Abstract
Objective
Wernicke encephalopathy and Korsakoff syndrome (the combined disorder is named Wernicke-Korsakoff syndrome [WKS]) are preventable, life-threatening neuropsychiatric syndromes resulting from thiamine deficiency. WKS has historically been associated with alcoholism; more recently, it has been recognized in patients who have anorexia nervosa or have undergone bariatric surgery for obesity. However, patients with nutritional deficiencies of any origin are at risk for WKS. We present clinical histories and neuroimaging data on 2 young adults with underlying psychiatric disorders who became malnourished and developed WKS.
Methods
A young woman with bipolar disorder and somatization disorder was hospitalized for intractable vomiting. A young man with chronic paranoid schizophrenia developed delusions that food and water were harmful, and was hospitalized after subsisting for 4 months on soda pop.
Results
Acute, life-threatening Wernicke encephalopathy was confirmed in both patients by brain magnetic resonance imaging showing classic thalamic injury. The patients were left with persistent cognitive and physical disabilities that were consistent with Korsakoff syndrome.
Conclusions
Failure to suspect a vitamin deficiency led to permanent cognitive and physical disabilities that may necessitate lifelong care for these patients. The neuropsychiatric consequences could have been prevented by prompt recognition of their thiamine deficiency.
Keywords: Wernicke, Korsakoff, malnutrition, thiamine, schizophrenia
Wernicke encephalopathy (WE), a life-threatening disorder caused by thiamine (vitamin B1) depletion, has historically been associated with a classic triad of acute neurological symptoms: confusion, ataxia, and ophthalmoplegia. Thiamine deficiency disease can affect primarily the central and peripheral nervous systems (dry beriberi) or the cardiovascular system (wet beriberi). When thiamine deficiency affects the nervous system, ocular findings such as ophthalmoplegia and nystagmus have been described as a hallmark of the disorder, affecting about 85% of patients.1 However, only 15% to 30% of patients present with the complete WE triad,1,2 and almost 20% of patients who develop persistent confabulatory amnesia, referred to as Korsakoff syndrome, demonstrate none of the 3 findings.1 Therefore, it can be challenging to base the diagnosis solely on physical signs.
Korsakoff syndrome, also called Korsakoff dementia, Korsakoff psychosis, or amnesic-confabulatory syndrome, is a life-altering, permanent neuropsychiatric condition characterized by anterograde and retrograde amnesia as well as frontal lobe dysfunction and affective disturbance.3 Korsakoff syndrome can follow an acute episode of WE, and may appear as an agitated delirium or delirium tremens. Korsakoff syndrome may affect up to 85% of patients who have suffered WE, and can result in persistent impairments ranging from mild memory deficit to stupor and coma.4,5 Confabulation and apathy are the classically reported sequelae of WE or Korsakoff syndrome. At least 60% of patients who survive WE also have residual physical impairments such as nystagmus and ataxia.6,7
A great majority of patients with acute WE and/or chronic Korsakoff syndrome (the combined disorder is termed Wernicke-Korsakoff syndrome [WKS]) are never recognized. Only 1% to 20% of cases are diagnosed clinically.5,8,9 Up to 80% are found on postmortem examination of the brain. Similarly, heart failure caused by beriberi may never be recognized, nor may thiamine deficiency’s multisystem effects.
WKS most often affects people who have a nutritional deficiency related to alcoholism. However, the syndrome can result from an inadequate supply of thiamine from reduced food intake or poor nutrition of any cause.1 For example, recent attention has been given to WKS in patients who have undergone gastric bypass surgery for obesity.10,11 WKS can affect patients with immunodeficiency syndromes,8 malignancies,12 hyperemesis gravidarum, 13 liver disease,9 or hyperthyroidism.14 Similarly, WKS has been described in patients who have received prolonged parenteral nutrition12 and in patients suffering with severe anorexia nervosa.15–18 In 1 study, 30% of patients hospitalized for anorexia nervosa were found to have thiamine deficiency on admission.19 Thiamine deficiency has been found in people who subsist primarily on white polished rice.20,21 Susceptibility to WKS may also be related to a hereditary deficiency of transketolase, an enzyme involved in thiamine metabolism.22,23
WKS is even more under-recognized among malnourished patients who have psychiatric disorders, but do not abuse alcohol or suffer from anorexia nervosa. Table 1 reviews previously reported non-alcohol–induced WKS arising primarily as a consequence of psychiatric illness.
TABLE 1.
Prior Reports of Wernicke-Korsakoff Syndrome (WKS) in Patients With Psychiatric Conditions
| Underlying Condition (Age at Onset) | Precipitator of WKS | MRI Result (Reference and Page Number) | Patient’s Condition at Latest Follow-up |
|---|---|---|---|
| Anorexia nervosa (15) | 18 mo of dieting; < 1000 calories/d and no bread for previous 3 mo | “Within normal limits except for a slight widening of the cerebellar sulcus” (Altinyazar et al,15 page 767) | Returned to full level of premorbid functioning after 9 mo |
| Anorexia nervosa (16) | 16 mo of dieting; 30 kg weight loss during previous 6 mo | “Cortical atrophy and bilateral subthalamic hyperdensities” (Peters et al,16 page 378) | Improved at time of discharge |
| Anorexia nervosa (17) | 10 y of severe dieting and 8y of drinking gin daily | “Increased signal/halo in the supratentorial periventricular region, increased T2 signal in the medial regions of the thalamus and in the central and periaqueductal midbrain. Enlarged perivascular spaces in the basal nuclei topography, a thinned corpus callosum and widened cerebellar sulci” (Saad et al,17 page 2) | Ophthalmoplegia resolved; memory deficits remained unaltered |
| Anorexia nervosa (18) | 10 wk of self-starvation | Within normal limits (Sharma et al18) | Completely resolved at 2 mo |
| Schizophrenia (26) | 4 mo of medication noncompliance and poor nutrition | None (Salawu and KwajafFa26) | Improved |
| Schizophrenia (27) | 3 mo of medication noncompliance and poor nutrition | “Increased signal at the medial aspect of the anterior thalamus extending into the hypothalamus and the massa intermedia” (Harrison et al,27 page C9) | Mental status back to baseline, but ongoing mild ataxia and persistent vertical nystagmus |
| Schizophrenia (28) | Long-term medication noncompliance and 48 d of self-starvation | None (Tsai et al28) | Incomplete recovery of short-term memory, nystagmus, and ataxia |
None of these studies provided biochemical confirmation of a WKS diagnosis.
This report describes magnetic resonance imaging (MRI)-confirmed acute WE and clinically confirmed persistent Korsakoff syndrome in 2 patients with preexisting psychiatric disorders. Because the patients were unwilling or unable to give accurate histories, we also obtained information from family members and medical records.
CASE REPORT 1: SW
Previous History
SW was a single, unemployed woman in her mid-20s. She was born at term, experienced no developmental delays, and was a high school graduate. Her biological parents separated shortly after her birth. Her father moved out of state, and she rarely saw him afterward. She was raised by her mother in a chaotic household. The mother remarried and had 2 more children. SW reported that her stepfather sexually abused her from ages 5 to 7 and that she was raped several times around the age of 16.
At age 9, SW saw a counselor for “rage attacks,” described as rapid shifts between being quiet and withdrawn, and being irritable and aggressive. She also struggled with depressed mood, decreased appetite, and lack of motivation. A local psychiatrist diagnosed her with bipolar disorder when she was 10. Her first psychiatric hospitalization was that same year, for “violent aggression toward her siblings, defiance, self-harm, suicidal ideation.” She was also hospitalized at ages 12 and 14, and was sent to live in a supervised group home for 6 months at age 15. Both mother and daughter were once hospitalized in the same hospital at the same time (the mother for a drug overdose in a suicide attempt). The mother and maternal grandmother were diagnosed with bipolar disorder.
When SW came to our attention, she was 20 years old, single, unemployed, and subsisting on food stamps and other public support. She had been living in a trailer with her unemployed fiancé, her 14-month-old daughter from another relationship, and the fiancé’s disabled mother. She had given birth to the child at the age of 18, after 35 weeks’ gestation. Her pregnancy had been complicated by persistent hyperemesis gravidarum that necessitated 3 hospital visits and central venous nutrition, because she refused any form of nutritional intake, including nasogastric tube feeding. A week after delivering her baby, SW was hospitalized twice in short succession for intractable vomiting. During these admissions, she underwent extensive gastroenterologic and psychiatric evaluations. After an upper endoscopy and all other tests proved normal, her symptoms were believed to be psychogenic and possibly caused by postpartum depression. Her psychiatrist began her on oxcarbazepine (Trileptal) and bupropion (Wellbutrin) for her depression and irritability.
For the 7 weeks before being admitted to our hospital, SW had reportedly eaten very little due to ongoing nausea and vomiting. She had been unable to take her regular medications. She had visited the local hospital emergency room about 10 times for nausea and vomiting, and had been treated with intravenous (IV) fluids, electrolyte repletion, antiemetics, antacids, and pain medications. She was transferred to our hospital for further evaluation.
Course During and After Index Hospitalization
On admission to our hospital, SW reported that she had not eaten anything for 7 weeks due to severe nausea, vomiting, and epigastric pain. She could not say how many times she had been vomiting each day, but she knew that she had lost approximately 30 pounds over the previous year due to intermittent episodes of nausea and vomiting. On physical exam during the triage process, she appeared thin but in no acute distress. She reported numbness, weakness, dizziness, fatigue, and decreased urine output; she also complained of a “locking up” of both of her hands and legs, lasting minutes, with increasing frequency. She was admitted to the general medicine service and given IV fluid maintenance of 5% dextrose in normal saline, with 20 mEq of potassium chloride.
The day after admission, SW told the consulting psychiatrist that she had recently lost her job, as well as custody of her child to the child’s biological father. The biological father also had a restraining order against SW because she had hit him and had threatened to hurt their child. While she was generally cooperative in answering questions, she seemed skeptical of the psychiatric service. She was given a diagnosis of undifferentiated somatoform disorder and was prescribed dissolvable mirtazapine (Remeron) to improve her appetite and mood. She denied having a history of an eating disorder or of abusing illicit substances, but admitted to binge drinking 3 or 4 times a month over the past 2 years, with up to 5 beers at a time.
A week later, she was transferred to a monitored bed for a trial of a continuous haloperidol (Haldol) infusion that might alleviate her nausea. She was able to eat a few crackers that day, but refused to eat anything afterward. The next day she complained of dizziness and developed double vision and nystagmus. The nystagmus became increasingly prominent over the next 2 days, to the point that she could not maintain lateral gaze. During these 3 days, her speech became slurred and her mentation slowed. Both mirtazapine and haloperidol were stopped because of the possibility that they were contributing to her delirium. The next day (10th day of her hospital stay), she was to be transferred to the medical-psychiatry unit, but was found during morning rounds to be almost completely unresponsive. Her extremities were cold and her pulses weak. Her vital signs were unstable, with a heart rate of 141 beats/min and blood pressure of 152/113 mm Hg. She was transferred immediately to the medical intensive care unit.
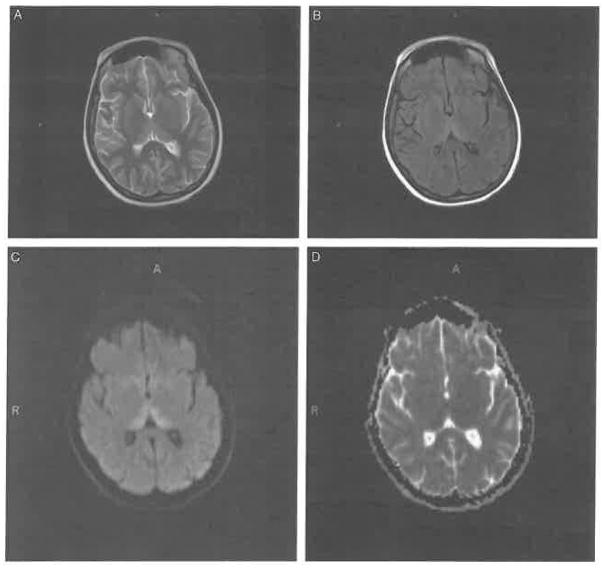
A brain MRI with contrast showed increased T2 signal and restricted diffusion of the thalamus, findings consistent with WE (Fig. 1). The Supplemental Digital Content shows full sets of the (A) fluid-attenuated inversion recovery (FLAIR) images (Supplemental Digital Content 1, http://links.lww.com/CBN/A15), (B) T2-weighted images (Supplemental Digital Content 2, http://links.lww.com/CBNA17), and (C) corresponding apparent diffusion coefficient (ADC) maps (Supplemental Digital Content 3, http://links.lww.com/CBN/A18). An electroencephalogram showed (1) an excess of interspersed slow waves during wakefulness, (2) a single diffuse sharp discharge with bifrontal dominance during drowsiness, and (3) a few brief episodes of rhythmic theta bursts from the right frontal region, indicating delirium. The cerebrospinal fluid and creatine kinase were normal.
FIGURE 1.
Upon her admission to the medical intensive care unit, patient SW’s brain magnetic resonance imaging scan is consistent with Wernicke encephalopathy. Axial (A) T2-weighted, (B) fluid-attenuated inversion recovery, and (C) high-intensity diffusion-weighted images, and (D) corresponding apparent diffusion coefficient map images show involvement of the dorsomedial thalamic nuclei.
SW was immediately started on 200 mg of IV thiamine daily, and a nasogastric tube was placed for regular tube feeding. Her condition improved markedly within the next 3 days, and she was transferred to the medical psychiatry unit. Although she remained weak and drowsy over the next 4 weeks, she continued to improve slowly. Her nasogastric tube was removed, and she was given a regular diet supplemented by high-protein, high-calorie milkshakes and 100 mg of oral thiamine daily.
Approximately 1 month later, she was transferred to a general psychiatry unit. At that time she could not walk or eat without assistance, and she had persistent cognitive deficits. Two months after the acute WE episode, she had a repeat brain MRI, which showed complete resolution of the abnormal signals in the thalamus.
SW spent the next 4 months on the general inpatient psychiatry unit. A number of antidepressant medications and psychosocial interventions were tried, with little success. She was eventually restarted on bupropion and started on carbamazepine (oxcarbazepine was not on formulary in the hospital). She also continued to take daily oral thiamine.
A battery of neuropsychological tests performed 1 month after her acute WE episode revealed severe impairment in her cognitive functioning. The Wechsler Adult Intelligence Scale-III short form verbal intelligence quotient was 72 (4th percentile).24 She had significant difficulty with short-term memory. Vertical nystagmus and a lack of right-hand dexterity affected her balance and coordination. Repeat neuropsychological and occupational therapy testing before her discharge showed a persistent moderate cognitive disorder. After 5 months in the hospital, SW was discharged to a nursing home, with a recommendation for indefinite 24-hour supervision.
SW spent the next 2 years in the nursing home. During those years she returned to our outpatient psychiatry clinic for 8 follow-up appointments. Her short-term memory remained significantly impaired, and she continued to have mild end-gaze nystagmus. She still needed help with eating, walking, and bathing. Although she had no nausea or vomiting, she sometimes refused food and medications. She was generally in good spirits. She said she was optimistic that she would eventually recover and be able to go home.
At the 2-year point, she was readmitted to our facility for new-onset seizures and delirium, secondary to a urinary tract infection. A new brain MRI showed no evidence of WKS, but for the first time did show focal restricted diffusion, with a high FLAIR signal intensity lesion in the splenium of the corpus callosum, most consistent with postictal changes (figure not shown). She was taken off carbamazepine and bupropion, and given levetiracetam (Keppra) and olanzapine (Zyprexa). She continued to take daily oral thiamine. She remained on the psychiatric inpatient unit for 3 more months because of ongoing nausea and vomiting, and was fed through a nasogastric tube. Because she could not resume voluntary eating, a percutaneous endoscopic gastrostomy tube was placed. She was then able to return to her nursing home.
SW currently carries diagnoses of WKS-related dementia and borderline personality disorder. She is involuntarily committed to a nursing home and regular psychiatric follow-up at our hospital. She still intermittently complains of nausea and vomiting, and has emotional outbursts whenever she cannot see her now 4-year-old child on a regular basis.
CASE REPORT 2: RF
Previous History
RF was an obese, single, unemployed man in his mid-30s, with a diagnosis of paranoid schizophrenia. He was the youngest of 3 sons. His parents are still married. RF dropped out of high school during his senior year after developing schizophrenia. For most of his adult life, he had been receiving disability insurance for his severe mental illness. He was treated for years with risperidone (Risperdol). He functioned well enough to live alone in a city about 100 miles from his parents. His mother would visit weekly to help him shop for groceries, and, when needed, gave him extra money. Apart from her visits, he had little contact with the outside world.
For the 10 months before his admission to our facility, RF had not been following up with his psychiatrist, and was thought to have stopped taking his risperidone. During this time, he had come to believe that local foods and water were “harmful.” Because of this belief, he had refused to eat and to drink water. He reported that for the 3 months immediately before admission, all that he had consumed was sugary soda pop. During that time he grew weak, reportedly lost about 25 pounds (from a previous weight of 300 pounds), and could no longer get out of bed. His mother reported that he looked “terrible” and that he had blurred vision. She obtained a court order to have him brought to a hospital for a medical and psychiatric evaluation.
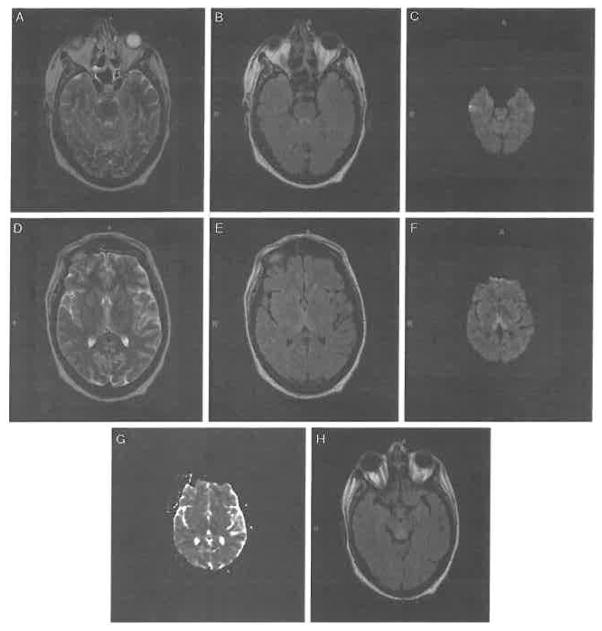
Upon admission to a local hospital, RF was found to be obtunded. He had elevated liver enzymes, anemia, folic acid deficiency, hypomagnesemia, and hypophosphatemia, all indicating malnutrition. He was also found to have hyperthyroidism. Electroencephalogram showed diffuse slow waves consistent with acute encephalopathy (delirium). Brain MRI revealed a high T2 signal and restricted diffusion along the periaqueductal gray region, and increased intensity in the bilateral dorsomedial thalamus and both mamillary bodies, consistent with WE (Fig. 2). The Supplemental Digital Content shows full sets of the (A) FLAIR images (Supplemental Digital Content 4, http://links.lww.com/CBN/A23), (B) T2-weighted images (Supplemental Digital Content 5, http://links.lww.com/CBN/A24), and (C) corresponding apparent diffusion coefficient (ADC) maps (Supplemental Digital Content 6, http://links.lww.com/CBN/A25).
FIGURE 2.
At the start of his local hospital admission, patient RF’s brain magnetic resonance imaging scan is consistent with Wernicke encephalopathy. Axial (A) T2-weighted, (B) fluid-attenuated inversion recovery (FLAIR), and (C) high-intensity diffusion-weighted imaging (DWI) images show involvement of the periaqueductal gray region; (D) T2-weighted, (E) FLAIR, (F) DWI, and (G) apparent diffusion coefficient map images show involvement of the bilateral posterior medial thalamus; and (H) FLAIR image shows mammillary body involvement.
RF began IV thiamine within 24 hours of admission. His agitation was treated as needed with IV lorazepam and risperidone. His course over the next 3 weeks was complicated by Pseudomonas pneumonitis, which required prolonged IV antibiotic treatment, intubation, and percutaneous tracheostomy placement. He also had a jejunostomy tube placed because he could neither swallow food on his own nor tolerate nasogastric tube feeding.
Course During and After Index Hospitalization
After 20 days at the local hospital, RF was transferred to our hospital for further treatment of his medical and psychiatric conditions. On admission, he denied headache, localized pain, numbness, tingling, paresthesias, diplopia, blurry vision, and bowel and bladder incontinence. On physical exam, he was an obese gentleman (weight = 260 pounds, body mass index = 32.5). A healing tracheostomy scar in the front center of his neck showed mild erythema but no exudate. Shortly after admission, neuropsychological testing with the Repeatable Battery for the Assessment of Neuropsychological Status25 revealed significant deficits in memory as well as in verbal and nonverbal skills. His total Repeatable Battery for the Assessment of Neuropsychological Status score was 52 (lower 1st percentile of intelligence quotient scores). His insight and judgment were poor. For example, although he could not sit upright on his own, he kept trying to get out of bed by himself. He had prominent vertical nystagmus and dysarthria. Both legs were significantly weak (2/5 strength). Sensation to light touch and pinprick was normal in his arms and legs. Motor coordination tests revealed bilateral dysdiadochokinesia and dysmetria. Reflexes were decreased at the biceps, brachioradialis, and patella, and were absent at the Achilles tendon. Plantar response was downgoing bilaterally.
RF was given enteral feedings through a jejunostomy tube, along with daily thiamine, folate, multivitamins, and risperidone. About 13 days after he restarted risperidone therapy, his paranoia over the toxicity of local food markedly diminished. A comparable response has been reported in similar cases of WKS in schizophrenia.26–28 Over the next 6 weeks of his hospitalization, RF received speech and physical therapy, and his jejunostomy tube was removed. However, he still could not walk on his own, and he continued having difficulty swallowing. He also had significant nystagmus and his dysarthric speech persisted, consistent with a diagnosis of WKS. RF was discharged to a skilled nursing facility and never returned to our hospital, so his follow-up course is unknown.
DISCUSSION
How could a deficiency in a vitamin cause so much damage? Thiamine is a water-soluble vitamin that can be stored in the body for only 9 to 18 days. Thiamine pyrophosphate, the metabolically active cofactor needed for glycolysis, is the brain’s primary energy producer. Cell membranes require thiamine to maintain normal osmotic gradients; thiamine deficiency can interfere with this homeostatic mechanism, leading to swelling in intracellular and extracellular spaces. In animal models, thiamine deficiency damages regions of the brain that depend particularly on thiamine-mediated glucose metabolism.29 In humans with prolonged thiamine deficiency, MRI scans like those in our patients show cytotoxic injury in the periventricular regions along the third ventricle, the medial thalamus, and mamillary bodies.30
Thiamine deficiency can be confirmed biochemically with a thiamine diphosphate test, which measures the level of active thiamine used in biochemical pathways, or with an erythrocyte transketolase assay, which provides an indirect measure of more recent thiamine utilization.31 However, because it can take days to weeks to get the results of these tests, they do not offer a practical way of diagnosing WE or WKS. Rather, it is important for clinicians to take a good clinical history and be familiar with both the risk factors for thiamine deficiency and its signs and symptoms.32
Nutritional deficits like thiamine deficiency can accompany many psychiatric disorders, and can affect anyone of any age who has poor eating habits.33,34 Vitamin deficiencies affect more patients who have comorbid psychiatric and medical conditions than patients who have medical conditions alone.35 This could be due in part to lack of knowledge about the importance of eating a balanced diet and/or limited resources and motivation to eat healthy food. Discussions about nutrition may have real value for patients with psychiatric disorders who may be or become at risk for nutritional deficiencies. There may also be a role for daily vitamin supplementation. Patients with thiamine deficiency should be encouraged to eat foods that contain high levels of thiamine, such as lentils, peas, brown (rather than white) rice, whole wheat bread, pork, organ meats, tuna, milk, eggs, nuts, and a variety of fruits and vegetables.
The standard of care in a patient with known alcoholism is to give IV thiamine before starting glucose-containing maintenance fluids or allowing the patient to eat 5,6,36–38 In general, physicians are more likely to give IV thiamine to patients with known or suspected alcohol dependence than to consider the broader spectrum of psychiatric disorders that can have related nutritional disturbances. As WE is exceedingly difficult to diagnose by physical exam, and as patients like SW and RF can be poor historians, we would argue for this standard of care to be extended to all patients who have even a remote likelihood of nutritional deficiency. The thiamine should be given IV because oral thiamine has only about 5% bioavailability but IV thiamine is virtually 100% bio-available.36 Acute treatment for WE should be thiamine hydrochloride 500 mg IV, diluted in normal saline, 3 times a day for at least 3 days, followed by 250 mg in normal saline, once a day for 3 days. Then the patient should be given oral thiamine 100 mg 3 times a day for the rest of the hospitalization and at least 100mg daily after discharge. Magnesium should be replaced simultaneously, because carbohydrate metabolism via thiamine-dependent pathways requires magnesium as a cofactor.
Physicians must be attuned to the likelihood that patients with psychiatric disorders have undiagnosed nutritional deficiencies. For example, our patient SW had a long history of psychosocial stressors that may have contributed to her nausea and vomiting. Her symptoms continued even after she delivered her child, suggesting a psychogenic component complicated by postpartum depression. Her ability to cope with early emotional trauma and later life stressors may have been further impaired by a combination of noncompliance in taking the psychotropic medications that had kept her psychiatric symptoms stable for the past year, and an inability to take her medications when she was nauseated and vomiting.
SW had a long history of poor nutrition that culminated in her being hospitalized for about 10 days before her physical signs of WE appeared. Early in thiamine deficiency, many patients have nausea and vomiting, leading to a vicious cycle of worsening nausea and vomiting and worsening thiamine depletion. Making matters worse, despite her extensive medical history SW was given glucose-containing IV fluids without supplemental thiamine. Giving glucose before thiamine is known to precipitate WE.38 Her condition was further exacerbated by the delayed start of IV thiamine.
Paranoia and poor insight led to RF’s medication noncompliance, worsening his psychosis and his odd eating habits. His soda-only diet led to his thiamine deficiency, Without a thorough history of this patient’s eating patterns, it could have been easy to miss the fact that his diet had contained little or no thiamine for at least 3 months.
The literature holds relatively few reports of nonalcoholic Korsakoff syndrome.39 SW’s case is a likely addition, based on her persistent cognitive and memory deficits after her acute episode of WE.40 It is plausible that delay in diagnosis and treatment of her WE put SW at higher risk of developing Korsakoff dementia. Because we were not able to follow RF after his discharge, we cannot confirm that he had Korsakoff syndrome. In patients who have undergone gastric bypass surgery, the development of Korsakoff dementia without earlier signs of WE may be explained by more insidious low-level thiamine deficiency.10,11
In summary, physicians should be aware of preventable vitamin deficiency-related neuropsychiatric syndromes, and should consider new signs and symptoms in patients with known psychiatric disorders as potential harbingers of reversible WE and irreversible WKS. Physicians should ask patients, relatives, and caregivers specifically about what patients have been eating—and not eating—and should thoroughly review the medical record for warning signs of nutritional deficiency. Physicians must exclude somatoform disorders, characterized by symptoms that mimic disease or injury but for which there is no identifiable physical cause; however, a patient with a psychiatric diagnosis can still develop serious medical conditions. If physicians assume that a patient’s symptoms stem wholly from a psychiatric disorder, they can miss the life-threatening emergency of WE. If they delay treating this reversible condition, the patients who survive may be left with long-term physical and cognitive sequelae. Patients at highest risk of developing non-alcoholic WE and WKS have a history of malnutrition, delusions related to food, or vomiting lasting longer than a week. These patients should be treated prophylactically with thiamine, especially before refeeding.
Acknowledgments
Dr McCormick was supported by NIH Career Development Award MHO83879-01. Dr Paradiso was supported by the Edward Mal-Iinckrodt Jr Foundation, the Dana Foundation, and NIH/NIA Career Development Award 5K23AG027837.
The authors thank Michael Brumm for his administrative assistance with the manuscript, and Dr Ameera Ismail for her assistance in interpreting the MRI images.
Footnotes
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.cogbehavneurol.com.
References
- 1.Ogershok PR, Rahman A, Nestor S, et al. Wernicke encephalopathy in nonalcoholic patients. Am J Med Sri. 2002;323:107–111. doi: 10.1097/00000441-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Munir A, Hussain SA, Sondhi D, et al. Wernicke’s encephalopathy in a non-alcoholic man: case report and brief review. Mt Sinai J Med. 2001;68:216–218. [PubMed] [Google Scholar]
- 3.Parkin AJ, Dunn JC, Lee C, et al. Neuropsychological sequelae of Wernicke’s encephalopathy in a 20-year-old woman: selective impairment of a frontal memory system. Brain Cogn. 1993;21:1–19. doi: 10.1006/brcg.1993.1001. [DOI] [PubMed] [Google Scholar]
- 4.Torvik A. Wernicke’s encephalopathy—prevalence and clinical spectrum. Alcohol Alcohol Suppl. 1991;1:381–384. [PubMed] [Google Scholar]
- 5.Day E, Bentham P, Callaghan R, et al. Thiamine for Wernicke-Korsakoff syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004:CD004033. doi: 10.1002/14651858.CD004033.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Kopelman MD, Thomson AD, Guerrini I, et al. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- 7.Zubaran C, Femandes JG, Rodnight R. Wernicke-Korsakoff syndrome. Postgrad Med J. 1997;73:27–31. doi: 10.1136/pgmj.73.855.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterworth RF, Gaudreau C, Vincelette J, et al. Thiamine deficiency and Wernicke’s encephalopathy in AIDS. Metab Brain Dis. 1991;6:207–212. doi: 10.1007/BF00996920. [DOI] [PubMed] [Google Scholar]
- 9.Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008;248:714–720. doi: 10.1097/SLA.0b013e3181884308. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Kumar A. Wernicke encephalopathy after obesity surgery: a systematic review. Neurology. 2007;68:807–811. doi: 10.1212/01.wnl.0000256812.29648.86. [DOI] [PubMed] [Google Scholar]
- 12.Francini-Pesenti F, Brocadello F, Famengo S, et al. Wernicke’s encephalopathy during parenteral nutrition. JPEN J Parenter Enteral Nutr. 2007;31:69–71. doi: 10.1177/014860710703100169. [DOI] [PubMed] [Google Scholar]
- 13.Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol. 2007;21:755–769. doi: 10.1016/j.bpg.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Bonucchi J, Hassan I, Policeni B, et al. Thyrotoxicosis associated Wernicke’s encephalopathy. J Gen Intern Med. 2008:23106–23109. doi: 10.1007/s11606-007-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altinyazar V, Kiylioglu N, Salkin G. Anorexia nervosa and Wernicke Korsakoff’s syndrome: atypical presentation by acute psychosis. Int J Eat Disord. 2010;43:766–769. doi: 10.1002/eat.20783. [DOI] [PubMed] [Google Scholar]
- 16.Peters TE, Parvin M, Petersen C, et al. A case report of Wernicke’s encephalopathy in a pediatric patient with anorexia nervosa—restricting type. J Adolesc Health. 2007;40:376–383. doi: 10.1016/j.jadohealth.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 17.Saad L, Silva LF, Banzato CE, et al. Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. J Med Case Reports. 2010;4:217. doi: 10.1186/1752-1947-4-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Sumich PM, Francis IC, et al. Wernicke’s encephalopathy presenting with upbeating nystagmus. J Clin Neurosci. 2002;9:476–478. doi: 10.1054/jocn.2002.1121. [DOI] [PubMed] [Google Scholar]
- 19.Winston AP, Jamieson CP, Madira W, et al. Prevalence of thiamine deficiency in anorexia nervosa. Int J Eat Disord. 2000;28:451–454. doi: 10.1002/1098-108x(200012)28:4<451::aid-eat14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Rao SN, Chandak GR. Cardiac beriberi: often a missed diagnosis. J Trop Pediatr. 2010;56:284–285. doi: 10.1093/tropej/fmp108. [DOI] [PubMed] [Google Scholar]
- 21.Rolfe M, Walker RW, Samba KN, et al. Urban beri-beri in The Gambia, west Africa. Trans R Soc Trop Med Hyg. 1993;87:114–115. doi: 10.1016/0035-9203(93)90449-z. [DOI] [PubMed] [Google Scholar]
- 22.Guerrini I, Thomson AD, Gurling HM. Molecular genetics of alcohol-related brain damage. Alcohol Alcohol. 2009;44:166–170. doi: 10.1093/alcalc/agn101. [DOI] [PubMed] [Google Scholar]
- 23.Coy JF, Dubel S, Kioschis P, et al. Molecular cloning of tissue-specific transcripts of a transketolase-related gene: implications for the evolution of new vertebrate genes. Genomics. 1996;32:309–316. doi: 10.1006/geno.1996.0124. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechskr Adult Intelligence Scale. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 25.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 26.Salawu F, Kwajaffa S. Wernicke’s encephalopathy in a Nigerian with schizophrenia. Ann Afr Med. 2007;6:200–202. doi: 10.4103/1596-3519.55695. [DOI] [PubMed] [Google Scholar]
- 27.Harrison RA, Trung Vu, Hunter AJ. Wernicke’s encephalopathy in a patient with schizophrenia. J Gen Intern Med. 2006;21:C8–C11. doi: 10.1111/j.1525-1497.2006.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai HY, Lieh Yeh T, Sheei-Meei W, et al. Starvation-induced Wernicke’s encephalopathy in schizophrenia. Psychiatry Clin Neurosci. 2004;58:338–339. doi: 10.1111/j.1440-1819.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- 29.Langlais PJ, Zhang SX. Cortical and subcortical white matter damage without Wernicke’s encephalopathy after recovery from thiamine deficiency in the rat. Alcohol Clin Exp Res. 1997;21:434–443. doi: 10.1111/j.1530-0277.1997.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 30.Zuccoti G, Santa Cruz D, Bertolini M, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171–176. doi: 10.3174/ajnr.A1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talwar D, Davidson H, St Cooney J, et al. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000;46:704–710. [PubMed] [Google Scholar]
- 32.Thomson AD, Marshall EJ, Guerrini I. Biomarkers for detecting thiamine deficiency—improving confidence and taking a comprehensive history are also important. Alcohol Alcohol. 2010;45:213. doi: 10.1093/alcalc/agq004. [DOI] [PubMed] [Google Scholar]
- 33.Lee N, Cheung RTF. A non-alcoholic case of Wernicke’s encephalopathy. Hong Kong Practitioner. 2000;22:457–461. [Google Scholar]
- 34.Saeki K, Saito Y, Komaki H, et al. Thiamine-deficient encephalopathy due to excessive intake of isotonic drink or overstrict diet therapy in Japanese children. Brain Dev. 2010;32:556–563. doi: 10.1016/j.braindev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Catalano G, Catalano MC, O’Dell KJ, et al. The utility of laboratory screening in medically III patients with psychiatric symptoms. Ann Clin Psychiatry. 2001;13:135–140. doi: 10.1023/a:1012229407218. [DOI] [PubMed] [Google Scholar]
- 36.Hack JB, Hoffman RS. Thiamine before glucose to prevent Wernicke encephalopathy: examining the conventional visdom. JAMA. 1998;279:583–584. doi: 10.1001/jama.279.8.583a. [DOI] [PubMed] [Google Scholar]
- 37.Thomson AD, Cook CC, Guerrini I, et al. Wernicke’s encephalopathy revisited. Translation of the case history section of the original manuscript by Carl Wernicke ‘Lehrbuch der Gehirnkrankheiten fur Aerzte and Studirende’ (1881) with a commentary. Alcohol Alcohol. 2008;43:174–179. doi: 10.1093/alcalc/agm144. [DOI] [PubMed] [Google Scholar]
- 38.Watson AJ, Walker JF, Tomkin GH, et al. Acute Wernickes encephalopathy precipitated by glucose loading. Ir J Med Sci. 1981;150:301–303. doi: 10.1007/BF02938260. [DOI] [PubMed] [Google Scholar]
- 39.Kopelman MD. The Korsakoff syndrome. Br J Psychiatry. 1995;166:154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- 40.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1–206. [PubMed] [Google Scholar]