Abstract
Embryonic exposure to the environmental contaminant and aryl hydrocarbon receptor agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin), disrupts cardiac development and function in fish, birds, and mammals. In zebrafish, the temporal window of sensitivity to the cardiotoxic effects of TCDD coincides with epicardium formation. We hypothesized that this TCDD-induced heart failure results from disruption of epicardial development. To determine whether embryonic TCDD exposure inhibits epicardium and proepicardium (PE) development in zebrafish, we used histology and fluorescence immunocytochemistry to examine the epicardium formation in fish exposed to TCDD. TCDD exposure prevented epicardium formation. Using live imaging and in situ hybridization, we found that TCDD exposure blocked the formation of the PE cluster. In situ hybridization experiments showed that TCDD exposure also prevented the expression of the PE marker tcf21 at the site where the PE normally forms. TCDD also inhibited expansion of the epicardial layer across the developing heart: Exposure after PE formation was completed prevented further expansion of the epicardium. However, TCDD exposure did not affect epicardial cells already present. Because TCDD blocks epicardium formation, but is not directly toxic to the epicardium once complete, we propose that inhibition of epicardium formation can account for the window of sensitivity to TCDD cardiotoxicity in developing zebrafish. Epicardium development is crucial to heart development. Loss of this layer during development may account for most if not all of the TCDD-induced cardiotoxicity in zebrafish.
Key Words: epicardium, TCDD, dioxin, zebrafish, heart defects, AHR.
Zebrafish are an established model for studying cardiovascular development and disease. Developing zebrafish hearts are exquisitely sensitive to cardiotoxicity induced by the environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin). Heart malformations caused by TCDD exposure in zebrafish include valve malformation, reduced heart size, and impaired development of the bulbus arteriosus (Antkiewicz et al., 2005; Grimes et al., 2008; Heideman et al., 2005; Mehta et al., 2008). TCDD exposure produces decreased cardiac output, reduced end diastolic volume, and decreased peripheral blood flow. This ultimately leads to heart failure, which includes ventricular standstill and total loss of circulation (Antkiewicz et al., 2005; Belair et al., 2001; Henry et al., 1997). One of the most interesting features of TCDD-induced cardiotoxicity is the fact that zebrafish carrying a lethal body burden of TCDD develop normal appearing hearts through heart tube formation and looping, with a functional circulation. It is only at approximately 48 hpf that the signs of TCDD toxicity become manifest. At this stage, the TCDD-exposed heart begins a process of unlooping, myocyte proliferation halts, cardiac output fails, the ventricle stops beating, valves are malformed, and the fish succumb with massive pericardial and yolk sac edema. Later in development, at between the second and third week of life, the zebrafish heart returns to a state of resistance to TCDD-induced cardiotoxicity (Lanham et al., 2012). It is known that cardiotoxicity is mediated by the receptor for TCDD, Ahr2 (Prasch et al., 2003). However, the mechanism underlying toxicity remains unknown, and in particular the basis for the developmental window of sensitivity remains a mystery.
Studies of the developing zebrafish heart initially identified only two cell layers, the myocardial layer and the endothelium lining the heart chambers, which is continuous with the vascular endothelium. However, recently Serluca (2008) showed that zebrafish develop an epicardium, which can first be observed during the third day after fertilization. The epicardium is derived from a transient cluster of cells termed the proepicardium (PE) that initially forms at the venous pole, or inflow tract, during heart development. PE cells migrate to the myocardium and spread out to form the epicardium, a simple squamous epithelium (Männer et al., 2001; Martinsen, 2005; Muñoz-Chápuli et al., 2002; Schlueter and Brand, 2012). A subset of epicardial cells undergo epithelial to mesenchymal transition, contribute to the development of coronary vasculature smooth muscle, and become perivascular and intermyocardial fibroblasts (Vincent and Buckingham, 2010). Epicardial derived cells are also involved in valve development, cardiomyocyte alignment, and proliferation and maturation of the cardiac conduction system (Gittenberger-de Groot et al., 2012; Lie-Venema et al., 2007; Muñoz-Chápuli et al., 2002).
We noted that the onset of sensitivity to TCDD cardiotoxicity in zebrafish coincides with the beginning of PE formation (Antkiewicz et al., 2005; Serluca, 2008). Cardiotoxicity begins at approximately 48 hpf, whereas the PE can first be clearly distinguished at approximately 50 hpf (Liu and Stainier, 2010; Serluca, 2008). Over the next few days, the epicardial cells migrate and envelope the zebrafish heart. TCDD cardiotoxicity begins to decline at about 5 dpf, a time that roughly coincides with completion of the initial epicardial cell layer. Over the next 2 weeks, the epicardium becomes thicker as epicardium formation is completed while TCDD sensitivity at the heart disappears (Lanham et al., 2012).
Because TCDD does not appear to interfere with initial heart formation, we hypothesized that TCDD inhibits a process that does not occur until after the heart chambers had formed. The timing of TCDD sensitivity suggested epicardium formation as a possible target. In addition, mutations that block epicardium formation produce cardiac malformations similar to those caused by TCDD (Gittenberger-de Groot et al., 2012; Lie-Venema et al., 2007; Olivey and Svensson, 2010; Ratajska et al., 2010; Serluca, 2008).
Here we report that TCDD exposure prevents PE formation and subsequent epicardium development. If the PE is allowed to form prior to TCDD exposure, TCDD halts further epicardial development but does not alter epicardial cells already present. Thus, TCDD has profound effects on the epicardium, which appear to be limited to the process of epicardium formation. This provides a model explaining the temporal pattern of sensitivity to TCDD-induced cardiotoxicity observed in the developing zebrafish.
MATERIALS AND METHODS
Zebrafish strains and exposure.
Adult zebrafish (Danio rerio) lines were maintained, and zebrafish embryos were reared and housed according to procedures described by Westerfield (2000). The AB wild-type line was used unless otherwise indicated. Transgenic lines, pard3:EGFP (Poon et al., 2010), tcf21:DsRed fish (Kikuchi et al., 2011), and ahr2 hu3335 (Goodale et al., 2012), were kindly provided by Drs Vladamir Korzh, Kenneth Poss, and Robert Tanguay, respectively. All procedures involving animals were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison and adhered to the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals.”
TCDD (> 99% purity; Chemsyn) was used as a 1mg/ml stock solution in dimethyl sulfoxide (DMSO). Fish were exposed to TCDD (1ng/ml) or vehicle (0.1% DMSO) for 1h in glass scintillation vials with gentle rocking (Antkiewicz et al., 2005). Ten embryos or larvae were present per milliliter of dosing solution, and each group of fish in a vial was considered n = 1 for statistics.
Control and TCDD-exposed embryos were dosed for 1h beginning at 4 hpf with either waterborne TCDD (1ng/ml) or an equivalent volume of 0.1% DMSO (control) and raised in 175 mmol/l mannitol in embryo water. This concentration was previously determined to prevent pericardial edema while allowing development of the embryo (Hill et al., 2004). The mannitol solution was replaced daily, and embryos were collected at 120 hpf.
Histology.
Larvae were fixed in 4% paraformaldehyde overnight at 4°C, dehydrated in a graded ethanol series, embedded in paraffin, and sectioned (4 μm) and stained with hematoxylin and eosin (H&E) (King Heiden et al., 2009). Sections were imaged using a Zeiss Axiocam digital camera mounted on a Zeiss Axioplan microscope.
Immunohistochemistry.
Antibody staining was performed as previously described (Dong et al., 2007). The antibody against activated leukocyte cell adhesion molecule (ALCAM) was used at a 1:50 dilution in PBS with 4% bovine albumin serum and 0.3% Triton (PBT). Secondary antimouse antibodies (Alexa Fluor 488, Alexa Fluor 568; Invitrogen) were used at 1:200 dilution in PBT. Embryos were mounted in Vectashield or Vectashield with DAPI (Vector Laboratories). Confocal images were collected on an Olympus Fluoview FV1000 microscope. Brightest point projections were made using Olympus Fluoview software, and images were processed using Adobe Photoshop.
PE imaging and scoring.
Live embryos (50 and 72 hpf) were imaged in 3% methylcellulose using a Nikon TE300 inverted microscope attached to a Princeton Instruments Micromax CCD camera. Ten-second videos showing the presumptive PE site were captured for each embryo using MotionScope software and analyzed using Metamorph software. Scores for PE development were assigned by an experimenter blinded to the treatment groups using a 0–3 scale (0 = PE clearly absent; 1 = slight evidence/very small PE; 2 = defined cluster, but smaller than normal; 3 = clearly present, normal PE). A two-tailed Student’s t-test assuming equal variance was used to determine statistical significance (p < 0.01).
In situ hybridization.
Whole mount in situ hybridization was preformed as previously described with minor modifications (Mehta et al., 2008). The tcf21 probe was kindly provided by Dr Fabrizio C. Serluca. Riboprobes were labeled with digoxigenin-UTP and visualized using anti-digoxigenin-AP Fab fragments (Roche) with BM purple (Roche). Hybridization was carried out at 60°C. Embryos were cleared in a 70% glycerol solution in PBS and imaged using an Olympus DP72 digital camera on an Olympus S2X16 microscope.
Epicardial development.
Individual fish carrying the pard3:EGFP reporter were exposed to TCDD at the indicated time, collected at 120 hpf, and fixed for confocal microscopy as described above. Individual fish were assessed for epicardium formation on the ventricle and the atrium. A chamber was scored as positive for epicardium formation if any EGFP-positive cell was detected overlying the myocardium. Between 45 and 54 fish were examined for each point, yielding a value of n = 45–54. Incidence data were analyzed using a Fisher’s Exact Test to determine statistical significance (p < 0.001).
RESULTS
Embryonic TCDD Exposure Prevents Epicardium Formation in Zebrafish
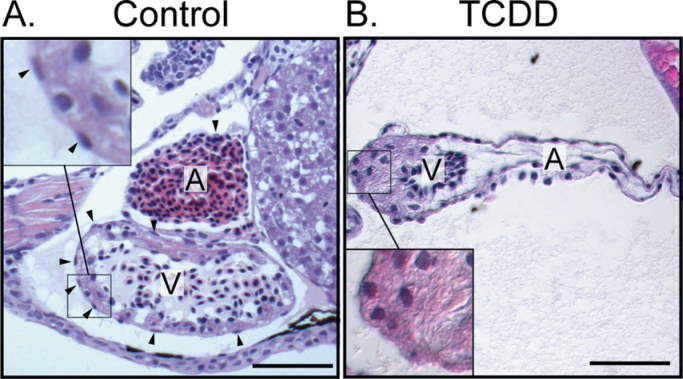
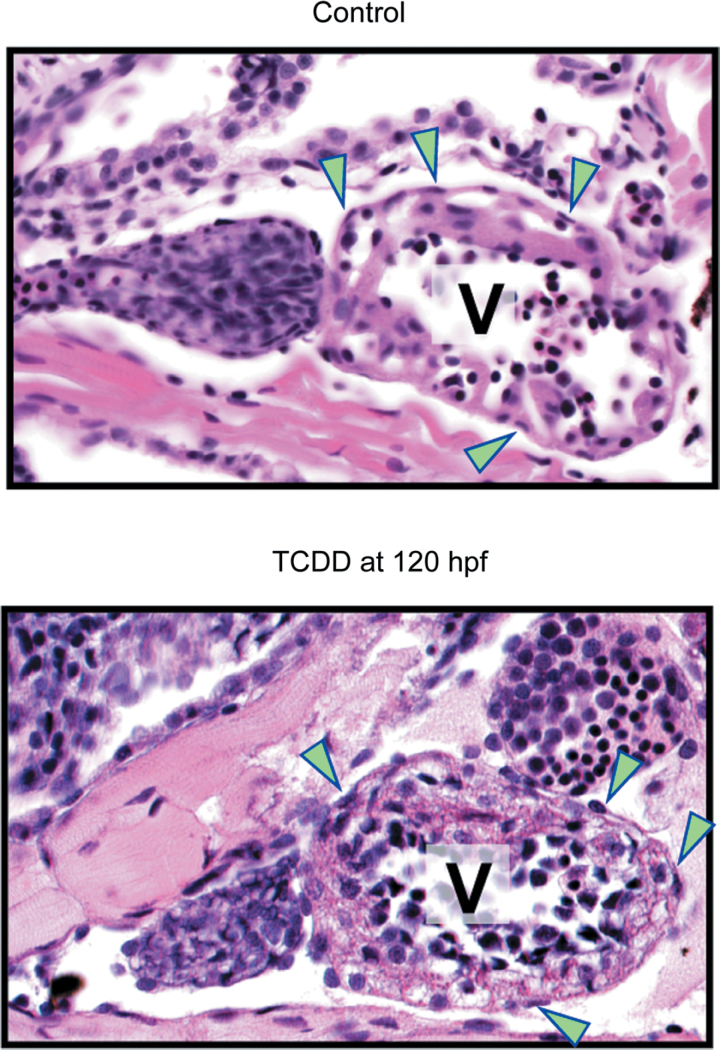
We exposed newly fertilized eggs to TCDD as described in the Materials and Methods section and collected larvae at 120 hpf to assess epicardium formation. H&E staining clearly showed oblong epicardial cells in control hearts and a consistent absence of these cells in TCDD-exposed hearts (Figs. 1A and B). The inset in Figure 1A shows the appearance of flattened cells creating a layer on the outside of the myocardium. The TCDD-exposed heart lacks these cells.
Fig. 1.
TCDD exposure prevents epicardium formation in zebrafish. Zebrafish were exposed to vehicle (A) or TCDD (B) immediately following fertilization as described in the Materials and Methods section and collected at 120 hpf. H&E-stained sagital sections show atrium and ventricle from lateral view, with anterior to the left. A, Atrium; V, Ventricle. Scale bars: 50 μm. Vehicle control hearts are on the left and corresponding TCDD-exposed hearts are shown at right. Insets show the flattened epicardial cells in the control ventricle and the corresponding region lacking epicardial cells in the TCDD-exposed heart. Arrowheads indicate epicardial cells.
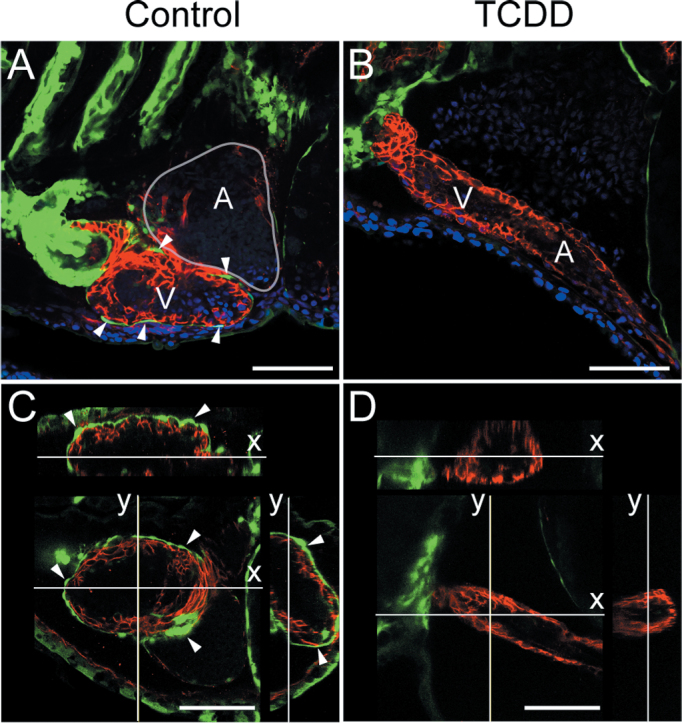
We used epicardial-specific reporter lines to follow epicardium development. Embryos carrying a pard3:EGFP reporter (Poon et al., 2010) were exposed to TCDD or vehicle as above, and hearts were examined using confocal microscopy at 120 hpf. Although epicardial cells expressing pard3:EGFP were consistently found on the ventricles in control hearts (Fig. 2A), we saw no EGFP epicardial cells in the TCDD-exposed hearts (Fig. 2B). Optical cross sections allowed a precise evaluation of the position of the pard3:EGFP-positive cells relative to the myocardium. In the control hearts, EGFP-positive cells clearly covered the myocardium (Fig. 2C); in the TCDD-exposed hearts, EGFP-positive cells were not detected on the myocardium (Fig. 2D).
Fig. 2.
Loss of pard3 reporter expression in TCDD-exposed hearts. Zebrafish were exposed to TCDD as in Figure 1. A and B) Fish were collected at 120 hpf, and lateral confocal images of pard3:EGFP control and exposed hearts are shown. ALCAM is counterstained as red, and EGFP is indicated in green. DAPI staining shows nuclei in outer pericardium in blue. White arrowheads indicate GFP-positive epicardial cells. (C and D). X and Y orthogonal optical slices through z-stacks (z-step = 0.52 μ) showing ventricle lumen. White arrowheads indicate GFP-positive epicardial cells. Scale bars: 50 μ.
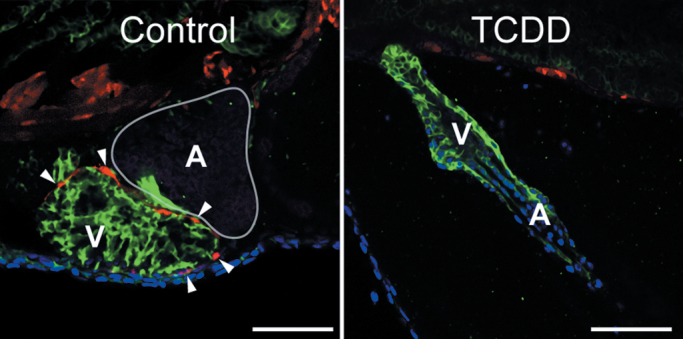
We also examined a tcf21:DsRed transgenic line expressing DsRed in epicardial cells (Kikuchi et al., 2011). As with the pard3:EGFP experiment, the DsRed signal clearly showed epicardial cells surrounding the control ventricle (Fig. 3, left) but not the ventricle in fish exposed to TCDD immediately after fertilization (Fig. 3, right). Taken together, the histological and reporter studies show that early exposure to TCDD prevents formation of the epicardium.
Fig. 3.
Expression of the tcf21 epicardium marker is lost in TCDD-exposed hearts. Zebrafish were exposed to TCDD immediately after fertilization and collected at 120 hpf as in the figures above. Confocal images show lateral views of hearts from the tcf21:DsRed transgenic line. Red indicates tcf21 expression; ALCAM expression is shown in green. DAPI in blue shows cell nuclei, generally at the pericardial surface. White arrowheads indicate DsRed-positive epicardial cells. Scale bars: 50 μ.
AHR2 Mediates Loss of Epicardium
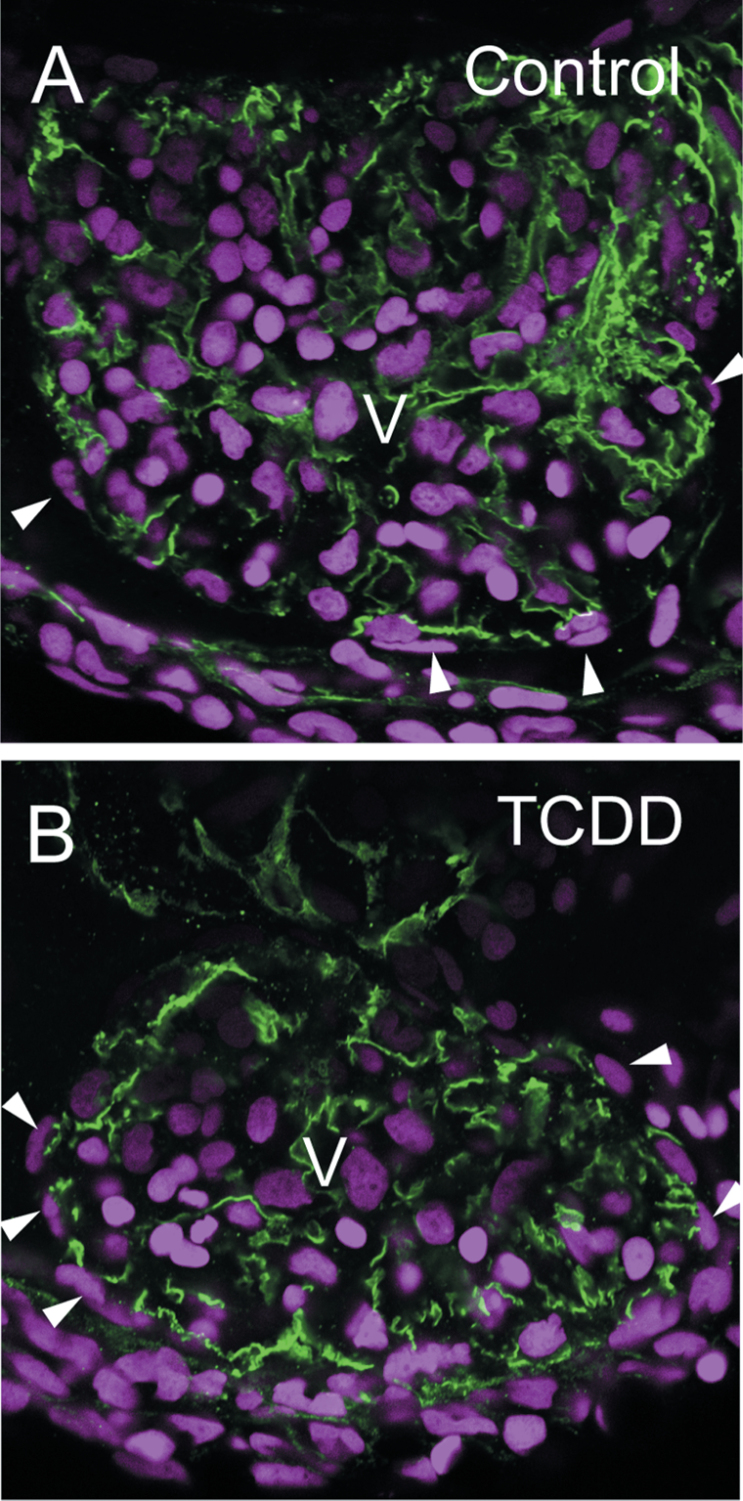
TCDD-induced cardiotoxicity has been shown to be dependent on the receptor for TCDD, Ahr2. Morpholino oligonucleotide (MO) knockdown of either Ahr2 or its dimerization partner Arnt1 is sufficient to protect zebrafish from developmental cardiotoxicity (Prasch et al., 2003 , 2006). To determine whether TCDD activation of Ahr2 mediates the loss of epicardium, we used a mutant lacking Ahr2 function (Goodale et al., 2012). Eggs were exposed to either DMSO as a vehicle control or TCDD immediately after fertilization as described in the Materials and Methods section. At 120 hpf, the fish were harvested and examined by confocal microscopy using antibodies against ALCAM to visualize the excitable myocardial cells. The DAPI staining showing cell nuclei allows visualization of epicardial cells on the outer surface of the myocardial layer (Fig. 4). As expected, TCDD had no apparent impact on epicardial coverage in these mutants.
Fig. 4.
TCDD has no effect on epicardium formation in ahr2 −/− mutants. Zebrafish homozygous for loss of functional ahr2 were exposed to TCDD immediately after fertilization and collected at 120 hpf as in the figures above. Confocal images show lateral views of hearts. ALCAM expression is shown in green and delineates the cytoplasm in myocardial cells. DAPI shows cell nuclei in blue. White arrowheads indicate flattened cells lying on the surface of the heart, outside of the myocardial layer. The ventricle center is labeled as V, and rounded erythrocyte nuclei are prominent within the ventricles.
Loss of the Epicardium Is Not a Secondary Effect of Pericardial Edema
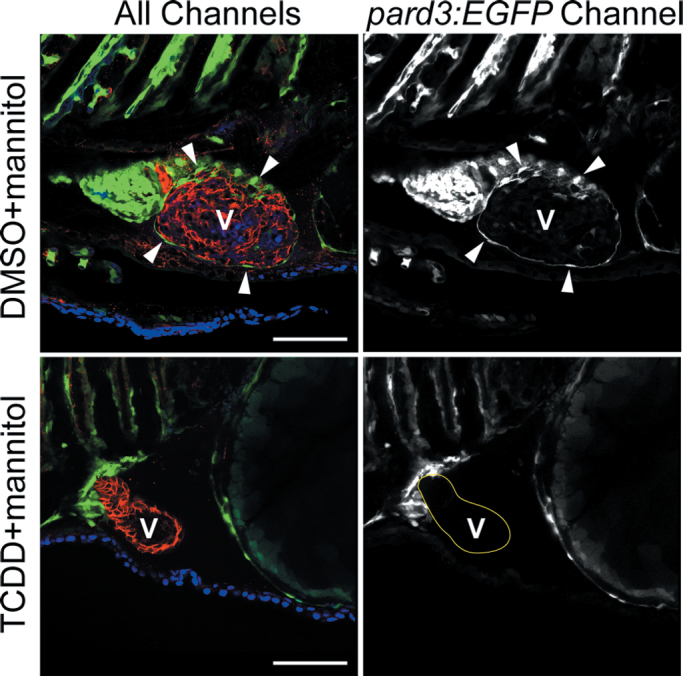
TCDD exposure alters heart shape and produces acute pericardial edema, beginning at approximately 72 hpf. This change in geometry alters the intrapericardial space, changing the environment in which the PE and epicardium are forming. Therefore, it is possible that the loss of epicardium was secondary to TCDD-induced pericardial edema. We used mannitol in the water as an osmotic support to prevent the TCDD pericardial edema produced (Hill et al., 2004). Although mannitol prevented TCDD-induced pericardial edema, the epicardium still failed to form in pard3:EGFP reporter embryos exposed to TCDD, whereas it clearly formed in the vehicle control larvae (Fig. 5). We conclude that the loss of the epicardium was not secondary to pericardial edema.
Fig. 5.
TCDD-induced pericardial edema is not linked to the loss of epicardium in TCDD-exposed larvae. Zebrafish carrying the pard3:EGFP reporter were exposed to TCDD or vehicle immediately following fertilization and moved into water with 175mM mannitol added as an osmotic support. Samples were collected at 120 hpf for confocal microscopy as described in the Materials and Methods section. Ventral views are shown with the anterior to the left. The left column of images shows all three channels of fluorescence together. ALCAM is counterstained in red, and EGFP is indicated in green. DAPI staining shows nuclei in outer pericardium in blue. White arrowheads indicate GFP-positive epicardial cells. The column of images at right shows the pard3:EGFP signal alone in white. Arrowheads show examples of pard3:EGFP-positive cells. A indicates atrium; V indicates ventricle. Scale bars: 50 μm.
TCDD Exposure Blocks PE Development in Zebrafish
TCDD is not readily metabolized nor excreted, and the half-life in adult trout is measured in months (Brambilla et al., 2007); consequently, we expect that our initial exposure at fertilization produces a persistent TCDD body burden in the developing fish lacking mature metabolism and excretion organs. Therefore, our first experiments do not point to disruption of any specific step in epicardium development. We first examined PE formation.
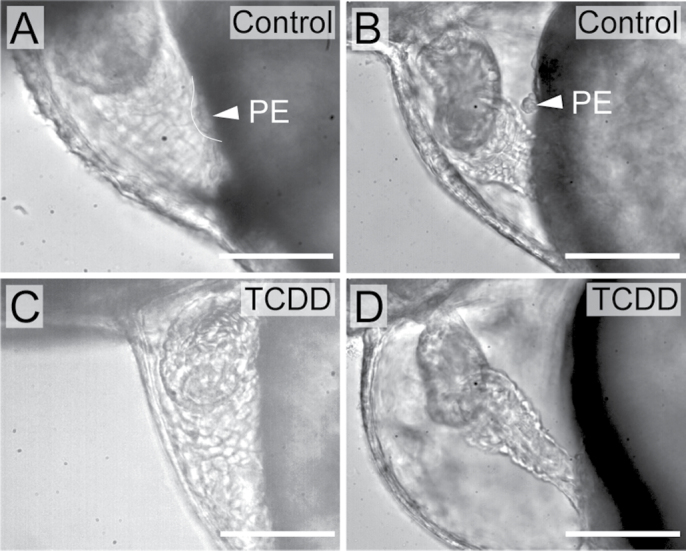
In zebrafish, the PE is first visible by brightfield microscopy around 50 hpf and increases in size from 50 to 72 hpf (Liu and Stainier, 2010; Serluca, 2008). This can be seen in the control images in Figures 6A and B. PE development was significantly impaired in TCDD-exposed fish at 50 hpf (Fig. 6C). PE development was significantly impaired in TCDD-exposed fish at 50 hpf. Exposure to TCDD retards growth; therefore, the loss of PE formation at 50 hpf might have been due to a developmental delay. We also examined PE formation in exposed and control embryos at 72 hpf. Even as late as 72 hpf, TCDD inhibited formation of the PE, indicating that the loss of the PE could not be explained by simple delay in formation (Fig. 6D).
Fig. 6.
TCDD exposure blocks PE development in zebrafish. Zebrafish were exposed to TCDD or vehicle immediately following fertilization as described in the Materials and Methods section. (A–D) Lateral views of hearts at 50 and 72 hpf are shown, with anterior to left in all panels. Vehicle control hearts are on the left, and corresponding TCDD-exposed hearts are shown at right. White arrowhead indicates the PE. Scale bars: 50 μm. (A) Vehicle control heart at 50 hpf. (B) Vehicle control heart at 72 hpf. (C) TCDD-exposed heart at 50 hpf. (D) TCDD-exposed heart at 72 hpf.
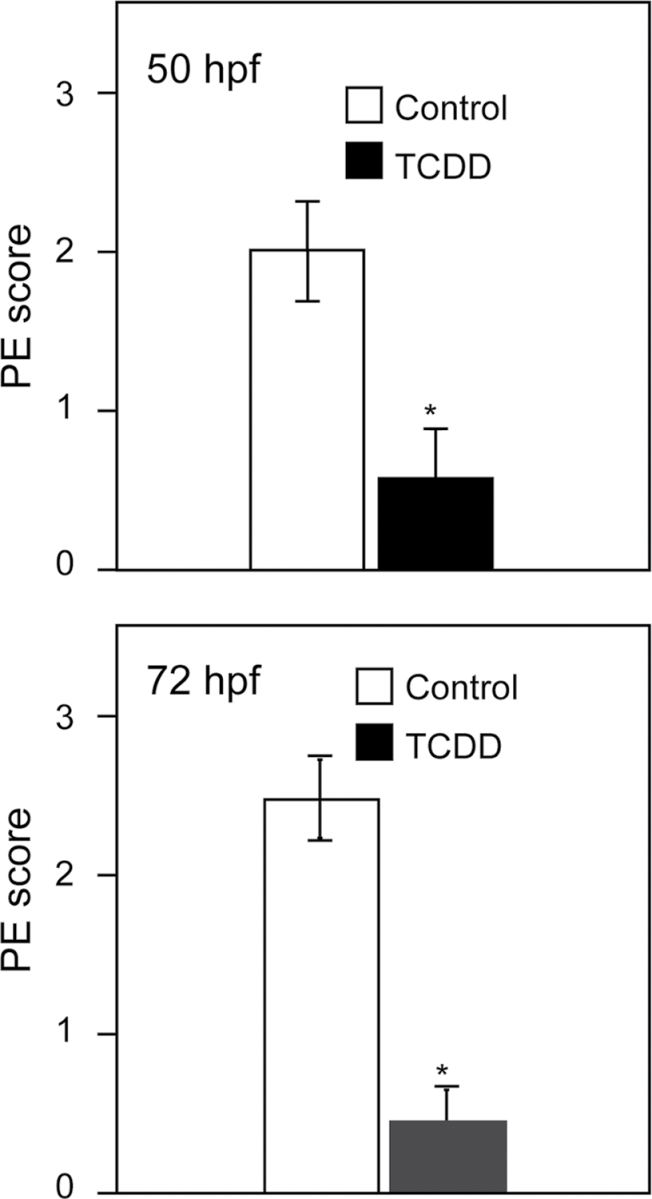
Because the PE is a small cluster of cells among other groups of cells, positive identification is difficult. To be certain that we were correctly identifying the PE, we scored PE formation in live fish, taking advantage of the fact that the real PE clusters remain relatively stationary, attached to the pericardium, whereas other cells move with the beat of the heart. We used video microscopy to allow observers, blind to the treatment groups, to score PE formation in treated and control fish as described in the Materials and Methods section. The results of these experiments at both 50 and 72 hpf show a significant loss of PE formation in TCDD-exposed fish (Fig. 7).
Fig. 7.
Scoring of PE formation. Graphs show incidence of PE formation at 50 and 72 hpf, respectively. Scoring is described in detail in Supplemental Methods. Briefly, embryos were scored using the following scale: 0, no PE; 1, slight evidence of PE; 2, moderate evidence for PE; 3, normal PE in full view. Experimenters were blind to the treatment groups scored. Asterisk indicates difference from control p < 0.01; Student’s t-test. Error bars indicate standard error.
Loss of PE Marker tcf21
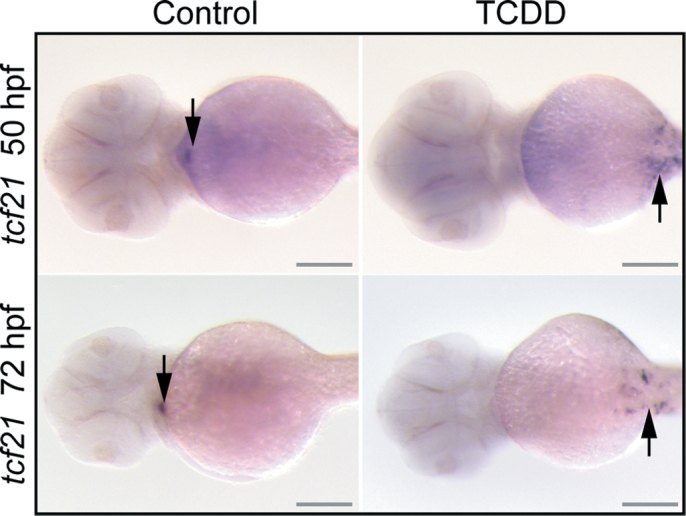
The PE is formed as a small cluster of cells that form in preparation for migration to the heart. TCDD might simply block the motility of these cells, producing a small region of PE-destined cells with failed adhesive properties, which are unable to form a cluster. If this were the case, we would expect to find a group of cells on the pericardial surface expressing PE marker genes. To test this, we used in situ hybridization to determine whether the PE marker gene tcf21 was still expressed in this region (Fig. 8). Although we were clearly able to discern the dot of tcf21 marking the PE in the control fish, TCDD exposure prevented the expression of tcf21 in this presumptive region of PE formation. This suggests that TCDD prevented not only the migration of cells to form the PE structure but also the formation of the group of cells expressing this PE marker.
Fig. 8.
TCDD alteration of PE and epicardium-specific marker. Zebrafish embryos were exposed to TCDD or vehicle at 24 hpf as described in the Materials and Methods section. Vehicle control hearts are on the left and corresponding TCDD-exposed hearts are shown at right. Fish were collected for in situ hybridization probing for tcf21 at either 50 or 72 hpf as indicated, and ventral views are shown with anterior end to the left. Arrows indicate regions of hybridization. Scale bars: 100 μm.
Timing of TCDD Exposure Differentially Affects Epicardial Development
The PE loss can readily explain the absence of an epicardium in larvae exposed to TCDD at the time of fertilization. However, does TCDD also affect the epicardium itself?
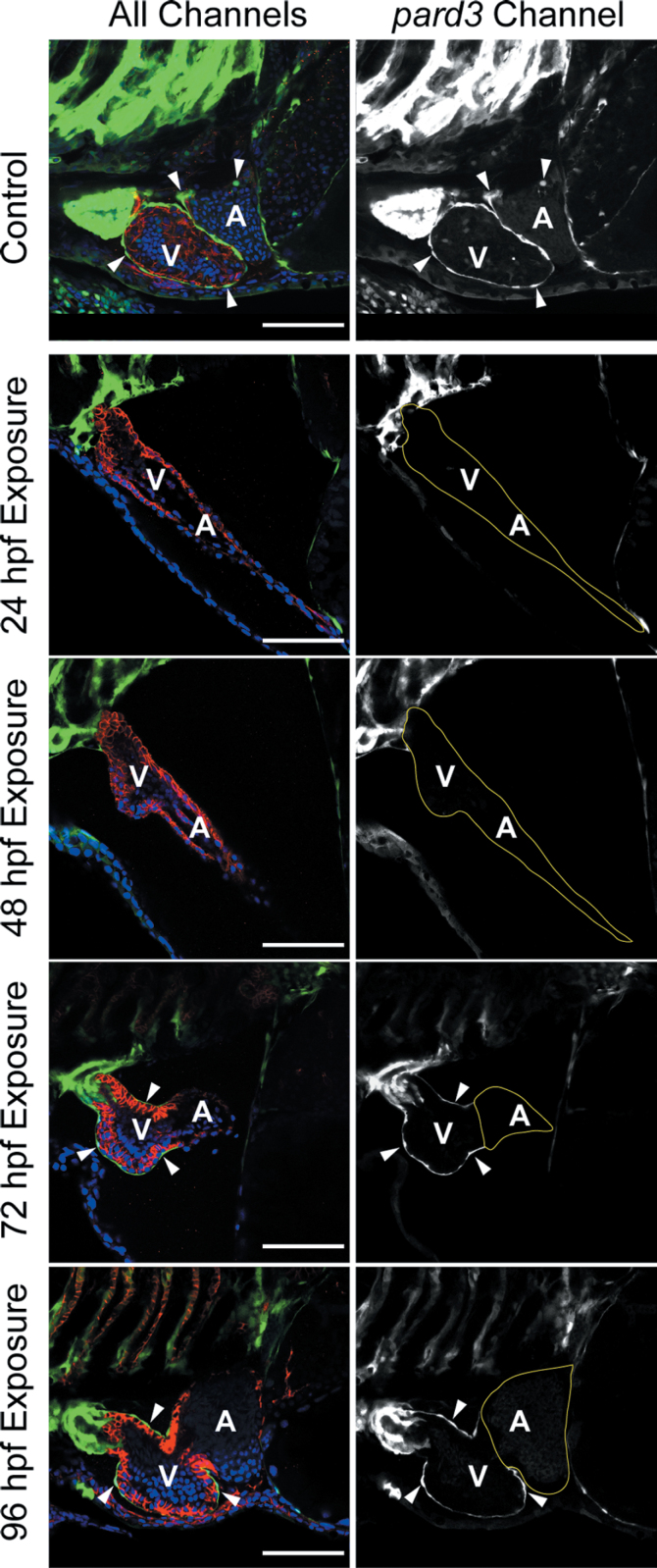
We tested this by delaying TCDD exposure until after the PE had formed. Zebrafish carrying the pard3:EGFP reporter were exposed to TCDD prior to or during PE formation at 24 and 48 hpf and after initial epicardium establishment at 72 or 96 hpf. All samples were collected at 120 hpf for examination of the epicardium by confocal microscopy (Fig. 9). The control shows the normal development of the epicardium across both ventricle and atrium at 120 hpf. As expected, embryos exposed at 24 and 48 hpf, prior to PE formation, had no epicardium at 120 hpf.
Fig. 9.
TCDD after PE formation halts further epicardium progression. Zebrafish carrying the pard3:EGFP reporter were exposed to TCDD at the indicated times. Samples were collected at 120 hpf for confocal microscopy as described in the Materials and Methods section. The left column of images shows all three channels of fluorescence together. ALCAM is counterstained in red, and EGFP is indicated in green. DAPI staining shows nuclei in outer pericardium in blue. White arrowheads indicate GFP-positive epicardial cells. The column of images at right shows the pard3:EGFP signal alone as white. The control was not exposed to TCDD and demonstrates normal epicardium formation at 120 hpf. Scale bars: 50 μm.
Complete epicardial formation was also inhibited in hearts in which TCDD exposure was delayed until the PE had formed: Exposure at 72 hpf produced hearts with epicardial cells covering the ventricle, but not the atrium; exposure at 96 hpf produced hearts with ventricular epicardium, but few if any epicardial cells on the atrium. These results show that even after the PE has formed, exposure to TCDD inhibits the normal advance of the epicardial layer.
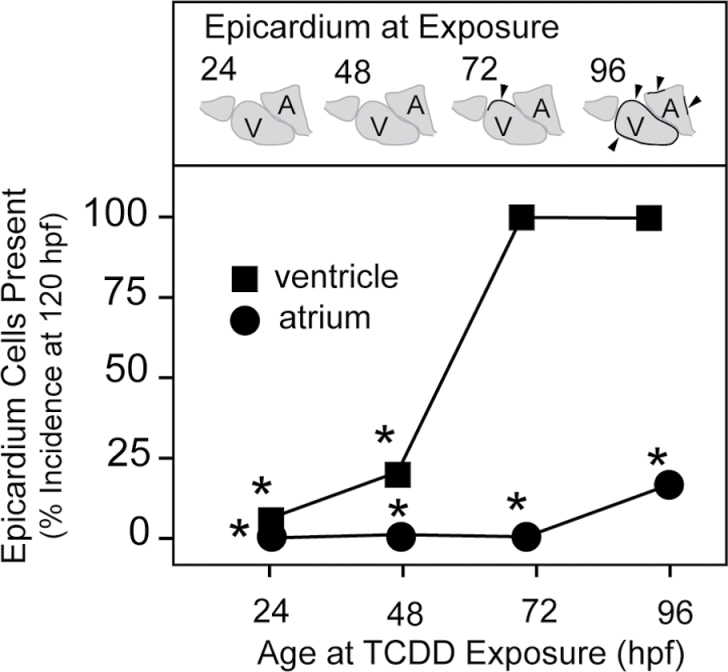
We used a binomial scoring system to assess the progression of the epicardial layer in hearts exposed to TCDD at the different times in the above experiments. Fish (approximately 50 individuals per point) were exposed at 24, 48, 72, and 96 hpf as described above and collected for examination at 120 hpf. Fish were scored for epicardial cells at the atrium and ventricle separately and were scored positive if even a single epicardial was found on the chamber. The incidence of epicardium cells found on the ventricle (squares) or the atrium (circles) is plotted on the y-axis in Figure 10.
Fig. 10.
TCDD exposure during epicardial expansion halts further epicardium development. Zebrafish carrying the pard3:EGFP reporter were exposed to TCDD at the times indicated on the x-axis and collected at 120 hpf for staining and confocal microscopy. The control was exposed to the DMSO vehicle alone. Incidence of the appearance of GFP-positive cells on either ventricle or atrium in the confocal images was counted at 120 hpf. The schematic figures above each time point represent the normal course of epicardium formation. Chambers with at least 1 EGFP-positive cell were scored as positive. If no cells were observed, the chamber was scored as negative. For these experiments, n = 1 represents individual fish, and the n for each treatment ranged from 45 to 54. The values shown are the percentages of positive scoring individuals in the treatment group. Scoring the percentage of individuals with an all or none response produces no error bars. Instead, Fisher’s Exact Test was used, and asterisks indicate difference from control at p < 0.001.
All control fish exposed to vehicle at any of the time points showed both ventricular and atrial coverage by epicardial cells. TCDD exposure at 72 hpf had little effect on the incidence of epicardial cells on the ventricle, probably due to the fact that epicardium formation has begun on the ventricle by about 72 hpf. However, epicardium coverage of the atrium was completely prevented. Even when exposure was delayed to 96 hpf, we found a significant inhibition of atrial coverage. These results show that TCDD exposure blocks epicardial expansion from the ventricle to the atrium, while apparently not reversing already formed epicardium.
To determine whether TCDD could affect the epicardium after it had been formed, we delayed TCDD exposure until 120 hpf, a point when epicardial cells have covered both atrium and ventricle, and collected hearts 2 days later at 168 hpf. H&E sections showed no difference in epicardium coverage between the exposed and control samples, indicating that although epicardium progenitors building the layer are sensitive, the established epicardium as formed is not a TCDD target (Fig. 11).
Fig. 11.
TCDD does not remove formed epicardium. The panels show H&E sections of 168 hpf larval hearts treated with DMSO (control) or TCDD at 120 hpf. Arrowheads indicate epicardial cells. In all panels, ventral views are shown with the anterior to the left. A indicates atrium; V indicates ventricle.
Discussion
Epicardium development involves specification of the PE, translocation of PE cells to the myocardium, and migration of epicardial cells across the heart chambers to form an epicardial sheet (Carmona et al., 2010; Männer et al., 2001; Ratajska et al., 2008). TCDD inhibited PE formation and disrupted the expression of PE markers. Ahr2 is required for the known cardiotoxic effects of TCDD in zebrafish embryos, and not surprisingly, we also found this true for the effects on PE and epicardial development: In ahr2 null mutants, the epicardium developed normally despite the presence of TCDD.
Pericardial Edema
An obvious effect of TCDD exposure is pronounced pericardial edema. This is thought to be secondary to heart failure and circulation loss (Incardona et al., 2004 , 2005). We have previously shown that relief of the pericardial edema with mannitol added as an osmotic support does not alter the course of heart failure, unlooping, and arrest induced by TCDD (Hill et al., 2004). We considered the possibility that pericardial edema could alter the concentrations of factors in the fluid surrounding the heart and the spatial relationships between tissues. This could conceivably alter epicardium formation. However, we have ruled this out with experiments in which the pericardial edema in TCDD-exposed embryos was prevented using mannitol as an osmotic support.
TCDD Exposure and PE Development
We found that TCDD produced a significant loss of PE formation in the developing zebrafish. We should note that our scoring system was set up to address problems in conclusively identifying the zebrafish PE. In contrast to species such as the chicken, it can be difficult to identify the PE with certainty, especially at early time points. The PE is a small cluster of perhaps a dozen cells. With many other groups of cells in the region, we found that the most effective method of scoring is to do so with live fish. In live fish, attachment of the PE to the pericardium distinguishes the more stable PE from groups of cells associated with the beating heart.
Even with this system and a trained eye, we found that the PE is variable in size. Often identification was easy and certain: These were scored as 3s. However, in other cases, the PEs in control fish were smaller and harder to identify with complete certainty. These groups of cells were scored as 2s. With this variation in size, the converse situation was sometimes encountered as well: In some cases, it was not possible to state with certainty that a clump of a few cells was not a small PE. These groups were scored as 1. It is noteworthy that by 72 hpf no control fish were scored below 2 and no TCDD-treated fish were scored higher than 1. We presume that all of the control fish indeed had PEs and speculate that the TCDD-exposed fish did not all score as zeros because of false positives.
One gene expressed in PE and epicardial cells is tcf21. It is not known exactly what role tcf21 plays in epicardial formation; however, it is associated with the PE and then the epicardial cells as they form. We consistently observed a loss of the PE structure. The in situ hybridization experiments reinforce the idea that the PE fails to form in the TCDD-exposed fish.
It seems unlikely that TCDD directly affects the genes marking the epicardium such as pard3 or tcf21 because once formed the epicardial cells continue to express these mRNAs in the presence of TCDD. Rather, TCDD appears to prevent these progenitor cells from properly forming, thereby indirectly blocking expression of these and possibly many other as yet undiscovered genes marking epicardium progenitors.
Although tcf21 expression was absent at the presumptive site of PE formation in TCDD-exposed fish, it was ectopically expressed elsewhere. Previous work has shown increased and delocalized expression of genes such as bmp4, noggin, and flk1 in TCDD-exposed zebrafish hearts, indicating a failure to maintain normal specification of gene expression (Mehta et al., 2008). How this occurs remains an important unanswered question.
TCDD Exposure and Heart Disease
Early embryonic exposure to TCDD disrupts cardiovascular development and function in a wide range of vertebrate species including fish, birds, and mammals. In humans, epidemiological studies have correlated long-term TCDD exposure with ischemic heart disease (Bertazzi et al., 1998; Flesch-Janys et al., 1995; Puga, 2011), and interestingly, sectioned and stained heart samples from patients with this disease lack epicardial cells (Di Meglio et al., 2010). In zebrafish, an established model for studying cardiovascular development and disease, TCDD exposure results in valve malformation (Mehta et al., 2008), reduced heart size (Antkiewicz et al., 2005), and impaired development of the bulbus arteriosus (Grimes et al., 2008; Mehta et al., 2008). TCDD-exposed zebrafish larvae have decreased cardiac output, reduced end diastolic volume, and decreased peripheral blood flow (Antkiewicz et al., 2005; Belair et al., 2001; Carney et al., 2006). Heart failure steadily worsens to ventricular standstill and total loss of circulation (Antkiewicz et al., 2005). The pericardial and yolk sac edema that ensues is thought to be secondary to circulation failure (Incardona et al., 2004).
We propose that much of these effects seen in the exposed zebrafish heart can be accounted for by the failure of the epicardial layer to form and mature; however, this must at present remain a working model because although the epicardium is assumed to be necessary for heart formation, the exact consequences of epicardium loss are difficult to define. Most of what is known about the loss of epicardium is based on mutations or other gene alterations that cause epicardium loss. For example, in the mouse, tcf21 null embryos (E9.5) have defects in PE migration, epicardial adhesion, and spreading. This partial loss of epicardium is accompanied by thinning of the myocardium and impaired atrioventricular valve formation. Loss of the wt1 gene in the mouse is embryonic lethal. In addition to epicardium defects, cardiac abnormalities such as thinning of the myocardial wall, pericardial edema, and pericardial hemorrhage were observed in these wt1 mutant mice. The function of wt1 conserved in zebrafish, and MO knockdown of wt1 expression causes a loss of the PE followed by the heart elongation and pericardial edema. These cardiac defects have all have been reported for zebrafish exposed to TCDD.
It is clear that bmp4 is critical in PE and cardiac development. In zebrafish, tcf21 and tbx18 expressions are lost in bmp4 and type I BMP receptor (acvrl1) mutants. Liu and Stainier (2010) went on to block BMP signalling using a heat shock–inducible dominant negative BMP receptor construct. Heat shock at 36 hpf produced significant reduction in tcf21 and tbx18 expression at the region of the presumptive PE. Perhaps more informative for our purposes, Liu and Stainier also found that the T-box transcription factor Tbx5a plays a role in heart development that appears to become critical around the time of PE formation. The tbx5a gene was originally discovered as the “heartstrings” mutation (Garrity et al., 2002). As with TCDD-exposed hearts, the hearts in zebrafish lacking tbx5a develop relatively normally until about the time of PE formation. At this stage, the PE and epicardial markers tbx18 and tcf21 are markedly reduced. After this, the heart deteriorates to become string like. Heat-shock expression of a dominant negative tbx5a at 10 hpf, long before PE formation, produces more dramatic effects than expression at 24 hpf, indicating that tbx5a is important in some early process needed later for PE and epicardium specification (Liu and Stainier, 2010).
Two Congruent Temporal Windows for TCDD Sensitivity
One of the most puzzling and interesting aspects of TCDD cardiotoxicity in zebrafish has been the narrow temporal window of sensitivity. When zebrafish are exposed to TCDD immediately after fertilization, the hearts form and develop normally, such that at 48 hpf, they are practically indistinguishable from normal control hearts. Blood flow is normal and chamber formation and looping proceeds, despite the presence of both TCDD and the AHR. However, after 48 hpf, the exposed hearts deteriorate: Looping is reversed, chamber morphology is altered, valve cushions fail to form, and with massive pericardial edema, circulation ceases. Remarkably, this sensitivity to TCDD drops off noticeably at around 120 hpf, and cardiotoxic effects entirely disappear after the second week of life (Lanham et al., 2012).
As closely as we can determine, this temporal window of TCDD sensitivity matches the time course of epicardium formation. However, the exact time course of epicardium formation has not been well defined. The PE is first visible at around 50 hpf, but an indistinct cluster of cells beginning to form a PE would be hard to identify, so the exact beginning of PE formation must remain an estimate. We find that the epicardium covers the ventricle first and then spreads onto the atrium at around 120 hpf. After that time, there is an apparent maturation of the epicardium all over the surface of the heart as it begins to contribute to heart formation. This coincides with the gradual complete loss of TCDD sensitivity at the heart.
It is noteworthy that TCDD does not appear to affect epicardial cells after they have formed. Therefore, if the epicardium formation is the target for TCDD cardiotoxicity, one would expect to see a loss of cardiotoxicity once the epicardial layer had been established. To the extent to which we can determine, this is the case. We propose that inhibition of epicardium formation can account for the window of sensitivity to TCDD cardiotoxicity in developing zebrafish. Because epicardium development is crucial to so many aspects of heart development, this toxic effect, inhibiting the development of a specific set of cells, may account for most of the TCDD-induced cardiotoxicity in developing zebrafish.
It is important perhaps not to oversimplify our interpretation. Replication of epicardial cells has been observed in adult zebrafish during periods of growth (Wills et al., 2008). Although we observed no overt toxicity in the adult heart (Lanham et al., 2012), subtle changes due to altered epicardial cell growth might not have been observed.
FUNDING
National Institutes of Health (NIH) grant R01 ES012716 from the National Institute of Environmental Health Sciences (NIEHS) (W.H. and R.E.P.) and the University of Wisconsin Sea Grant Institute, National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce grant number NA 16RG2257, Sea Grant Project Numbers R/BT-22 and R/BT-25 (W.H. and R.E.P.). The contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIEHS, NIH.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank Dorothy Nesbit, B. David Campbell, and Rickie L. Jones for assistance in the lab.
References
- Antkiewicz D. S., Burns C. G., Carney S. A., Peterson R. E., Heideman W. (2005). Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377 [DOI] [PubMed] [Google Scholar]
- Belair C. D., Peterson R. E., Heideman W. (2001). Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 222, 581–594 [DOI] [PubMed] [Google Scholar]
- Bertazzi P. A., Bernucci I., Brambilla G., Consonni D., Pesatori A. C. (1998). The Seveso studies on early and long-term effects of dioxin exposure: A review. Environ. Health Perspect. 106,(Suppl. 2)625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla G., Dellatte E., Fochi I., Iacovella N., Miniero R., di Domenico A. (2007). Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): A hint for safer fish farming. Chemosphere. 66, 1019–1030 [DOI] [PubMed] [Google Scholar]
- Carmona R., Guadix J. A., Cano E., Ruiz-Villalba A., Portillo-Sánchez V., Pérez-Pomares J. M., Muñoz-Chápuli R. (2010). The embryonic epicardium: An essential element of cardiac development. J. Cell. Mol. Med. 14, 2066–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. A., Chen J., Burns C. G., Xiong K. M., Peterson R. E., Heideman W. (2006). Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 70, 549–561 [DOI] [PubMed] [Google Scholar]
- Di Meglio F., Castaldo C., Nurzynska D., Romano V., Miraglia R., Montagnani S. (2010). Epicardial cells are missing from the surface of hearts with ischemic cardiomyopathy: A useful clue about the self-renewal potential of the adult human heart?. Int. J. Cardiol. 145, e44–e46 [DOI] [PubMed] [Google Scholar]
- Dong P. D., Munson C. A., Norton W., Crosnier C., Pan X., Gong Z., Neumann C. J., Stainier D. Y. (2007). Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat. Genet. 39, 397–402 [DOI] [PubMed] [Google Scholar]
- Flesch-Janys D., Berger J., Gurn P., Manz A., Nagel S., Waltsgott H., Dwyer J. H. (1995). Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am. J. Epidemiol. 142, 1165–1175 [DOI] [PubMed] [Google Scholar]
- Garrity D. M., Childs S., Fishman M. C. (2002). The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development. 129, 4635–4645 [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot A. C., Winter E. M., Bartelings M. M., Jose Goumans M., Deruiter M. C., Poelmann R. E. (2012). The arterial and cardiac epicardium in development, disease and repair. Differentiation; Research in Biological Diversity. 84, 41–53 [DOI] [PubMed] [Google Scholar]
- Goodale B. C., La Du J. K., Bisson W. H., Janszen D. B., Waters K. M., Tanguay R. L. (2012). AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS ONE. 7, e29346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes A. C., Erwin K. N., Stadt H. A., Hunter G. L., Gefroh H. A., Tsai H. J., Kirby M. L. (2008). PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol. Sci. 106, 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman W., Antkiewicz D. S., Carney S. A., Peterson R. E. (2005). Zebrafish and cardiac toxicology. Cardiovasc. Toxicol. 5, 203–214 [DOI] [PubMed] [Google Scholar]
- Henry T. R., Spitsbergen J. M., Hornung M. W., Abnet C. C., Peterson R. E. (1997). Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 142, 56–68 [DOI] [PubMed] [Google Scholar]
- Hill A. J., Bello S. M., Prasch A. L., Peterson R. E., Heideman W. (2004). Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol. Sci. 78, 78–87 [DOI] [PubMed] [Google Scholar]
- Incardona J. P., Carls M. G., Teraoka H., Sloan C. A., Collier T. K., Scholz N. L. (2005). Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 113, 1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona J. P., Collier T. K., Scholz N. L. (2004). Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 196, 191–205 [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Gupta V., Wang J., Holdway J. E., Wills A. A., Fang Y., Poss K. D. (2011). tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 138, 2895–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Heiden T. C., Spitsbergen J., Heideman W., Peterson R. E. (2009). Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol. Sci. 109, 75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham K. A., Peterson R. E., Heideman W. (2012). Sensitivity to dioxin decreases as zebrafish mature. Toxicol. Sci. 127, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie-Venema H., van den Akker N. M., Bax N. A., Winter E. M., Maas S., Kekarainen T., Hoeben R. C., deRuiter M. C., Poelmann R. E., Gittenberger-de Groot A. C. (2007). Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. ScientificWorldJournal. 7, 1777–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Stainier D. Y. (2010). Tbx5 and Bmp signaling are essential for proepicardium specification in zebrafish. Circ. Res. 106, 1818–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männer J., Pérez-Pomares J. M., Macías D., Muñoz-Chápuli R. (2001). The origin, formation and developmental significance of the epicardium: A review. Cells. Tissues. Organs (Print). 169, 89–103 [DOI] [PubMed] [Google Scholar]
- Martinsen B. J. (2005). Reference guide to the stages of chick heart embryology. Dev. Dyn. 233, 1217–1237 [DOI] [PubMed] [Google Scholar]
- Mehta V., Peterson R. E., Heideman W. (2008). 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicol. Sci. 104, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Chápuli R., Macías D., González-Iriarte M., Carmona R., Atencia G., Pérez-Pomares J. M. (2002). The epicardium and epicardial-derived cells: Multiple functions in cardiac development. Rev. Esp. Cardiol. 55, 1070–1082 [DOI] [PubMed] [Google Scholar]
- Olivey H. E., Svensson E. C. (2010). Epicardial-myocardial signaling directing coronary vasculogenesis. Circ. Res. 106, 818–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K. L., Liebling M., Kondrychyn I., Garcia-Lecea M., Korzh V. (2010). Zebrafish cardiac enhancer trap lines: New tools for in vivo studies of cardiovascular development and disease. Dev. Dyn. 239, 914–926 [DOI] [PubMed] [Google Scholar]
- Prasch A. L., Tanguay R. L., Mehta V., Heideman W., Peterson R. E. (2006). Identification of zebrafish ARNT1 homologs: 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 69, 776–787 [DOI] [PubMed] [Google Scholar]
- Prasch A. L., Teraoka H., Carney S. A., Dong W., Hiraga T., Stegeman J. J., Heideman W., Peterson R. E. (2003). Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 76, 138–150 [DOI] [PubMed] [Google Scholar]
- Puga A. (2011). Perspectives on the potential involvement of the AH receptor-dioxin axis in cardiovascular disease. Toxicol. Sci. 120, 256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajska A., Czarnowska E., Ciszek B. (2008). Embryonic development of the proepicardium and coronary vessels. Int. J. Dev. Biol. 52, 229–236 [DOI] [PubMed] [Google Scholar]
- Ratajska A., Kołodzińska A., Ciszek B., Wasiutyński A. (2010). Relationship between heart development and pathogenesis of congenital heart defects in current literature. Kardiol. Pol. 68,(Suppl. 5)S418–S427 [PubMed] [Google Scholar]
- Schlueter J., Brand T. (2012). Epicardial progenitor cells in cardiac development and regeneration. J. Cardiovasc. Transl. Res. 5, 641–653 [DOI] [PubMed] [Google Scholar]
- Serluca F. C. (2008). Development of the proepicardial organ in the zebrafish. Dev. Biol. 315, 18–27 [DOI] [PubMed] [Google Scholar]
- Vincent S. D., Buckingham M. E. (2010). How to make a heart: The origin and regulation of cardiac progenitor cells. Curr. Top. Dev. Biol. 90, 1–41 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed. Univ. of Oregon Press, Eugene, OR: [Google Scholar]
- Wills A. A., Holdway J. E., Major R. J., Poss K. D. (2008). Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 135, 183–192 [DOI] [PubMed] [Google Scholar]