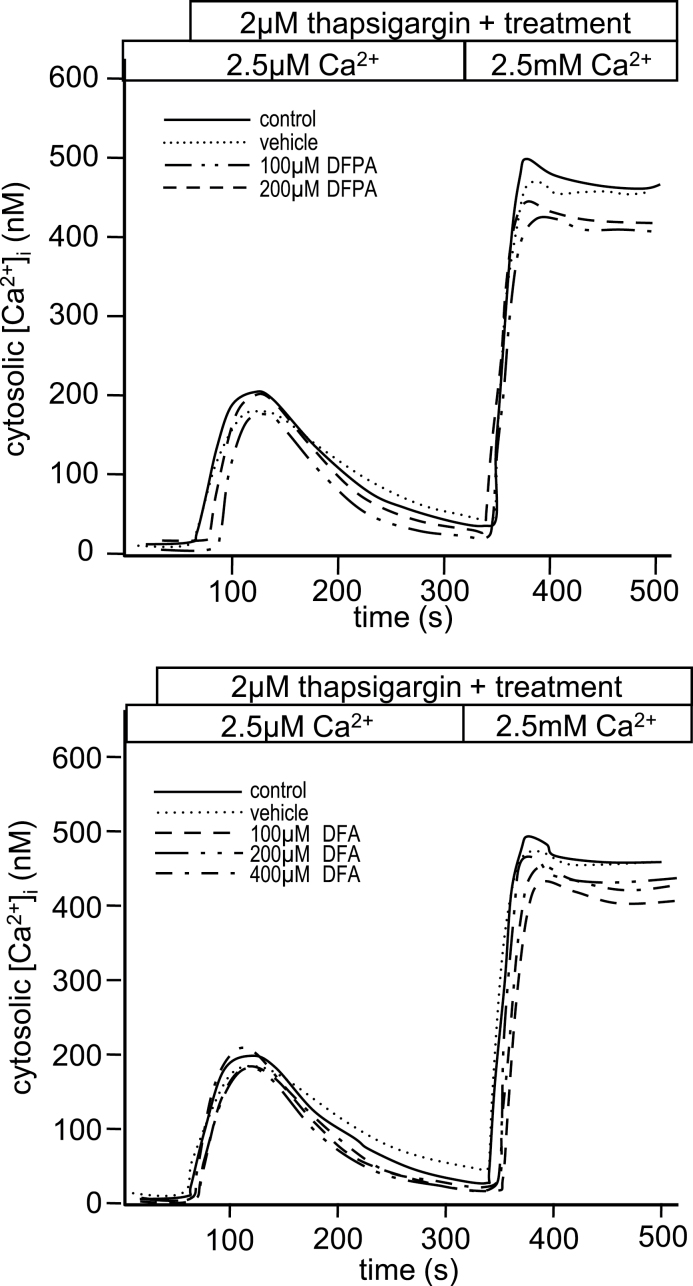
To assess the role that chlorines, in the 3 and 4 positions on DCPA, may have on the immunotoxic effects in T cells, we synthesized the fluorine analog of DCPA, DFPA (see Materials and Methods section) and used DFA, a commercially available fluorine analog of DCA. Viability assays indicate that exposure to DFPA and DFA is nontoxic compared with their chlorine counterparts. Concentrations up to 400µM of both DFPA and DFA were not cytotoxic to Jurkat T cells (Supplementary figs. 3A and 3B). Proliferation assays were also conducted and no changes in proliferation after 24h were observed at concentration up to 400µM (data not shown). To determine the effect of DFPA and DFA on IL-2 secretion, Jurkat T cells were stimulated and IL-2 was measured as described above. No changes in IL-2 secretion were detected when exposed to DFPA or DFA with increasing concentrations up to 400µM (Figs. 5A and 5B). These results indicate the presence of chlorine at the 3 and 4 positions plays an important role in the inhibition of IL-2 in DCPA- and DCA-exposed Jurkat T cells. To confirm the apparent inert effects when fluorine is substituted for chlorine, NFAT expression and Ca2+ influx were also examined as described above. Consistent with the viability, proliferation, and IL-2 secretion data, Jurkat T cells exposed to DFPA and DFA did not alter NFAT expression (Figs. 5C and 5D) or Ca2+ homeostasis (Figs. 6A and 6B). These data provide clear evidence that the immunotoxic effects of DCPA and its metabolites on Jurkat T cells can be attributed, in large part, to the presence of chlorine.
Fig. 5.
Fluorine analogs do not alter IL-2 secretion and NFAT expression in Jurkat cells. Jurkat T cells were treated with DFPA or DFA at the concentrations indicated. Additional treatment groups included a vehicle (veh) control and a no treatment control (labeled “cells”). IL-2 secretion by treated and control Jurkat T cells after stimulation with anti-CD3 and anti-CD28 for 24h was measured by standard ELISA techniques. Error bars reflect ± SEM and asterisks (*) indicate statistically significant results, p < 0.05 using ANOVA with a Student-Newman-Keuls post hoc test. NFAT expression in treated and control Jurkat T cells transfected with a NFAT luciferase plasmid was assessed after treatment as previously described. Results are representative of three separate experiments performed in triplicate.
Fig. 6.
Fluorine analogs do not alter the calcium flux in Jurkat cells. Jurkat T cells loaded with fluo-3 were assessed for changes in calcium influx in (A) DFPA and (B) DFA-treated samples as previously described. All results are representative of at least three different experiments, and statistical analysis was done collectively on all experimental units (n = 4 for each of DFPA, n = 3 for DFA).
Discussion
The effects of DCPA on the immune system have been well documented (reviewed by Salazar et al., 2008). In mouse models, in vivo and in vitro exposure to DCPA results in an anti-inflammatory effect by macrophage inhibition. DCPA also decreases IL-2 secretion by activated T cells by altering NFAT translocation and calcium homeostasis (Lewis et al., 2008).
The focus of this report was to determine whether NOH-DCA and 6OH-DCA, reactive metabolites of DCPA (McMillan et al., 1990b), as well as fluorine-based analogs also inhibit IL-2 production by reducing NFAT expression and Ca2+ influx. Previously known toxic effects of both NOH-DCA and 6OH-DCA include binding to hemoglobin leading to methemoglobinemia, a serious medical condition that results in the formation of methemoglobin adducts that do not bind or transport oxygen. NOH-DCA has also been reported as a nephrotoxicant and can induce hemolytic anemia (McMillan et al., 1991; Rankin et al., 2008). There are no reports on the effect of DFPA or DFA on T-cell function.
We report here the first evidence of the immunotoxic effects of the DCPA metabolites, DCA, NOH-DCA, and 6OH-DCA on Jurkat T-cell function. Jurkat T cells have been used for over 20 years as a model for human T-cell signaling (Jain et al., 1995). Previous research has demonstrated that exposure of DCPA to human Jurkat T cells inhibited IL-2 secretion in a concentration and Ca2+-dependent manner (Lewis et al., 2008). Like DCPA, DCA inhibits IL-2 secretion, NFAT expression, and calcium homeostasis, although higher concentrations than that of DCPA were required (Figs. 2B, 3A, and 4B). This is in agreement with others who have reported that immune parameters including T-cell–dependent antibody production, myelotoxic effects, and IL-6 response in mouse T cells were altered by DCPA but required higher concentrations of DCA to produce similar effects (Barnett et al., 1992; Malerba et al., 2002).
Activation of T cells requires the release of Ca2+ into the cytosol from ER stores, which leads to the activation of calcineurin and dephosphorylation of NFAT resulting in secretion of IL-2. However, depletion of ER stores is not sufficient for the sustained IL-2 production required for a robust immune response. Calcium release–activated calcium (CRAC) channels on the plasma membrane are activated by the Ca2+ released from ER stores and these CRAC channels allow the influx of extracellular Ca2+ to ensure a sustained IL-2 production. Depletion of the ER stores results in the aggregation of STIM1 proteins that reside on the ER membrane and the further activation of CRAC channels through Orai1, an important CRAC protein. We recently reported that DCPA inhibits STIM puncta (aggregates) formation in HEK293 cells (Zhou et al., 2011). We have previously demonstrated that DCPA does not alter the release of Ca2+ from ER stores (Lewis et al., 2008) so decreases in Ca2+ influx are likely occurring through inhibition of Ca2+ influx through CRAC channels. Inhibition of STIM1 by DCPA or DCA would result in decreased Ca2+ influx leading to downstream consequences including decreased levels of dephosphorylated NFAT and decreased IL-2 production. It is reasonable to conclude that DCPA may have similar effects in Jurkat T cells.
Of the metabolites tested NOH-DCA appears most overtly cytotoxic to T cells (Supplementary fig. 1) and inhibits IL-2 secretion more potently, with a 90% reduction in IL-2 at 50µM, whereas at 50µM DCPA, IL-2 is decreased by 50% (Fig. 2A). Further, cells treated with 50µM NOH-DCA resulted in a 54% decrease in NFAT expression (Fig. 3B) when activated with antihuman CD3 and CD28 antibodies and to a similar degree when stimulated with PMA/A23187 (Supplementary fig. 2). Our data also show a toxic effect of 6OH-DCA on T-cell function, although 6OH-DCA required higher concentrations in order to observe a similar effect with that seen with NOH-DCA. Importantly, no change in Ca2+ influx was seen with either NOH-DCA or 6OH-DCA (Figs. 4C and 4D). The effect of these metabolites on calcium homeostasis suggests that the mechanism by which NOH-DCA and 6OH-DCA inhibit IL-2 secretion is different from that of DCPA (Figs. 4A and 4C). NOH-DCA and 6OH-DCA appeared to inhibit IL-2 in a Ca2+-independent manner, whereas the effects of DCPA and DCA on T-cell function are elicited in a Ca2+-dependent manner. Although the mechanism(s) for the decreases in IL-2 and NFAT expression caused by 6OH-DCA and NOH-DCA cannot be ascertained from these experiments, it is possible that the hydroxylated DCA metabolites alter Jurkat T-cell activation by directly interfering with activation of calcineurin. This could allow normal changes in cytosolic Ca2+ but prevent activation of NFAT and thus, production of IL-2. Several direct inhibitors of NFAT activation have been identified and these include cyclosporine A (Li et al., 2011) and the immunophilin FKBP12 (Siamakpour-Reihani et al., 2011). These molecules bind directly to calcineurin and inhibit its phosphatase activity, and thus, the translocation of NFAT (Li et al., 2011; Siamakpour-Reihani et al., 2011).
Approximately 45% of all herbicides contain a carbon- chlorine bond and 17% of all organochlorines require special safety precautions for use in the workplace (Naumann, 2000). In many cases the biological activity of the compound is conferred by the presence of the chlorines. The best known example is DDT, as removal of specific chlorines renders this pesticide inactive. Other toxic chlorine-containing herbicides include chlordane and heptachlor. In order to determine the role that chlorine substituents have on DCPA-exposed T cells, we substituted fluorines for the chlorines found in both DCPA and DCA. In the last 20 years, the number of fluorinated chemicals has increased significantly and now 28% of all halogenated agrochemicals are fluorinated (Jeschke, 2004). The position and number of fluorines in agrochemicals determine the activity of many pesticides. Unlike DCPA and DCA, DFPA and DFA are not cytotoxic (up to 400µM) (Supplementary fig. 3) and do not alter IL-2 secretion, NFAT expression, or calcium homeostasis (Figs. 5 and 6). This indicates that chlorine substitution plays an important role in exerting immunotoxic effects in T cells. Several possibilities exist for the differential effects observed with chlorine and fluorine. First, fluorines are highly electronegative and act only as hydrogen acceptors, whereas chlorine and other halogens act as both hydrogen acceptors and donors (Purser et al., 2008). This increase in electronegativity could alter the distribution of charge so that the fluorine analogs cannot interact with its target in the same way as DCPA and DCA. Second, trifluoro-substitution can increase lipophilicity but mono- or difluoro-substitution have been known to decrease it (Purser et al., 2008). Changes in the lipophilicity may also alter interaction with the target of DCPA and because DCPA is targeted to the cytosol, increases in lipophilicity may prevent access into the cell (Hanson et al., 2010). Lastly, fluorines are similar in size to hydrogen and may not produce the 3D confirmation required to elicit the effects observed with DCPA. Further studies are required to determine the mechanism of DCPA, in particular, how the chlorine location and position alter T-cell function. These specific properties of fluorine appear to be responsible for the apparent reversal of effects observed in DFPA- and DFA-treated cells.
In conclusion, the hydroxylated metabolites of DCPA display differential effects on T cells. Although DCA elicits similar Ca2+-dependent effects on IL-2 secretion, the parent compound, DCPA, appears to be more active. However, DCPA is reported to be quickly metabolized in the body to DCA and persists in the body, possibly allowing DCA to accumulate to concentrations greater than that of DCPA (Roberts et al., 2009). Further exposure to DCA, as a breakdown of product of other herbicides such as linuron and diuron, may also increase overall exposure to DCA and its hydroxylated metabolites, resulting in alterations in human T-cell function. In addition, our data demonstrate that the position and location of the chlorines are critical in eliciting these effects. The hydroxylation of DCA occurs in mammals and has been reported for its role in met hemoglobinemia (McMillan et al., 1990b), but its effects on the immune system are unknown. This is the first reported study of the immune effects of the hydroxylated metabolites of DCPA. Our data suggest that NOH-DCA is the most toxic metabolite of DCPA, with significant functional consequences on human T-cell function at the concentrations used for this study. However, given the relatively high concentrations required to induce an effect with even NOH-DCA, the immunotoxic risk to humans of exposure to DCPA appears fairly minimal. Yet, to our knowledge, none of the reported human studies measured levels of either NOH-DCA or 6OH-DCA. Thus, further studies are needed to determine the nature and mechanism of exposure to DCPA and its metabolites and its potential adverse effects on human health.
Supplementary Material
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
Funding
National Institute of Health (ES011311 to J.B.B.); the U.S. Environmental Protection Agency Greater Research Opportunity Fellowship (MA-91684801 to T.L.L.).
Supplementary Material
Acknowledgments
The authors would like to thank Dr Kathleen Brundage for her help with the experiments using flow cytometry, which were performed in the West Virginia University (WVU) Flow Cytometry Core Facility and supported in part by NIH grants RR016440 and RR020866. The authors also thank Dr Karen Martin for her assistance with experiments in the WVU Imaging Facility, which is also supported in part by NIH grants RR016440 and RR020866. The authors would also like to thank Dr Gary Rankin for his gift of the 6OH-DCA and to Dr Peter Gannett who synthesized the DFPA and NOH-DCA.
Although the research described in the article has been funded wholly or in part by the U.S. Environmental Protection Agency’s STAR program through grant MA-91684801 to T.L.L., it has not been subjected to any EPA review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred.
References
- Barnett J. B., Gandy J., Wilbourn D., Theus S. A. (1992). Comparison of the immunotoxicity of propanil and its metabolite, 3,4-dichloroaniline, in C57Bl/6 mice. Fundam. Appl. Toxicol. 18, 628–631 [DOI] [PubMed] [Google Scholar]
- Bauer E. R., Meyer H. H., Stahlschmidt-Allner P., Sauerwein H. (1998). Application of an androgen receptor assay for the characterisation of the androgenic or antiandrogenic activity of various phenylurea herbicides and their derivatives. Analyst. 123, 2485–2487 [DOI] [PubMed] [Google Scholar]
- Brundage K. M., Schafer R., Barnett J. B. (2004). Altered AP-1 (activating protein-1) activity and c-jun activation in T cells exposed to the amide class herbicide 3,4-dichloropropionanilide (DCPA). Toxicol. Sci. 79, 98–105 [DOI] [PubMed] [Google Scholar]
- Corsini E., Codeca I., Mangiaratti S., Birindelli S., Minoia C., Turci R., Viviani B., Facchi A., Vitelli N., Lucchi L., et al. (2007). Immunomodulatory effects of the herbicide propanil on cytokine production in humans: In vivo and in vitro exposure. Toxicol. Appl. Pharmacol. 222, 202–210 [DOI] [PubMed] [Google Scholar]
- Crinnion W. J. (2009). Chlorinated pesticides: Threats to health and importance of detection. Altern. Med. Rev. 14, 347–359 [PubMed] [Google Scholar]
- European Union Risk Assessment Report (2006). 3,4-Dichloroaniline. Vol. 65 Available at: http://esis.jrc.ec.europa.eu/doc/risk_assessment/REPORT/ 34dichloroaniline_DCAreport048.pdf Accessed September 19, 2012
- Gaynor J. J., Still C. C. (1983). Subcellular localization of rice leaf aryl acylamidase activity. Plant Physiol. 72, 80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- Hanson M. L., Peer C. J., Brundage R., Callery P. S., Brundage K., Schafer R., Eremin S., Barnett J. B. (2010). Subcellular localization of the amide class herbicide 3,4-dichloropropionanilide (DCPA) in T cells and hepatocytes. J. Toxicol. Environ. Health Part A. 73, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., Loh C., Rao A. (1995). Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 7, 333–342 [DOI] [PubMed] [Google Scholar]
- Jeschke P. (2004). The unique role of fluorine in the design of active ingredients for modern crop protection. Chembiochem. 5, 571–589 [DOI] [PubMed] [Google Scholar]
- Kiely T., Donaldson D., Grube A. (2004). Pesticide Industry Sales and Usage. 2000 and 2001 Market Estimates Available at: http://www.epa.gov/pesticides/pestsales/01pestsales/market_estimates2001.pdf Accessed October 25, 2012.
- Lerman L., Weinstock-Rosin M., Nudelman A. (2005). An Improved Synthesis of Hydroxyindoles. ChemInform. 36.,doi: 10.1002/chin.200521120 [Google Scholar]
- Lewis T. L., Brundage K. M., Brundage R. A., Barnett J. B. (2008). 3,4-Dichloropropionanilide (DCPA) inhibits T-cell activation by altering the intracellular calcium concentration following store depletion. Toxicol. Sci. 103, 97–107 [DOI] [PubMed] [Google Scholar]
- Li H., Rao A., Hogan P. G. (2011). Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok R., Leone R. E., Williams A. J. (1996). Facile rearrangements of alkynylamino heterocycles with noble metal cations. J. Org. Chem. 61, 3289–3297 [Google Scholar]
- Malerba I., Castoldi A. F., Parent-Massin D., Gribaldo L. (2002). In vitro myelotoxicity of propanil and 3,4-dichloroaniline on murine and human CFU-E/BFU-E progenitors. Toxicol. Sci. 69, 433–438 [DOI] [PubMed] [Google Scholar]
- McMillan D. C., Bradshaw T. P., Hinson J. A., Jollow D. J. (1991). Role of metabolites in propanil-induced hemolytic anemia. Toxicol. Appl. Pharmacol. 110, 70–78 [DOI] [PubMed] [Google Scholar]
- McMillan D. C., Leakey J. E., Arlotto M. P., McMillan J. M., Hinson J. A. (1990a). Metabolism of the arylamide herbicide propanil. II. Effects of propanil and its derivatives on hepatic microsomal drug-metabolizing enzymes in the rat. Toxicol. Appl. Pharmacol. 103, 102–112 [DOI] [PubMed] [Google Scholar]
- McMillan D. C., McRae T. A., Hinson J. A. (1990b). Propanil-induced methemoglobinemia and hemoglobin binding in the rat. Toxicol. Appl. Pharmacol. 105, 503–507 [DOI] [PubMed] [Google Scholar]
- Naumann K. (2000). Influence of chlorine substituents on biological activity of chemicals: A review. Pest Manag. Sci. 56, 3–21 [Google Scholar]
- Purser S., Moore P. R., Swallow S., Gouverneur V. (2008). Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 [DOI] [PubMed] [Google Scholar]
- Rankin G. O., Racine C., Sweeney A., Kraynie A., Anestis D. K., Barnett J. B. (2008). In vitro nephrotoxicity induced by propanil. Environ. Toxicol. 23, 435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. M., McClure G. Y., Lavy T. L., Mattice J. D., Keller R. J., Gandy J. (2001). Propanil (3,4-dichloropropionanilide) particulate concentrations within and near the residences of families living adjacent to aerially sprayed rice fields. Arch. Environ. Contam. Toxicol. 41, 112–116 [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Heilmair R., Buckley N. A., Dawson A. H., Fahim M., Eddleston M., Eyer P. (2009). Clinical outcomes and kinetics of propanil following acute self-poisoning: A prospective case series. BMC Clin. Pharmacol. 9, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar K. D., Ustyugova I. V., Brundage K. M., Barnett J. B., Schafer R. (2008). A review of the immunotoxicity of the pesticide 3,4-dichloropropionanalide. J. Toxicol. Environ. Health B Crit. Rev. 11, 630–645 [DOI] [PubMed] [Google Scholar]
- Siamakpour-Reihani S., Caster J., Bandhu Nepal D., Courtwright A., Hilliard E., Usary J., Ketelsen D., Darr D., Shen X. J., Patterson C., et al. (2011). The role of calcineurin/NFAT in SFRP2 induced angiogenesis—A rationale for breast cancer treatment with the calcineurin inhibitor tacro limus. PLoS ONE. 6, e20412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentovic M. A., Lo H. H., Brown P. I., Rankin G. O. (1995). 3,5-Dichloroaniline toxicity in Fischer 344 rats pretreated with inhibitors and inducers of cytochrome P450. Toxicol. Lett. 78, 207–214 [DOI] [PubMed] [Google Scholar]
- Zhang B., Lin S. (2009). Effects of 3,4-dichloroaniline on testicle enzymes as biological markers in rats. Biomed. Environ. Sci. 22, 40–43 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Lewis T. L., Robinson L. J., Brundage K. M., Schafer R., Martin K. H., Blair H. C., Soboloff J., Barnett J. B. (2011). The role of calcium release activated calcium channels in osteoclast differentiation. J. Cell Physiol. 226, 1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.