Abstract
The anticancer drug (2-[4-amino-3-methylphenyl]-5-fluorobenzothiazole lysylamide dihydrochloride) (NSC 710305, Phortress) is a metabolically activated prodrug that causes DNA adduct formation and subsequent toxicity. Preclinically, it was found that hepatic, bone marrow, and pulmonary toxicity presented challenges to developing this drug. An ex vivo precision-cut lung slice (PCLS) model was used to search for concentration dependent effects of NSC 710305 (10, 25, 50, and 100µM) on cytokine content, protein content, and immuno/histological endpoints. Preparation and culture of PCLS caused an initial spike in proinflammatory cytokine expression and therefore treatment with NSC 710305 was delayed until 48h after initiating the slice cultures to avoid confounding the response to slicing with any drug response. PCLSs were evaluated after 24, 48, and 72h exposures to NSC 710305. Reversibility of toxicity due to the 72-h treatment was evaluated after a 24-h recovery period. NSC 710305 caused a concentration-dependent cytokine response, and only the toxicity caused by a 72-h exposure to 25µM reversed during the 24-h recovery period. Immuno/histological examination and quantitation of tissue protein levels indicated that tissue destruction, ED-1 (activated macrophage) staining, and protein levels were associated with the levels of proinflammatory cytokines in the tissue. In conclusion, the concentration- and time-dependent inflammatory response of PCLS to NSC 710305 preceded relevant tissue damage by a few days. The no-observable adverse effect level (NOAEL) for 24, 48, and 72h exposures was established as 10µM NSC 710305.
Key Words: NSC 710305, Phortress, PCLS, lung slices, cytokines, lung injury, inflammation, in vitro, pulmonary toxicity.
Mechanical, microbial, or chemical (e.g., drug) lung injuries are associated with increased concentrations of several proinflammatory cytokines and chemokines, including interleukin 1 beta (IL-1β), interleukin 5 beta (IL-5β), interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and cytokine-induced neutrophil chemoattractant (CINC) (Cannizzaro et al., 2011; Card et al., 2003; Delclaux and Azoulay, 2003; Heise et al., 2011; Hoshino et al., 2009; Kirkman and Watts, 2011; Palmer et al., 2011; Song et al., 2009; Strieter et al., 2003; Yoshida et al., 2010). Bleomycin, methotrexate, and BCNU are examples of drugs which increase proinflammatory cytokine production in association with adverse pulmonary effects. The pulmonary toxicity exhibited by these drugs is typically unresolved inflammation, which, in humans, leads to idiopathic pulmonary fibrosis months or even years later (Degryse et al., 2010; Patten et al., 1980; Reinhart et al., 1996; Strieter and Mehrad, 2009). Amiodarone also increases proinflammatory cytokine production, but induces acute pulmonary toxicity (Ben-Noun, 2000; Papiris et al., 2010). The proinflammatory cytokines, IL-1β and TNF-α, and CINC are part of the initial response in the wound repair mechanism (Crosby and Waters, 2010; Geiser et al., 2000). However, these cytokines are also found in the bronchoalveolar lavage fluids of patients with acute lung injury or acute respiratory distress syndrome (Goodman et al., 1996; Park et al., 2001), and they play a part in the evolution of fibrosis (Kelly et al., 2003). Inflammation, acute or chronic, that does not resolve leads to the destruction of the lung architecture (Keane and Strieter, 2001).
NSC 710305 (2-[4-amino-3-methylphenyl]-5-fluorobenzothiazole lysylamide dihydrochloride) is a P450 CYP1A1-activated antitumor prodrug (Bradshaw et al., 2009). Cytotoxicity in sensitive cell lines, such as the human breast cancer cell line MCF-7, is governed by CYP1A1 metabolism and the subsequent production of reactive metabolites that form DNA adducts. Insensitive cell lines, such as MDA-MB-435 (derived from M14 melanoma cells), lack CYP1A1 and are resistant to NSC 710305 toxicity (Bradshaw et al., 2002a ,b; Leong et al., 2003). In rats, dogs, monkeys, and humans, CYP1A1 expression in the lung is inducible (Graham et al., 2002 ; Harrigan et al., 2006 ; Lee et al., 1998; Martignoni et al., 2006; Schulz et al., 1996 ; Wei et al., 2002), which leads to concern about the possibility of pulmonary toxicity with NSC 710305.
In mice, the maximal tolerated dose (MTD) of 120mg/m2 NSC 710305 (20mg/kg/day on days 1 and 8) decreased lung:body and kidney:body weight ratios and increased serum alkaline phosphatase, indicating hepatic toxicity (Bradshaw et al., 2009). In dogs, a 1-h infusion of 80mg/m2 achieving plasma drug levels within the efficacious range based on in vitro data caused gastrointestinal toxicity and neutropenia. However, a dose 3.5 times higher produced substantial pulmonary, bone marrow, and hepatic toxicity. In nonhuman primates, 1-h infusions of 60mg/m2 produced no evidence of pulmonary toxicity, but increasing the dose to 80mg/m2 resulted in severe pulmonary toxicity and death within 24h (http://dtp.nci.nih.gov/timeline/posters/Aminoflavone.pdf). These findings prompted additional studies to determine if pulmonary toxicity could be monitored by clinical pulmonary function tests, but no clear distinction was observed between the control, low-dose and high-dose NSC-710305 groups.
Precision-cut lung slices (PCLSs) have emerged recently as a useful in vitro model to study inflammation-mediated pulmonary damage, including that resulting from ex vivo exposure to pulmonary toxicants (Amin et al., 2006; Behrsing et al., 2004 , 2006; Henjakovic et al., 2008; Le Prieur et al., 2000; Switalla et al., 2010; Tyson et al., 2005; Wohlsen et al., 2003). The PCLS system is uniquely suited to examine molecular inflammatory responses to toxicant exposures and compare species differences, because the slices contain target cell types within relevant tissue architecture, exposure conditions can be tightly controlled, and human lung tissue can be evaluated. Previous work demonstrated NSC 710305-induced cytokine response (TNF-α and IL-1β) and injury in rat and human PCLS, and importantly human PCLSs were more susceptible to toxicity than rat PCLSs (Amin et al., 2006). This report extends the previous study of NSC 710305 in rat PCLS to characterize drug-induced lung inflammation and damage. The method of preparing PCLS induced a cytokine response itself; so, PCLS cultures were allowed a recovery period for cytokine levels to stabilize prior to starting ex vivo NSC 710305 treatment.
MATERIALS AND METHODS
Animal care and use.
All vertebrate animal work was conducted at the Frederick National Laboratory for Cancer Research (FNLCR) facility in Frederick, MD, which is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. All animal work was done in accordance with institutional and governmental guiding principles in the care and use of rats, in compliance with procedures outlined in the “Guide for Care and Use of Laboratory Animals” (ILAR et al., 1996).
Chemicals.
Glutathione, bovine serum albumin (BSA), hydrocortisone 21-acetate, retinyl acetate, low-melting temperature agarose, dimethyl sulfoxide (DMSO) were obtained from Sigma (St Louis, MO); Humulin N (Human insulin, rDNA origin) was obtained from Eli Lilly (Indianapolis, IN); Medium 199 with l-glutamine without phenol red, Ca+- and Mg+-free PBS, and antibiotic-antimycotic solution were obtained from Invitrogen/Gibco (Carlsbad, CA); Viaspan (Belzer–University of Wisconsin cold storage solution) supplemented with glutathione (3mM) was obtained from Fisher Scientific (Pittsburgh, PA); and NSC 710305 was obtained from the Developmental Therapeutics Program repository (Rockville, MD). MSD Tris Lysis Buffer was obtained from MesoScale Discovery (MSD) (Gaithersburg, MD). Complete Mini, EDTA-free Protease Inhibitor Cocktail was obtained from Roche Applied Science (Indianapolis, IN).
Equipment.
Tissue-coring press and titanium inserts (Vitron; Tucson, AZ); Krumdieck slicer (Alabama Research and Development; Munford, AL); 0.45-μm surfactant-free hydroanalysis mixed cellulose ester triton-free (HATF) filter paper used to support the PCLSs in culture (Millipore; Bedford, MA); polytetrafluoroethylene (PTFE) membrane TF-200 and 0.2-μm filters (Pall Life Sciences; Ann Arbor, MI); low-background glass scintillation vials (Research Products International; Mt Prospect, IL); and TC-8 roller drum units (New Brunswick Scientific; Edison, NJ).
PCLS preparation.
Male Fischer 344 rats were anesthetized with isoflurane inhalation. The lungs were removed and filled with 0.4% low-melting agarose in PBS at ~37°C and then placed in a beaker of ice-cold PBS to solidify the agarose. After 5min, the lungs were transferred to a container of ice-cold Viaspan supplemented with 3mM glutathione. The lobes were separated with a scalpel and cored into cylinders of 8mm in diameter. The cores were then sliced into ∼500-μm-thick disks using a Krumdieck slicer and collected into ice-cold Viaspan supplemented with glutathione. Uniformly shaped slices were selected for experiments. Using a sterile cotton swab, the slices were placed on sterile HATF paper inside the titanium inserts.
Slice equilibration and initiation of slice cultures.
The titanium inserts with the slices were placed in sterile scintillation vials, each containing a 1.7-ml culture medium consisting of Medium 199 supplemented with 288U/l Humulin N, 100 µg/l hydrocortisone 21-acetate (unless noted otherwise), and 100 µg/l retinoic acid. The vials were then capped with sterilized open-end caps containing PTFE membrane filters, each held in place by a hole-punched Teflon liner to allow gas exchange with the external atmosphere. Vials were placed in the roller drum inside a humidified incubator at 37°C under a 95% air and 5% CO2 atmosphere (culture day 1). The roller drum containing the vials was rotated at 6–7rpm. After equilibration for 3h, the conditioned medium was harvested and replaced with fresh, prewarmed media. After 21 additional hours (culture day 2) and every 24h after that, conditioned medium in each vial was harvested and replaced with fresh medium until the slices were harvested for analysis.
Drug treatment of PCLS.
Treatment with NSC 710305 at designated concentrations began at time = 0h on culture day 3, i.e., 48h after initiating the PCLS cultures. NSC 710305 was introduced into the cell culture medium using 1:1000 dilutions from individual working stock solutions all made as serial dilutions in DMSO from the highest concentration of 1000× stock. Vehicle control groups were exposed to 0.1% DMSO prepared by similar 1:1000 dilution into medium. Each treatment group comprised multiple replicate slices cultured individually in separate tubes. Every 24h, conditioned medium was harvested from each culture vial and replaced with fresh medium containing NSC 710305 or DMSO vehicle until slices were harvested for analysis.
Biomarker analysis.
At the indicated time points, medium and slice samples were collected for processing and analysis. The collected medium (1ml/vial) from every slice culture within an experimental group was pooled and stored at −70°C for subsequent biochemical analysis. PCLSs on the filter paper were removed from their titanium inserts and rinsed in PBS (room temp) by briefly submerging the slice (still on its HATF paper) into a vessel containing PBS. For cytokine and protein analyses, each PCLS was transferred into its own 1.5-ml Eppendorf tube containing 0.5ml lysis buffer (MSD Tris Lysis Buffer supplemented with Complete Mini, EDTA-free Protease Inhibitor Cocktail Tablets) on ice. The sections were then homogenized and briefly sonicated at ice-cold temperatures. The resulting lysates were centrifuged at 9000 × g for 5min to remove particulate matter. The resulting supernatants were stored at −70°C until analysis. To examine the biomarker content, conditioned medium, PCLS lysates, and control medium were sent on ice to the Clinical Services Program of the FNLCR for analysis of content of the cytokines (IL-β, IL-4, IL-5, TNF-α, IFN-γ, and IL-13) and the chemokine CINC, using the MSD platform, according to the manufacturer’s protocols. Protein concentration of the lysate was analyzed using a Pierce BCA protein assay kit (VWR International; West Chester, PA) and BSA standards in lysis buffer.
Histology.
The faces of slice sections designated for morphological examination were covered by lens paper (wetted in 10% buffered formalin) and placed between two foam inserts. This “sandwich” was first placed in a histological cassette, fixed in 10% buffered formalin for 18–24h, then transferred to 70% alcohol, and finally submitted to the FNLCR Pathology/Histotechnology Laboratory for embedding in paraffin and sectioning at 4 μm. Cut sections from the paraffin blocks were stained with hematoxylin-eosin (H&E) and then thoroughly examined under low (×25 and ×50) and medium (×100 and ×200) magnifications to assess viability. Following examination, a viability percentage score was assigned to both alveolar and bronchial cells. Additional cut sections were immunostained with ED-1 antibody (AbD Serotec, NC), diluted 1:50, according to the manufacturer’s instructions. ED-1(+) cells were counted in multiple slices from two separate experiments. Five representative sections from each slice were selected based on the highest density of positive cells, and a manual cell count performed. A mean ED-1(+) score was assigned for each group based on the count.
Data analysis.
Data sets obtained from in-house Pierce protein analysis of tissue lysates, pathology scores from histological assessment, and cytokine results from MSD platform analysis of multiplex plates were consolidated, and data sets corresponding to the same tissue lysate were matched. Slices corresponding to the same treatment had individual slice data sets averaged for further comparison. Treatment group data sets were compared with respective vehicle control groups and significant differences (p < 0.01 and p < 0.05) were calculated using single-tailed, homoskedastic Student’s t-test. Lower limits of detection for MSD platform-derived cytokine results were obtained from the Discovery Workbench analysis software package that accompanied the MSD Sector 6000 imager purchase.
RESULTS
Before using the PCLS to investigate drug-induced inflammation and lung damage, the method of preparing PCLS cultures itself was evaluated for effects on inflammatory cytokine response and lung histology. The aim of this experiment was to make sure that the tissue damage caused by PCLS methodology would not confound the study of lung damage caused by NSC 710305.
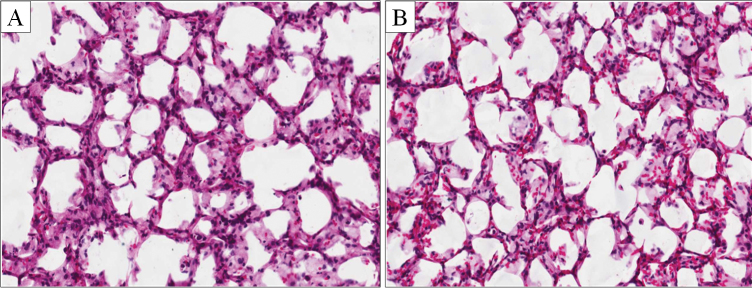
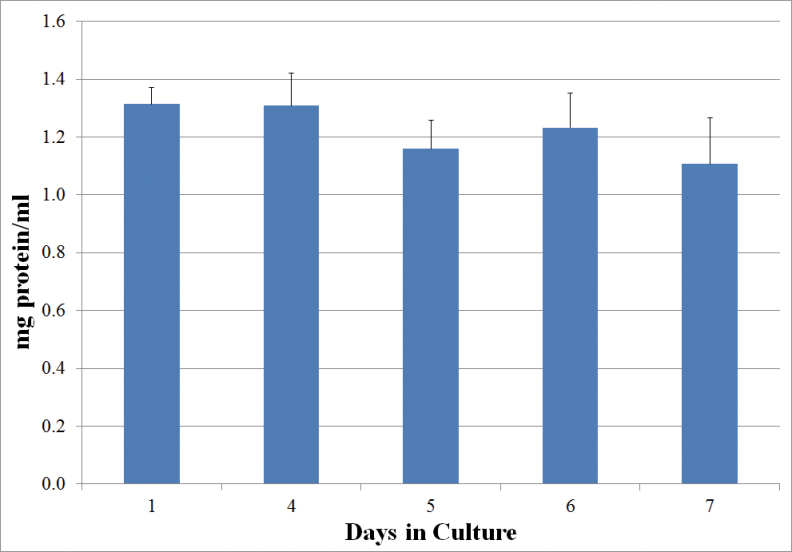
PCLS cultured for 1 week in medium alone or in the DMSO vehicle retained much of the normal architectural structure of freshly prepared slices, although a loss of cellularity and minor changes in morphology did occur (Fig. 1). On average, untreated slices retained ~100% of their protein content during the first 3 days of culture, and > 80% over the entire 7-day culture period (Fig. 2). The histological findings and the stable protein content were similar to those reported for previous studies of rat’s PCLSs (Amin et al., 2006; Behrsing et al., 2004 , 2006; Tyson et al., 2005).
FIG. 1.
Morphology of precision-cut lung slices (A) freshly prepared and (B) after 1 week of culture in 0.1% DMSO vehicle. Histological evaluation indicates rat lung PCLSs maintain highly viability and tissue architecture over 7 days in culture (H&E staining, ×200 magnification).
FIG. 2.
Protein content (mg/ml + SEM) of PCLS after the indicated days of culture in vehicle (0.1% DMSO).
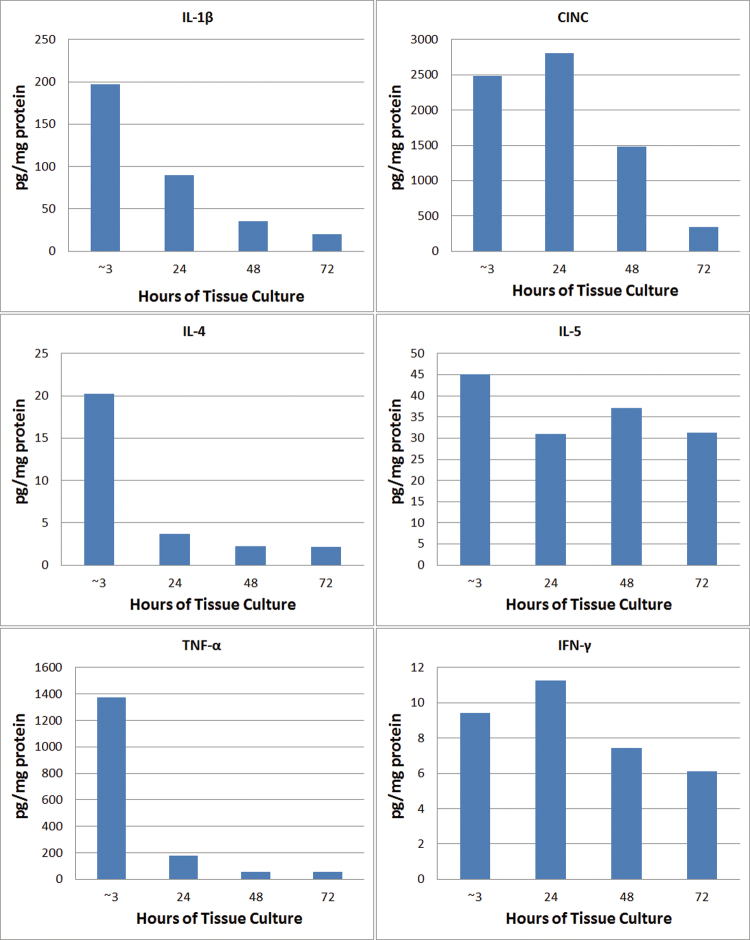
The tissue content of proinflammatory cytokines was measured in untreated PCLS cultured for 1 week in vitro and compared with levels in freshly isolated lung tissue fragments that were not sliced into PCLS. After ~3h of tissue culture, the PCLS content of IL-1β, CINC, and TNF-α increased to 200–2500 pg/mg protein, significantly above levels in lung tissue fragments (Fig. 3). One of these cytokines, CINC, remained elevated 48h after slicing (culture day 3). In contrast, preparing PCLS caused only a small increase in IL-4, IL-5, IFN-γ, and IL-13 (not shown) content relative to the lower limit of detection (LLOD) of the assays (~1–10 pg/ml). The average tissue content of IL-4 and IL-5 reached only ~20 and 45 pg/mg protein, respectively, whereas the content of IL-13 and IFN-γ remained near the LLOD of the assays (typically 1–10 pg/ml). Despite the large increases in PCLS content of IL-1β, CINC, and TNF-α, the only detectable cytokine in conditioned medium was CINC (~90 pg/mg protein/ml at 24h after the start of the culture period). Repeated measurements of untreated PCLS on culture day 1 (the day of slicing) and culture days 2–6 established that the cytokine response to the method of preparing PCLS required 48h of culture to subside (Fig. 3). Therefore, the in vitro treatment of PCLS with NSC 710305 was initiated on time 0h of culture day 3, i.e., after this 48-h recovery period.
FIG. 3.
Proinflammatory cytokine content of untreated PCLS lysates after ~3, 24, 48, and 72h of tissue culture. The tissue slicing procedure induced large increases in IL-1β, CINC, and TNF-α content that subsided by 48–72h of culture. Preparing PCLS caused only a small increase in IL-4, IL-5, IFN-γ, and IL-13 (not shown) content relative to the LLOD of the assays (~1–10 pg/ml).
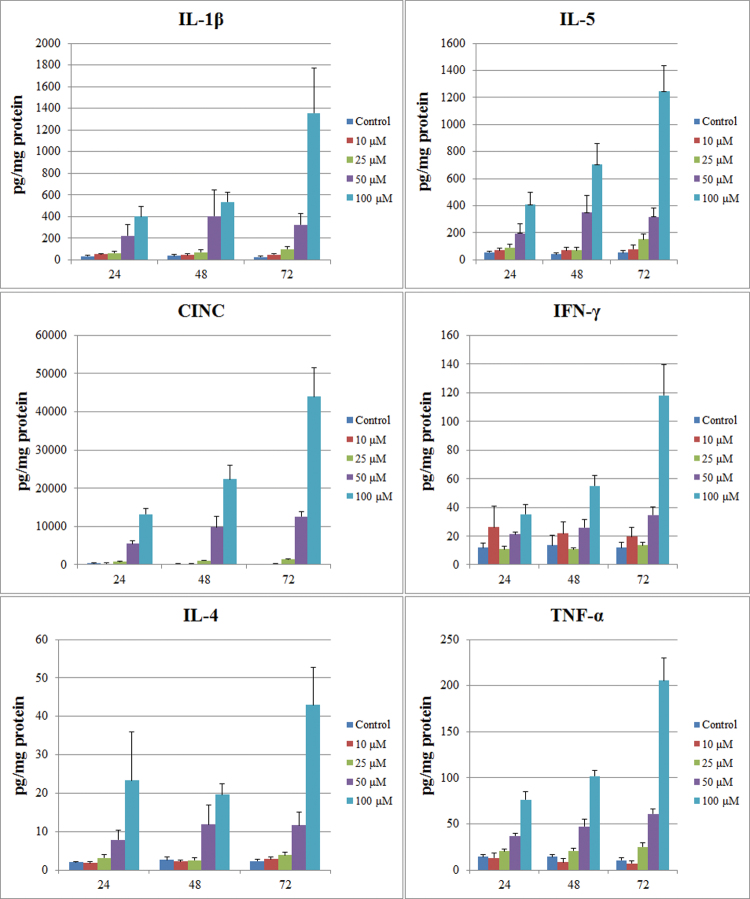
On culture day 3, PCLSs were exposed to a range of concentrations of NSC 710305, and tissue cytokine levels were evaluated after 24, 48, and 72h of drug exposure (Fig. 4). TNF-α and IFN-γ levels could not be detected (< LLOD) at any time point in vehicle controls, and there was no significant change (p > 0.05) in the level of any cytokine (IL-β, IL-4, IL-5, TNF-α, IFN-γ, or CINC) after exposure to 10μM NSC 710305. Exposure to 25μM NSC 710305 modestly elevated TNF-α within 24h, with average levels of TNF-α in PCLS reaching 25 pg/mg, just above assay LLOD. Exposure to 25μM NSC 710305 for 72h increased tissue levels of IL-β, IL-5, and CINC. IL-4 levels were not quantifiable, as they usually did not exceed the 4 pg/mg protein LLOD of the assay. Exposure to 50 and 100μM caused significant increases in PCLS content for all six cytokines analyzed (Fig. 4). CINC levels showed the largest fold and absolute increase from ~165 pg/mg protein in vehicle-treated controls, reaching ~44,000 pg/mg protein after 72h exposure to 100μM NSC 710305. IL-β and IL-5 showed the next largest fold increases, as well as absolute increases. Released cytokines in the conditioned medium were only measurable in the 100μM NSC 710305 treatment group, and IL-13 levels were below assay LLOD (~10 pg/mg protein) in all treatment groups.
FIG. 4.
Concentration- and time-dependent changes in PCLS cytokine content due to NSC 710305 treatment. Data with error bars (average + SEM) were compiled from n = 4–8 experiments, and each experiment consisted of pooled lysates from four slices per data point. The x-axis indicates the exposure time (h) to NSC 710305, which was added to the slices on culture day 3.
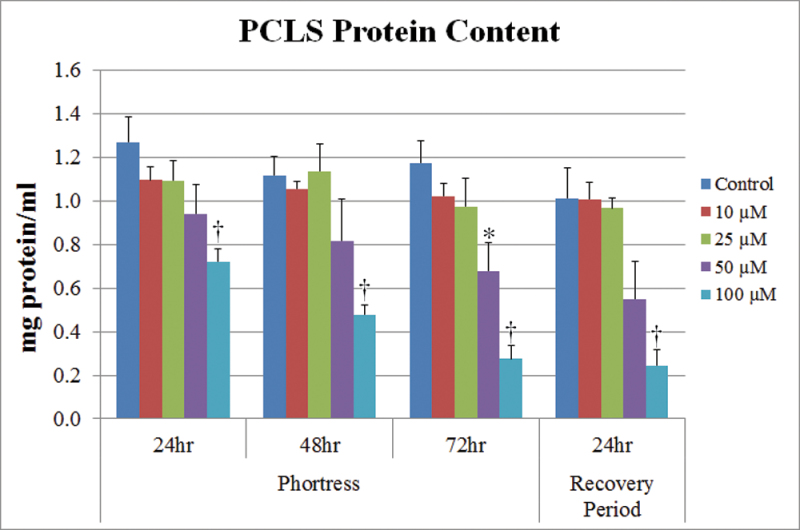
Because the cytokine content of PCLS cultures was normalized to protein levels, the protein content of PCLS was also analyzed during this same time period (Fig. 5). Treatment with 10 and 25µM had no more than minimal effect on protein content. However, treatment with 50 or 100µM NSC 710305 caused a significant decrease in PCLS protein content, with the maximum decrease of approximately fivefold occurring in PCLS exposed to 100µM NSC 710305 for 72h. The extent of decreases in protein content accounted for some of the increases in IL-4, TNF-α, and IFN-γ content, but was too small to contribute to the large increases in IL-β, IL-5, and CINC levels.
FIG. 5.
Concentration- and time-dependent changes in PCLS protein content due to treatment with NSC 710305 for 24, 48, and 72h, as well as after a 24-h recovery period without drug following a 72-h exposure. The x-axis indicates the exposure time to NSC 710305, which was added to the slices on culture day 3. Error bars, SEM; *p < 0.05; † p < 0.01.
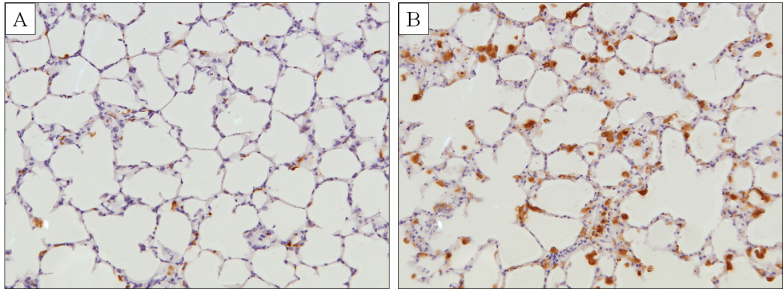
The rise in tissue cytokine levels and decline in tissue protein content were paralleled by adverse changes in histopathology. A 72-h exposure of PCLS to NSC 710305 caused concentration-dependent destruction of tissue and concomitant decreases in alveolar and bronchiole viability (Table 1). Overall changes in tissue included decreased cellularity compared with the control and the presence of small, dark pyknotic nuclei in the cells lining the alveoli, which are indicative of cell injury. In these same specimens, there was also a significant increase in ED-1(+) activated macrophages compared with vehicle controls, including the PCLS treated with 25µM NSC 710305 (Fig 6; Table 1). No increase in number of activated macrophages above control was seen with 10μM NSC 710305 (data not shown).
TABLE 1.
Histological Assessment of PCLS Exposed to Phortress (Average ± SEM)
| Day | NSC 710305 | %Viability | ED-1 scores | |
|---|---|---|---|---|
| Alveoli | Bronchi | |||
| 1 | 0 | 89.2±2.0 | 95.0±0.0 | 32.0±3.0 |
| 7 | 0 | 81.0±1.0 | 90.0± a | 41.5±2.8 |
| 25 | 69.2±1.5 | 86.7±2.5 | 53.8±4.5 | |
| 50 | 45.0±6.2 | 51.7±12.8 | 53.1±4.2 | |
| 100 | 26.0±2.4 | 21.7±16.7 | 57.3±4.2 | |
aControl group bronchi % viability at day 7 had only two slices evaluable for bronchi viability (no SEM possible).
FIG. 6.
PCLS stained with an activated macrophage marker (ED-1) following 72-h exposure to (A) vehicle control or (B) 100μM of NSC 710305. Note that NSC 710305 not only increased the number of ED-1+ macrophages, but also induced abundant vacuolated cytoplasm consistent with activation (×200 magnification).
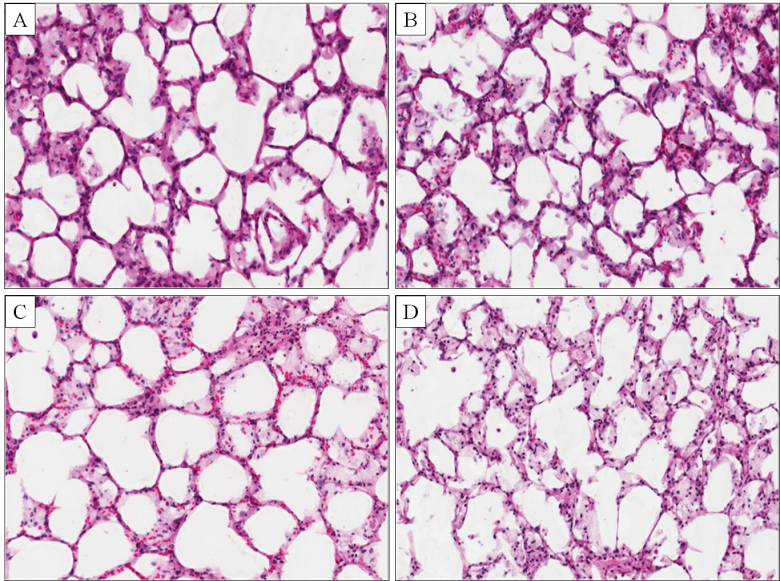
A key question that could be addressed with the PCLS model is whether the adverse effects caused by 72h exposure to NSC 710305 were reversible. To evaluate the reversibility of toxicity, PCLSs were harvested for histological and cytokine analyses after a 24-h recovery period from a 72-h drug treatment. Even after the recovery period, concentration-dependent cytotoxicity was evident in histological sections (Fig. 7). PCLS cytokine content was compared at the beginning and end of the 24-h recovery period (Table 2). High PCLS cytokine content caused by drug treatment was reversible only in the PCLS cultures treated with 25µM NSC 710305 (IFN-γ could not be evaluated because 25µM did not significantly increase its level). IL-5 and CINC levels decreased significantly (p < 0.05) during the 24-h recovery period, and IL-5 content fell to control PCLS levels of ~60 pg/mg protein. Importantly, PCLS cytokine levels in the groups treated with 50 and 100µM NSC 710305 for 72h continued to rise during the 24-h recovery period (Table 2).
FIG. 7.
Histology of control and NSC 710305–treated PCLS after a 24-h recovery period from 72-h exposures to NSC 710305. Untreated PCLS controls (A) show well-preserved lung morphology with minimal loss of cellularity. PCLS treated for 72h with NSC 710305 at 25μM (B), 50μM (C), and 100μM (D) show distortions in the alveolar architecture, nuclear shrinkage, and a progressive loss of cellularity, indicating continued cytotoxic effects of the drug (H&E staining, ×200 magnification).
TABLE 2 .
Assessment of NSC 710305 Removal on Cytokine Levels at Exposure Concentrations That Caused Significant Increases Over Control Levels
| NSC 710305 | Time point | IL-1β | CINC | IL-4 | IL-5 | TNF-α | IFN-γ |
|---|---|---|---|---|---|---|---|
| 25μM | 72-h exposure | 99.1 | 1421.8 | 3.9 | 150.9 | 25.1 | 13.5 |
| 24-h recovery | 60.6 | 899.6 | 2.9 | 63.1 | 20.0 | 16.0 | |
| Δ | −38.6 | −522.2 | −1.0 | −87.8 | −5.1 | 2.5 | |
| % Change | −39 | −37 | −25 | −58 | −20 | 19 | |
| 50μM | 72-h exposure | 323.4 | 12623.1 | 11.6 | 317.0 | 61.0 | 34.6 |
| 24-h recovery | 479.4 | 17445.4 | 44.0 | 700.7 | 87.2 | 40.4 | |
| Δ | 156.0 | 4822.3 | 32.3 | 383.7 | 26.2 | 5.8 | |
| % Change | 48 | 38 | 278 | 121 | 43 | 17 | |
| 100μM | 72-h exposure | 1356.2 | 43908.0 | 43.0 | 1243.3 | 205.5 | 118.1 |
| 24-h recovery | 2925.8 | 91368.7 | 89.5 | 2004.8 | 372.8 | 232.0 | |
| Δ | 1569.6 | 47460.7 | 46.6 | 761.5 | 167.3 | 113.9 | |
| % Change | 116 | 108 | 108 | 61 | 81 | 96 |
All values are expressed as pg/mg protein except % Change (the % increase or decrease, relative to the 72-h value) measured after 24-h drug removal. Bold values indicate a reduction or increase of tissue cytokine levels, respectively.
DISCUSSION
In this study, inflammatory cytokine responses in PCLS, resulting from slice production and from treatment with the cytotoxic agent, NSC 710305, were separated so as to isolate drug effects from methodological consequences. Immediately following tissue slicing, several mediators of inflammation increased, plateaued, and subsided within 2 days of the beginning of in vitro tissue culture. Although slight increases in PCLS levels of IL-4 and IL-5 were noted, tissue slicing of PCLS cultures resulted in marked elevations in IL-1β, CINC, and TNF-α, which can most likely be attributed to the mechanical tissue injury, similar to an in vivo wound-healing response (Crosby and Waters, 2010; Eming et al., 2007; Grimstad et al., 2011). Therefore, in order to assess PCLS cytokine response to NSC 710305 exposure, drug addition was delayed until the recovery period allowed cytokine levels to return to baseline (on day 3 of culture).
Treating slices with NSC 710305 resulted in concentration-dependent cytokine responses. Exposure to 25µM NSC 710305 restimulated IL-4 (albeit at very low levels) and IL-5, in addition to IL-1β, TNF-α, and CINC. The inflammatory cytokine response to 25µM NSC 710305 was reversible during a 24-h recovery period. However, at higher concentrations (50 and 100µM), the PCLS cytokine level response was much more robust and continued to progress during the recovery period in the absence of drug. Exposure at the highest concentrations resulted in the induction of three different classes of cytokines: IFN-γ, a macrophage activator considered more of a chronic inflammatory mediator; IL-4, an antiinflammatory cytokine; and TNF-α. The acute inflammatory response to tissue slicing is reduced with the addition of hydrocortisone, a known antiinflammatory agent, in the medium (Adcock and Caramori, 2001; Barnes, 1998). Hydrocortisone was also included in the cell culture model used in this study, but did not block the inflammatory cytokine response to NSC 710305.
This induction may indicate a broader activation of the inflammatory process in response to a higher level of tissue injury caused by NSC 710305. The ability of the PCLS system to demonstrate a limited inflammatory response in the absence of a functioning vascular system that would allow inflammatory cells to be continuously recruited into the lungs can be attributed to the alveolar macrophages, neutrophils, and lymphocytes that remain entrapped in the tissue following slice preparation and by lung parenchymal cells that allow the slices to produce proinflammatory cytokines. Among the various cytokines analyzed in this study, CINC is a neutrophil chemoattractant, IL-1β and TNF-α are neutrophil activators and hallmarks of acute inflammation (Goodman et al., 2003), and IFN-γ is a macrophage activator considered to be a mediator of chronic inflammation. This PCLS inflammatory response was further confirmed by finding a significantly increased number of activated macrophages in NSC 710305–treated slices. In vivo, it is possible that the tissue damage may be greater than what was determined in vitro (Grommes and Soehnlein, 2011). Production and secretion of CINC and IL-1β in an in vivo setting would likely amplify the inflammatory response due to neutrophil recruitment to the injury site (Shimabukuro et al., 2003). Further, pharmacokinetics of Phortress administration to rats would provide in vivo serum levels, so that a correlation between rat NOAEL and the determined PCLS NOAEL could be made. A review of literature identified a more recent research article (Bradshaw et al., 2009) that compared Phortress exposure between rats and mice but serum levels are only disclosed for mice. It was noted that mice were the most sensitive species of the two (MTD = 20mg/kg/day) and an iv dose of 10mg/kg yielded a C max of 2.3μM/h, with no significant toxic effects. If the pharmacokinetics of Phortress in mouse and rat are similar, the determined NOAEL of 10μM in rat PCLS seems plausible.
However, it must also be considered that a host of antiinflammatory cytokine interplay, also derived from various nonpulmonary sources, is also at work in vivo. This inherent limitation of the model prevents the study of complex pro- and antiinflammatory signaling cascades and interactions that occur in an in vivo system. Notwithstanding well-known limitations of in vitro systems: the dynamic roller PCLS system provides a unique model that is amenable for long-term cultures and allows for the examination of tissue containing a full complement of organ-specific heterologous cell types. The tissue remains cytokine responsive to chemical insults in a controlled environment where the culture medium or PCLS tissue itself can be directly examined to elucidate the potential adverse effects of experimental compounds. NSC 710305 exposure resulted in the production of many of the same proinflammatory cytokines seen in acute pulmonary injury and in the activation of resident macrophages. These findings offer a plausible explanation for the cause of the pulmonary toxicity seen in the preclinical study.
FUNDING
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
ACKNOWLEDGMENTS
The authors would like to thank Danielle Mesa, John Hamre III, Kristine Robillard, and Ryan Richards for their technical expertise in conducting the slice work. The pathological evaluation of slice tissue was conducted by Dr Khalid Amin, consultant for SAIC-Frederick. F344 rat lung tissue was obtained from Dr Melinda Hollingshead and Suzie Borgel of the Biological Testing Branch at NCI-Frederick. The MSD-based cytokine and chemokine measurements were conducted by Helen Rager and Yanu Wang in the Lymphocyte Testing Section of the Clinical Support Laboratory, SAIC-Frederick. Embedding, fixing, and staining of the PCLS tissue were conducted by Tammy Beachly and Donna Butcher of the NCI-Frederick Pathology/Histotechnology Laboratory. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- Adcock I. M., Caramori G. (2001). Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79, 376–384 [DOI] [PubMed] [Google Scholar]
- Amin K., Ip C., Le T., Tomaszewski J. E, Green C. E, Tyson C. A., Behrsing H. (2006). Human and rat lung tissue exhibit differential sensitivity to phortress toxicity: Use of precision-cut lung slices. Toxicologist 90, 404 [Google Scholar]
- Barnes P. J. (1998). Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. 94, 557–572 [DOI] [PubMed] [Google Scholar]
- Behrsing H. P, Amin K., Ip C., Tyson C. A.(2004). Induction of fibrosis by bleomycin and carmustine in rat lung slices. Toxicologist 78, 52 [Google Scholar]
- Behrsing H. P, Ip C., Le T., Tomaszewski J., Green C., Tyson C., Amin K. (2006). Demonstration of differential toxicity induced by aminoflavone prodrug in human and rat precision-cut lung slices. Toxicologist 90, 403 [Google Scholar]
- Ben-Noun L. (2000). Drug-induced respiratory disorders: Incidence, prevention and management. Drug Saf. 23, 143–164 [DOI] [PubMed] [Google Scholar]
- Bradshaw T. D., Bibby M. C., Double J. A., Fichtner I., Cooper P. A., Alley M. C., Donohue S., Stinson S. F., Tomaszewjski J. E., Sausville E. A., et al. (2002a). Preclinical evaluation of amino acid prodrugs of novel antitumor 2-(4-amino-3-methylphenyl)benzothiazoles. Mol. Cancer Ther. 1, 239–246 [PubMed] [Google Scholar]
- Bradshaw T. D., Chua M. S., Browne H. L., Trapani V., Sausville E. A., Stevens M. F. (2002b). In vitro evaluation of amino acid prodrugs of novel antitumour 2-(4-amino-3-methylphenyl)benzothiazoles. Br. J. Cancer 86, 1348–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw T. D., Wren J. E., Bruce M., Barrett D. A., Leong C. O., Gaskell M., Wright E. K., Farmer P. B., Henderson C. J., Wolf R, et al. (2009). Preclinical toxicokinetic evaluation of phortress [2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride] in two rodent species. Pharmacology 83, 99–109 [DOI] [PubMed] [Google Scholar]
- Cannizzaro V., Hantos Z., Sly P. D., Zosky G. R. (2011). Linking lung function and inflammatory responses in ventilator-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 300, L112–L120 [DOI] [PubMed] [Google Scholar]
- Card J. W., Racz W. J., Brien J. F., Margolin S. B., Massey T. E. (2003). Differential effects of pirfenidone on acute pulmonary injury and ensuing fibrosis in the hamster model of amiodarone-induced pulmonary toxicity. Toxicol. Sci. 75, 169–180 [DOI] [PubMed] [Google Scholar]
- Crosby L. M., Waters C. M. (2010). Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 298, L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse A. L., Tanjore H., Xu X. C., Polosukhin V. V., Jones B. R., McMahon F. B., Gleaves L. A., Blackwell T. S., Lawson W. E. (2010). Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L442–L452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclaux C., Azoulay E. (2003). Inflammatory response to infectious pulmonary injury Eur. Respir. J. 22, 10s–14s [DOI] [PubMed] [Google Scholar]
- Eming S. A., Krieg T., Davidson J. M. (2007). Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 [DOI] [PubMed] [Google Scholar]
- Geiser T., Jarreau P. H., Atabai K., Matthay M. A. (2000). Interleukin-1beta augments in vitro alveolar epithelial repair. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1184–L1190 [DOI] [PubMed] [Google Scholar]
- Goodman R. B., Pugin J., Lee J. S., Matthay M. A. (2003). Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14, 523–535 [DOI] [PubMed] [Google Scholar]
- Goodman R. B., Strieter R. M., Martin D. P., Steinberg K. P., Milberg J. A., Maunder R. J., Kunkel S. L., Walz A., Hudson L. D., Martin T. R. (1996). Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 154(3 Pt 1)602–611 [DOI] [PubMed] [Google Scholar]
- Graham R. A., Downey A., Mudra D., Krueger L., Carroll K., Chengelis C., Madan A., Parkinson A. (2002). In vivo and in vitro induction of cytochrome P450 enzymes in beagle dogs. Drug Metab. Dispos. 30, 1206–1213 [DOI] [PubMed] [Google Scholar]
- Grimstad O., Sandanger O., Ryan L., Otterdal K., Damaas J. K., Pukstad B., Espevik T. (2011). Cellular sources and inducers of cytokines present in acute wound fluid. Wound Repair Regener. 19,, 337–347 [DOI] [PubMed] [Google Scholar]
- Grommes J., Soehnlein O. (2011). Contribution of neutrophils to acute lung injury. Mol. Med. 17, 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan J. A., McGarrigle B. P., Sutter T. R., Olson J. R. (2006). Tissue specific induction of cytochrome P450 (CYP) 1A1 and 1B1 in rat liver and lung following in vitro (tissue slice) and in vivo exposure to benzo(a)pyrene. Toxicol. In Vitro 20, 426–438 [DOI] [PubMed] [Google Scholar]
- Heise R. L., Stober V., Cheluvaraju C., Hollingsworth J. W., Garantziotis S. (2011). Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 286, 17435–17444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henjakovic M., Sewald K., Switalla S., Kaiser D., Müller M., Veres T. Z., Martin C., Uhlig S., Krug N., Braun A. (2008). Ex vivo testing of immune responses in precision-cut lung slices. Toxicol. Appl. Pharmacol. 231, 68–76 [DOI] [PubMed] [Google Scholar]
- Hoshino T., Okamoto M., Sakazaki Y., Kato S., Young H. A., Aizawa H. (2009). Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice. Am. J. Respir. Cell Mol. Biol. 41, 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, Commission on Life Sciences, and National Research Council (1996). Guide for the care and use of laboratory animals. Washington DC, National Academy Press [Google Scholar]
- Keane M. P., Strieter R. M. (2002). The importance of balanced pro-inflammatory and anti-inflammatory mechanisms in diffuse lung disease. Respir. Res. 3, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Kolb M., Bonniaud P., Gauldie J. (2003). Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr. Pharm. Des. 9, 39–49 [DOI] [PubMed] [Google Scholar]
- Kirkman E., Watts S. (2011). Characterization of the response to primary blast injury. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 366, 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prieur E., Vaz E., Bion A., Dionnet F., Morin J. P. (2000). Toxicity of diesel engine exhausts in an in vitro model of lung slices in biphasic organotypic culture: Induction of a proinflammatory and apoptotic response. Arch. Toxicol. 74, 460–466 [DOI] [PubMed] [Google Scholar]
- Lee C., Watt K. C., Chang A. M., Plopper C. G., Buckpitt A. R., Pinkerton K. E. (1998). Site-selective differences in cytochrome P450 isoform activities. Comparison of expression in rat and rhesus monkey lung and induction in rats. Drug Metab. Dispos. 26, 396–400 [PubMed] [Google Scholar]
- Leong C. O., Gaskell M., Martin E. A., Heydon R. T., Farmer P. B., Bibby M. C., Cooper P. A., Double J. A., Bradshaw T. D., Stevens M. F. (2003). Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. Br. J. Cancer 88, 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M., Groothuis G. M., de Kanter R. (2006). Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2, 875–894 [DOI] [PubMed] [Google Scholar]
- Palmer S. M., Flake G. P., Kelly F. L., Zhang H. L., Nugent J. L., Kirby P. J., Foley J. F., Gwinn W. M., Morgan D. L. (2011). Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS ONE 6, e17644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiris S. A., Triantafillidou C., Kolilekas L., Markoulaki D., Manali E. D. (2010). Amiodarone: Review of pulmonary effects and toxicity. Drug Saf. 33, 539–558 [DOI] [PubMed] [Google Scholar]
- Park W. Y., Goodman R. B., Steinberg K. P., Ruzinski J. T., Radella F., 2nd, Park D. R., Pugin J., Skerrett S. J., Hudson L. D., Martin T. R. (2001). Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 164, 1896–1903 [DOI] [PubMed] [Google Scholar]
- Patten G. A., Billi J. E., Rotman H. H. (1980). Rapidly progressive, fatal pulmonary fibrosis induced by carmustine. JAMA 244, 687–688 [PubMed] [Google Scholar]
- Reinhart P. G., Lai Y. L., Gairola C. G. (1996). Amiodarone-induced pulmonary fibrosis in Fischer 344 rats. Toxicology 110, 95–101 [DOI] [PubMed] [Google Scholar]
- Schulz T. G., Neubert D., Davies D. S., Edwards R. J. (1996). Inducibility of cytochromes P-450 by dioxin in liver and extrahepatic tissues of the marmoset monkey (Callithrix jacchus). Biochim. Biophys. Acta 1298, 131–140 [DOI] [PubMed] [Google Scholar]
- Shimabukuro D. W., Sawa T., Gropper M. A. (2003). Injury and repair in lung and airways. Crit. Care Med. 31, S524–S531 [DOI] [PubMed] [Google Scholar]
- Song Y., Li X., Du X. (2009). Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 34, 559–567 [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Belperio J. A., Keane M. P. (2003). Host innate defenses in the lung: The role of cytokines. Curr. Opin. Infect. Dis. 16, 193–198 [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Mehrad B. (2009). New mechanisms of pulmonary fibrosis. Chest 136, 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalla S., Knebel J., Ritter D., Krug N., Braun A., Sewald K. (2010). Effects of acute in vitro exposure of murine precision-cut lung slices to gaseous nitrogen dioxide and ozone in an air-liquid interface (ALI) culture. Toxicol. Lett. 196, 117–124 [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Amin K., Ip C., Behrsing H. P. (2005). Comparison of BCNU and SarCNU toxicity in long-term cultures of precision-cut lung slices. Toxicologist 84, 240 [Google Scholar]
- Wei C., Caccavale R. J., Weyand E. H., Chen S., Iba M. M. (2002). Induction of CYP1A1 and CYP1A2 expressions by prototypic and atypical inducers in the human lung. Cancer Lett. 178, 25–36 [DOI] [PubMed] [Google Scholar]
- Wohlsen A., Martin C., Vollmer E., Branscheid D., Magnussen H., Becker W. M., Lepp U., Uhlig S. (2003). The early allergic response in small airways of human precision-cut lung slices. Eur. Respir. J. 21, 1024–1032 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Yoshioka Y., Fujimura M., Kayamuro H., Yamashita K., Higashisaka K., Nakanishi R., Morishita Y., Nabeshi H., Yamashita T, et al. (2010). Urban aerosols induce pro-inflammatory cytokine production in macrophages and cause airway inflammation in vivo. Biol. Pharm. Bull. 33, 780–783 [DOI] [PubMed] [Google Scholar]