Abstract
Dysfunctional lipid and glucose metabolism contribute to metabolic syndrome—a major public health concern that enhances cardiovascular disease risk. Arsenic (As(III)) exposure may increase metabolic syndrome and cardiovascular disease risk by impairing adipose tissue differentiation, function, and insulin sensitivity through pathogenic mechanisms that remain unclear. We hypothesized that As(III) signals through the Pertussis toxin (Ptx) sensitive, Gi protein–coupled receptor (GPCR) to impair adipogenesis, as previously demonstrated for its stimulation of vascular oxidant generation, angiogenesis, and remodeling. Because both As(III) and GPCR ligands inhibit progenitor cell differentiation into adipocytes, we investigated the hypothesis in a model of low-passage human mesenchymal stem cells (hMSC). As(III) (0.1–1.0µM) suppressed dexamethasone/insulin-induced hMSC adipogenesis, as indicated by decreased transcriptional promoters of differentiation, decreased fat droplet formation, and decreased expression of differentiated adipocyte markers, such as adiponectin and perilipin. Preincubating hMSC with Ptx prevented 90% of the suppressive effect of As(III). Selective competitive antagonists of Gi-coupled endothelin-1 type A and B receptors were ~60% effective in blocking As(III) inhibition and combination of antagonists to both receptors were 85% effective. In contrast, antagonists to the sphingosine-1-phosphate type 1 receptor (previously shown to mediate As(III) vascular effects) or the angiotensin II type 1 receptor were ineffective in blocking As(III) effects. These studies suggest a majority of arsenic-inhibited adipocyte differentiation, and metabolism requires endothelin-1 GPCRs and that As(III) effects on GPCR signaling are tissue and context specific. This may represent a significant mechanism for the contribution of arsenic exposure to increased metabolic and cardiovascular diseases.
Key Words: arsenic, endothelin-1, adipose, adipocyte, mesenchymal stem cell, G protein–, coupled receptor.
Chronic environmental exposure to As(III) in drinking water and food is a major public health concern that may affect the health of more than 140 million people worldwide. In addition to promoting a number of cancers, arsenic-promoted cardiovascular disease may be a leading cause of death from As(III) exposures (Smith and Steinmaus, 2011) and even low-to-moderate levels of drinking water arsenic increase risk of cardiac mortality (Chen et al., 2011) and hypertension (Abhyankar et al., 2012). In addition, metabolic disease has been implicated in this As(III)-promoted increase in cardiovascular disease risk (Navas-Acien et al., 2005). In spite of As(III) being implicated in promoting disease risk, the pathogenic mechanisms underlying As(III)-induced disease etiology remain unresolved, and there has been a recent call for more mechanistic research to determine why As(III) poses such a high risk for cardiovascular or metabolic toxicity (Smith and Steinmaus, 2011).
Adipose tissue is a highly dynamic endocrine organ that is critical for maintaining energy balance. Even in nonexpanding white adipose tissue, adipocytes renew frequently (~10% annual turnover) to maintain healthy tissue (Cawthorn et al., 2012). Dysregulation of adipose tissue that affects tissue renewal or affects lipid storage is pathogenic (Cawthorn et al., 2012; Janke et al., 2002; Vigouroux et al., 2011). The mesenchymal progenitor cells (mesenchymal stem cells [MSC]) in the adipose stromal/vascular niche of tissue are critical in adipocyte renewal, and loss of adipogenic differentiation from these cells is an important contributor to systemic insulin resistance in metabolic disease (Cawthorn et al., 2012; Janke et al., 2002; Vigouroux et al., 2011). The MSC can differentiate into multiple cell types including adipocytes, osteoblasts, chondrocytes, and smooth muscle cells depending on changes in the niche, as well as stimulation by a number of hormones and paracrine factors (Cawthorn et al., 2012; Tang and Lane, 2012). Thus, environmental exposures that target the health or function of these cells, as well as the milieu of the progenitor niche, can produce a broad range of pathogenic processes.
The adipogenic differentiation program is well defined. Initial actions of adipogenic hormones, such as insulin and glucocorticoids, lead to a cascade of transcriptional activation with enhanced expression of SREBP-1, C/EBPβ, C/EBPδ, and peroxisome proliferative-activated receptor-γ (PPARγ) upstream of C/EBPα and additional PPARγ (Cawthorn et al., 2012; Vigouroux et al., 2011; Wauson et al., 2002). Expression of adipocytes, such as the secreted metabolic regulator adiponectic (ADIPOQ), and fat droplet coat proteins, such as perilipin (PLIN1), is essentially absent in the MSC, but increases several orders of magnitude as the transcriptional cascade advances the cells to mature adipocytes. The hallmark of the mature adipocyte is the characteristic accumulation of PLIN1-coated, triglyceride-rich fat droplets. A number of studies demonstrate that As(III) inhibits adipogenic differentiation from stem and progenitor cells and suggests that As(III) actions on adipogenesis contribute to metabolic disease (Cheng et al., 2011; Wang et al., 2005; Wauson et al., 2002). The mechanism for this inhibition is unclear; however, it does not involve cytotoxic effects at environmentally relevant As(III) levels. Instead, As(III) suppresses transcriptional programs essential for differentiation, including suppressing CCAAT enhancer–binding protein α (C/EBPα) and possibly PPARγ (Cheng et al., 2011; Wauson et al., 2002). Expression of these transcription factors is several steps downstream in the pathway for inducing adipogenesis, and the location of proximal rate–limiting actions of arsenic in the pathway is unknown.
Interactions between receptor tyrosine kinases and Gi protein–coupled receptor (GPCR) provide context-specific signaling that can be cooperative or inhibitory in regulating adipose function and the stem cell niche. Mature abdominal adipose tissue upregulates endothelin-1 expression and the renin-angiotensin system to actively suppress differentiation in the niche, actions that require Gi-coupled endothelin-1 receptor type A or B (ENDRA, ENDRB) and the angiotensin II type 1 receptor (AGTR1), respectively (Bhattacharya and Ullrich, 2006; Eriksson et al., 2009; Hauner et al., 1994; Janke et al., 2002; Tomono et al., 2008; van Harmelen et al., 2008). Receptors for sphingosine-1-phosphate may impair adipose regeneration by inducing differentiation of adipose tissue–derived MSC toward smooth muscle cells (Nincheri et al., 2009). GPCR-mediated repression of insulin signaling for differentiation may result from disrupting serine/threonine protein kinase Akt (AKT/PKB) signaling (Bhattacharya and Ullrich, 2006), disrupting signaling protein interactions with the insulin receptor (Elbaz et al., 2000), or decreasing expression of the receptor and its immediate downstream signal amplifiers (van Harmelen et al., 2008). ENDRA or ENDRB suppression of adipogenesis required constant and prolonged exposure to ligand, suggesting signaling shifts for transcriptionally repressive programs (Eriksson et al., 2009; van Harmelen et al., 2008).
We demonstrated that As(III) activates Gi-coupled S1PR1 to increase vascular cell oxidative signaling and vascular remodeling (Straub et al., 2009). We hypothesized that As(III) may act through a similar Ptx-sensitive GPCR-mediated mechanism on stromal/vascular progenitor cells to alter the stem cell niche and limit adipocyte differentiation. The role of the S1PR1 in suppressing adipogenesis is unclear, as sphingosine-1-phosphate has been shown to maintain multipotency and proliferation in the niche (He et al., 2010) and to induce adipose-derived mesenchymal cell differentiation to smooth muscle (Nincheri et al., 2009). Nonetheless, we investigated whether this receptor or other GPCR was required for As(III) suppression of adipogenesis.
MATERIALS AND METHODS
Experimental paradigm.
To demonstrate arsenic effects on MSC and potential mechanisms initiating these effects, confluent mouse stromal-vascular cells (SVC) or adipose-derived human MSC (hMSC) were stimulated to differentiate with a standard dexamethasone, isobutylmethylxanthine (IBMX), and insulin medium for 2 days in the presence or absence of sodium arsenite (As(III); 0.1–5.0µM). This was followed by a 9-day incubation in insulin-containing medium with or without As(III) to allow maximal expression of mature adipocyte markers (adiponectin, perilipin, and Nile red–positive lipid droplets). Pertussis toxin (Ptx) was added 24h prior to induction of differentiation to irreversibly inhibit Gi coupling to GPCR. Competitive GPCR antagonists were added 30min prior to As(III) at the time of induction and at each medium change during induction/maturation.
Mouse SVC isolation.
SVCs were isolated from epididymal fat pads and cultured essentially as described (Hausman et al., 2008). Fat pads from two mice were removed, weighed, minced in 0.75ml of Kreb’s-Ringer Buffer (KRB), and transferred to a sterile 2ml tube. After adding 0.75ml of 2× collagenase solution (final concentration, 2mg/ml, type I collagenase [Worthington]), tubes were incubated at 37°C for 1h in a rotator. Tubes were vortexed three times during that hour. The fat mixture was filtered through 100 µm cell strainers and centrifuged at 500 × g for 10min. The cell pellet was brought up in 5ml of red blood cell lysis buffer (Sigma) and incubated at room temperature for 5min. Ten milliliters of KRB were added, and cells were centrifuged again. The pellet was brought up in 1ml of Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 media (10% fetal bovine serum [FBS] and 1% penicillin, streptomycin), and cell counts were performed on a Countess (Invitrogen). Typical yield was 1500–2500 cells/mg fat. Two days after plating, SVC were switched to DMEM/F12 with 5% FBS. At confluence, cells were switched to adipocyte induction media (17nM insulin, 0.1mM dexamethasone, 250mM IBMX, and 60mM indomethacin in DMEM/F12 with 5% FBS) for 2 days. Maintenance medium (17nM insulin, then DMEM/F12 with 10% FBS) was then added for the remaining time in culture.
hMSC culture.
Separate primary adipose–derived hMSC lines from young female donors (PromoCell GmbH or LifeLine Cell Technology; passages 4–8) were grown to confluence in StemLife MSC medium. Differentiation was induced with AdipoLife DfKt-1 adipogenesis medium for 2 days and then switched to the AdipoLife Maintenance media for an additional 9 days. During medium exchange adipogenesis, only 75% of the media was removed and to prevent cell exposure to air. All cultures were maintained at 37°C in 5% CO2.
Arsenic and inhibitor treatments.
Unless otherwise noted, cells were treated with sodium arsenite (ThermoFisher Scientific, Pittsburgh, PA) at confluence while inhibitors were added 30min prior to As(III) during initiation and maintenance media exchange. Pertussis toxin (Sigma Aldrich) was added 24h prior to induction. Other chemical inhibitors used included: VPC 23019 (S1PR1/3 competitive antagonist: Avanti Polar Lipids, Inc.), L158,809 (AGTR1 competitive antagonist: a kind gift from Merck Research Laboratories), and BQ610 and RES-701-1 (competitive antagonists of ENDRA and ENDRB, respectively: Enzo Life Sciences).
shRNA EDNR knockdown.
Seventy percent confluent hMSC were transfected with 50nM of pools of shRNA specific for EDNRA and EDNRB or with 25nM of both specific siRNA pools (siGENOME SMART pool, ThermoScientific, Pittsburgh, PA) using Dharmafect 1 reagent (ThermoScientific) according to the supplier’s protocol. As a negative control, cells were transfected with 50nM of Non-Specific Pool no. 2 siRNA (siGENOME SMART pool, ThermoScientific). Four days post-transfection, the cells were placed in the differentiation protocol in the presence or absence of As(III), and RNA was extracted on at the end of 8 days (2 days of induction and 6 days of maintenance).
Click-iT cell proliferation assay.
Cell proliferation was measured using the Click-iT EdU Alexa Fluor 488 Flow Cytometry cell proliferation assay (Invitrogen, Grand Island, NY). 5-Ethynyl-2′-deoxyuridine was added to the cells for the first 24h of the 48h adipogenesis induction. Cells were then trypsinized for release from the culture dish, and the Click-iT dye conjugation reaction was conducted according to assay protocol. Cells were also stained with the DNA dye 633 Red, and mean fluorescent intensity of the two dyes was measured on a FACSCanto (BD Biosciences, San Jose, CA).
Western analysis.
Western analysis for changes in protein abundance was performed, as previously described (Barchowsky et al., 1999). Briefly, 12 days postdifferentiation, cells were rinsed twice with cold stop buffer (10mM Tris-HCl, pH 7.4, 10mM EDTA, 5mM ethylene glycol tetraacetic acid, 0.1M NaF, 0.20M sucrose, 100µM Na-orthovanadate, and 5mM pyrophosphate) and then scraped in a Tris SDS lysis buffer. The cell suspension was sonicated 3 × 8 s and then centrifuged at 10,000 × g at 4°C for 5min. Protein concentration was assayed using Pierce Coomassie Plus reagent according to the manufacturer’s instructions, and 25 µg of protein was loaded into gels for separation by polyacrylamide gel electrophoresis. The proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P, EMD Millipore Corporation, Billerica, MA) and probed with antibodies to perilipin (Rabbit mAb no. 9349, Cell Signaling Technology, Danvers, MA) and anti-β-actin (mouse monoclonal, Sigma, St Louis, MO). Reacted bands were detected by horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence substrates (PerkinElmer, Boston, MA). Band densities were quantified using ImageJ software v1.38x (National Institutes of Health, Bethesda, MD) and normalized to the density of β-actin in the same sample.
Microscopy and quantitative imaging.
Quantitative fluorescent imaging was used to measure lipid droplet formation and perilipin protein expression. hMSC were cultured on gelatin-coated coverslips and induced to differentiate in the absence or presence of As(III). After 9 days, the cells were fixed in 2.5% paraformaldehyde for fat droplet and coat protein staining. Nile red (Sigma) was used to stain triglycerides. Antibody to perilipin (Rabbit mAb no. 9349, Cell Signaling Technology) followed by Alexa Fluor 488–conjugated goat anti-rabbit secondary antibody (Invitrogen) was used for immunostaining. 4′,6-Diamidino-2-phenylindole (DAPI) was used to stain nuclei. Four fields of cells from each coverslip were imaged at ×40 using an Olympus Fluoview 1000 confocal microscope. The percent of thresholded pixel density per unit area for Nile red or perilipin staining was calculated using Metamorph v7.5.2 software and normalized to the percent thresholded pixel density for DAPI staining. The mean value was calculated for the four fields of each coverslip (n = 1) and the mean and SE values from at least three coverslips from two separate experiments were calculated for group values.
Quantitative RT-PCR.
Total RNA was harvested from undifferentiated and differentiated mouse SVC and hMSC using TRIzol (Invitrogen) and analyzed for mRNA levels of ADIPOQ, PPARγ, cEBPα, cEBPβ, cEBPγ, PLIN1, EDN1, EDNRA, EDNRB1, EDNRB2, and the housekeeping gene RPL13A, as previously described (Gao et al., 2004). RNA extracts from mouse cells were analyzed for ADIPOQ, PPARγ, and the housekeeping gene RPL41. Primer pairs used to amplify the specific cDNAs are provided in Supplementary table 1. Gene transcripts were quantified using standard curves for the respective cDNA products, and changes in resulting inducible cDNA levels were normalized to changes in RPL13A or RPL41 cDNA levels to determine the picogram of normalized product per milliliter of reaction.
Statistics.
Statistical analysis was performed on data from three to four replicate cultures from multiple experiments. Standard unpaired t-tests were used to test significance between single treatments and comparisons. One-way or two-way ANOVA was used to identify significant differences between treatment multiple groups and controls. The degree of significance between groups was compared using Bonferroni’s post hoc test. All statistics were performed using GraphPad Prism, v 5.02 software (GraphPad Software, San Diego, CA). Data are presented as means ± SEM of quantified values or fold control.
RESULTS
As(III) Inhibits Differentiation of Primary Adipose-Derived SVC and MSC
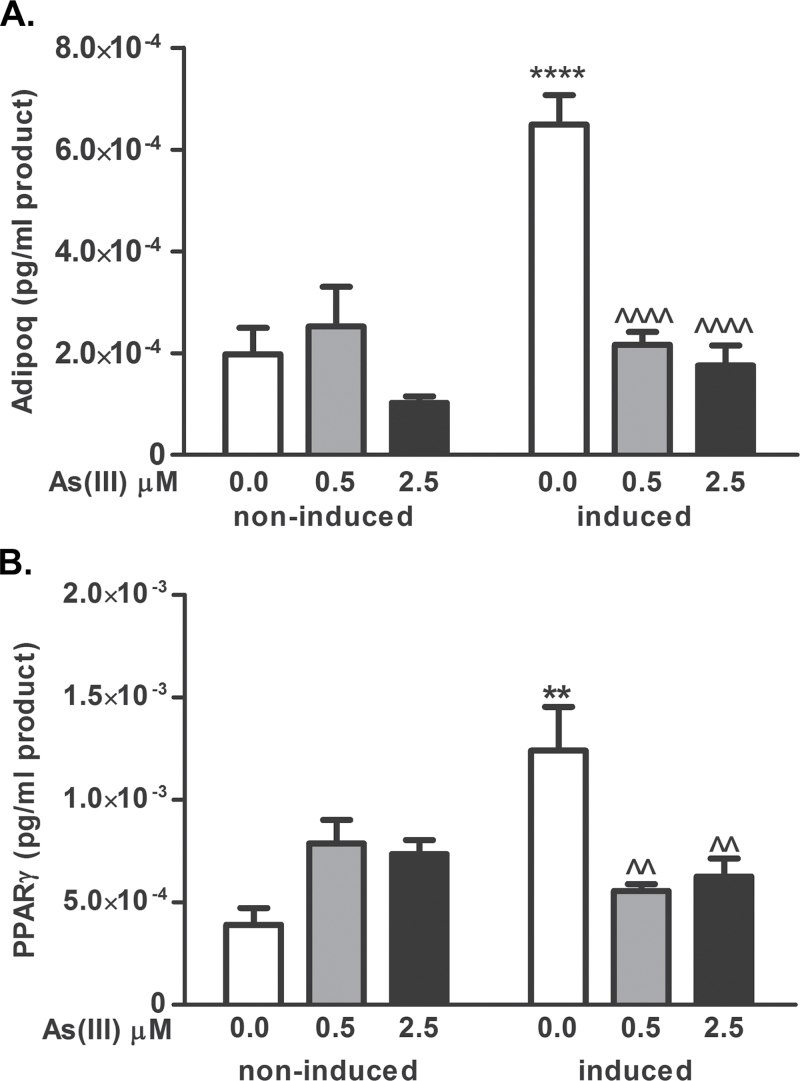
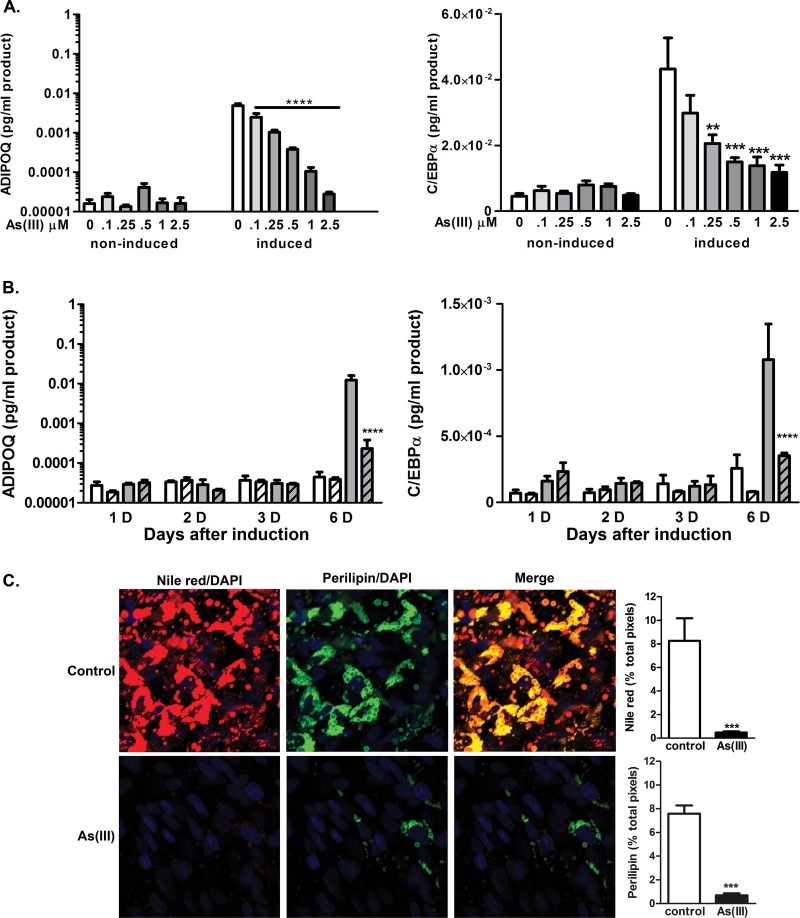
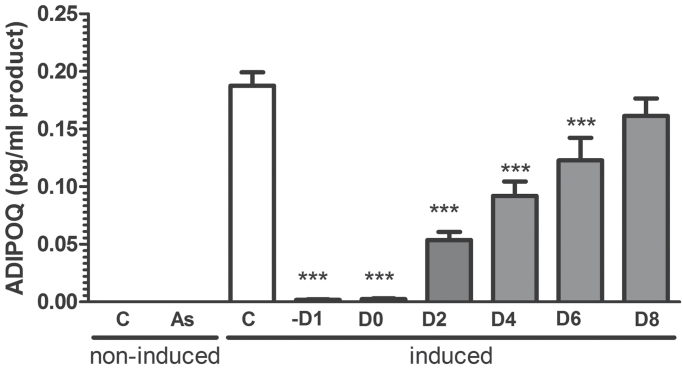
As(III) inhibits adipogenesis and adipose function in well-characterized fibroblast cell lines (Wauson et al., 2002). To confirm that As(III) inhibits differentiation in primary cells derived from the stromal/vascular niche, SVC isolated from mouse epididymal fat pads were induced to differentiate in the presence or absence of As(III) and assessed for extent of differentiation by measuring induction of the adipose marker ADIPOQ and the transcription factor PPARγ. The SVC stimulated with induction medium in the presence of 500nM As(III) were not induced to express either of the differentiation markers (Fig. 1). hMSC isolated from the comparable adipose niche did not differ from the SVC in sensitivity to As(III) inhibition, as 500nM As(III) produced a near maximal block-induced differentiation (Fig. 2A) and expression of the of C/EBPα or ADIPOQ (Fig. 2B). Cells exposed to As(III) failed to change morphology (data not shown) or accumulate Nile red–positive lipid droplets bound by the coat protein perilipin (PLIN1, Fig. 2A). As(III)-attenuated adipose marker transcripts were not due to cytotoxicity, because a high dose of 2.5µM As(III) slightly increased proliferation of the induced cells (Supplementary fig. 1). Comparing the early program of adipogenic regulator expression revealed that expression of proximal factors, such as C/EBPβ or C/EBPδ, was not different in control or arsenic exposed hMSC (Supplementary fig. 2). Examination of when the effect of As(III) occurs in the adipogenic program demonstrated that As(III) does not decrease the basal levels of adipogenic gene expression, but blocks induction of the program as it is manifest on day 6 after adding induction medium (Fig. 2C). Addition of As(III) before, at the same time, or several days after adding induction medium significantly inhibited ADIPOQ expression (Fig. 3). However, adding arsenic after day 6 failed to block adipocyte marker expression, indicating that As(III) must be present before the critical switch in the differentiation program is activated.
FIG. 1.
As(III) inhibits expression of the adipogenic transcriptional program in primary mouse SVC. SVC isolated from epididymal fat pads were cultured in basal or differentiation/maintenance medium in the presence or absence of As(III) for 12 days to allow full differentiation to adipocytes. Total RNA was then isolated and transcript levels for the differentiation markers adiponectin (ADIPOQ), and PPARγ were measured by QRT-PCR. The data are presented as mean ± SEM of the pg/ml of PCR product normalized to the housekeeping gene RPL13 and analyzed by two-way ANOVA with a Bonferroni’s post hoc test. ** or *** designates difference from noninduced control at p < 0.01 or p < 0.0001, respectively. ^^ and ^^^^ designate significance from control at p < 0.01 and p < 0.0001, respectively (n = 4).
FIG. 2.
As(III) causes concentration-dependent inhibition of hMSC differentiation to adipocytes. (A) hMSC were cultured in basal medium or differentiation/maintenance medium for 12 days in the presence of 0–2.5µM of As(III). (B) Cells were cultured for 1, 2, 3, or 6 days after initiation to differentiate (noninduced white bars, induced grey bars) in the absence or presence of 1µM As(III) (striped bars). Total RNA was then isolated and transcript levels for the differentiation markers adiponectin (ADIPOQ) and PPARγ were measured by QRT-PCR. The data are presented as mean ± SEM of the pg/ml of PCR product normalized to the housekeeping gene RPL13 and analyzed by two-way ANOVA with a Bonferroni’s post hoc test. ** or **** designates significance from control at p < 0.01 or p < 0.0001, respectively (n = 4 and data are representative of two replicate experiments). (C) hMSC grown on coated glass coverslips were induced to differentiate in the presence or absence of 1µM As(III). After 12 days of differentiation, the cells were fixed and processed for imaging fat droplet (Nile red stain), perilipin (green immunofluorescence), and nuclear (DAPI stain, blue) content. Images of four fields per coverslip were captured at ×40 and the thresholded fluorescence quantified and averaged. The data in the graphs present mean ± SEM percentage of positive pixels normalized to DAPI staining in four separate cultures. Differences were measured using a standard unpaired t-test. ** designates significance from control of p < 0.01.
FIG. 3.
As(III) must be present early to inhibit adipocyte differentiation. Cells were incubated in basal medium (noninduced) or differentiation/maintenance medium (induced) for 12 days. As(III) (1.0µM) was added at the indicated times before or during induction. Total RNA was then isolated, and transcript levels for the differentiation markers, adiponectin (ADIPOQ) and C/EBPα, were measured by QRT-PCR. The data are presented as mean ± SEM of the pg/ml of PCR product normalized to the housekeeping gene RPL13 and analyzed by one-way ANOVA with a Bonferroni’s post hoc test. *** designates significance from control at p < 0.001 (n = 4 and data are representative of two replicate experiments).
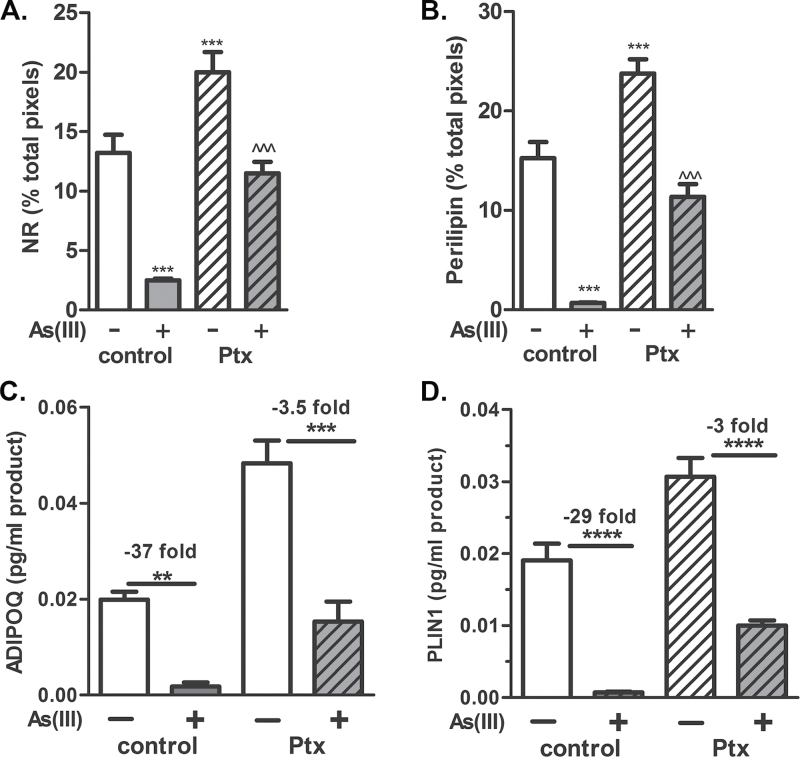
As(III) Inhibition of Adipogenesis Is Prevented by Ptx
We next asked whether As(III) signals for repressed adipogenesis by activating GPCR, as we previously found for As(III)-induced vascular remodeling (Straub et al., 2009). Adding Ptx to the undifferentiated hMSC 24h before changing to induction medium slightly enhanced adipocyte differentiation, but prevented As(III) from inhibiting differentiation of hMSC to adipose cells with PLIN1-coated fat droplets (Fig. 4A and Supplementary fig. 3). Ptx treatment also protected expression of ADIPOQ mRNA or PLIN1 mRNA (Fig. 4) and protein (Supplementary fig. 3) from As(III). These data suggest that As(III) acts through a Gi-linked GPCR to inhibit adipogenesis.
FIG. 4.
Ptx inhibition of Gi prevents As(III) inhibition of adipogenesis. Ptx (1µM) was added to the indicated groups of cells 24h prior to the start of induction of differentiation in the absence or presence of 1µM As(III). Eleven days after induction, the cells were fixed and stained with Nile red and immunostained for perilipin (A and B) or extracted for RNA. In (A) and (B), fluorescence intensity was quantified, and the data are presented as mean ± SEM percentage of positive pixels normalized to DAPI staining in four separate cultures. In (C) and (D), RNA was analyzed for transcript expression by QRT-PCR and normalized to RPL13 expression. The data in the graphs are presented as mean ± SEM of the normalized measures and all were analyzed by one-way ANOVA (noninduced samples are not plotted on graphs) with a Bonferroni’s post hoc test. In (A) and (B), *** designates significance from control and ^^^ designates significance from Ptx alone (p < 0.001). In (C) and (D), *** designates significance from control or Ptx with fold inhibition calculated (p < 0.001, n = 4). Calculation of fold difference from respective controls was made by dividing the mean values for control groups by the mean of As(III)-exposed groups.
Endothelin-1 Receptors (ENDRA and ENDRB) mediate As(III)-inhibited adipogenesis
There are at least three types of Gi-linked receptors that affect adipocyte differentiation, including S1PR1/3, AGTR1, and ENDRA/ENDRB (Bhattacharya and Ullrich, 2006; Hashimoto et al., 2009; Tomono et al., 2008; van Harmelen et al., 2008). In contrast to As(III) stimulation of endothelial cells signaling through S1PR1, blocking S1PR1 with VPC23019 decreased adipogenesis regardless of the presence of As(III) and As(III) did not add to this blockade (Supplementary fig. 4A). Competitive antagonism of AGTR1 also failed to prevent As(III) from inhibiting ADIPOQ and PLIN1 expression (Supplementary fig. 4B). However, addition of competitive antagonists of either ENDRA (BQ610) or ENDRB (RES-701-1) partially prevented As(III)-inhibited expression of the maturation markers (Fig. 5). Addition of both antagonists together was comparable to Ptx in preventing As(III) from inhibiting adiponectin expression (Fig. 5B).
FIG. 5.
Endothelin-1 receptors mediate As(III) inhibition of adipogenesis. hMSC were incubated with 1µM ET-1 receptor antagonists, BQ610 (ENDRA) or RES-701-1 (ENDRB1) for 30min prior to adding induction cocktail and 1µM As(III) and at each medium change through differentiation. On day 12 of differentiation, total RNA (A) or protein (B) was isolated from cells and probed for adiponectin (ADIPOQ) transcript or perilipin (PLIN1) expression. In (A), data are presented as mean ± SEM of pg/ml of QRT-PCR product normalized to RPL13 transcript expression. In (B), data are presented as mean ± SEM of band density from Western analysis of PLIN1 expression normalized to β-actin. Numbers from densitometry are plotted for both westerns on the same graph. Differences between groups were analyzed by (A) two-way ANOVA with a Bonferroni’s post hoc test for significance or (B) unpaired t-test. *** or **** designates significance from control at p < 0.001 or p < 0.0001, respectively (n = 4 and representative of two or more replicate experiments).
Using shRNA to knock down the two receptors individually or together demonstrated a slightly different profile for receptor-dependent actions (Fig. 6). Knocking down EDNRA did not prevent As(III)-inhibited differentiation (ADIPO expression). However, knocking down ENDRB reduced differentiation in the absence of As(III), but was partially protective in reducing As(III)-inhibited differentiation (Fig. 6). Knocking down both receptors simultaneously also reduced control differentiation, but to a lesser extent than knocking down EDNRB alone. Simultaneous knockdown was protective relative to differentiation in cells transfected with nonspecific shRNA or shRNA to EDNRA, but less protective than knocking down EDNRB alone (Fig. 6).
FIG. 6.
shRNA knockdown of EDNR attenuates As(III)-inhibited hMSC differentiation. hMSC were transfected with pools of shRNA to nonspecific sequence (NS), to EDNRA or EDNRB, or to both EDNRA and EDNRB. After 4 days, the cells were induced to differentiated in the presence or absence of 1µM As(III), and total RNA was extracted at the end of 8 days of differentiation. Data are presented as mean ± SEM of pg/ml of QRT-PCR product normalized to RPL13 transcript expression. *, **, and *** designate significant difference from between the groups indicated by bars at p < 0.05, p <0.01, and p < 0.0001, respectively. In (D), * or ** designates significance from respective control groups and ^ designates difference between NS plus As(III) and shEDNRA-treated cells plus As(III) at p < 0.05 (n = 4).
As(III) Decreases hMSC Endothelin-1 Expression, But Induces ENDRB1
The effect of As(III) on expression of endothelin-1 or its receptors was examined to determine whether As(III) acts by increasing levels of the receptor ligand (endothelin-1) or the receptors, respectively. Adding As(III) with differentiation and maintenance media decreased endothelin-1 transcript levels by ~60% (Fig. 7A). In contrast, As(III) induced expression of transcripts for both ENDRBs by more than threefold (Fig. 7B). Western analysis of protein extracts from the cells with an antibody that does not discriminate between the ENDRB subtypes indicated that protein expression was also increased (Fig. 7C). ENDRA transcript and protein expression were unchanged (Fig. 7B). The data indicate that induction of endothelin-1 expression may not account for As(III) actions its cognate receptors and that induction of the ENDRBs may facilitate As(III) inhibition of adipogenesis.
FIG. 7.
As(III) differentially affects endothelin-1 and endothelin-1 receptor expression. hMSC were induced to differentiate in the absence or presence of 1µM As(III). After 12 days of differentiation, total RNA (A and C) or protein (B) was isolated from cells and probed for expression of ET-1 or its cognate receptors. Data are presented as mean ± SEM of pg/ml of QRT-PCR product normalized to RPL13 transcript expression. Differences between groups were analyzed by unpaired t-test (A) or one-way ANOVA with a Bonferroni’s post hoc test (B) for significance. *, **, and *** designate significance from control at p < 0.05, p < 0.01, and p < 0.001, respectively (n = 3).
As(III) exposures to hMSC transfected with the EDNR-specific shRNA pools revealed a complex relationship of receptor-dependent expression of the individual endothelin receptors. The data in Figure 6B confirm the low level of expression of hMSC EDNRA transcript and that addition of shRNA to EDNRA decreases the transcript level. However, knocking down EDNRB caused a greater than 12-fold increase in EDNRA transcript. This increase was inhibited by As(III). The addition of shRNA to EDNRB was effective in reducing both EDNRB1 and EDNRB2 transcripts by ~86% and As(III) was effective in inducing the two transcripts in cells transfected with nonspecific shRNA. However, knocking down EDNRA completely blocked As(III) induction of EDNRB1 and attenuated As(III)-induced EDNRB2 transcripts (Figs. 6C and 6D).
DISCUSSION
The proximal site of arsenic action is often not identified when its pathogenic mechanisms are investigated. The data presented in this study and our previous report on As(III) activation of vascular S1PR1 (Straub et al., 2009) indicate that continual activation of surface membrane, Gi-linked GPCR transmits pathogenic As(III) signaling. By acting through these receptors, As(III) can generate a repressive signaling program that inhibits adipose differentiation and potentially functions important for normal adipose metabolism and insulin sensitivity. In addition to demonstrating a mechanism for As(III) effects on adipose regeneration, the studies provide insight into tissue-specific response to As(III) that is dictated by the endogenous regulatory actions of the GPCR in different cell types. The important observation is that the signals generated from the respective receptors in response to As(III) are consistent with repressive or pathogenic signaling generated in response to high expression of their cognate endogenous ligands.
Suppression of adipogenesis by Ptx-sensitive GPCRs is well established (Hauner et al., 1994; Janke et al., 2002; Shinohara et al., 1992; Tomono et al., 2008; van Harmelen et al., 2008). As seen previously (Hauner et al., 1994) and in Figure 5, addition of Ptx to progenitor cells derived from human adipose tissues increases adipogenesis, suggesting tonic suppression of adipogenesis by Gi-linked receptors. Ptx ribosylation of Gi prevented ~90% of the inhibitory effect of As(III), as indicated by the fold repression of adiponectin or perilipin transcript in cells treated with Ptx and As(III) relative to the nearly 30-fold repression observed in cells exposed to As(III) (Fig. 5). The fact that 10% repression remained indicated that Gi proteins are required for the majority, but not all of the As(III) inhibition.
None of the competitive inhibitors of individual Gi-linked receptors produced the same level of blockade as Ptx although inhibitors against ENDRA and ENDRB provided more than 60% protection individually (Fig. 5A). Combining the antagonists produced essentially the same efficacy in blocking As(III) as Ptx (Fig. 5B). This might be expected, because these two receptors act cooperatively and heterodimerize to provide sustained signaling (Evans and Walker, 2008; Watts, 2010; Zuccarello et al., 1999). However, knocking down the receptors with shRNA revealed the complexity of the receptor interactions and As(III) action through the receptors. It appears that the receptors inhibit each other’s actions, as well as cooperate. For example, EDNRB suppresses expression of EDNRA, and EDNRA mediates As(III) induction of EDNRB. It is surprising that expression of EDNRA is inhibited by As(III) in the absence of EDNRB, but not when EDNRB is present (Figs. 6B and 7). This may suggest that As(III) stimulates EDNRA downregulation by acting through EDNRA, as well as requires EDNRA to increase EDNRB. There does appear to be a requirement for both receptors for the majority of As(III) inhibition of the adipocyte differentiation program, but their complex relationship with each other and possibly other GPCRs (Watts, 2010) in mediating As(III) inhibition remains to be resolved.
The residual 10% of As(III)-inhibited adipogenesis that is not blocked by Ptx may be mediated by As(III) acting on another receptor, such as the epidermal growth factor receptor (EGFR). As(III) has been shown to activate EGFR signaling in bladder (Simeonova et al., 2002) and lung (Andrew et al., 2009) epithelial cells although the activation may not be by mimicking ligand binding (Simeonova et al., 2002). Instead, this activation may result from other receptors transactivating EGFR through Src-dependent phosphorylation of the receptor (Simeonova et al., 2002). Ligand activation of EGFR is less effective than endothelin-1 receptor activation in blocking adipogenesis (Bhattacharya and Ullrich, 2006). However, activating EGFR potentiates endothelin-1-inhibited adipogenesis and signaling to disrupt insulin responsiveness in adipocytes (Bhattacharya and Ullrich, 2006). Additional studies are needed to confirm the possibility that As(III) stimulates both receptors to give full inhibition of adipogenesis.
The repressive pathway activated by As(III) stimulation of EDNRA or EDNRB is not clear because the blockade of the differentiation pathway appears to be below normal induction of C/EBPβ and C/EBPδ (Supplementary fig. 2). However, the data in Figure 2 confirm previous reports in mouse cell lines and hMSC that As(III) inhibits C/EBPα expression and the adipogenic program was previously reported (Cheng et al., 2011; Wauson et al., 2002). The data in Figure 3 indicate that As(III) has to be present during the initial 6 days of the induction program before C/EBPα expression increases to prevent adipocyte differentiation measured by ADIPOQ expression. It is plausible that As(III) activation of EDNR stimulates a phosphorylation cascade that results in a repressive transcriptional complex to form at the level of the C/EBPα promoter or that stimulates repressive epigenetic modification of the promoter. However, as the differentiation program advances from day 2–6, the As(III)-stimulated repressive program becomes ineffective in preventing adipogenesis. This again suggests epigenetic regulation as once the chromatin has opened to allow expression of the adipose marker genes, As(III) is no longer inhibitory. Identifying this receptor-initiated repressive pathway will be important for revealing a mechanism for As(III)-promoted insulin insensitivity, as well as important information for how environmental exposures can influence the stem cell niche to impair adipose regeneration.
In conclusion, the findings presented in these studies demonstrate that As(III) stimulates a specific repression of the transcriptional program for adipogenesis. This repression may involve activation of cell-signaling cascades that recruit repressive complexes to promoters of regulatory genes, such as C/EBPα, but not initiation of the adipogenic program by differentiating factors such as glucocorticoids or insulin. Instead, As(III) initiates signaling primarily through the Gi protein–coupled endothelin-1 receptors to repress expression of differentiation regulators. Thus, like high expression of endothelin-1, As(III) may suppress adipogenesis and impair adipocyte function by selective receptor-mediated signal amplification.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (R01ES013781, R01ES013781-S1); National Institutes of Health.
REFERENCES
- Abhyankar L. N., Jones M. R., Guallar E., Navas-Acien A. (2012). Arsenic exposure and hypertension: A systematic review. Environ. Health Perspect. 120, 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew A. S., Mason R. A., Memoli V., Duell E. J. (2009). Arsenic activates EGFR pathway signaling in the lung. Toxicol. Sci. 109, 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A, Roussel R. R, Klei L. R, James P. E, Ganju N, Smith K. R, Dudeck E .J. (1999). Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol. Appl. Pharmacol. 159, 65–75 [DOI] [PubMed] [Google Scholar]
- Bhattacharya I., Ullrich A. (2006). Endothelin-1 inhibits adipogenesis: Role of phosphorylation of Akt and ERK1/2. FEBS Lett. 580, 5765–5771 [DOI] [PubMed] [Google Scholar]
- Cawthorn W. P., Scheller E. L., MacDougald O. A. (2012). Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 53, 227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Graziano J. H., Parvez F., Liu M., Slavkovich V., Kalra T., Argos M., Islam T., Ahmed A., Rakibuz-Zaman M, et al. (2011). Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 342, d2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Qiu L., Zhang H., Cheng M., Li W., Zhao X., Liu K., Lei L., Ma J. (2011). Arsenic trioxide promotes senescence and regulates the balance of adipogenic and osteogenic differentiation in human mesenchymal stem cells. Acta Biochim. Biophys. Sin. (Shanghai) 43, 204–209 [DOI] [PubMed] [Google Scholar]
- Elbaz N., Bedecs K., Masson M., Sutren M., Strosberg A. D., Nahmias C. (2000). Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol. Endocrinol. 14, 795–804 [DOI] [PubMed] [Google Scholar]
- Eriksson A. K., van Harmelen V., Stenson B. M., Aström G., Wåhlén K., Laurencikiene J., Rydén M. (2009). Endothelin-1 stimulates human adipocyte lipolysis through the ET A receptor. Int. J. Obes. (Lond). 33, 67–74 [DOI] [PubMed] [Google Scholar]
- Evans N. J., Walker J. W. (2008). Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys. J. 95, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Barchowsky A, Nemec A. A., Fabisiak J. P. (2004). Microbial stimulation by Mycoplasm fermentans synergistically amplifies IL-6 release by human lung fibroblasts in response to residual oil fly ash (ROFA) and nickel. Toxicol. Sci. 81, 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Igarashi J., Kosaka H. (2009). Sphingosine kinase is induced in mouse 3T3-L1 cells and promotes adipogenesis. J. Lipid Res. 50, 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H., Petruschke T., Gries F. A. (1994). Endothelin-1 inhibits the adipose differentiation of cultured human adipocyte precursor cells. Metab. Clin. Exp. 43, 227–232 [DOI] [PubMed] [Google Scholar]
- Hausman D. B., Park H. J., Hausman G. J. (2008). Isolation and culture of preadipocytes from rodent white adipose tissue. Methods Mol. Biol. 456, 201–219 [DOI] [PubMed] [Google Scholar]
- He X., H’ng S. C., Leong D. T., Hutmacher D. W., Melendez A. J. (2010). Sphingosine-1-phosphate mediates proliferation maintaining the multipotency of human adult bone marrow and adipose tissue-derived stem cells. J. Mol. Cell Biol. 2, 199–208 [DOI] [PubMed] [Google Scholar]
- Janke J., Engeli S., Gorzelniak K., Luft F. C., Sharma A. M. (2002). Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes 51, 1699–1707 [DOI] [PubMed] [Google Scholar]
- Navas-Acien A., Sharrett A. R., Silbergeld E. K., Schwartz B. S., Nachman K. E., Burke T. A., Guallar E. (2005). Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol. 162, 1037–1049 [DOI] [PubMed] [Google Scholar]
- Nincheri P., Luciani P., Squecco R., Donati C., Bernacchioni C., Borgognoni L., Luciani G., Benvenuti S., Francini F., Bruni P. (2009). Sphingosine 1-phosphate induces differentiation of adipose tissue-derived mesenchymal stem cells towards smooth muscle cells. Cell. Mol. Life Sci. 66, 1741–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara O., Murata Y., Shimizu M. (1992). Enhancement of differentiation of cultured adipogenic cells (TA1) by pertussis toxin. Biochem. Cell Biol. 70, 650–655 [DOI] [PubMed] [Google Scholar]
- Simeonova P. P., Wang S., Hulderman T., Luster M. I. (2002). c-Src-dependent activation of the epidermal growth factor receptor and mitogen-activated protein kinase pathway by arsenic. Role in carcinogenesis. J. Biol. Chem. 277, 2945–2950 [DOI] [PubMed] [Google Scholar]
- Smith A. H., Steinmaus C. M. (2011). Arsenic in drinking water. BMJ 342, d2248 [DOI] [PubMed] [Google Scholar]
- Straub A. C., Klei L. R., Stolz D. B., Barchowsky A. (2009). Arsenic requires sphingosine-1-phosphate type 1 receptors to induce angiogenic genes and endothelial cell remodeling. Am. J. Pathol. 174, 1949–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q. Q., Lane M. D. (2012). Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 81, 715–736 [DOI] [PubMed] [Google Scholar]
- Tomono Y., Iwai M., Inaba S., Mogi M., Horiuchi M. (2008). Blockade of AT1 receptor improves adipocyte differentiation in atherosclerotic and diabetic models. Am. J. Hypertens. 21, 206–212 [DOI] [PubMed] [Google Scholar]
- van Harmelen V., Eriksson A., Aström G., Wåhlén K., Näslund E., Karpe F., Frayn K., Olsson T., Andersson J., Rydén M, et al. (2008). Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes 57, 378–386 [DOI] [PubMed] [Google Scholar]
- Vigouroux C., Caron-Debarle M., Le Dour C., Magré J., Capeau J. (2011). Molecular mechanisms of human lipodystrophies: From adipocyte lipid droplet to oxidative stress and lipotoxicity. Int. J. Biochem. Cell Biol. 43, 862–876 [DOI] [PubMed] [Google Scholar]
- Wang Z. X., Jiang C. S., Liu L., Wang X. H., Jin H. J., Wu Q., Chen Q. (2005). The role of Akt on arsenic trioxide suppression of 3T3-L1 preadipocyte differentiation. Cell Res. 15, 379–386 [DOI] [PubMed] [Google Scholar]
- Watts S. W. (2010). Endothelin receptors: What’s new and what do we need to know? Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R254–R260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauson E. M., Langan A. S., Vorce R. L. (2002). Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol. Sci. 65, 211–219 [DOI] [PubMed] [Google Scholar]
- Zuccarello M., Boccaletti R., Rapoport R. M. (1999). Does blockade of endothelin B1-receptor activation increase endothelin B2/endothelin A receptor-mediated constriction in the rabbit basilar artery? J. Cardiovasc. Pharmacol. 33, 679–684 [DOI] [PubMed] [Google Scholar]