Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) can cause serious gastrointestinal (GI) injury including jejunal/ileal mucosal ulceration, bleeding, and even perforation in susceptible patients. The underlying mechanisms are largely unknown, but they are distinct from those related to gastric injury. Based on recent insights from experimental models, including genetics and pharmacology in rodents typically exposed to diclofenac, indomethacin, or naproxen, we propose a multiple-hit pathogenesis of NSAID enteropathy. The multiple hits start with an initial pharmacokinetic determinant caused by vectorial hepatobiliary excretion and delivery of glucuronidated NSAID or oxidative metabolite conjugates to the distal small intestinal lumen, where bacterial β-glucuronidase produces critical aglycones. The released aglycones are then taken up by enterocytes and further metabolized by intestinal cytochrome P450s to potentially reactive intermediates. The “first hit” is caused by the NSAID and/or oxidative metabolites that induce severe endoplasmic reticulum stress or mitochondrial stress and lead to cell death. The “second hit” is created by the significant subsequent inflammatory response that would follow such a first-hit injury. Based on these putative mechanisms, strategies have been developed to protect the enterocytes from being exposed to the parent NSAID and/or oxidative metabolites. Among these, a novel strategy already demonstrated in a murine model is the selective disruption of bacteria-specific β-glucuronidases with a novel small molecule inhibitor that does not harm the bacteria and that alleviates NSAID-induced enteropathy. Such mechanism-based strategies require further investigation but provide potential avenues for the alleviation of the GI toxicity caused by multiple NSAID hits.
Key Words: NSAIDs, enteropathy, glucuronides, intestinal microbiome, β-glucuronidase
Small intestinal injury induced by nonsteroidal anti- inflammatory drugs (NSAIDs) has been increasingly recognized as a frequent and serious adverse drug reaction associated with the majority of the nonselective cyclooxygenase (COX)–1/2 inhibitors. The underlying mechanisms are incompletely understood, and currently, there are no clinically approved effective therapies available. This review critically evaluates recent insights into the mechanisms and proposes novel strategies to alleviate NSAID enteropathy by mechanism-based therapeutic approaches.
CLINICAL SIGNIFICANCE OF NSAID ENTEROPATHY AND CURRENT EXPERIMENTAL MODELS
Clinical Significance
NSAID-associated enteropathy features high morbidity and mortality rates and is therefore a significant clinical challenge, causing a major burden on the health care system (Davies et al., 2000; Scarpignato and Hunt, 2010). In the United States, the annual number of hospitalizations for serious NSAID-related gastrointestinal (GI) complications has been estimated to be greater than 100,000 patients and associated with approximately 16,500 deaths (Wolfe et al., 1999). This is understandable as NSAIDs are among the most widely consumed pharmaceuticals in the world (Scarpignato and Hunt, 2010). The most commonly appreciated GI complications are gastric injury, but the more distal parts of the GI tract are also frequently affected. For example, serious injury to the small intestine has been estimated to account for one third of all NSAID-associated complications (Scarpignato and Hunt, 2010). These can manifest as ulceration and bleeding of the mucosa, inflammation, and, in rare cases, perforation (Allison et al., 1992; Bjarnason et al., 1993; Wolfe et al., 1999). Typically, ulcers present as necrotic or apoptotic injury of enterocytes that may involve deeper layers of the mucosa, with loss of villi and an acute inflammatory cell infiltrate. These adverse drug reactions in the duodenum, jejunum, and ileum are collectively termed enteropathy. (NSAIDs can also cause colonopathy [Davies, 1995], but this is beyond the scope of this review.) The increasing use of novel diagnostic tools including video capsule endoscopy has revealed that, unexpectedly, approximately two thirds of both long-term (> 3 months) and short-term (< 1 week) NSAID users exhibit mild or more severe forms of drug-induced lesions in the small intestine (Björnsson et al., 2008; Fortun and Hawkey, 2007; Maiden, 2009). In addition, many GI lesions in apparent “control subjects” were found to be attributable to non-prescription use of NSAIDs (Sidhu et al., 2010). In spite of its high incidence, there are currently no approved effective therapies to prevent or treat NSAID enteropathy.
This lack of enteric-protective therapies is in part due to an incomplete understanding of the underlying mechanisms of NSAID-induced GI damage (Whittle, 2004). Importantly, the successfully used current therapies to treat gastric injury do not protect from small intestinal injury. For example, the widely used proton pump inhibitors (PPIs; e.g., omeprazole, lansoprazole) or histamine H2-receptor antagonists (e.g., cimetidine, famotidine) are not effective in protecting from intestinal injury in patients. In fact, in animal models and in patients, these therapies have been shown to aggravate the extent of enteropathy (Daniell, 2012; Satoh et al., 2012; Wallace, 2012).
Gastrointestinal injury is primarily associated with the traditional, nonselective COX-1/2 inhibitors, whereas the selective COX-2 inhibitors were initially thought to be GI-safe. However, a number of reports based on capsule endoscopy suggest that the incidence of jejunal/ileal injury induced by chronic use of selective COX-2 inhibitors may be equally high as that caused by certain nonselective COX inhibitors (Maehata et al., 2012; Maiden, 2009; Maiden et al., 2007). The reasons are not completely understood but could include a key role of COX-2 in maintaining small bowel mucosal integrity.
Experimental Models
Although NSAIDs have been clinically used for decades and their potential to induce GI toxicity has long been recognized, the current understanding of the mechanisms responsible for these adverse drug effects is still incomplete. More recently, experimental animal models have shed more light on the mechanistic pathways. Both rats and mice have been extensively used to emulate the human disease although rodents are clearly more sensitive than humans to NSAID-induced GI toxicity. The reasons underlying this high susceptibility are not entirely clear but may be due to the fact that in rodents, the hepatobiliary excretion of NSAID and metabolite conjugates and the extent of enterohepatic cycling are higher than in humans (Boelsterli and Ramirez-Alcantara, 2011). Another reason is that rodents harbor more enteric bacteria than humans (DeSesso and Jacobson, 2001), which could play a role in bacteria-catalyzed hydrolysis of NSAID conjugates (see below). Specifically, in rodents, bacteria are more abundant throughout the small intestine, whereas in humans they are confined to the very distal parts of the small bowel. In addition, changes in the diet can alter the composition of the gut microbiota both in humans and mice (Satoh and Takeuchi, 2012; Tremaroli and Bäckhed, 2012). Therefore, one can assume that laboratory rodents, having a well-defined homogeneous diet compared with humans, will exhibit less interindividual variability in the microbiota profile caused by this factor than patients.
There are other differences between the human and the rodent GI tract; for example, in rodents, the ileum is much shorter (~2% of the total length of the small intestine) than the jejunum, whereas in humans, the ileum is more than half of the length of the small intestine (DeSesso and Jacobson, 2001). In spite of these structural and functional differences, the pathogenesis of ulcer formation features many similarities across these species. For example, the distribution of ulcers along the small intestine shows the same pattern in humans, rats, and mice; the lesions appear focally and are more abundant in the distal parts of the intestine (Fujimori et al., 2010). In fact, both in rats (Atchison et al., 2000; Seitz and Boelsterli, 1998) and mice (Ramirez-Alcantara et al., 2009), most of the ulcers develop in quartile 3 and to a lesser extent in quartile 4, whereas the more proximal quartiles 1 and 2 are mostly free of lesions.
In most experimental studies, the NSAIDs were administered po (to mimic the clinical situation). However, ip injection of diclofenac caused quantitatively and qualitatively very similar results as po administration (Seitz and Boelsterli, 1998). This can be explained by pharmacokinetic behavior; the weakly acidic NSAIDs are protonated and hence primarily absorbed in the stomach. Thus, both after ip and po administration, diclofenac will be delivered to the liver via the portal system, where the drug is conjugated to glucuronic acid (and also to sulfate or taurine), and large amounts excreted into bile. Consequently, the small intestine is exposed to the drug (plus metabolites) from the luminal side (see below). Interestingly, even topical administration of NSAIDs can lead to GI injury, as shown recently for diclofenac in hairless (but immunocompetent) mice (~600 µg, thrice per week). In these mice, peptic ulcers, esophageal ulcers, and GI bleeding occurred and greatly increased mortality (Lerche et al., 2011).
The most frequently used NSAIDs for modeling enteropathy include diclofenac (Atchison et al., 2000; LoGuidice et al., 2010, 2012; Ramirez-Alcantara et al., 2009; Reuter et al., 1997; Seitz and Boelsterli, 1998), indomethacin (Anthony et al., 1993; Dial et al., 2008; Duggan et al., 1975; Fukumoto et al., 2011; Harusato et al., 2011; Watanabe et al., 2008; Wright et al., 1997; Takeuchi et al., 2010c; Yamada et al., 2011), and naproxen (Wallace et al., 2011). All three compounds are weakly acidic (pK a, 4.0–4.5) nonselective COX-1/2 inhibitors, featuring a carboxylic acid moiety that is crucial for hepatic glucuronidation, hepatobiliary excretion, and enterohepatic cycling (see below). Therefore, most of the mechanistic insights into NSAID-induced intestinal injury stems from studies involving diclofenac, indomethacin, naproxen, or their congeners.
MECHANISMS OF MUCOSAL INJURY TO JEJUNUM AND ILEUM
Multi-Hit Concept
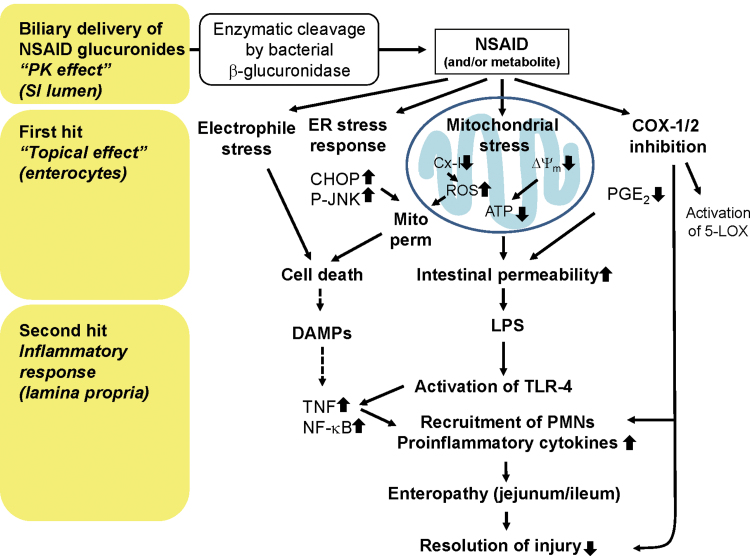
The molecular and cellular mechanisms underlying NSAID-induced toxicity to the small intestinal mucosa are clearly distinct from those in the stomach (Whittle, 2004); a detailed discussion of these differences can be found in a recent review (Wallace, 2012). The major mechanisms underlying enteropathy are summarized in Figure 1. On the one hand, and similar to the situation in the gastric mucosa, inhibition of COX-1 and/or COX-2 (the therapeutic target) may contribute to GI toxicity (Hotz-Behofsits et al., 2010; Sigthorsson et al., 2002; Tanaka et al., 2002b). On the other hand, there are also significant off-target effects involved. Collectively, these adverse effects are largely a consequence of locally high concentrations of NSAIDs present in the intestinal lumen and have been described as “topical effects” (Somasundaram et al., 1997). The exact concentrations of NSAIDs and/or their metabolites in the lumen are not known; however, biliary concentrations of parent drug plus glucuronide conjugates, following administration of a single ulcerogenic dose of diclofenac to rats, were found to be in the low millimolar range (Seitz and Boelsterli, 1998) and therefore clearly higher than the corresponding peak plasma concentrations, which are in the low micromolar range (LoGuidice et al., 2012). These topical (luminal) effects, which are still largely hypothetical, include uptake of the drug (and/or metabolites) into the enterocytes, possible bioactivation to reactive metabolites, and induction of endoplasmic reticulum (ER) stress (Tanaka et al., 2005; Tsutsumi et al., 2004) and mitochondrial stress (LoGuidice et al., 2010; Ramirez-Alcantara et al., 2009; Somasundaram et al., 2000; Watanabe et al., 2011) and can be considered as a “first hit.”
Fig. 1.
Putative mechanisms in the multiple-hit pathogenesis of NSAID-induced enteropathy. Dotted arrows, not supported by experimental evidence. Cx-1, mitochondrial complex I; ∆Ψm, mitochondrial transmembrane potential; Mito perm, mitochondrial permeabilization; SI, small intestine.
After this initial insult to enterocytes, the mucosal epithelia become more permeable, and bacterial lipopolysaccharide (LPS), present in the lumen, can penetrate deeper into the mucosa and activate toll-like receptor 4 (TLR4) on macrophages abundant in the lamina propria. This can lead to tumor necrosis factor–mediated cell injury and secondary activation of the innate immune system and recruitment of inflammatory cells to the site of injury (Watanabe et al., 2008). This inflammatory response can be considered a “second hit.”
A number of experimental approaches (mostly using chemical inhibitors or targeted gene deletion techniques) have provided evidence for the involvement of multiple mechanisms (involved in both first and second hit). This conclusion was primarily based on experimental findings in a variety of knockout mouse models that proved to be protected against NSAID enteropathy (see below).
Adverse Effects Mediated via COX Inhibition
The pharmacological target of NSAIDs is COX (prostaglandin endoperoxide synthase), which results in decreased levels of prostaglandins (Vane, 1971). Indeed, several studies in rodents have shown that, in the small intestine, the concentrations of PGE2 (which is the predominant prostaglandin in the intestinal mucosa) are substantially decreased after NSAID administration; for example, PGE2 concentrations were maximally decreased at 3h after a single dose of indomethacin in mice, clearly preceding the development of ulcers (Takeuchi et al., 2010a). Because prostaglandins have been implicated in a number of critical functions in the intestine, including maintenance of blood flow, turnover of epithelial cells, and resolution of inflammation, the question may be raised whether the inhibition of prostaglandin synthesis by NSAIDs may be causally related to the enteric toxicity via loss of critical function. For example, endogenous PGE2 promotes the healing of small intestinal lesions by stimulating angiogenesis, mediated by the activation of the prostaglandin subtype receptor EP4. Thus, small doses of indomethacin, given after a large ulcerogenic dose of indomethacin, significantly impaired ulcer healing for days; this was reversed by coadministration of an EP4 agonist (Takeuchi et al., 2010c).
As to a causative role of prostaglandin synthesis inhibition in NSAID enteropathy, the two COX isoforms (COX-1 and COX-2, encoded by Ptgs1 and Ptgs2, respectively) could play different roles because these isoforms have distinct functions. Specifically, COX-1 is a constitutive form and is related to the development of inflammation, whereas COX-2 is an inducible form, involved in the resolution of inflammation. However, the respective role of the COX isoforms in the pathogenesis of small intestinal ulceration has remained controversial. Originally, it was hoped that the use of specific COX-1- or COX-2 knockout mice could help clarify this problem; however, homozygous Ptgs1 −/− knockout mice, although having greatly decreased intestinal PGE2 levels, did not develop enteropathy. On the other hand, homozygous Ptgs2 −/− mice did develop enteropathy (although different from the type induced by NSAIDs) yet had normal intestinal PGE2 levels (Langenbach et al., 1999; Sigthorsson et al., 2002), suggesting that prostaglandins may not play a critical role in NSAID enteropathy. In line with this were the findings that, in rats, neither selective COX-1 inhibitors (e.g., SC-560) nor selective COX-2 inhibitors (e.g., rofecoxib, celecoxib) were able to induce intestinal damage. Meanwhile, it has become clear that small intestinal ulceration does only develop if an NSAID inhibits both COX-1 and COX-2 (as is the case for, e.g., diclofenac or indomethacin) (Tanaka et al., 2002a, b). Unfortunately, Ptgs1/2 double knockout mice are not viable and die shortly after birth; therefore, a combination of genetic deletion and pharmacological knockdown had to be utilized to provide the proof of concept. For example, both wild-type and homozygous Ptgs1 −/− knockout mice reacted similarly to an ulcerogenic dose of indomethacin. However, the selective COX-2 inhibitor, celecoxib (which did not cause enteropathy in wild-type animals), caused ulceration in the small intestine of Ptgs1 −/− mice (Sigthorsson et al., 2002). The underlying mechanisms for a role of dual inhibition of COX-1 and COX-2 are not entirely clear; however, the data are compatible with the concept that inhibition of COX-1 will upregulate COX-2 and that PGE2 (derived from COX-2) prevents the adverse effects caused by COX-1 inhibition, helping maintain mucosal integrity (Takeuchi et al., 2010b); therefore, if both isoforms are inhibited together, enteropathy will develop.
The concept that inhibition of both COX isoforms leads to enteropathy was corroborated by a recent clinical report on a functional deficiency (several SNPs) of the gene coding for the cytosolic phospholipase A2-α (Adler et al., 2009). This enzyme normally releases arachidonic acid from membrane phospholipids, which then is the substrate for COX (and for LOX), which converts arachidonic acid into prostaglandins (or leukotrienes, respectively). The patient carrying this mutation (and who was not taking any NSAIDs) repeatedly developed several sites of ulceration in the jejunum and ileum. These clinical findings of this rare mutation leading to a rare phenotype support the conclusion that loss of protective function of prostaglandins can lead to small intestinal ulceration. Experimental data support this conclusion; cPla 2 α knockout mice exhibited small intestinal ulcers although they were not severe (Sapirstein and Bonventre, 2000).
Alternatively, inhibition of the COX pathway by NSAIDs may shunt the metabolism of arachidonic acid into the other direction, i.e., activating the lipoxygenase (5-LOX) pathway. This could lead to oxidative stress due to increased production of superoxide from the 5-LOX-catalyzed peroxyradical formation and/or decrease in glutathione (GSH) due to reduction of the peroxyradicals. This mechanism has been disputed because certain NSAIDs that cause enteropathy do not only inhibit COX but also regulate 5-LOX. For example, diclofenac decreased leukotriene and 5-HETE formation in polymorphonuclear leukocytes (PMNs; Ku et al., 1986); however, this effect observed in vitro occurred at high concentrations only and did not seem to be related to a direct inhibition of 5-LOX but rather due to a disturbance of lipid metabolism. More recent evidence has provided clear evidence that diclofenac does not inhibit 5-LOX in rats (Maier et al., 2008). Therefore, increased oxidant stress from activation of the 5-LOX pathway is still a possible mechanism that could contribute to NSAID enteropathy. Although there is some evidence that leukotrienes could contribute to gastric mucosal injury (Burnett and Levy, 2012), the role of the 5-LOX pathway in NSAID enteropathy is still unclear.
FIRST HIT: TOPICAL EFFECTS
A topical effect is defined as a COX-independent impact caused by direct contact of intestinal epithelia with a drug present in the lumen. Topical effects can be produced by a number of distinct mechanisms and include generation of reactive metabolites by enterocyte cytochrome P450s (CYP), ER stress, oxidative stress, or mitochondrial injury. Topical effects not only play a key role after po administration of an NSAID but are also equally important after ip or other parenteral routes due to hepatobiliary excretion of metabolites and enterohepatic cycling.
Interactions With Biomembranes
One of the mechanisms by which NSAIDs can produce a topical effect is a direct interaction with cellular membranes, altering their biophysical properties. For example, due to electrostatic and hydrophobic interactions between the anionic NSAIDs and the positively charged nitrogen in phosphatidylcholine, the biophysical properties of the membrane can be altered, leading to changes in membrane fluidity, membrane instability, and increased permeability to protons and bacterial toxins (Lichtenberger et al., 2012). Although this is an attractive mecha nism in cultured cells, it is not clear to what extent this mechanism contributes to NSAID enteropathy in vivo.
Mitochondrial Injury
Most (but not all) NSAIDs that have been associated with enteropathy are uncouplers of oxidative phosphorylation (OXPHOS) in mitochondria, dissociating respiration from energy production. This feature, which is related to their molecular structure and typical for lipophilic weak acids, suggests that mitochondrial energy depletion could be a mechanism contributing to enteropathy; indeed, it has been suggested that mitochondrial dysfunction could be the basis for the topical effect component of NSAID enteropathy (Mahmud et al., 1996; Somasundaram et al., 1997). However, most functional studies on the effects of NSAIDs on oxygen consumption were performed in isolated mitochondria, and the in vivo relevance is difficult to assess. In addition, NSAIDs have also been shown to inhibit certain complexes of the electron transport chain.
Inhibition of Complex I.
A recent study demonstrated that certain NSAIDs, including diclofenac and indomethacin, inhibit rotenone-sensitive complex I activity (measured by NADH consumption) in isolated mitochondria from rat duodenal mucosa or Caco-2 cells (Sandoval-Acuña et al., 2012). For example, the IC50 for diclofenac was 13µM, i.e., clearly in a noncytotoxic concentration range. This inhibition was reversible in the presence of quercetin, a ubiquinone (coenzyme Q, coQ) mimetic, suggesting that NSAIDs may block the ubiquinone binding site. These data are in contrast to earlier studies (Nadanaciva et al., 2007) where diclofenac did not inhibit complex I (but inhibited complex V) as determined by immunocapture mAb techniques; the discrepancy could be explained by the different experimental systems used.
Inhibition of complex I activity invariably leads to increased superoxide production in mitochondria, which could contribute to redox-sensitive signaling and cell death. Thus, these novel data provide an attractive hypothesis to explain both oxidant stress and toxicity of NSAIDs. However, the in vivo relevance of complex I inhibition is still unclear. Similarly, the relevance of recent findings in Saccharomyces cerevisiae yeast cells where diclofenac specifically inhibited complex III through interaction with subunit Rip1p (van Leeuwen et al., 2011) for the mammalian GI tract remains unknown.
Uncoupling of OXPHOS.
Another inherent characteristic of many NSAIDs is their ability to act as uncouplers of OXPHOS. Interestingly, drug-induced mitochondrial uncoupling alone is not sufficient to induce overt small intestinal injury, but in combination with COX inhibition, enteropathy can develop. For example, an elegant series of experiments has revealed that the profen analog, R-2-phenylpropionic acid, which is not a COX inhibitor but which is an uncoupler of OXPHOS, does not cause enteropathy in mice when given alone (Hotz-Behofsits et al., 2010). However, in combination with genetic or pharmacologic inhibition of COX-2 (e.g., by celecoxib administration), R-2-phenylpropionic acid was able to induce small intestinal ulcers independently of mucosal prostaglandin levels. Similarly, the uncoupler 2,4-dinitrophenol, when instilled into the small bowel in rats, alone did not cause any apparent damage to the small intestine nor decrease intestinal prostaglandin levels. However, in combination with aspirin, which is not excreted via bile and which does not cause enteropathy when administered alone, the uncoupler caused ulcers in the small intestine to a similar extent as those induced by indomethacin (Somasundaram et al., 2000). These experimental findings indicate that a combination of COX inhibition and topical effects, both of which uncouple mitochondrial OXPHOS, has a high potential of triggering small intestinal ulceration.
The mechanisms are incompletely understood, but uncoupling, which causes a dissipation of the mitochondrial inner transmembrane potential, ∆Ψm, could have two consequences for enterocytes. First, the futile cycling of protons across the inner mitochondrial membrane leads to an attenuation of mitochondrial ATP production and a gradual depletion of cellular ATP. Second, a collapse of ∆Ψm could trigger the opening of the mitochondrial permeability transition (mPT) pore, potentially leading to cell death if it happens in a sufficiently large number of mitochondria.
Mitochondrial Permeability Transition.
Again, most experimental evidence that NSAIDs can induce mitochondrial permeabilization, followed by release of apoptogenic factors from the mitochondrial intermembrane space into the cytosol, stems from studies with isolated mitochondria; hence, the in vivo relevance is difficult to estimate. Increased mitochondrial (outer) membrane permeability can be triggered by different stimuli and executed by different mechanisms. One mechanism is the opening of the mPT pore, involving both the inner and outer membrane, which can be triggered by increased [Ca2+], oxidant stress, and/or a collapse of the mitochondrial membrane potential (∆Ψm) (for recent reviews, see Baines [2009]; Halestrap [2009]). A number of NSAIDs indeed cause increases in cellular [Ca2+] and oxidant stress (Tanaka et al., 2005), and many of them are uncouplers of OXPHOS, resulting in a dissipation of ∆Ψm (Al-Nasser, 2000; Masubuchi et al., 2000, 2002; Tay et al., 2005; Uyemura et al., 1997). However, direct evidence that NSAIDs can cause mPT pore opening in the small intestinal mucosa has been limited to in vitro studies with cultured enterocytes, where high concentrations of diclofenac were able to cause cyclosporin A–sensitive changes in calcein/Co2+ fluorescence, an accepted indicator of the mPT (LoGuidice et al., 2010).
To ascertain whether the induction of mPT is causally involved in NSAID-induced ulceration, one would have to inhibit the mPT pore and monitor whether this would protect from the subsequent development of enteropathy. This hypothesis has been tested in vivo by targeting cyclophilin D (CypD), a mitochondrial matrix protein and key regulator of the mPT. Indeed, pretreatment of mice with alisporivir (Debio 025), a nonimmunosuppressive cyclosporin A analog that avidly binds to CypD and prevents it from interacting with mPT regulatory proteins, fully protected from enteropathy induced by diclofenac (LoGuidice et al., 2010). Similarly, diclofenac was not associated with small intestinal ulceration if administered to CypD-deficient (Ppif knockout) mice (LoGuidice et al., 2010), suggesting that the mitochondrial mPT plays a critical role in the development of NSAID ulceration.
Increased Intestinal Permeability.
Another consequence of NSAID-induced decreases in mitochondrial ATP production is a loss of the gut barrier function. This can be quantitatively assessed by, e.g., orally administering dextran, a poorly absorbable high–molecular weight glycan, to mice. If the dextran molecule is labeled with the fluorescent marker FITC, then the increased intestinal permeability can be easily measured by determining the increases in FITC-specific fluorescence in the blood after NSAID administration (LoGuidice et al., 2012). It has been suggested that decreased enterocytic ATP levels, rather than the loss of prostaglandins, may be responsible for the increased mucosal permeability (Montrose et al., 2010). In accordance, in vivo exposure of the intestinal mucosa to an uncoupler (2,4-dinitrophenol) resulted in increased tight junction permeability (Nazli et al., 2004; Somasundaram et al., 2000).
Reactive Metabolites
Among the possible topical effects, toxicity due to reactive metabolites could also contribute to NSAID enteropathy. For example, reactive, electrophilic NSAID metabolites could be generated either in the liver and reach the small intestine via hepatobiliary transport or directly in the intestinal epithelia.
CYP-Mediated Oxidative Metabolites.
Although the small intestine expresses a number of CYP forms (Kaminsky and Zhang, 2003; Komura and Iwaki, 2011), the role of oxidative metabolites generated in situ has not been clearly elucidated. In humans, it is primarily CYP2C8/9/19 that is involved in the oxidative biotransformation of many NSAIDs. Interestingly, rats lack Cyp2C in the intestine (although they express it abundantly in the liver) (Bruyère et al., 2009); therefore, it is tempting to speculate that the intestinal epithelial cells do not produce reactive NSAID intermediates via Cyp2C or that the topical effects are either mediated by the parent compound or by liver-derived metabolites.
In mice, however, there is evidence that intestinal CYPs may contribute to the “topical effect.” In an elegant recent study utilizing an intestinal epithelium-specific CYP reductase knockout mouse (IE-Cpr-null mouse) to genetically eliminate all CYP-mediated oxidative metabolism (Zhu and Zhang, 2012), an ulcerogenic dose of diclofenac produced fewer ulcers than in wild-type animals. Because these mice were unable to generate the 5-hydroxy- or 4′-hydroxy metabolites from diclofenac (as well as the subsequent formation of reactive iminoquinones), the enterocytes exhibited much less electrophile stress (protein-reactive intermediates and formation of reactive oxygen species [ROS]) than the wild-type mice. Accordingly, there were less diclofenac glutathione S-adducts found in enterocytes of these mice, and staining with an anti-diclofenac antibody revealed that there were less adducts present. Furthermore, pretreatment with grapefruit juice, which inactivates certain CYPs in the small intestine, similarly protected mice from diclofenac enteropathy. Taken together, these novel data suggest that CYP-mediated reactive intermediates, generated in enterocytes, may be involved in diclofenac enteropathy in mice.
However, the relevance of CYP-mediated NSAID bioactivation in the GI in humans is still unclear. A study in nonhuman primates revealed that the expression levels of intestinal CYPs varied along the different segments of the small intestine. Specifically, in the cynomolgus monkey, transcript levels of a number of CYPs including CYP2C75 and 3A4 were higher in the proximal jejunum than in the distal ileum (Nakanishi et al., 2010). Similarly, diclofenac-4′-hydroxylase acitivity was higher in the proximal parts of the jejunum (where ulcer formation is minimal) than in the more distal areas (where the majority of ulcers develop) (Nakanishi et al., 2011). If CYP-mediated bioactivation of NSAIDs in the intestine is indeed causally related to the formation of ulcers, then this segmental distribution pattern does not reflect the incidence of ulceration.
Acyl Glucuronides and Iso-Glucuronides.
It has been a matter of debate for many years whether acyl glucuronides (formed in the liver from carboxylic acid–containing NSAIDs) or iso-glucuronides (secondary metabolites formed after acyl migration of the aglycone along the sugar ring) (Dickinson, 2011) may be causally involved in the topical effects associated with NSAID enteropathy (Boelsterli and Ramirez-Alcantara, 2011). Although acyl glucuronides are electrophilic protein-reactive intermediates and although covalent adducts have been detected on the enterocyte plasma membrane after in vivo administration of diclofenac (Atchison et al., 2000), the fact that the adduct distribution along the small intestine is identical with the severity of ulceration could be merely correlative, rather than causal. In fact, recent data in which the enzyme-catalyzed hydrolysis of diclofenac acyl glucuronide by intestinal bacteria was inhibited with a bacteria-specific β-glucuronidase inhibitor, and which resulted in protection from ulceration, suggest that the aglycone, rather than the glucuronide, may be involved in the topical effects associated with enteropathy (LoGuidice et al., 2012).
Oxidant Stress
There is insufficient and only indirect evidence that oxidative stress is involved in NSAID enteropathy. For example, indomethacin significantly increased the expression of heme oxygenase (HO-1) in mouse small intestinal mucosal tissue (Harusato et al., 2011). (HO-1 is an antioxidant enzyme that is induced by oxidant stress.) A recent study revealed that genetic deletion of Bach1, a transcriptional repressor of HO-1, not only led to highly increased upregulation of HO-1 but also fully protected mice from an ulcerogenic dose of indomethacin (10mg/kg, sc) (Harusato et al., 2011); the underlying mechanisms, however, have remained unclear. Another indirect pathway that is activated by oxidant stress is the MAPK pathway (via JNK phosphorylation). Administration of the specific JNK inhibitor, SP600125, protected from diclofenac-induced enteropathy in an established mouse model of enteropathy (Ramirez-Alcantara et al., 2009). These effects could be induced by mitochondrial dysfunction, resulting in increased oxidant stress (see above); alternatively, oxidative stress could be a secondary effect triggered by the inflammatory response and oxidative burst of cells of the innate immune system.
ER Stress Response
Studies based on molecular biomarkers revealed that NSAIDs induce an ER stress response in a number of cellular and in vivo models. For example, a recent toxicoproteomics study in mice has confirmed that NSAIDs can cause ER stress in the GI (Ohyama et al., 2012). Mice were administered a high dose of diclofenac (100mg/kg, ip), and pieces of the pyloric stomach were analyzed 6h postdose. FD-LC-MS/MS analysis revealed that the ER stress marker protein GRP78 was highly increased in the diclofenac group; other increased protein levels were found for gastrin and HSP27 (both involved in apoptosis). Another biomarker of ER stress is CHOP (C/EBP homologous protein, Gadd153), a transcription factor that becomes phosphorylated following ER stress and that induces mitochondria-mediated cell death (via inhibition of anti-apoptotic Bcl-2 family proteins). For example, in cultured guinea pig gastric mucosal cells, NSAIDs induced apoptosis via Chop induction, whereas deletion of Chop by transfection of cells with a dominant-negative form of Chop, or isolation of peritoneal macrophages from Chop-null mice, completely blocked NSAID-induced cell death (Tsutsumi et al., 2004). In vivo, Chop induction not only preceded ulcer formation in mice that were given diclofenac, but homozygous Chop-knockout mice were refractory to diclofenac enteropathy (LoGuidice et al., 2010). Taken together, these data suggest a critical role of ER stress in the early topical effects of NSAID enteropathy.
SECOND HIT: INNATE IMMUNE SYSTEM AND INFLAMMATORY RESPONSE
The second stage of NSAID-induced injury to the small intestine is an inflammatory response. This is triggered by bacteria and bacteria-derived proinflammatory mediators invading mucosal layers beyond the epithelium. As a consequence, TLR-mediated signaling pathways are activated, and neutrophils begin to infiltrate into the damaged areas. For example, neutrophils accumulate as early as 6h after a challenge with indomethacin (Stadnyk et al., 2002). Also, proinflammatory mediators are activated in the small intestinal mucosa (Watanabe et al., 2008). These events are a clear inflammatory response involving cells of the innate immune system. In contrast, the adaptive immune system does not seem to play a critical role in NSAID enteropathy as mice deficient in mature T and B cells (Rag2 −/− mice) exhibited indomethacin-induced small intestinal injury comparable with wild-type control mice (Beck et al., 2000).
LPS/TLR4
TLRs recognize specific molecular patterns on pathogens including bacteria, and they trigger an inflammatory response. One of them, TLR4, is a receptor for LPS (endotoxin), a major component of gram-negative bacteria (Poltorak et al., 1998), whereas other types of bacteria in the gut produce agonists for other TLRs. TLR4, which is expressed on monocytes and macrophages in the lamina propria, has an extracellular leucine-rich repeat domain and an intracellular interleukin-1R (IL-1R) signaling domain (Medzhitov et al., 1997). Upon activation by LPS, TLR4 signals via the accessory protein, MyD88, and activates NF-κB (Medzhitov et al., 1998), followed by activation of the proinflammatory cytokines tumor necrosis factor (TNF) and IL-1β.
To explore the role of TLR4 in the pathogenesis of NSAID enteropathy, a number of approaches have been used (Watanabe et al., 2008). First, pretreatment of mice with antibiotics that inhibit gram-negative enterobacteria but leave gram-positive bacteria intact (e.g., aztreonam), decreased indomethacin (10mg/kg, po)-induced enteropathy by 95%. Second, TLR4-mutant mice (C.C3H-Tlr4 LPS-d) (Vogel et al., 1994) treated with indomethacin or diclofenac (60mg/kg, po) exhibited markedly decreased small intestinal injury (by 80% for diclofenac). Finally, TLR4-knockout mice or MyD88-knockout mice were protected from indomethacin-induced enteropathy (by 79 and 68%, respectively). These findings indicate that the TLR4-MyD88-dependent signaling pathway is involved in amplifying NSAID-induced enteropathy.
High Mobility Group Box 1
High mobility group box 1 (HMGB1) belongs to a family of proteins that are released from cells undergoing necrosis (but not from apoptotic cells) (Wang et al., 1999). HMGB1 is also actively secreted from macrophages during inflammation, where the protein acts as an inflammatory cytokine. HMGB1 is an endogenous ligand for TLR4 and other TLRs. A recent study has provided evidence that this pathway could be involved in NSAID-induced enteropathy (Nadatani et al., 2012). Immunoneutralizing HMGB1 or preventing its release from immune cells by chemical inhibitors attenuated indomethacin-induced enteropathy in mice, whereas exogenous HMGB1 potentiated the ulcerogenic effects. This modulation occurred through TLR4 activation (see above). This is clearly a contributing factor during the second hit, and it is currently not known to what extent this mechanism is critical for NSAID-induced enteropathy.
TNF
There is compelling evidence that TNF-α is causally involved in the pathogenesis of NSAID enteropathy. Normally, prostaglandins, and in particular the intestinal PGE2, inhibit TNF biosynthesis, whereas decreased levels of prostaglandins caused by NSAID exposure induce TNF synthesis (Bertrand et al., 1998). TNF appears to have multiple roles in NSAID enteropathy, but the exact mechanisms are not clear. On the one hand, a number of studies using TNF (or TNF receptor) knockout mice have clearly demonstrated that TNF is involved in enterocyte apoptosis and the subsequent inflammatory response in the small intestine. For example, indomethacin administration to TNF-null mice resulted in significant attenuation of small intestinal injury (Fukumoto et al., 2011); in particular, the acute inflammatory response (PMN infiltration and upregulation of chemokines) was significantly reduced. Similarly, administration of an anti-TNF antibody to rats treated with indomethacin greatly protected from injury (Cury et al., 2008). On the other hand, TNF has been indirectly linked with providing cytoprotective functions in the intestinal mucosa by inducing COX-2 expression, which is mediated by transactivation of EGFR (Hobbs et al., 2011).
Apart from TNF, other cytokines/chemokines are also involved in the pathogenesis of NSAID enteropathy. For example, genetic deletion of IL-17A, a cytokine produced by T cells in the lamina propria that regulates the production of proinflammatory cytokines and chemokines, greatly protected from indomethacin enteropathy in mice, suggesting a role for IL-17A (Yamada et al., 2011).
Neutrophils
Massive infiltration of neutrophils at the areas of ulceration is a characteristic feature of NSAID enteropathy. This is not only evident from histopathology but also from a time-dependent increase in myeloperoxidase activity (a biomarker for neutrophils) following challenge with NSAIDs (Watanabe et al., 2008). The question arises whether these neutrophils are actually contributing to the injury by aggravating the damage via ROS or proteases production or whether they are rather involved in resolving the tissue damage. A number of reports indicate that they might play a key role in aggravating the ulcerative injury. For example, neutrophil depletion by antineutrophil serum resulted in massive reduction of indomethacin-induced enteropathy (Watanabe et al., 2008). Similarly, administration of indomethacin to fucosyltransferase VII-knockout mice (featuring impaired neutrophil recruitment) significantly decreased intestinal injury (Beck et al., 2000). One mechanism by which these neutrophils could damage the tissue is by triggering an oxidative burst; indeed, homozygous gp91phox-knockout mice (lacking the catalytic subunit of a phagocyte plasma membrane NADPH oxidase) were less susceptible to NSAID-induced damage than wild-type mice (Beck et al., 2000). One contributing mechanism of leukocyte recruitment is the NSAID-mediated inhibition of COX-2; normally, COX-2 plays a role in protecting from infiltrating leukocytes, but a deficiency in COX-2 may open the avenue for leukocytes infiltrating into the mucosa and causing inflammation (Wallace et al., 2000).
Role of Underlying Inflammatory Disease
Because many patients who are treated with NSAIDs have an underlying inflammatory condition (the indication for drug treatment in the first place), the question may be asked whether this altered physiological state itself may contribute to the susceptibility to NSAIDs (Boelsterli, 2003). Supporting evidence for this concept stems from a rat model of adjuvant-induced arthritis, in which diclofenac- or indomethacin-induced enteropathy was aggravated after indomethacin or diclofenac, compared with wild-type rats (Kato et al., 2007). Interestingly, TLR4 mRNA and protein levels were both increased in the arthritic rats under basal conditions, and the number of macrophages in the lamina propria also was significantly increased in the arthritic rats. However, without NSAIDs exposure, no intestinal ulcers developed, likely because of normal prostaglandin levels. Thus, it is possible, but not well explored, that the disease against which the NSAIDs are prescribed can modulate the risk of developing enteropathy.
PHARMACOKINETIC DETERMINANTS
An interesting feature of NSAID enteropathy is the highly selective target tissue toxicity. Most NSAIDs are weak acids (pK a 3–6) and, depending on their degree of lipophilicity, mostly absorbed in the stomach (McCormack and Brune, 1987; Rainsford and Bjarnason, 2012). Thus, the distal part of the small intestinal mucosa is not the primary tissue exposed to the drugs after po administration. After both po and ip administration, the carboxylic acid–containing NSAIDs reach the liver, where they are glucuronidated and excreted into the biliary tree. The conjugated NSAIDs (or conjugated oxidative metabolites) then reach the small intestine, where the glucuronide moiety is cleaved off by bacterial β-glucuronidase, exposing the enterocytes to the aglycones (free parent NSAID or oxidative metabolites). Thus, the pharmacokinetics is a major determinant that sets the stage for the primary interaction of NSAIDs with the mucosa (first hit) (Boelsterli and Ramirez-Alcantara, 2011).
Hepatobiliary Excretion and Enterohepatic Cycling of NSAID Glucuronides
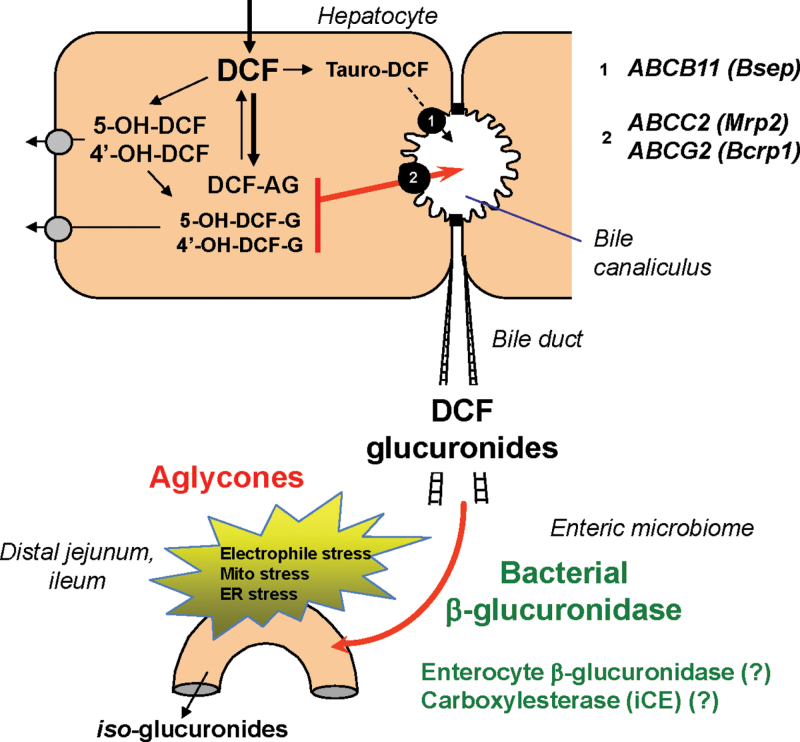
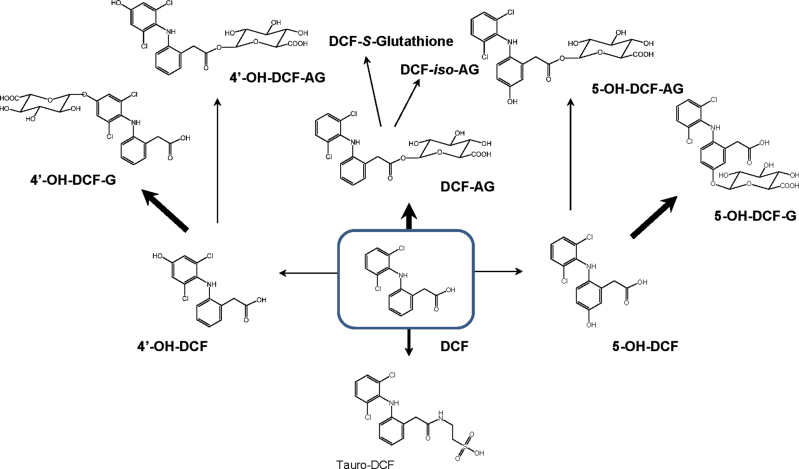
In rodent models, NSAIDs (both parent drug and oxidative metabolites) are conjugated in the liver to glucuronic acid (King et al., 2001; Peris-Ribera et al., 1991) or taurine (Mohri et al., 1998) and exported into bile against a concentration gradient via ATP-dependent transporters (Fig. 2). For example, diclofenac glucuronides are transported across the apical membrane of hepatocytes into the bile canaliculus primarily by Mrp2 (ABCC2) (Seitz and Boelsterli, 1998) or Bcrp1 (ABCG2) (Lagas et al., 2010), whereas the specific carrier(s) for the tauroconjugates are less well defined. However, the exact metabolite profile in bile has not yet been determined. In mice, only ~4% of an orally administered dose of diclofenac was excreted in bile as acyl glucuronide (Lagas et al., 2010). Currently, it is not known whether and to what extent other conjugates (acyl glucuronides or phenol glucuronides of the 4′-hydroxy and 5-hydroxy diclofenac, or even diglucuronides) are excreted through bile. However, a recent study using high-resolution mass spectrometric analysis of the metabolite profile has revealed that there are a number of distinct diclofenac glucuronide species present and that phenol (ether) glucuronides and taurine conjugates are the most abundant metabolites in mouse plasma (Sarda et al., 2012) (Fig. 3). In addition, a portion of the acyl glucuronides can be converted into iso-glucuronides, isomers formed after transacylation and migration of the aglycone along the sugar ring (Dickinson, 2011).
Fig. 2.
Hepatic glucuronoconjugation of diclofenac (DCF), biliary export of glucuronides, and site-selective jejunal hydrolysis of glucuronides by bacterial β-glucuronidase. The local release of the aglycones causes electrophile stress and ER/mitochondrial stress. AG, acyl glucuronide; G, ether glucuronide.
Fig. 3.
Metabolic pathways of diclofenac (DCF) conversion to its oxidative (CYP-mediated) and tauro- or glucuronoconjugates. Thick arrows, prevalent pathways. AG, acyl glucuronide; G, ether glucuronide; iso-AG, transacetylated isomers of DCF acyl glucuronide.
Early studies had indicated that NSAIDs undergoing enterohepatic circulation are more likely to cause enteropathy than those without enterohepatic circulation, due to repeated and prolonged exposure of the gut mucosa to the drugs (Reuter et al., 1997; Wax et al., 1970). The key to this observation is the fact that, in the small intestine, the glucuronides must be cleaved to be reabsorbed. For example, for diclofenac, the bulk of the drug reaching the small intestinal lumen is conjugated, whereas the proportion of free parent drug was negligible (Lagas et al., 2010). One mechanism for the release of the aglycone is spontaneous hydrolysis of the glucuronide, which is pH-dependent. For example, acyl glucuronides are unstable under alkaline conditions, whereas ether (phenolic) glucuronides are generally more stable (Brunelle and Verbeeck, 1993). In humans, the pH of the distal small intestine has been reported to be ~7.5 (Evans et al., 1988), but for rodents, the intestinal pH is clearly lower; in fact, mouse jejunum and ileum have a pH of 4.8, and rat jejunum and ileum have a pH of 5.1 and 5.9, respectively (McConnell et al., 2008). Interestingly, in the fasted state, the pH was slightly higher, i.e., 5.0 and 5.2 in mouse jejunum and ileum, respectively, than in the fed state. Thus, it appears that, at least in rodents, the contribution of spontaneous hydrolysis of the glucuronides is small; therefore, other mechanisms are more prevalent, the most relevant one being enzymatic cleavage of the glucuronides by bacterial β-glucuronidase.
Role of Enteric Bacteria and Bacterial β-Glucuronidase
Approximately 100 trillion bacterial cells stably reside in the mammalian intestine (Vaishnava et al., 2011). They are important for normal health, but they could also potentially induce harmful immune responses. Although in the colon a mucus layer acts as a physical barrier separating the mucosa from the bacteria, in the small intestine, such a thick mucus layer would interfere with absorption of nutrients. Instead, an antimicrobial peptide, RegIIIg, that is secreted by enterocytes, keeps the bacteria at a distance of ~50 µm (Vaishnava et al., 2011).
Enteric bacteria, in particular gram-negative bacteria, play an important role as a causative factor in NSAID enteropathy. Early studies with germ-free rodents or rodents given antibiotics clearly revealed that the elimination of the intestinal microflora significantly protected from NSAID-associated ulceration (Kent et al., 1969; Robert and Asano, 1977; Uejima et al., 1996). More recently, the key role of the enteric microflora, and in particular the specific composition, has been further highlighted in the following study. Rats treated with naproxen together with a PPI (omeprazole or lansoprazole) developed more severe intestinal ulceration and bleeding than naproxen alone–treated rats. This was primarily due to shifts in the numbers and types of enteric bacteria, including an 80% reduction of jejunal Actinobacteria and Bifidobacteria (Wallace et al., 2011). When germ-free mice were colonized with jejunal bacteria from PPI-treated rats, the severity of enteropathy was enhanced compared with colonization with bacteria from vehicle control rats.
The reasons why bacteria can contribute to NSAID enteropathy are twofold. First, bacteria can invade deeper layers of the mucosa when the tight junctions become more permeable after a toxic insult and subsequently activate TLRs (see above). Second, and importantly, bacteria can metabolically convert NSAID glucuronides into aglycones by the action of β-glucuronidase, an enzyme that bacteria employ to scavenge for sugars. β-Glucuronidase activity, however, is not found in all enteric bacterial strains. For example, in an analysis of human colonic bacteria, the gene gus, which encodes for β-glucuronidase, was found in only nine strains of a total of 40 strains screened (including Roseburia hominis and Roseburia intestinalis, and Faecalibacterium prausnitzii [Dabek et al., 2008]) and correlated well with screens for activity measurements. However, other data indicate that ~50% of the human intestinal symbiotic bacteria of known sequence encode for a β-glucuronidase (Wallace et al., 2010).
An analysis of the relative activity of β-glucuronidase in the proximal part of the small intestine in comparison with that in the distal part of the small intestine across different species revealed two major findings. Firstly, the distal part exhibited higher bacterial β-glucuronidase activity than the proximal part (Hawksworth et al., 1971). Secondly, rodents (especially mice) have much higher intestinal β-glucuronidase activity than humans (Hawksworth et al., 1971). These data could perhaps help explain the higher severity of ulceration in the distal parts of the small intestine and provide a plausible explanation for the observed greater susceptibility of rodents to NSAIDs compared with humans. However, the relative contribution of bacterial β-glucuronidase (in the lumen) as opposed to the mammalian host β-glucuronidase (in enterocyte ER and lysosomes) has not yet been clearly determined genetically.
NOVEL STRATEGIES AND PHARMACOLOGICAL APPROACHES TO PROTECT FROM NSAID ENTEROPATHY
Some current approaches to avoid GI damage following NSAID therapy include the use of selective COX-2 inhibitors, the development of NO- or H2S-releasing NSAIDs, and the coadministration of PPIs (to reduce gastropathy only). However, currently, there are no approved pharmacological interventions that can treat or fully prevent NSAID-induced enteropathy (Park et al., 2011; Wallace, 2012); compounds aimed at reducing the inflammatory response or to stimulate prostaglandin-mediated effects have been used, but with limited efficacy and/or adverse effects (Park et al., 2011; Wallace, 2012). Importantly, most experimental and therapeutic approaches to alleviate enteropathy have focused on the second hit, the inflammatory component, whereas approaches to protect from the first hit (mitochondrial stress, ER stress, electrophile stress) or even upstream mechanisms (delivery of glucuronides and local release of the aglycones) have not been fully explored.
Due to the multiple mechanisms involved in NSAID enteropathy, several strategies can be applied, including prostaglandin cotherapy and inhibition of mitochondrial signaling of cell death. However, a novel and promising approach is the targeted inhibition of aglycone release in the small intestine by specific bacterial enzyme inhibitors.
Prostaglandin Cotherapy and Other Mucosa-Protective Agents
Because of the postulated protective effects of some prostaglandins, cotherapy with PGs or PG analogs theoretically should result in an at least partial protection from NSAID-induced enteropathy (Takeuchi et al., 2010a). Clinical data demonstrating efficacy were lacking until the recent introduction of capsule endoscopy. A study using this novel diagnostic technique has demonstrated that coadministration in patients of misoprostol, a PGE1 analog, reduced small intestinal mucosal lesions associated with diclofenac therapy (3 × 25mg/day for 2 weeks) by 75% (Fujimori et al., 2009). Although this is a promising avenue for further investigations, certain adverse effects, including diarrhea, abdominal pain, and the inconvenience of multiple daily dosing, may limit this approach. In addition, misoprostol could not be used in women of child-bearing potential due to its abortifacient activity.
An alternative clinical approach has been the use of rebamipide, a mucosa-protective agent used for treating gastric ulcers, in NSAID-induced enteropathy (Fujimori et al., 2011). Although rebamipide has certain ulcer-preventive effects, the mechanisms are not understood.
Pharmacological Inhibition of Mitochondrial Stress
Mitochondrial permeabilization and release of mitochondrial mediators signaling in cell death pathways are regulated by a number of factors; a key regulator of the mPT pore seems to be CypD (see above). Cotreatment of mice with an ulcerogenic dose of diclofenac and the selective mitochondrial CypD inhibitor, alisporivir (Debio 025), afforded significant protection against the NSAID-induced intestinal permeability increase and enteropathy (LoGuidice et al., 2010). Because alisporivir is undergoing clinical trials as an agent to treat hepatitis C through preventing mitochondrial dysfunction, its enteric-protective potential should also be investigated in patients (Quarato et al., 2012).
Pharmacological Inhibition of Bacterial β-Glucuronidase
Site-specific delivery of bile-derived NSAID glucuronides and local release of the aglycones in the jejunal/ileal lumen by bacterial enzymes are key mechanisms in the pathogenesis of enteropathy (see above). The recent demonstration that the selective inhibition of bacterial β-glucuronidases with a bacteria-specific chemical inhibitor alleviates diclofenac-induced small intestinal ulceration in mice strongly supports this concept (LoGuidice et al., 2012). These novel small molecule bacterial β-glucuronidase inhibitors belong to a class of compounds that have proven effective in protecting against the toxicity of CPT-11 (irinotecan) through the same mechanism (Ahmad et al., 2011; Wallace et al., 2010). The bacterial β-glucuronidase inhibitor employed has no effects on the mammalian β-glucuronidase and is not lethal to either human or bacterial cells. Thus, it is considered selective and nontoxic in studies conducted to date. Because this pharmacokinetic interaction apparently did not alter the systemic exposure to the pharmacologically active parent diclofenac (the majority of biliary glucuronides are ring-hydroxylated metabolites, rather than the parent drug) (LoGuidice et al., 2012), it is likely that the desired anti-inflammatory and analgesic effect of the NSAID would remain unaffected. Further pharmacokinetic and pharmaco-/toxicodynamic characterization of these small molecule inhibitors is currently under way.
Conclusions
The prescription of nonselective COX-1/2 inhibitors against rheumatoid disorders is likely to increase with aging populations and because many health professionals are reluctant to prescribe selective COX-2 inhibitors due to their potential for cardiovascular complications. Furthermore, NSAIDs find increasing clinical application as anticancer drugs or to treat Alzheimer’s disease. Therefore, it is likely that NSAID-induced enteropathy will be increasingly diagnosed. Recent advances in understanding the mechanisms of small bowel ulceration have clearly indicated that the pathogenesis is distinct from that in the upper GI tract. It has become equally clear that hepatobiliary delivery of NSAID glucuronides to the jejunum/ileum, followed by site-selective release of the aglycones by bacterial β-glucuronidase, is a major determinant of enteropathy. This is followed by enteric bioactivation, electrophile stress, and mitochondrial injury to enterocytes (first hit), followed by an inflammatory response (second hit). Thus, bacterial β-glucuronidase has a high potential to becoming a potentially novel therapeutic target in treating NSAID-induced enteropathy.
FUNDING
Boehringer Ingelheim Endowed Chair in Mechanistic Toxicology at the University of Connecticut; research grant from Helsinn Healthcare, SA, Switzerland (419542 to U.A.B.); National Institutes of Health (CA98468 to M.R.R.).
Acknowledgments
The authors disclose no conflicts.
References
- Adler D. H., Phillips J. A., 3rd, Cogan J. D., Iverson T. M., Schnetz-Boutaud N., Stein J. A., Brenner D. A., Milne G. L., Morrow J. D., Boutaud O., et al. (2009). The enteropathy of prostaglandin deficiency. J. Gastroenterol. 44(Suppl. 19)1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Hughes M. A., Lane K. T., Redinbo M. R., Yeh L. A., Scott J. E. (2011). A high throughput assay for discovery of bacterial β-glucuronidase inhibitors. Curr. Chem. Genomics 5, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nasser I. A. (2000). Ibuprofen-induced liver mitochondrial permeability transition. Toxicol. Lett. 111, 213–218 [DOI] [PubMed] [Google Scholar]
- Allison M. C., Howatson A. G., Torrance C. J., Lee F. D., Russell R. I. (1992). Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 327, 749–754 [DOI] [PubMed] [Google Scholar]
- Anthony A., Dhillon A. P., Nygard G., Hudson M., Piasecki C., Strong P., Trevethick M. A., Clayton N. M., Jordan C. C., Pounder R. E. (1993). Early histological features of small intestinal injury induced by indomethacin. Aliment. Pharmacol. Ther. 7, 29–39 [DOI] [PubMed] [Google Scholar]
- Atchison C. R., West A. B., Balakumaran A., Hargus S. J., Pohl L. R., Daiker D. H., Aronson J. F., Hoffmann W. E., Shipp B. K., Treinen-Moslen M. (2000). Drug enterocyte adducts: Possible causal factor for diclofenac enteropathy in rats. Gastroenterology 119, 1537–1547 [DOI] [PubMed] [Google Scholar]
- Baines C. P. (2009). The molecular composition of the mitochondrial permeability transition pore. J. Mol. Cell. Cardiol. 46, 850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P. L., Xavier R., Lu N., Nanda N. N., Dinauer M., Podolsky D. K., Seed B. (2000). Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology 119, 699–705 [DOI] [PubMed] [Google Scholar]
- Bertrand V., Guimbaud R., Tulliez M., Mauprivez C., Sogni P., Couturier D., Giroud J. P., Chaussade S., Chauvelot-Moachon L. (1998). Increase in tumor necrosis factor-alpha production linked to the toxicity of indomethacin for the rat small intestine. Br. J. Pharmacol. 124, 1385–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., MacPherson A. J., Russell A. S. (1993). Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104, 1832–1847 [DOI] [PubMed] [Google Scholar]
- Björnsson E., Westgaard G., Bjarnason I. (2008). Severe injury to the small bowel associated with a short course of diclofenac. Scand. J. Gastroenterol. 43, 759–760 [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A. (2003). Disease-related determinants of susceptibility to drug-induced idiosyncratic hepatotoxicity. Curr. Opin. Drug Discov. Devel. 6, 81–91 [PubMed] [Google Scholar]
- Boelsterli U. A., Ramirez-Alcantara V. (2011). NSAID acyl glucuronides and enteropathy. Curr. Drug Metab. 12, 245–252 [DOI] [PubMed] [Google Scholar]
- Brunelle F. M., Verbeeck R. K. (1993). Glucuronidation of diflunisal by rat liver microsomes. Effect of microsomal beta-glucuronidase activity. Biochem. Pharmacol. 46, 1953–1958 [DOI] [PubMed] [Google Scholar]
- Bruyère A., Declevès X., Bouzom F., Proust L., Martinet M., Walther B., Parmentier Y. (2009). Development of an optimized procedure for the preparation of rat intestinal microsomes: Comparison of hepatic and intestinal microsomal cytochrome P450 enzyme activities in two rat strains. Xenobiotica. 39, 22–32 [DOI] [PubMed] [Google Scholar]
- Burnett B. P., Levy R. M. (2012). 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 29, 79–98 [DOI] [PubMed] [Google Scholar]
- Cury D. H., Costa J. E., Irika K., Mijji L., Garcez A., Buchiguel C., Silva I., Sipahi A. (2008). Protective effect of octreotide and infliximab in an experimental model of indomethacin-induced inflammatory bowel disease. Dig. Dis. Sci. 53, 2516–2520 [DOI] [PubMed] [Google Scholar]
- Dabek M., McCrae S. I., Stevens V. J., Duncan S. H., Louis P. (2008). Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495 [DOI] [PubMed] [Google Scholar]
- Daniell H. W. (2012). NSAID-PPI enteropathy in humans. Gastroenterology 142, e20 [DOI] [PubMed] [Google Scholar]
- Davies N. M. (1995). Toxicity of nonsteroidal anti-inflammatory drugs in the large intestine. Dis. Colon Rectum 38, 1311–1321 [DOI] [PubMed] [Google Scholar]
- Davies N. M., Saleh J. Y., Skjodt N. M. (2000). Detection and prevention of NSAID-induced enteropathy. J. Pharm. Pharm. Sci. 3, 137–155 [PubMed] [Google Scholar]
- DeSesso J. M., Jacobson C. F. (2001). Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 39, 209–228 [DOI] [PubMed] [Google Scholar]
- Dial E. J., Darling R. L., Lichtenberger L. M. (2008). Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J. Gastroenterol. Hepatol. 23(8 Pt 2)e384–e389 [DOI] [PubMed] [Google Scholar]
- Dickinson R. G. (2011). Iso-glucuronides. Curr. Drug Metab. 12, 222–228 [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Hooke K. F., Noll R. M., Kwan K. C. (1975). Enterohepatic circulation of indomethacin and its role in intestinal irritation. Biochem. Pharmacol. 24, 1749–1754 [DOI] [PubMed] [Google Scholar]
- Evans D. F., Pye G., Bramley R., Clark A. G., Dyson T. J., Hardcastle J. D. (1988). Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29, 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun P. J., Hawkey C. J. (2007). Nonsteroidal antiinflammatory drugs and the small intestine. Curr. Opin. Gastroenterol. 23, 134–141 [DOI] [PubMed] [Google Scholar]
- Fujimori S., Gudis K., Takahashi Y., Seo T., Yamada Y., Ehara A., Kobayashi T., Mitsui K., Yonezawa M., Tanaka S., et al. (2010). Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur. J. Clin. Invest. 40, 504–510 [DOI] [PubMed] [Google Scholar]
- Fujimori S., Seo T., Gudis K., Ehara A., Kobayashi T., Mitsui K., Yonezawa M., Tanaka S., Tatsuguchi A., Sakamoto C. (2009). Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: A pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest. Endosc. 69, 1339–1346 [DOI] [PubMed] [Google Scholar]
- Fujimori S., Takahashi Y., Gudis K., Seo T., Ehara A., Kobayashi T., Mitsui K., Yonezawa M., Tanaka S., Tatsuguchi A., et al. (2011). Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: A double-blind, randomized, controlled trial evaluated by capsule endoscopy. J. Gastroenterol. 46, 57–64 [DOI] [PubMed] [Google Scholar]
- Fukumoto K., Naito Y., Takagi T., Yamada S., Horie R., Inoue K., Harusato A., Hirata I., Omatsu T., Mizushima K., et al. (2011). Role of tumor necrosis factor-α in the pathogenesis of indomethacin-induced small intestinal injury in mice. Int. J. Mol. Med. 27, 353–359 [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. (2009). What is the mitochondrial permeability transition pore?. J. Mol. Cell. Cardiol. 46, 821–831 [DOI] [PubMed] [Google Scholar]
- Harusato A., Naito Y., Takagi T., Uchiyama K., Mizushima K., Hirai Y., Yamada S., Tuji T., Yoriki H., Horie R., et al. (2011). Suppression of indomethacin-induced apoptosis in the small intestine due to Bach1 deficiency. Free Radic. Res. 45, 717–727 [DOI] [PubMed] [Google Scholar]
- Hawksworth G., Drasar B. S., Hill M. J. (1971). Intestinal bacteria and the hydrolysis of glycosidic bonds. J. Med. Microbiol. 4, 451–459 [DOI] [PubMed] [Google Scholar]
- Hobbs S. S., Goettel J. A., Liang D., Yan F., Edelblum K. L., Frey M. R., Mullane M. T., Polk D. B. (2011). TNF transactivation of EGFR stimulates cytoprotective COX-2 expression in gastrointestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G220–G229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz-Behofsits C., Simpson R. J., Walley M., Bjarnason I. T. (2010). Role of COX-2 in nonsteroidal anti-inflammatory drug enteropathy in rodents. Scand. J. Gastroenterol. 45, 822–827 [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Zhang Q. Y. (2003). The small intestine as a xenobiotic-metabolizing organ. Drug Metab. Dispos. 31, 1520–1525 [DOI] [PubMed] [Google Scholar]
- Kato S., Ito Y., Nishio H., Aoi Y., Amagase K., Takeuchi K. (2007). Increased susceptibility of small intestine to NSAID-provoked ulceration in rats with adjuvant-induced arthritis: Involvement of enhanced expression of TLR4. Life Sci. 81, 1309–1316 [DOI] [PubMed] [Google Scholar]
- Kent T. H., Cardelli R. M., Stamler F. W. (1969). Small intestinal ulcers and intestinal flora in rats given indomethacin. Am. J. Pathol. 54, 237–249 [PMC free article] [PubMed] [Google Scholar]
- King C., Tang W., Ngui J., Tephly T., Braun M. (2001). Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of diclofenac. Toxicol. Sci. 61, 49–53 [DOI] [PubMed] [Google Scholar]
- Komura H., Iwaki M. (2011). In vitro and in vivo small intestinal metabolism of CYP3A and UGT substrates in preclinical animals species and humans: Species differences. Drug Metab. Rev. 43, 476–498 [DOI] [PubMed] [Google Scholar]
- Ku E. C., Lee W., Kothari H. V., Scholer D. W. (1986). Effect of diclofenac sodium on the arachidonic acid cascade. Am. J. Med. 80(4B)18–23 [DOI] [PubMed] [Google Scholar]
- Lagas J. S., Sparidans R. W., Wagenaar E., Beijnen J. H., Schinkel A. H. (2010). Hepatic clearance of reactive glucuronide metabolites of diclofenac in the mouse is dependent on multiple ATP-binding cassette efflux transporters. Mol. Pharmacol. 77, 687–694 [DOI] [PubMed] [Google Scholar]
- Langenbach R., Loftin C., Lee C., Tiano H. (1999). Cyclooxygenase knockout mice: Models for elucidating isoform-specific functions. Biochem. Pharmacol. 58, 1237–1246 [DOI] [PubMed] [Google Scholar]
- Lerche C. M., Philipsen P. A., Poulsen T., Wulf H. C. (2011). High death rate in mice treated topically with diclofenac. Exp. Dermatol. 20, 336–338 [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Zhou Y., Jayaraman V., Doyen J. R., O’Neil R. G., Dial E. J., Volk D. E., Gorenstein D. G., Boggara M. B., Krishnamoorti R. (2012). Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: Characterization of interaction of NSAIDs with phosphatidylcholine. Biochim. Biophys. Acta 1821, 994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGuidice A., Ramirez-Alcantara V., Proli A., Gavillet B., Boelsterli U. A. (2010). Pharmacologic targeting or genetic deletion of mitochondrial cyclophilin D protects from NSAID-induced small intestinal ulceration in mice. Toxicol. Sci. 118, 276–285 [DOI] [PubMed] [Google Scholar]
- LoGuidice A., Wallace B. D., Bendel L., Redinbo M. R., Boelsterli U. A. (2012). Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J. Pharmacol. Exp. Ther. 341, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehata Y., Esaki M., Morishita T., Kochi S., Endo S., Shikata K., Kobayashi H., Matsumoto T. (2012). Small bowel injury induced by selective cyclooxygenase-2 inhibitors: A prospective, double-blind, randomized clinical trial comparing celecoxib and meloxicam. J. Gastroenterol. 47, 387–393 [DOI] [PubMed] [Google Scholar]
- Mahmud T., Scott D. L., Bjarnason I. (1996). A unifying hypothesis for the mechanism of NSAID related gastrointestinal toxicity. Ann. Rheum. Dis. 55, 211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden L. (2009). Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J. Gastroenterol. 44(Suppl. 19)64–71 [DOI] [PubMed] [Google Scholar]
- Maiden L., Thjodleifsson B., Seigal A., Bjarnason I. I., Scott D., Birgisson S., Bjarnason I. (2007). Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 5, 1040–1045 [DOI] [PubMed] [Google Scholar]
- Maier T. J., Tausch L., Hoernig M., Coste O., Schmidt R., Angioni C., Metzner J., Groesch S., Pergola C., Steinhilber D., et al. (2008). Celecoxib inhibits 5-lipoxygenase. Biochem. Pharmacol. 76, 862–872 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y., Nakayama S., Horie T. (2002). Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology 35, 544–551 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y., Yamada S., Horie T. (2000). Possible mechanism of hepatocyte injury induced by diphenylamine and its structurally related nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 292, 982–987 [PubMed] [Google Scholar]
- McConnell E. L., Basit A. W., Murdan S. (2008). Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 60, 63–70 [DOI] [PubMed] [Google Scholar]
- McCormack K., Brune K. (1987). Classical absorption theory and the development of gastric mucosal damage associated with the non-steroidal anti-inflammatory drugs. Arch. Toxicol. 60, 261–269 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr (1997). A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr (1998). MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- Mohri K., Okada K., Benet L. Z. (1998). Stereoselective metabolism of benoxaprofen in rats. Biliary excretion of benoxaprofen taurine conjugate and glucuronide. Drug Metab. Dispos. 26, 332–337 [PubMed] [Google Scholar]
- Montrose D. C., Kadaveru K., Ilsley J. N., Root S. H., Rajan T. V., Ramesh M., Nichols F. C., Liang B. T., Sonin D., Hand A. R., et al. (2010). cPLA2 is protective against COX inhibitor-induced intestinal damage. Toxicol. Sci. 117, 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaciva S., Bernal A., Aggeler R., Capaldi R., Will Y. (2007). Target identification of drug induced mitochondrial toxicity using immunocapture based OXPHOS activity assays. Toxicol. In Vitro 21, 902–911 [DOI] [PubMed] [Google Scholar]
- Nadatani Y., Watanabe T., Tanigawa T., Machida H., Okazaki H., Yamagami H., Watanabe K., Tominaga K., Fujiwara Y., Arakawa T. (2012). High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am. J. Pathol. 181, 98–110 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Matsushita A., Matsuno K., Iwasaki K., Utoh M., Nakamura C., Uno Y. (2010). Regional distribution of cytochrome p450 mRNA expression in the liver and small intestine of cynomolgus monkeys. Drug Metab. Pharmacokinet. 25, 290–297 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Matsushita A., Matsuno K., Iwasaki K., Utoh M., Nakamura C., Uno Y. (2011). Regional distribution of drug-metabolizing enzyme activities in the liver and small intestine of cynomolgus monkeys. Drug Metab. Pharmacokinet. 26, 288–294 [DOI] [PubMed] [Google Scholar]
- Nazli A., Yang P. C., Jury J., Howe K., Watson J. L., Söderholm J. D., Sherman P. M., Perdue M. H., McKay D. M. (2004). Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 164, 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Shiokawa A., Ito K., Masuyama R., Ichibangase T., Kishikawa N., Imai K., Kuroda N. (2012). Toxicoproteomic analysis of a mouse model of nonsteroidal anti-inflammatory drug-induced gastric ulcers. Biochem. Biophys. Res. Commun. 420, 210–215 [DOI] [PubMed] [Google Scholar]
- Park S. C., Chun H. J., Kang C. D., Sul D. (2011). Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J. Gastroenterol. 17, 4647–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Ribera J. E., Torres-Molina F., Garcia-Carbonell M. C., Aristorena J. C., Pla-Delfina J. M. (1991). Pharmacokinetics and bioavailability of diclofenac in the rat. J. Pharmacokinet. Biopharm. 19, 647–665 [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., et al. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- Quarato G., D’Aprile A., Gavillet B., Vuagniaux G., Moradpour D., Capitanio N., Piccoli C. (2012). The cyclophilin inhibitor alisporivir prevents hepatitis C virus-mediated mitochondrial dysfunction. Hepatology 55, 1333–1343 [DOI] [PubMed] [Google Scholar]
- Rainsford K. D., Bjarnason I. (2012). NSAIDs: Take with food or after fasting?. J. Pharm. Pharmacol. 64, 465–469 [DOI] [PubMed] [Google Scholar]
- Ramirez-Alcantara V., LoGuidice A., Boelsterli U. A. (2009). Protection from diclofenac-induced small intestinal injury by the JNK inhibitor SP600125 in a mouse model of NSAID-associated enteropathy. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G990–G998 [DOI] [PubMed] [Google Scholar]
- Reuter B. K., Davies N. M., Wallace J. L. (1997). Nonsteroidal anti-inflammatory drug enteropathy in rats: Role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 112, 109–117 [DOI] [PubMed] [Google Scholar]
- Robert A., Asano T. (1977). Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins 14, 333–341 [DOI] [PubMed] [Google Scholar]
- Sandoval-Acuña C., Lopez-Alarcón C., Aliaga M. E., Speisky H. (2012). Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem. Biol. Interact. 199, 18–28 [DOI] [PubMed] [Google Scholar]
- Sapirstein A., Bonventre J. V. (2000). Specific physiological roles of cytosolic phospholipase A(2) as defined by gene knockouts. Biochim. Biophys. Acta 1488, 139–148 [DOI] [PubMed] [Google Scholar]
- Sarda S., Page C., Pickup K., Schulz-Utermoehl T., Wilson I. (2012). Diclofenac metabolism in the mouse: Novel in vivo metabolites identified by high performance liquid chromatography coupled to linear ion trap mass spectrometry. Xenobiotica. 42, 179–194 [DOI] [PubMed] [Google Scholar]
- Satoh H., Amagase K., Takeuchi K. (2012). Exacerbation of nonsteroidal anti-inflammatory drug-induced small intestinal lesions by antisecretory drugs in rats: The role of intestinal motility. J. Pharmacol. Exp. Ther. 343, 270–277 [DOI] [PubMed] [Google Scholar]
- Satoh H., Takeuchi K. (2012). Role of food and enterobacteria in the formation and prevention of small intestinal damage induced by NSAIDs. In Cell/Tissue Injury and Cytoprotection/Organoprotection in the Gastrointestinal Tract: Mechanisms, Prevention and Treatment (Filaretova L. P., Takeuchi K, Eds.), pp. 52–60 Karger: Basel, Switzerland: [Google Scholar]
- Scarpignato C., Hunt R. H. (2010). Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: Clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. North Am. 39, 433–464 [DOI] [PubMed] [Google Scholar]
- Seitz S., Boelsterli U. A. (1998). Diclofenac acyl glucuronide, a major biliary metabolite, is directly involved in small intestinal injury in rats. Gastroenterology 115, 1476–1482 [DOI] [PubMed] [Google Scholar]
- Sidhu R., Brunt L. K., Morley S. R., Sanders D. S., McAlindon M. E. (2010). Undisclosed use of nonsteroidal anti-inflammatory drugs may underlie small-bowel injury observed by capsule endoscopy. Clin. Gastroenterol. Hepatol. 8, 992–995 [DOI] [PubMed] [Google Scholar]
- Sigthorsson G., Simpson R. J., Walley M., Anthony A., Foster R., Hotz-Behoftsitz C., Palizban A., Pombo J., Watts J., Morham S. G., et al. (2002). COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122, 1913–1923 [DOI] [PubMed] [Google Scholar]
- Somasundaram S., Rafi S., Hayllar J., Sigthorsson G., Jacob M., Price A. B., Macpherson A., Mahmod T., Scott D., Wrigglesworth J. M., et al. (1997). Mitochondrial damage: A possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 41, 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S., Sigthorsson G., Simpson R. J., Watts J., Jacob M., Tavares I. A., Rafi S., Roseth A., Foster R., Price A. B., et al. (2000). Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment. Pharmacol. Ther. 14, 639–650 [DOI] [PubMed] [Google Scholar]
- Stadnyk A. W., Dollard C., Issekutz T. B., Issekutz A. C. (2002). Neutrophil migration into indomethacin induced rat small intestinal injury is CD11a/CD18 and CD11b/CD18 co-dependent. Gut 50, 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kato S., Amagase K. (2010a). Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J. Pharmacol. Sci. 114, 248–261 [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Tanaka A., Kato S., Amagase K., Satoh H. (2010b). Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clin. Chim. Acta 411, 459–466 [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Tanigami M., Amagase K., Ochi A., Okuda S., Hatazawa R. (2010c). Endogenous prostaglandin E2 accelerates healing of indomethacin-induced small intestinal lesions through upregulation of vascular endothelial growth factor expression by activation of EP4 receptors. J. Gastroenterol. Hepatol. 25(Suppl. 1)S67–S74 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Hase S., Miyazawa T., Ohno R., Takeuchi K. (2002a). Role of cyclooxygenase (COX)-1 and COX-2 inhibition in nonsteroidal anti-inflammatory drug-induced intestinal damage in rats: Relation to various pathogenic events. J. Pharmacol. Exp. Ther. 303, 1248–1254 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Hase S., Miyazawa T., Takeuchi K. (2002b). Up-regulation of cyclooxygenase-2 by inhibition of cyclooxygenase-1: A key to nonsteroidal anti-inflammatory drug-induced intestinal damage. J. Pharmacol. Exp. Ther. 300, 754–761 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tomisato W., Hoshino T., Ishihara T., Namba T., Aburaya M., Katsu T., Suzuki K., Tsutsumi S., Mizushima T. (2005). Involvement of intracellular Ca2+ levels in nonsteroidal anti-inflammatory drug-induced apoptosis. J. Biol. Chem. 280, 31059–31067 [DOI] [PubMed] [Google Scholar]
- Tay V. K., Wang A. S., Leow K. Y., Ong M. M., Wong K. P., Boelsterli U. A. (2005). Mitochondrial permeability transition as a source of superoxide anion induced by the nitroaromatic drug nimesulide in vitro. Free Radic. Biol. Med. 39, 949–959 [DOI] [PubMed] [Google Scholar]
- Tremaroli V., Bäckhed F. (2012). Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 [DOI] [PubMed] [Google Scholar]
- Tsutsumi S., Gotoh T., Tomisato W., Mima S., Hoshino T., Hwang H. J., Takenaka H., Tsuchiya T., Mori M., Mizushima T. (2004). Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 11, 1009–1016 [DOI] [PubMed] [Google Scholar]
- Uejima M., Kinouchi T., Kataoka K., Hiraoka I., Ohnishi Y. (1996). Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol. Immunol. 40, 553–560 [DOI] [PubMed] [Google Scholar]
- Uyemura S. A., Santos A. C., Mingatto F. E., Jordani M. C., Curti C. (1997). Diclofenac sodium and mefenamic acid: Potent inducers of the membrane permeability transition in renal cortex mitochondria. Arch. Biochem. Biophys. 342, 231–235 [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Yamamoto M., Severson K. M., Ruhn K. A., Yu X., Koren O., Ley R., Wakeland E. K., Hooper L. V. (2011). The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen J. S., Orij R., Luttik M. A., Smits G. J., Vermeulen N. P., Vos J. C. (2011). Subunits Rip1p and Cox9p of the respiratory chain contribute to diclofenac-induced mitochondrial dysfunction. Microbiology (Reading, Engl.) 157(Pt 3)685–694 [DOI] [PubMed] [Google Scholar]
- Vane J. R. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature New Biol. 231, 232–235 [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Wax J. S., Perera P. Y., Padlan C., Potter M., Mock B. A. (1994). Construction of a BALB/c congenic mouse, C.C3H-Lpsd, that expresses the Lpsd allele: Analysis of chromosome 4 markers surrounding the Lps gene. Infect. Immun. 62, 4454–4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. D., Wang H., Lane K. T., Scott J. E., Orans J., Koo J. S., Venkatesh M., Jobin C., Yeh L. A., Mani S., et al. (2010). Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. (2012). NSAID gastropathy and enteropathy: Distinct pathogenesis likely necessitates distinct prevention strategies. Br. J. Pharmacol. 165, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., McKnight W., Reuter B. K., Vergnolle N. (2000). NSAID-induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119, 706–714 [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Syer S., Denou E., de Palma G., Vong L., McKnight W., Jury J., Bolla M., Bercik P., Collins S. M., et al. (2011). Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141, 1314–22, 1322.e1 [DOI] [PubMed] [Google Scholar]
- Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., et al. (1999). HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Higuchi K., Kobata A., Nishio H., Tanigawa T., Shiba M., Tominaga K., Fujiwara Y., Oshitani N., Asahara T., et al. (2008). Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 57, 181–187 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tanigawa T., Nadatani Y., Otani K., Machida H., Okazaki H., Yamagami H., Watanabe K., Tominaga K., Fujiwara Y., et al. (2011). Mitochondrial disorders in NSAIDs-induced small bowel injury. J. Clin. Biochem. Nutr. 48, 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax J., Clinger W. A., Varner P., Bass P., Winder C. V. (1970). Relationship of the enterohepatic cycle to ulcerogenesis in the rat small bowel with flufenamic acid. Gastroenterology 58, 772–780 [PubMed] [Google Scholar]
- Whittle B. J. (2004). Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur. J. Pharmacol. 500, 427–439 [DOI] [PubMed] [Google Scholar]
- Wolfe M. M., Lichtenstein D. R., Singh G. (1999). Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 340, 1888–1899 [DOI] [PubMed] [Google Scholar]
- Wright M. R., Davies N. M., Jamali F. (1997). Toxicokinetics of indomethacin-induced intestinal permeability in the rat. Pharmacol. Res. 35, 499–504 [DOI] [PubMed] [Google Scholar]
- Yamada S., Naito Y., Takagi T., Mizushima K., Hirai Y., Horie R., Fukumoto K., Inoue K., Harusato A., Yoshida N., et al. (2011). Reduced small-intestinal injury induced by indomethacin in interleukin-17A-deficient mice. J. Gastroenterol. Hepatol. 26, 398–404 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhang Q. Y. (2012). Role of intestinal cytochrome p450 enzymes in diclofenac-induced toxicity in the small intestine. J. Pharmacol. Exp. Ther. 343, 362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]