1. Introduction
Reconstructive surgeons often face significant challenges when repairing craniofacial bony defects arising from traumatic injury and tumor resection among other causes. Currently, these defects are addressed clinically by using synthetic graft materials such as ceramics, polymers, and metals; autologous bone tissue; or allogeneic and xenogeneic demineralized bone matrix.[1–4] However, there are various inherent drawbacks associated with the use of these including a lack of biodegradability for most metals and for some ceramics, limited supply and donor site morbidity with autologous bone tissue, and the potential for disease transmission with allogeneic and xenogeneic tissue.[3,5,6]
Tissue engineers seek to overcome these drawbacks by developing osteogenic materials that support the capacity of the body to regenerate bone and integrate with the surrounding bone tissue. Prior approaches have focused on mimicking isolated components of native bone, such as the nanoscale extracellular matrix (ECM) architecture by using nanofibrous materials; the bioactive moieties by coating surfaces with cell-adhesive peptide sequences; the inorganic matrix elements by incorporating hydroxyapatite (HAp); and the signaling molecules by releasing growth factors from the materials.[7–10] However, natural bone ECM is a composite material consisting of fibrous collagen, hydroxyapatite, proteoglycans, and growth factors.[11] Recent approaches have evolved to generate scaffold/extracellular matrix hybrid constructs integrating the multiple components found in native bone matrix with synthetic biomaterials. These approaches typically include the use of porous scaffolds or polymeric carriers combined with various ECM components. The synthesis and characterization, cellular attachment and proliferation, in vitro osteogenicity, and the biological response to in vivo implantation at ectopic and orthotopic sites of hybrid constructs incorporating multiple components of native bone matrix will be discussed in this review.
2. Synthesis of Scaffold/ECM Hybrid Constructs
Natural bone consists of collagen, HAp, and noncollagenous proteins such as proteoglycans, matrix metalloproteinases, and growth factors. [11] The collagen present within bone is organized into fibers approximately 300 nm in length and 1.5 nm in diameter.[11, 12] The fibers are mineralized with biological HAp, a calcium-deficient apatite with carbonate ion substitutions. The plate-like HAp crystals grow preferentially along the collagen fibers and have very small crystallite sizes with a lower crystallinity than synthetic HAp.[12] They are nucleated in the 40 nm gaps present between the collagen fibers and initiated by trace proteins bound to the collagenous ECM. In addition to initiating HAp crystals, trace proteins are important for binding growth factors, remodeling the ECM, promoting angiogenesis, and inducing new bone formation.[11]
There are many approaches for generating hybrid constructs for bone tissue engineering that can incorporate the key aspects of the composition of bone (Table 1). One method includes coating a polymeric scaffold of micro- or nano-scaled architecture with collagen and calcium phosphate.[13, 14] This type of construct is beneficial because of its simplicity and resemblance to bone ECM. The coating of both collagen and calcium phosphate may provide stem cells cultured within the construct an environment that encourages osteogenic differentiation and bone-like ECM deposition. On the other hand, such constructs may lack the complex organization and composition needed to provide cells with the biological cues to form bone. The fibrillar structure of collagen or the inclusion of growth factors may be necessary to form bone correctly. Another approach is to incorporate components of decellularized tissues within a polymeric carrier or scaffolding material.[15, 16] The use of decellularized tissue may allow for the inclusion of many of the proteins and minerals found in the biological tissue. These decellularized tissues also retain much of their original structure and may provide cells with the correct template for tissue regeneration. Nevertheless, some devitalization processes may irreversibly damage the proteases and growth factors found in the native tissue, rendering them inactive. In addition, this approach requires donor tissue, although at lower amounts with respect to autologous, allogeneic, and xenogeneic bone grafts. The same disadvantages observed with these grafts are also found in hybrid constructs incorporating biological tissues. The last method covered in this review is the creation of a cell-generated ECM coating on the surfaces of the scaffold that mimics the composition of native bone.[17,18] Osteoblasts or osteogenically differentiated stem cells are typically used to generate the ECM coating. This cell-generated ECM coating may potentially have all the components that can regulate the composition and organization of the ECM similar to that of native bone. However, disadvantages to this method are that the biological components of this construct are difficult to characterize and the optimal cell culture time to generate an osteogenic ECM coating must be elucidated. In addition, it is difficult to provide the cells with the correct distribution and environment to evenly deposit the osteogenic ECM coating throughout the scaffold.
Table 1.
Methods of synthesis for the three different types of hybrid constructs discussed within this manuscript, including polymeric constructs incorporating collagen and calcium phosphate, biological tissue ECM-based constructs, and cell-generated ECM-based constructs.
| Construct Type | Methods of Synthesis | References |
|---|---|---|
| Polymeric constructs incorporating collagen and calcium phosphate |
Sequential deposition of collagen or gelatin and hydroxyapatite onto scaffold |
|
|
[13, 14, 22, 26, 27] |
|
|
Simultaneous incorporation of collagen or gelatin and calcium phosphate within scaffold |
[29–33] | |
|
||
|
Incorporation of collagen or gelatin within scaffold followed by deposition of calcium phosphate |
||
|
[34–36] | |
|
Incorporation of calcium phosphate within scaffold followed by deposition of collagen or gelatin |
||
|
||
| Biological tissue ECM-based construct |
|
[15, 16, 37–39] |
| Cell-generated ECM-based construct |
|
[17, 18, 51–57, 63–77, 79–82, 84, 88–94, 115, 116] |
2.1 Polymeric constructs incorporating collagen and calcium phosphate
These hybrid constructs seek to mimic bone ECM by combining porous polymeric scaffolds that have nano- and micro-sized features with collagen and calcium phosphate (Figure 1). Also under consideration are hybrid constructs incorporating gelatin instead of collagen. Although, gelatin is a hydrolyzed form of collagen, it has a similar composition, allows for cell adhesion, and is biodegradable in vivo.[19–21]
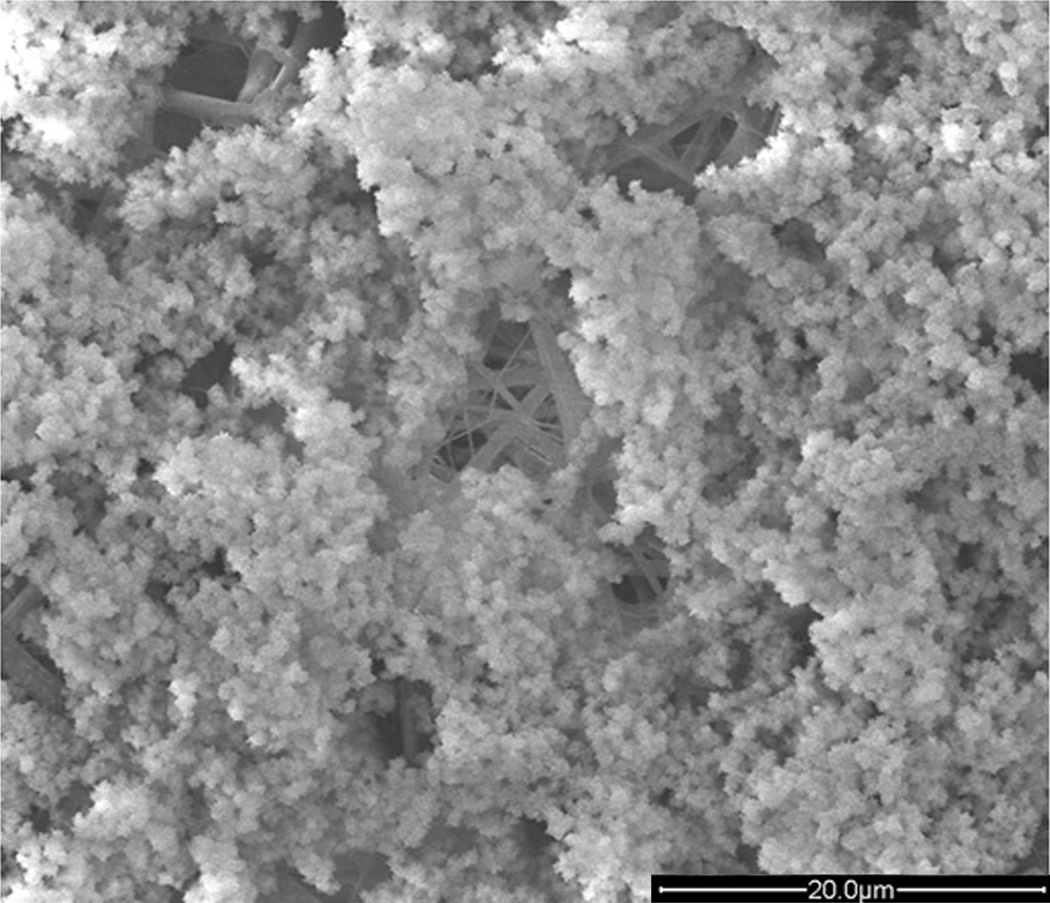
Figure 1.
Scanning electron micrograph of a hybrid construct combining a synthetic material with collagen and nanohydroxyapatite. The construct was generated by initially electrospinning a PLLA/PBLG/collagen solution followed by 3 cycles of soaking in a calcium chloride solution then in sodium phosphate dibasic solution. The result was hydroxyapatite crystals covering collagen-like fibers. Reproduced with permission.[34] 2012, Elsevier.
Three major methods of construct synthesis have been reported in the literature. The first method involves a polymer scaffold coated initially with collagen and subsequently with hydroxyapatite. The second method uses a combination of polymer, collagen, and calcium phosphate in suspension to generate a hybrid construct after the removal of the solvent. The third method combines the two prior methods by incorporating either the collagen or the calcium phosphate into the polymer solution, removing the solvent, and then coating the surface of the composite scaffold with the other component.
In the first method, the generation of the synthetic portions of several hybrid constructs was accomplished in several ways, including electrospinning, 3D printing, and freeze-drying of polymer solutions.[13,14,22] Electrospinning creates a non-woven fiber mesh mat with controllable fiber diameter, porosity, and thickness.[23] With 3D printing, it is possible to generate a scaffold with a pre-determined macrostructure and microstructure.[24] Freeze-drying of polymer solutions can create a porous sponge with a controlled pore structure.[25] Each of these methods is capable of creating a highly porous scaffold that allows for the penetration of the coating solutions throughout the scaffold.
Following fabrication, the scaffold may be submerged within a collagen or gelatin solution and subsequently in simulated body fluid (SBF) solution to generate a coating of collagen or gelatin and HAp on the polymer surface. A layer-by-layer method may be used to control the thickness of the coating.[26] As an example, Li et al. coated a poly(ε-caprolactone) (PCL) scaffold several times, first with gelatin followed by a number of layers of poly(styrene sulfonate) and finally with gelatin.[13] The amount of HAp present on the scaffold may also be controlled by varying the incubation time within the SBF solution. SBF has nearly the same ionic concentration as human plasma but is highly supersaturated with respect to apatite.[27] As a result, SBF forms bone-like HAp crystals on bioactive surfaces such as collagen or gelatin.[27] However, SBF with the same concentration as human plasma (1X SBF) may take more than 16 days to fully coat a surface with HAp.[28] Therefore, in order to decrease the mineralization time, SBF with up to 10 times the concentration of ions found in 1X SBF may be used. To further decrease the construct preparation time, it is possible to soak scaffolds in a combined collagen and SBF solution. Yun et al. used this combined method and were able to remove a fully coated construct after 24 hours.[14]
The second method uses electrospinning, lyophilization, or vacuum evaporation to remove the solvent from a polymer, collagen, and calcium phosphate suspension. As an example, Zhang et al. dispersed chitosan, bovine collagen, and HAp nanoparticles in dimethyl sulfoxide and acetic acid and created a nanofibrous scaffold by electrospinning.[29] Lyophilization was used to generate sponges from a suspension of micro-sized HAp with chitosan and gelatin or a suspension of alginate, porcine gelatin, and β-tricalcium phosphate (TCP).[30,31] In another method, a porous sponge was generated via lyophilization using ice microparticles as a porogen.[32] Specifically, Li et al. created ice microparticles from a solution of bovine collagen with dispersed 500 nm sized HAp particles frozen in liquid nitrogen. The ice microparticles were combined with poly(L-lactic acid) (PLLA) dissolved in dioxane at −5°C, kept in liquid nitrogen for 12 hours, and lyophilized to remove the solvents. Li et al. also created porous scaffolds through vacuum evaporation. They combined HAp nanoparticles with bovine collagen and paraffin microspheres in water and malonic acid, allowed the mixture to air dry, and followed by cross-linking of the collagen using formaldehyde. Subsequently, poly(lactic-co-glycolic acid) (PLGA) dissolved in pyridine was drop-cast into the interspace between the paraffin microspheres. The pyridine solvent was allowed to evaporate under low vacuum and then the paraffin microspheres were dissolved with cyclohexane, resulting in composite scaffolds.[33]
In the third method, constructs are typically fabricated by electrospinning or 3D printing of a polymer and either collagen or calcium phosphate solution followed by the coating of the scaffold with calcium phosphate or collagen, respectively. A nanofibrous polymer scaffold was generated by electrospinning a combination of PLLA, poly(benzyl-L-glutamate) (PBLG), and collagen dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP).[34] The nanofibrous scaffold was then coated with nano-sized crystals of HAp using 3 cycles of dipping in calcium chloride followed by dipping in sodium pyrophosphate. Another hybrid construct was generated by electrospinning PLGA combined with amorphous calcium phosphate and collagen I.[35] The collagen within the scaffolds was cross-linked with glutaraldehyde and then incubated in SBF to create a HAp coating. 3D printing was used to generate a PLGA and TCP composite thumb-shaped scaffold, with multiple 1 mm by 1 mm channels present throughout.[36] The scaffold was subsequently coated with a collagen-based hydrogel containing human mesenchymal stem cells (MSCs).
2.2 Biological tissue ECM-based construct
Biological tissue ECM-based constructs generally consist of a polymeric carrier material and acellular biological tissue (Figure 2). Most of these hybrid constructs incorporate demineralized bone matrix (DBM) because of the osteogenic factors known to be present within DBM.[16,37,38] However, some constructs use acellular bone matrix (ABM) or acellular urinary bladder submucosa (UBS).[15,39]
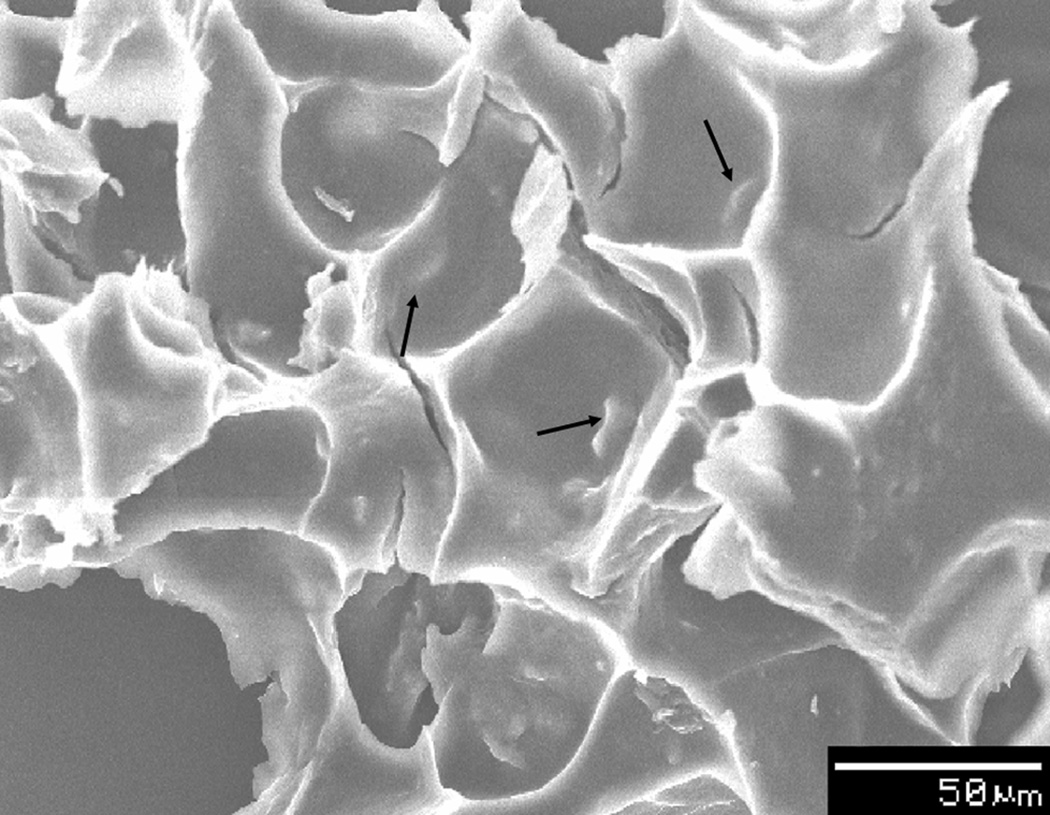
Figure 2.
Scanning electron micrograph of a hybrid construct composed of biological tissue ECM and a polymer, which incorporates ABM with PEG-PCLPEG at 20 wt%. The arrows indicate a few of the ABM particles present within the construct. Reproduced with permission.[39] 2011, John Wiley and Sons.
DBM is an osteoinductive material that is generated by decellularizing and demineralizing cortical bone.[40] A typical method of generating powdered DBM involves first cleaning of cortical bone to remove any remaining soft tissue followed by rinsing with a saline solution.[40,41] The bone is subsequently cut into small fragments and defatted and dehydrated using a 1:1 chloroform-methanol solution. The resulting fragments are frozen and pulverized into particles of sizes in the sub-millimeter range using a mill or mortar and pestle. The particles are then demineralized using hydrochloric acid ranging from 0.1 – 0.6 N at 4°C and sterilized using ethylene oxide.[41] ABM is generated in a similar manner as DBM, but there is no demineralization step. Instead it is generated by sterilizing the bone particulates with ethylene oxide immediately following the pulverization step.[39]
UBS is generated from the submucosal layer of the smooth muscle layer of the bladder. One method of creating UBS is by mechanical delamination of the submucosa from the smooth muscle followed by a treatment with dilute peracetic acid and deionized water to render the tissue acellular.[15] The acellular tissue is subsequently lyophilized and pulverized using a mortar and pestle to create a powder of particulates in the range of 100 to 500 µm.[15]
Each of these decellularized tissues provides many of the native components of the tissue. UBS is composed mainly of collagen, but also retains fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF).[42] ABM contains both the organic matrix and the mineral components of bone whereas DBM only retains the organic matrix. However, it has been shown that demineralization of bone matrix increases access to the bone morphogenic proteins (BMP) bound to the organic matrix.[43] The presence of BMPs have been shown to induce bone formation at an ectopic site.[44] Thus, DBM should provide any seeded cells with access to BMPs. Nevertheless, it is possible to excessively demineralize the tissue and deplete the BMPs from DBM.[45]’ In addition, it has been shown that particulate size can affect the osteogenicity of DBM.[46]–[48]
The formed particulates from different tissues are combined with either a liquid polymeric carrier that later solidifies to form a gel or combined with a polymer to form a film. In the liquid polymeric carrier case, there are several types of polymers used and different methods of solidification employed. For example, poly(ethylene glycol)-PCL-poly(ethylene glycol) (PEG-PCL-PEG) co-polymer was dissolved in water at 60°C, mixed with ABM, and cooled to form a composite gel.[39] As another example, Kurkalli et al. combined rat DBM with Pluronic F-127, a reverse thermo-responsive polymer, and placed the solution in vivo to gel.[38] Reverse thermo-responsive polymers display low viscosity at room temperature, but form a gel at body temperature.[49] In another study, porcine UBS and a sucrose polymer were combined with PLGA in solution, polymerized, and the sucrose polymer was dissolved away to form a porous structure.[15]
In order to generate a film, a polymer and the acellular tissue particulates are combined and placed at the bottom of a well to generate a 2D surface that is a composite of the two materials. Thomas et al. combined DBM particles with PLLA beads ranging from 0.52 mm to 1.91 mm in size at varying ratios to generate a 2D substrate.[16] A film was generated by combining human ABM or human DBM with PLGA in chloroform. The suspension was then cast as a thin layer in a petri dish and subsequently dried under air flow for 24 hours to create a composite thin film.[37]
2.3 Cell-generated ECM-based construct
Cell-generated ECM-based constructs are generated by culturing stem cells, osteoblasts, or pre-osteoblastic cells on porous scaffolds. The goal of this approach is to create a cell-generated ECM coating on the surfaces of the scaffold that mimics the composition of native bone (Figure 3). The cell culture to generate the ECM has been performed under static conditions, flow conditions, electromagnetic stimulation, or dynamic strain.
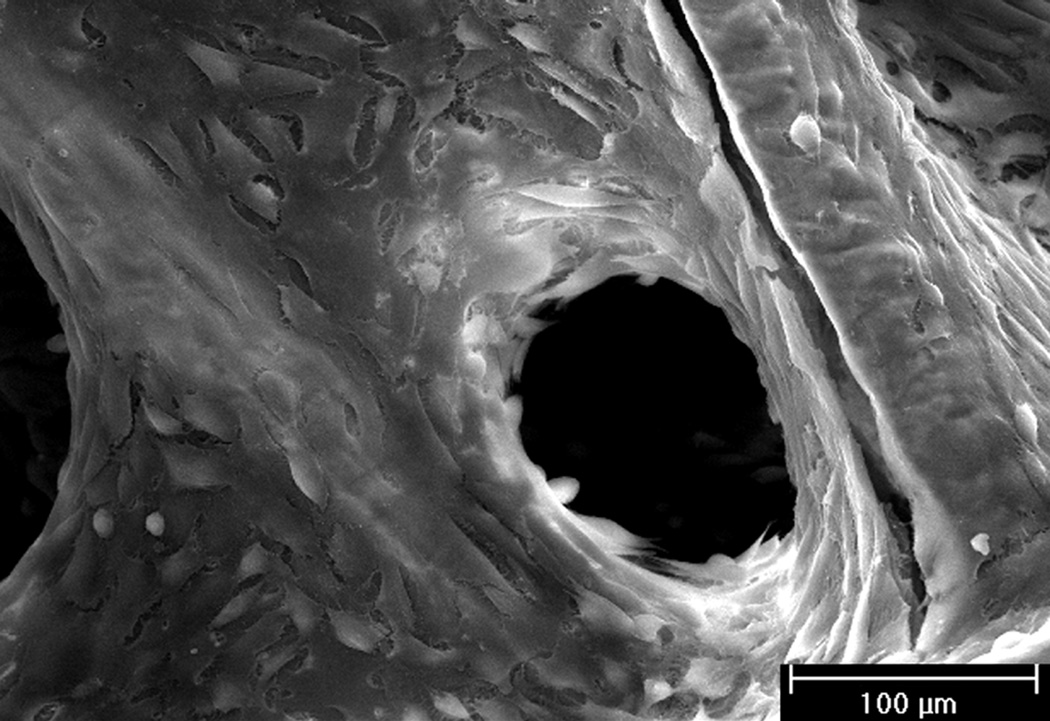
Figure 3.
Scanning electron micrograph of a hybrid construct which has a cellgenerated ECM coating on a fiber mesh scaffold. Rat MSCs were seeded onto titanium fiber mesh scaffolds and cultured in osteogenic media for 16 days to generate the ECM visibly coating the fibers and filling the space in between. Reproduced with permission.[68] 2005, John Wiley and Sons.
Static culture has been used to generate ECM coatings on scaffolds because of the ease of culture. This method of culture works well for small scaffolds, where diffusional limitations of nutrients are less significant.[50] The varieties of scaffolds that have been used in static culture include mineral pellets, porous polymer scaffolds, gelatin cryogels, and fiber mesh scaffolds ranging in thicknesses from 0.8 mm to 5 mm.[17,18,51]–[57] Osteoblast-like cell lines such as SaOS-2 cells, bone marrow stromal cells (BMSCs) from human and rat sources, and primary rat osteoblasts have been cultured on these scaffolds from a minimum of 16 days up to 6 weeks to generate the bone mimetic ECM.
However, in large constructs, portions of the scaffold may encounter a lack in nutrients due to diffusional limitations, causing cells present in these areas to become less active.[58] Bioreactor culture addresses the diffusional limitations by enhancing the mass transfer of nutrients and oxygen and the removal of metabolic waste products using fluid flow around or through the scaffold.[58] In addition, fluid flow through the pores of constructs stimulates seeded cells in the form of shear stress, which has been shown to enhance osteogenic differentiation of stem and progenitor cells.[59,60]
Bioreactors involving flow culture conditions include a flow perfusion bioreactor, a rotational oxygen-permeable bioreactor, and a spinner flask bioreactor. A flow perfusion bioreactor consists of a pump that perfuses constructs with media through a confined fluid path at a controlled flow rate.[61,62] A variety of porous scaffold types have been placed within a flow perfusion bioreactor including foam and fiber mesh scaffolds.[63]–[77] The cells cultured under flow perfusion conditions are similar to those cultured under static conditions and include SaOS-2 cells and BMSCs from human, rat, and goat sources and have been cultured from 15 days up to 40 days. A rotational oxygen-permeable bioreactor consists of a rotating apparatus and a chamber which allows for gas exchange.[78] Cell seeded constructs and media are placed within the chamber and rotated at a controllable rate, which causes the constructs to be continuously in free fall and thus subjected to constant fluid flow.[58] Electrospun polymer fiber mesh scaffolds and polymer foam scaffolds have been cultured with rat BMSCs, rabbit amniotic MSCs, and porcine bone marrow progenitor cells using the rotational oxygen-permeable bioreactor for durations ranging from 10 days to 34 weeks.[79]–[82] The spinner flask bioreactor generates fluid flow by suspending constructs within a media reservoir and placing a stir bar at the bottom to stir the media at a controlled rate.[83] A cell-generated ECM construct was created from silk fibroin scaffolds seeded with human BMSCs and cultured within a spinner flask that was stirred for 5 weeks.[84]
Analogous to shear stress, pulsed electromagnetic fields (PEMF) have been shown to stimulate osteogenic differentiation of stem cells and ECM mineralization.[85,86] Additionally, dynamic loading has been shown to enhance matrix production and osteogenic differentiation.[87,88] Both PEMF and dynamic loading have been used to generate an ECM coating on constructs without the addition of any osteogenic cell culture supplements.[88]–[94] Polymer foams, gelatin cryogels, and titanium disks were used as scaffolds and cultured with such cells as the SaOS-2 cell line and human BMSCs.[89]–[93] These cell-seeded constructs were statically cultured for 22 days or 6 weeks in the presence of an electromagnetic field and, in some cases, with additional ultrasonic stimulation. Investigators have also cultured polymer foam scaffolds with cells such as human MSCs and an osteoblastic cell line, MLO-A5, under 5% strain for 19 or 20 days to enhance ECM production.[88,94]
Also of note, several of the cell-generated ECM-based constructs were decellularized prior to analysis. Most hybrid constructs underwent 3 cycles of freezing in liquid nitrogen and thawing in 37°C water followed by ultrasonication for 10 minutes.[18,51,64,73,74,76] An alternate method of decellularization was accomplished by treating the constructs with 0.5% Triton X-100 and 20 mM ammonium hydroxide for 3 minutes at 37°C.[57]
2.4 Compositional and physical characterization of hybrid constructs
While the method of synthesis for these various hybrid constructs drastically differs, the manner of characterization is quite similar. Construct characterization has been approached using i) visualization of the distribution of cells, proteins, and minerals through the construct, ii) analysis of the protein and mineral composition, and iii) determination of physical characteristics.
Scanning electron microscopy (SEM) allows for the visualization of micro- and nano-scaled features on the surface of the construct. However, SEM does not allow for ready observation of the distribution or identification of the cellular, protein, and mineral components within the interior of hybrid constructs. A combination of fluorescent staining and confocal microscopy has been used to demonstrate the distribution of cells throughout the construct.[52,66] For further characterization of the biological factor distribution within the construct, several histological stains have been used, including methylene blue and hematoxylin & eosin for cells, alcian blue and Safranin O for proteins and glycosaminoglycans (GAGs), and alizarin red and von Kossa for minerals, while immunohistochemistry has been used to visualize the distribution of specific biological components.[17,30,82,84]
In order to determine more precisely the composition of the hybrid constructs, the amount of proteins and GAGs have been determined using colorimetric assays and their identification has been established using enzyme-linked immunosorbent assay (ELISA), western blotting, and mass spectrometry. Typically, to determine the amount of proteins and GAGs in the construct, a detergent or chaotropic solution has been used to solubilize the proteins.[17,55,92] Once the proteins are in solution, colorimetric assays such as the bicinchoninic acid (BCA) assay, coomassie blue assay, 1,9-dimethylmethylene blue (DMMB) assay, and chloramine T assay have been used to determine protein and GAG amounts.[14,57,92] Precise identification of the proteins and GAGs present within the protein solution can be performed via ELISA and western blotting using antibodies.[95,96] Another method for precise identification of proteins in the solution combines a liquid chromatography column along with a tandem mass spectrometer (LC-MS/MS) and a protein database search engine.[97] The protein solution first undergoes trypsinization and is then run through the liquid chromatography column, followed by injection into a tandem mass spectrometer. The resulting spectrum is analyzed by a protein database search engine and matched to specific proteins.[97] If known amounts of the specific protein are analyzed in a similar manner, a standard curve can be created from the LC-MS/MS spectra and the amount of the specific protein can be calculated.[98]
However, each of these methods has a threshold necessary to correctly identify and determine the amount of a protein and a GAG. The chloramine T, DMMB, and BCA assays are typically able to detect molecular concentrations in the micromolar ranges, although submicromolar ranges have been reached using various modifications.[99]–[101] The coomassie blue staining typically can only detect greater than 10 ng of protein, but has been recently improved to detect greater than 2 ng of protein.[102] Meanwhile, the techniques that use antibodies allow for the detection of proteins at much lower levels. Conventional ELISA has a detection limit in the picomolar range, but using a modified technique, detection in the subfemtomolar range has been accomplished.[103] Western blotting can detect greater than 100 pg of protein while LC-MS/MS permits detection of proteins on the order of subpicograms.[104,105]
When determining the mineral amount and composition within the hybrid constructs, colorimetric and spectroscopic assays have been applied. Quantifying the amount of calcium phosphate mineral present within the construct has been accomplished by the colorimetric calcium and phosphate assays.[51,76] However, these assays cannot accurately determine what form of calcium phosphate is present. This issue is addressed with several techniques including X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), NMR, energy dispersive X-ray spectroscopy (EDX), or X-ray photoelectron spectroscopy (XPS) to determine the chemical composition and crystallinity of the minerals (Figure 4).[13,22,39] Each type of mineral presents a unique spectrum or diffractogram in each of these analyses, thus allowing for identification of the specific minerals present.[106]
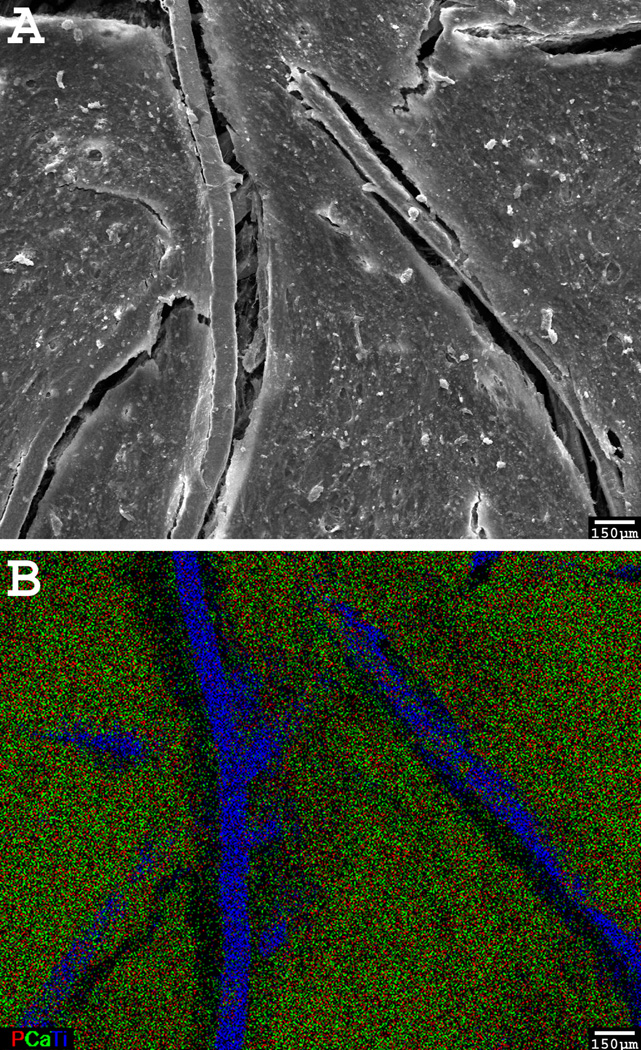
Figure 4.
A SEM micrograph (A) of the surface of a cell-generated ECM-based construct and its corresponding EDX elemental mapping (B). The overlay of calcium, phosphorous, and titanium demonstrates that the calcium and phosphorous are co-localized on the titanium construct. A color version can be found in the original article.[73] Reproduced with permission.[73] 2009, John Wiley and Sons.
Several methods have been used to determine the physical characteristics of the constructs. Contact angle measurements can be used to quantitatively demonstrate that the surface has been altered. Water placed onto the surface of a construct forms a droplet, and the hydrophobicity of the surface can be predicted based on the angle that the droplet of water makes with the surface. This is especially useful when a hydrophobic scaffold material is coated with a hydrophilic substance such as HAp or collagen. Micro-computed tomography (µCT) and fluid replacement methodologies have been used to determine the porosity of the constructs.[14,54,65] µCT uses X-rays to visualize sections of radio-opaque materials and uses a computer to reassemble the sections into a 3-D rendering of the construct.[107] Using this representation, the interconnectivity of the pores, pore size, and porosity can be calculated.[107] The fluid replacement methodologies, such as mercury porosimetry, gas pycnometry, and liquid intrusion, measure the change in initial fluid volume when the pores of the constructs are filled by the various fluids, which in turn is used to calculate the construct pore size and porosity.[108]
3. In Vitro Cell Culture on Hybrid Constructs
Following the synthesis and characterization of a hybrid construct, the assessment of its bone repair potential is an important step in establishing the cytocompatibility and osteogenicity of the material. Typically, the initial characterization is through in vitro cell culture, since it is a less intensive method of determining the bone repairing potential as compared to in vivo implantation. Through the attachment and proliferation of cells onto the hybrid constructs, the cytocompatibility of the material can be elucidated. Increased cellular attachment and proliferation may suggest that if the hybrid construct is implanted, osteoblasts and stem cells will be able to successfully invade and colonize it. Furthermore, an increase in osteoblastic gene expression, protein secretion, and construct mineralization can demonstrate its osteogenicity. The osteogenic differentiation of the cells within the hybrid construct in vitro suggests that once it is implanted, stem cells migrating from the surrounding tissue may be able to differentiate down an osteogenic pathway and contribute to bone regeneration in vivo.
3.1 Cellular attachment and viability on hybrid constructs
Several cell types have been cultured on hybrid constructs to determine their overall cytocompatibility. These cell types include cell lines such as MC3T3-E1, mouse marrow stromal cells (D1 cell line), human fetal osteoblasts (hFOBs), and SaOS-2 or stem cells such as adipose derived stem cells (ADSCs) and MSCs from human, rat, and mouse sources. An advantage of cell lines is that they are easily procured and cultured. However, with the exception of MC3T3-E1 and D1 cells, the cell lines are already differentiated into osteoblasts and thus can only provide limited information regarding the osteoinductivity of the hybrid construct. Notwithstanding, certain stem cell populations present the potential to differentiate down the osteoblastic lineage and may be useful in assessing the osteogenicity of a hybrid construct.
The viability and proliferation of the cultured cells can be determined by a variety of assays. For instance, the lactate dehydrogenase assay quantifies the number of dead cells, while the PicoGreen assay determines the total amount of dsDNA which then can be used to calculate the number of cells if the amount of dsDNA has been measured for a known number of cells. In addition, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and glucose consumption assays measure the metabolic activity of the viable cells.
Cell lines cultured on hybrid constructs generally demonstrate a higher attachment and proliferation as compared to cell lines cultured on the corresponding scaffold material in the absence of an ECM component.[13,14,16,29,32] Li et al. determined that MC3T3-E1 cells cultured on electrospun PCL coated with collagen and calcium phosphate demonstrated significant enhancement in proliferation at day 7 when compared to cells seeded onto unmodified electrospun PCL fiber meshes.[13] Additionally, the surface of the construct was covered with multiple layers of cells due to proliferation. MC3T3-E1 cells were also shown to have faster attachment, higher degree of cell extension, and flattened morphology after 30 minutes of incubation and a higher increase in cell number after 7 days of culture when seeded onto a mesoporous bioactive glass (MBG)-PCL-ECM coated construct as compared to a plain PCL scaffold.[14]
Similarly, stem cells are generally able to attach and proliferate on hybrid constructs.[18,30,31,34,35,37,51,64] Human MSCs seeded onto hybrid chitosan/gelatin/HAp constructs displayed high cell proliferation and deep cell penetration under flow perfusion conditions.[31] When mouse MSCs were cultured on films of PLGA/DBM or PLGA/ABM, they expressed a higher level of attachment than that of cells cultured on PLGA film alone.[37] Also, rat MSCs seeded onto hybrid titanium and cell-generated ECM constructs and hybrid PCL and cell-generated ECM constructs exhibited cell proliferation until day 18 or day 8, respectively, with a subsequent drop at later timepoints.[18,51,64] The investigators explained both drops at the later timepoints as being caused by the MSCs encasing themselves in matrix, preventing the DNA from being released into the analysis solution and being detected.
The information gleaned from the cell attachment and viability studies can be used to guide hybrid construct composition. For example, low cell viability may suggest that a component of the construct is cytotoxic and should be excluded, whereas cell attachment, maintenance of viability, and proliferation generally indicate cytocompatibility. Each type of hybrid construct reviewed herein has been observed generally to demonstrate cellular attachment, viability, and proliferation. Accordingly, these hybrid constructs demonstrate cytocompatibility and may support cell infiltration and survival in vivo.
3.2 Osteogenic differentiation of stem and pre-osteoblastic cells on hybrid constructs
The differentiation of stem cells such as ADSCs and MSCs from human and rat sources and pre-osteoblastic cells such as MC3T3-E1 cells cultured on the constructs can be used to determine their osteogenicity. Use of media containing osteogenic supplements has been shown to cause osteogenic differentiation of the stem and pre-osteoblastic cells.[109] Thus, the true test of the osteogenicity of the construct is through culture in media without osteogenic supplements, such as dexamethasone. This can be measured by the alkaline phosphatase (ALP) activity, an early stage marker of osteoblastic differentiation; gene expression of osteoblastic markers of the seeded cells; and the amount of osteocalcin, osteopontin, as well as mineral deposition present in the construct, all of which are late stage markers of osteoblastic differentiation.[110]–[112]
The alkaline phosphatase activity of cells seeded onto several hybrid constructs were seen to be increased when compared to cells seeded on the base material lacking the biological components.[14,18,31,34,51,64] However, even with osteogenic supplementation, Thomas et al. demonstrated that D1 cells cultured on the mixture of PLLA beads and DBM particles had a significantly lower ALP activity as compared to the cells cultured on PLLA beads alone.[16] Additionally, Hild et al. showed that human MSCs cultured on composite calcium phosphate/collagen/PLGA films displayed no significant difference in ALP activity when compared to PLGA films.[35]
The upregulation in the production of osteopontin has also been observed in MSCs cultured onto chitosan/gelatin/HAp constructs.[31] Similarly, MSCs cultured onto hybrid TCP/alginate/gelatin constructs exhibited enhanced osteopontin and osteocalcin production, and this was observed in both the presence and absence of the osteogenic supplement, dexamethasone.[30]
Significantly higher gene expression of osteogenic markers such as runt-related transcription factor 2 (RUNX2), Collagen I, ALP, osteopontin, BMP-2, VEGF, FGF, aggrecan, and matrix metalloproteinase 9 (MMP-9) was observed for MC3T3-E1 cells and MSCs cultured on hybrid constructs in media with or without dexamethasone as compared to cells cultured on their respective base materials alone.[14,74] However, in the case of a construct composed of PLLA beads and DBM particles, there was significantly lower gene expression by the seeded D1 cells for bone sialoprotein (BSP), RUNX2 and osteocalcin as compared to the cells cultured on PLLA beads alone.[16]
Many of the hybrid constructs also demonstrated a significantly higher amount of calcium mineralization than their base material counterparts.[18,30,34,35,51,64] In particular, the TCP/alginate/gelatin constructs showed an increase in calcium mineralization even in the absence of the osteogenic supplement dexamethasone.[30] The presence or lack of osteogenic differentiation of stem cells observed for the varying hybrid constructs does not seem to implicate any specific combination of components. Interestingly, even the presence of the osteogenic supplement dexamethasone does not guarantee differentiation. However, the one study that resulted in lower gene expression of osteogenic markers and ALP activity did not contain any form of calcium phosphate.[16] The existence of a form of calcium phosphate may be necessary for proper osteogenic differentiation of the stem cells.
The implications from the osteogenic differentiation studies can also be used to improve hybrid construct composition for bone repair. A decrease in activity and amount of early and late osteogenic markers may signify that the construct components are not osteogenic, while an increase might suggest the potential of the constructs to promote bone repair in vivo. Evaluation of the hybrid constructs reviewed herein demonstrates that all of the cell-generated ECM-based hybrid constructs have an increase in osteogenic markers as compared to their base material. Meanwhile a few of the polymeric constructs incorporating collagen and calcium phosphate and the biological tissue ECM hybrid constructs demonstrate decreased osteogenic markers compared to their respective base materials. The results of the in vitro studies indicate that the cell-generated ECM-based hybrid constructs may enhance bone formation and osteointegration, while those with decreased osteogenic markers may perform less favorably.
In Vivo Implantation of Hybrid Constructs
Although an in vitro evaluation reveals important information regarding the cytocompatibility and osteogenicity of the constructs, the in vivo implantation of the hybrid constructs allows for the evaluation of their performance in the ultimately desired environment. Implantation of these hybrid constructs can take place at an ectopic site, such as in muscle or under the skin, or at an orthotopic site, including sites in the cranium or on the femur. Ectopic implantation occurs at a site where the tissue is not normally found and can be used to evaluate cytotoxic and inflammatory responses as well as the osteoinductivity of the construct.[113] Orthotopic implantation occurs at a site where the tissue is normally found and provides information regarding the integration of the construct with surrounding tissue along with how well it assists in the union of an otherwise non-healing bone defect.[114]
4.1 Ectopic implantation
Analysis of a construct implanted at an ectopic site provides insight regarding the response of the body to the foreign object. Implants are typically excised, fixed, sectioned, and stained for the identification and quantification of inflammatory cells. Bone formation within the constructs has also been visualized using radiological imaging, by histology, and by immunohistochemistry for bone markers.[36,69,81] Hybrid constructs incorporating biological tissue ECM and constructs containing cell-generated ECM have been implanted ectopically and analyzed for bone formation and inflammatory response.[15,36,39,69,70,79,81]
4.1.1 Acellular hybrid constructs
Biological tissue ECM hybrid constructs have tended to exhibit a high initial inflammatory response that drops at later timepoints.[15,39] ABM and PEG-PCL-PEG hybrid constructs were injected subcutaneously into the back of mice and excised at 1, 2 and 4 weeks.[39] The number of inflammatory cells was high in both PEG-PCL-PEG scaffolds and ABM/PEG-PCL-PEG constructs at weeks 1 and 2, but by week 4, the number had dropped significantly. Similarly, composite UBS and PLGA constructs were placed into a subcutaneous pocket on the backs of mice for 7, 14, 28, and 56 days.[15] The implants showed an inflammatory response with mixed cell populations at day 7, but by days 28 and 56, the inflammatory response was much less, with only the presence of mononuclear cells detected. By the end of the implantation period, the PLGA portion of the construct was retained, although the UBS component was not identifiable.
4.1.2 Cellular hybrid constructs
Meanwhile, constructs containing cell-generated ECM have been typically analyzed for bone formation.[69,70,79,81] Cell-generated ECM coated biphasic calcium phosphate constructs containing human BMSCs were implanted in subcutaneous pockets on the backs of mice for 6 weeks.[69,70] The constructs demonstrated de novo bone formation with areas of mineralized bone and osteoids as well as the presence of osteocytes, osteoblasts, blood vessels, bone marrow, and fat cells in close proximity to newly formed bone.
Another study investigated the implantation of composite tooth and bone constructs composed of porcine cell-generated ECM-coated PLGA onto the omentum of rats for 8 weeks.[81] The implants consisted mainly of alveolar bone-like tissue found near the tooth portion, precursor osteoid tissue, and compact bone found at a distance from the tooth portion. Collagen I, BSP, and osteocalcin were detected throughout the compact bone-like tissue, while only collagen I was found in the alveolar bone-like tissue, similar to that found in native porcine alveolar bone tissue.
The hybrid construct incorporating tricalcium phosphate, PLGA, and gelled collagen was investigated for bone formation in a subcutaneous implantation model.[36] The channels present in the construct were found to contain fibrous tissue, but the constructs were also surrounded and penetrated by new cortical bone.
4.2 Orthotopic implantation
Analysis of a construct implanted at an orthotopic site allows for investigation of the integration of the implant material with the surrounding bone tissue and the ability of the construct to promote healing of the defect.[114] Similar to ectopic implantations, orthotopic implants are typically excised, fixed, sectioned, and analyzed for the presence of inflammatory cells and bone formation. The presence of inflammatory cells and bone formation within hybrid constructs has also been visualized by histology and immunohistochemistry.[38,57] Additionally, bone formation within hybrid constructs has been determined by staining with fluorescent dyes and X-rays of the implant.[80,82,115] Cell-generated ECM constructs and constructs incorporating biological tissue ECM have been implanted orthotopically and analyzed for inflammatory response and bone formation in both small and large animals.[38,57,80,82,115,116]
4.2.1 Acellular hybrid constructs
Tour et al. investigated the bone formation and inflammatory response of acellular cell-generated ECM constructs. HAp and rat calvarial osteoblast-generated ECM constructs were implanted in critical-sized calvarial defects in rats for 12 weeks.[57] The composite HAp and ECM constructs contained more new bone formation than HAp scaffolds alone, with the bone forming at the margins and in the central portion of the scaffold on the dural side. However, no construct had completely restored the defect. Each construct had similar staining patterns for BSP, osteopontin, and periostin, a cell-adhesion molecule for pre-osteoblasts.[117] BSP and osteopontin were found in HAp particles incorporated within the newly formed bone, whereas the periostin was found between the non-integrated particles. There was still a large active inflammatory response at 12 weeks, but the composite HAp and ECM constructs demonstrated larger amounts of macrophages present near the non-integrated HAp particles than in the HAp scaffold alone.
In another study, biological tissue ECM hybrid constructs were examined by Kurikalli et al. for their orthotopic bone formation and inflammatory response.[38] A hybrid construct composed of Pluronic F-127, DBM, and rat MSCs was implanted into a critical-sized cranial defect in rats for 1 month. At the study endpoint, the implants displayed a continuous layer of bone throughout the defect and integration with the defect edges. The shape of the newly formed bone also showed exact conformity with the missing bone fragment, suggesting that the implanted MSCs remodeled the scaffolding. There was no visible sign of inflammatory cells reported within the implant.
4.2.2 Cellular hybrid constructs
Titanium constructs containing rat MSCs along with their ECM coating were implanted in a critical-sized cranial defect in rats for periods of 1 week and 1 month.[116] After excision of the1 week implants, a thin fibrous capsule was seen surrounding the implant and exhibited no macroscopic sign of bone formation. Mineralized matrix was observed at the implant edges and at the periosteal side. Additionally, fibrous tissue with capillary infiltration was seen to be present throughout the implant. In the 1 month implants, there were osteocytes embedded within a mineralized matrix that had osteoids, osteoblasts covering the surface, and bone marrow present within the titanium fiber mesh. Some of the implants were seen to have a connection of bone across the defect, but a large variability in the amount of bone formation was found in each implant.
Implantation in animals larger than rats was explored by Steigman et al. through the implantation of electrospun PLLA constructs containing rabbit amniotic MSCs and their ECM coating into sternal defects of rabbits.[80] X-ray images of the implant at 8 weeks showed that there was radio-opaque material covering the constructs with complete closure of the defect. The constructs demonstrated substantial engraftment and typical bone morphology, with very little inflammatory cells present. The implants demonstrated similar amounts of mineralization before implantation and after the 8 week implantation period, but there was an increase in ALP activity in the 8 week post-implantation constructs when compared to pre-implantation constructs.
Even larger animals than rabbits have received hybrid construct implants. Zhang et al. implanted composite tooth and bone constructs comprised of porcine cell-generated ECM coated PLGA into the mandible of pigs for 12 weeks or 20 weeks.[82] Radiographs and ultra high-resolution volume computed tomography (VCT) images of the excised implants were taken to determine the density of the regenerated tissue. Radiographs and VCT images demonstrated that the 20 week implants had denser bone than the 12 week implants. They also demonstrated that the scaffolds without the cell-generated ECM or cells did not exhibit any mineralization. Histology showed complete bony bridge formation on both the buccal and lingual sides of the implant after 12 weeks. Nevertheless, there was disorganized bone formation within the centers of the implants. Immunohistochemistry demonstrated that the bony portion of the construct contained BSP and osteocalcin.
Similar to the in vitro studies, a varying biological response was observed for the hybrid constructs in both small and large animals. Nonetheless, the incorporation of MSCs within the hybrid constructs displayed greater bone defect closure than the hybrid constructs lacking cells. Accordingly, these studies warrant further investigation into the use of hybrid constructs as a cell transportation vehicle for enhanced repair of bone defects.
5. Conclusions
The human body presents a limited natural ability to fully repair large bony tissue defects. To improve current clinical treatments of non-healing bone defects, tissue engineers have been researching materials that can successfully integrate with the native bone and promote tissue repair. This review discussed current approaches that have included the incorporation of several components found in native bone matrix in conjunction with a biomaterial that might otherwise be non-osteogenic. Methods of combining these components into a hybrid construct include coating a scaffold with collagen and a form of calcium phosphate, combining acellular biological tissue with a polymer, and creating a cell-generated ECM coating on a scaffold. These hybrid constructs have demonstrated an increase in overall performance in cell viability and proliferation, in vitro differentiation, and in vivo bone formation over synthetic materials alone. Yet, the studies on hybrid constructs suggest that additional investigation into the essential components of a construct and the potential inclusion of cells within a construct will be necessary to improve their biocompatibility, osteogenicity, and repair potential.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01 AR057083). R.A.T. also acknowledges the Ruth L. Kirschstein National Research Service Award (F31 AR055874) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Abbreviations
- µCT
micro-computed tomography
- ABM
acellular bone matrix
- ADSC
adipose derived stem cell
- ALP
alkaline phosphatase
- BCA
bicinchoninic acid
- BMP
bone morphogenetic protein
- BMSC
bone marrow stromal cell
- BSP
bone sialoprotein
- DBM
demineralized bone matrix
- DMMB
1, 9-dimethylmethylene blue
- ECM
extracellular matrix
- EDX
energy dispersive X-ray spectroscopy
- ELISA
enzyme-linked immunosorbent assay
- FGF
fibroblast growth factor
- FTIR
Fourier transform infrared spectroscopy
- GAG
glycosaminoglycan
- HAp
hydroxyapatite
- HFIP
1,1,1,3,3,3-hexafluoro-2-propanol
- hFOB
human fetal osteoblast
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MBG
mesoporous bioactive glass
- MMP-9
matrix metalloproteinase 9
- MSC
mesenchymal stem cell
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NMR
nuclear magnetic resonance
- PBLG
poly(benzyl-L-glutamate)
- PCL
poly(ε-caprolactone)
- PEG
poly(ethylene glycol)
- PEMF
pulsed electromagnetic field
- PLGA
poly(lactic-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- RUNX2
runt-related transcription factor 2
- SBF
simulated body fluid
- SEM
scanning electron microscopy
- TCP
β-tricalcium phosphate
- UBS
acellular urinary bladder submucosa
- VCT
ultra high-resolution volume computed tomography
- VEGF
vascular endothelial growth factor
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
Contributor Information
Richard A. Thibault, Department of Bioengineering, Rice University, P.O. Box 1892, MS-142, Houston, TX, USA 77251-1892, rat1@rice.edu
Antonios G. Mikos, Department of Bioengineering, Rice University, P.O. Box 1892, MS-142, Houston, TX, USA 77251-1892, mikos@rice.edu
F. Kurtis Kasper, Department of Bioengineering, Rice University, P.O. Box 1892, MS-142, Houston, TX, USA 77251-1892, kasper@rice.edu
References
- 1.Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, Iannetti G. Surg Neurol. 2003;60:71. doi: 10.1016/s0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 2.Khan SN, Fraser JF, Sandhu HS, Cammisa FP, Jr, Girardi FP, Lane JM. J Am Acad Orthop Surg. 2005;13:129. doi: 10.5435/00124635-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Kawashita M. J Artif Organs. 2011;14:163. doi: 10.1007/s10047-011-0585-5. [DOI] [PubMed] [Google Scholar]
- 4.Rah DK. Yonsei Med J. 2000;41:756. doi: 10.3349/ymj.2000.41.6.756. [DOI] [PubMed] [Google Scholar]
- 5.Ge Z, Jin Z, Cao T. Biomed Mater. 2008;3:022001. doi: 10.1088/1748-6041/3/2/022001. [DOI] [PubMed] [Google Scholar]
- 6.Giannoudis PV, Dinopoulos H, Tsiridis E. Injury. 2005;36(Suppl 3):S20. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Ho MH, Wang DM, Hsieh HJ, Liu HC, Hsien TY, Lai JY, Hou LT. Biomaterials. 2005;26:3197. doi: 10.1016/j.biomaterials.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Biomaterials. 2005;26:5158. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Zheng L, Zhao H, Miao J, Sun C, Ren N, Wang J, Liu H, Tao X. Tissue Eng Part A. 2011;17:1341. doi: 10.1089/ten.TEA.2010.0497. [DOI] [PubMed] [Google Scholar]
- 10.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V. Biomaterials. 2009;30:3551. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Bilezikian JP, Raisz LG, Rodan GA. In: Principles of Bone Biology. Bilezikian JP, Raisz LG, Rodan GA, editors. Vol. 1. San Diego, USA: Academic Press; 2002. [Google Scholar]
- 12.Tampieri A, Sprio S, Sandri M, Valentini F. Trends Biotechnol. 2011;29:526. doi: 10.1016/j.tibtech.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Xie J, Yuan X, Xia Y. Langmuir. 2008;24:14145. doi: 10.1021/la802984a. [DOI] [PubMed] [Google Scholar]
- 14.Yun HS, Kim SH, Khang D, Choi J, Kim HH, Kang M. Int J Nanomedicine. 2011;6:2521. doi: 10.2147/IJN.S25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Exp Hematol. 2001;29:1310. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- 16.Thomas CB, Maxson S, Burg KJ. J Biomater Sci Polym Ed. 2011;22:589. doi: 10.1163/092050610X488232. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. Biomaterials. 2005;26:4442. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Tissue Eng Part A. 2010;16:431. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington W, Vonhippel P. Adv Protein Chem. 1961;16:1. doi: 10.1016/s0065-3233(08)60028-5. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Smith LA, Hu J, Ma PX. Biomaterials. 2009;30:2252. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Adv Drug Deliv Rev. 2009;61:1007. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Mak AF, Wang M, Li J. J Biomed Mater Res B Appl Biomater. 2006;77:315. doi: 10.1002/jbm.b.30356. [DOI] [PubMed] [Google Scholar]
- 23.Doshi J, Reneker DH. J Electrostat. 1995;35:151. [Google Scholar]
- 24.Yang S, Leong KF, Du Z, Chua CK. Tissue Eng. 2002;8:1. doi: 10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 25.Schoof H, Apel J, Heschel I, Rau G. J Biomed Mater Res. 2001;58:352. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 26.Schaaf P, Voegel JC, Jierry L, Boulmedais F. Adv Mater. 2012;24:1001. doi: 10.1002/adma.201104227. [DOI] [PubMed] [Google Scholar]
- 27.Kokubo T, Takadama H. Biomaterials. 2006;27:2907. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Jayasuriya AC, Kibbe S. J Mater Sci Mater Med. 2009;21:393. doi: 10.1007/s10856-009-3874-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Reddy VJ, Wong SY, Li X, Su B, Ramakrishna S, Lim CT. Tissue Eng Part A. 2010;16:1949. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- 30.Eslaminejad MB, Mirzadeh H, Mohamadi Y, Nickmahzar A. J Tissue Eng Regen Med. 2007;1:417. doi: 10.1002/term.49. [DOI] [PubMed] [Google Scholar]
- 31.Sellgren KL, Ma T. J Tissue Eng Regen Med. 2012;6:49. doi: 10.1002/term.396. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Chen Y, Mak AF, Tuan RS, Li L, Li Y. Acta Biomater. 2010;6:2013. doi: 10.1016/j.actbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Yuan X, He F, Mak AF. J Biomed Mater Res B Appl Biomater. 2008;86:381. doi: 10.1002/jbm.b.31031. [DOI] [PubMed] [Google Scholar]
- 34.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S. Biomaterials. 2012;33:846. doi: 10.1016/j.biomaterials.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Hild N, Schneider OD, Mohn D, Luechinger NA, Koehler FM, Hofmann S, Vetsch JR, Thimm BW, Muller R, Stark WJ. Nanoscale. 2010;3:401. doi: 10.1039/c0nr00615g. [DOI] [PubMed] [Google Scholar]
- 36.Weinand C, Gupta R, Weinberg E, Madisch I, Neville CM, Jupiter JB, Vacanti JP. Tissue Eng Part A. 2009;15:2605. doi: 10.1089/ten.TEA.2008.0467. [DOI] [PubMed] [Google Scholar]
- 37.Jayasuriya AC, Ebraheim NA. J Mater Sci Mater Med. 2009;20:1637. doi: 10.1007/s10856-009-3738-9. [DOI] [PubMed] [Google Scholar]
- 38.Kurkalli BG, Gurevitch O, Sosnik A, Cohn D, Slavin S. Curr Stem Cell Res. Ther. 2010;5:49. doi: 10.2174/157488810790442831. [DOI] [PubMed] [Google Scholar]
- 39.Ni PY, Fan M, Qian ZY, Luo JC, Gong CY, Fu SZ, Shi S, Luo F, Yang ZM. J Biomed Mater Res A. 2011;100:171. doi: 10.1002/jbm.a.33262. [DOI] [PubMed] [Google Scholar]
- 40.Iwata H, Sakano S, Itoh T, Bauer TW. Clin Orthop Relat Res. 2002;395:99. doi: 10.1097/00003086-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Hagino T, Hamada Y. J Orthop Res. 1999;17:232. doi: 10.1002/jor.1100170212. [DOI] [PubMed] [Google Scholar]
- 42.Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. J Pediatr Surg. 2000;35:1097. doi: 10.1053/jpsu.2000.7834. [DOI] [PubMed] [Google Scholar]
- 43.Sampath TK, Reddi AH. Biochem Biophys Res Commun. 1984;119:949. doi: 10.1016/0006-291x(84)90865-9. [DOI] [PubMed] [Google Scholar]
- 44.Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 45.Pietrzak WS, Ali SN, Chitturi D, Jacob M, Woodell-May JE. Cell Tissue Bank. 2011;12:81. doi: 10.1007/s10561-009-9168-6. [DOI] [PubMed] [Google Scholar]
- 46.Syftestad G, Urist MR. Clin Orthop Relat Res. 1979;141:281. [PubMed] [Google Scholar]
- 47.Holt DJ, Grainger DW. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.04.002. in press. [DOI] [PubMed] [Google Scholar]
- 48.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohn D, Sosnik A, Levy A. Biomaterials. 2003;24:3707. doi: 10.1016/s0142-9612(03)00245-x. [DOI] [PubMed] [Google Scholar]
- 50.Lovett M, Lee K, Edwards A, Kaplan DL. Tissue Eng Part B Rev. 2009;15:353. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Biomaterials. 2005;26:971. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Fassina L, Saino E, Visai L, Avanzini MA, Cusella De Angelis MG, Benazzo F, Van Vlierberghe S, Dubruel P, Magenes G. Conf Proc IEEE Eng Med Biol Soc. 2010;247 doi: 10.1109/IEMBS.2010.5627475. [DOI] [PubMed] [Google Scholar]
- 53.Hagenmuller H, Hofmann S, Kohler T, Merkle HP, Kaplan DL, Vunjak-Novakovic G, Muller R, Meinel L. Ann Biomed Eng. 2007;35:1657. doi: 10.1007/s10439-007-9338-2. [DOI] [PubMed] [Google Scholar]
- 54.Martins AM, Pham QP, Malafaya PB, Sousa RA, Gomes ME, Raphael RM, Kasper FK, Reis RL, Mikos AG. Tissue Eng Part A. 2009;15:295. doi: 10.1089/ten.tea.2008.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saino E, Grandi S, Quartarone E, Maliardi V, Galli D, Bloise N, Fassina L, De Angelis MG, Mustarelli P, Imbriani M, Visai L. Eur Cell Mater. 2011;21:59. doi: 10.22203/ecm.v021a05. [DOI] [PubMed] [Google Scholar]
- 56.Thimm BW, Wust S, Hofmann S, Hagenmuller H, Muller R. Acta Biomater. 2011;7:2218. doi: 10.1016/j.actbio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Tour G, Wendel M, Tcacencu I. Tissue Eng Part A. 2011;17:127. doi: 10.1089/ten.TEA.2010.0175. [DOI] [PubMed] [Google Scholar]
- 58.Ikada Y. Tissue Engineering Fundamentals Applications. Vol. 8. Elsevier; San Diego, USA: 2006. [Google Scholar]
- 59.Kreke MR, Huckle WR, Goldstein AS. Bone. 2005;36:1047. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL. Am J Physiol. 1997;273:C810. doi: 10.1152/ajpcell.1997.273.3.C810. [DOI] [PubMed] [Google Scholar]
- 61.Bancroft GN, Sikavitsas VI, Mikos AG. Tissue Eng. 2003;9:549. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 62.Dahlin RL, Meretoja VV, Ni M, Kasper FK, Mikos AG. Tissue Eng Part C Methods. 2012 doi: 10.1089/ten.tec.2012.0037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Proc Natl Acad Sci USA. 2002;99:12600. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. Proc Natl Acad Sci USA. 2006;103:2488. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fassina L, Visai L, Asti L, Benazzo F, Speziale P, Tanzi MC, Magenes G. Tissue Eng. 2005;11:685. doi: 10.1089/ten.2005.11.685. [DOI] [PubMed] [Google Scholar]
- 66.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. J Biomed Mater Res A. 2003;67:87. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 67.Holtorf HL, Datta N, Jansen JA, Mikos AG. J Biomed Mater Res A. 2005;74:171. doi: 10.1002/jbm.a.30330. [DOI] [PubMed] [Google Scholar]
- 68.Holtorf HL, Jansen JA, Mikos AG. J Biomed Mater Res A. 2005;72:326. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 69.Janssen FW, Oostra J, Oorschot A, van Blitterswijk CA. Biomaterials. 2006;27:315. doi: 10.1016/j.biomaterials.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 70.Janssen FW, van Dijkhuizen-Radersma R, Van Oorschot A, Oostra J, de Bruijn JD, Van Blitterswijk CA. J Tissue Eng Regen Med. 2010;4:12. doi: 10.1002/term.197. [DOI] [PubMed] [Google Scholar]
- 71.Li D, Tang T, Lu J, Dai K. Tissue Eng Part A. 2009;15:2773. doi: 10.1089/ten.TEA.2008.0540. [DOI] [PubMed] [Google Scholar]
- 72.Martins AM, Saraf A, Sousa RA, Alves CM, Mikos AG, Kasper FK, Reis RL. J Biomed Mater Res A. 2010;94:1061. doi: 10.1002/jbm.a.32785. [DOI] [PubMed] [Google Scholar]
- 73.Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, Mikos AG. J Biomed Mater Res A. 2009;88:295. doi: 10.1002/jbm.a.31875. [DOI] [PubMed] [Google Scholar]
- 74.Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. Biomaterials. 2008;29:2729. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 75.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Proc Natl Acad Sci USA. 2003;100:14683. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thibault RA, Mikos AG, Kasper FK. Biomacromolecules. 2011;12:4204. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Dolder J, Bancroft GN, Sikavitsas VI, Spauwen PH, Jansen JA, Mikos AG. J Biomed Mater Res A. 2003;64:235. doi: 10.1002/jbm.a.10365. [DOI] [PubMed] [Google Scholar]
- 78.Terai H, Hannouche D, Ochoa E, Yamano Y, Vacanti JP. Mater Sci Eng C. 2002;20:3. [Google Scholar]
- 79.Shin M, Yoshimoto H, Vacanti JP. Tissue Eng. 2004;10:33. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 80.Steigman SA, Ahmed A, Shanti RM, Tuan RS, Valim C, Fauza DO. J Pediatr Surg. 2009;44:1120. doi: 10.1016/j.jpedsurg.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue Eng. 2005;11:1599. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Abukawa H, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Methods. 2009;47:122. doi: 10.1016/j.ymeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. AIChE J. 1996;42:850. [Google Scholar]
- 84.Hofmann S, Hagenmuller H, Koch AM, Muller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Biomaterials. 2007;28:1152. doi: 10.1016/j.biomaterials.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Jansen JH, van der Jagt OP, Punt BJ, Verhaar JA, van Leeuwen JP, Weinans H, Jahr H. BMC Musculoskelet Disord. 2010;11:188. doi: 10.1186/1471-2474-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun LY, Hsieh DK, Lin PC, Chiu HT, Chiou TW. Bioelectromagnetics. 2010;31:209. doi: 10.1002/bem.20550. [DOI] [PubMed] [Google Scholar]
- 87.Mauney JR, Sjostorm S, Blumberg J, Horan R, O'Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Calcif Tissue Int. 2004;74:458. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 88.Sittichokechaiwut A, Edwards JH, Scutt AM, Reilly GC. Eur Cell Mater. 2010;20:45. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- 89.Fassina L, Saino E, Sbarra MS, Visai L, Cusella De Angelis MG, Mazzini G, Benazzo F, Magenes G. Tissue Eng Part C Methods. 2009;15:233. doi: 10.1089/ten.tec.2008.0398. [DOI] [PubMed] [Google Scholar]
- 90.Fassina L, Saino E, Visai L, Magenes G. Conf Proc IEEE Eng Med Biol Soc. 2008:3582. doi: 10.1109/IEMBS.2008.4649980. [DOI] [PubMed] [Google Scholar]
- 91.Fassina L, Saino E, Visai L, Silvani G, Cusella De Angelis MG, Mazzini G, Benazzo F, Magenes G. J Biomed Mater Res A. 2008;87:750. doi: 10.1002/jbm.a.31827. [DOI] [PubMed] [Google Scholar]
- 92.Fassina L, Visai L, Benazzo F, Benedetti L, Calligaro A, De Angelis MG, Farina A, Maliardi V, Magenes G. Tissue Eng. 2006;12:1985. doi: 10.1089/ten.2006.12.1985. [DOI] [PubMed] [Google Scholar]
- 93.Saino E, Fassina L, Van Vlierberghe S, Avanzini MA, Dubruel P, Magenes G, Visai L, Benazzo F. Int J Immunopathol Pharmacol. 2011;24:1. doi: 10.1177/03946320110241S201. [DOI] [PubMed] [Google Scholar]
- 94.Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC. Bone. 2009;44:822. doi: 10.1016/j.bone.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 95.Engvall E, Perlmann P. Immunochemistry. 1971;8:871. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 96.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci U S A. 1979;76:4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Electrophoresis. 1999;20:3551. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 98.Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M. Mol Cell Proteomics. 2005;4:1265. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 99.Goldberg RL, Kolibas LM. Connect Tissue Res. 1990;24:265. doi: 10.3109/03008209009152154. [DOI] [PubMed] [Google Scholar]
- 100.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Anal Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 101.Szymanowicz G, Laurain G. Anal Biochem. 1981;113:58. doi: 10.1016/0003-2697(81)90043-9. [DOI] [PubMed] [Google Scholar]
- 102.Kang D, Gho Y, Suh M, Kang C. Bull Korean Chem Soc. 2002;23:1511. [Google Scholar]
- 103.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC. Nat Biotechnol. 2010;28:595. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li M, Alnouti Y, Leverence R, Bi H, Gusev AI. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:152. doi: 10.1016/j.jchromb.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 105.Walker JM. In: Protein Protocols Handbook (Methods in Molecular Biology) Walker JM, editor. Vol. 1. Humana Press; Totowa, USA: 2002. [Google Scholar]
- 106.Yang F, Wolke JGC, Jansen JA. Chem Eng J. 2008;137:154. [Google Scholar]
- 107.Schladitz K. J Microsc. 2011;243:111. doi: 10.1111/j.1365-2818.2011.03513.x. [DOI] [PubMed] [Google Scholar]
- 108.Ho ST, Hutmacher DW. Biomaterials. 2006;27:1362. doi: 10.1016/j.biomaterials.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 109.Vater C, Kasten P, Stiehler M. Acta Biomater. 2010;7:463. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 110.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Physiol Rev. 1989;69:990. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 111.Haylock DN, Nilsson SK. Br J Haematol. 2006;134:467. doi: 10.1111/j.1365-2141.2006.06218.x. [DOI] [PubMed] [Google Scholar]
- 112.Stein GS, Lian JB, Owen TA. FASEB J. 1990;4:3111. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 113.McCullen SD, Chow AG, Stevens MM. Curr Opin Biotechnol. 2011;22:715. doi: 10.1016/j.copbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Conn PM. In: Sourcebook of models for biomedical research. Conn PM, editor. Vol. 1. Humana Press; Totowa, USA: 2008. [Google Scholar]
- 115.Rodrigues MT, Gomes ME, Viegas CA, Azevedo JT, Dias IR, Guzon FM, Reis RL. J Tissue Eng Regen Med. 2011;5:41. doi: 10.1002/term.287. [DOI] [PubMed] [Google Scholar]
- 116.Sikavitsas VI, van den Dolder J, Bancroft GN, Jansen JA, Mikos AG. J Biomed Mater Res A. 2003;67:944. doi: 10.1002/jbm.a.10126. [DOI] [PubMed] [Google Scholar]
- 117.Ruan K, Bao S, Ouyang G. Cell Mol Life Sci. 2009;66:2219. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]