Abstract
It is well known that BMPs induce bone formation and that some BMPs, including BMP2 and BMP7, are clinically used in orthopedics. Signaling by BMPs plays an important role in a variety of cell-types in bone such as osteoblasts, chondrocytes, and osteoclasts. It is recently reported using an osteoblast-targeted deletion of BMP signaling that BMP signaling in osteoblasts physiologically induces bone resorption by enhancing osteoclastogenesis via the RANKL-OPG pathway and reduces bone mass. In this review, The physiological function of BMP signaling in bone will be focused, and the current outcomes from mouse genetic studies will be discuss.
Keywords: BMP signaling, osteoblast, chondrocyte, osteoclast, Wnt signaling, bone mass
1. Introduction
Bone morphogenetic proteins (BMPs) were discovered and named in 1965 by Marshall Urist, who initially identified the ability of an unknown factor in bone to induce ectopic bones in muscle [1]. In the last 45 years, the osteogenic function of BMPs has been extensively examined, mainly using osteoblasts in culture with exogenous treatments of BMPs [2]. Based on their potent osteogenic abilities, clinical trials have been initiated to use BMP2 and BMP7 to improve fracture repair [2]. After successful completion of the trials, the FDA has approved BMP2 and BMP7 for clinical use in long bone open-fractures, non-union fractures and spinal fusion. Similarly to osteogenic BMPs in vitro, studies of human mutations also suggested the importance of BMP signaling for skeletogenesis and bone-related diseases such as chondrodysplasia and fibrodysplasia ossificans progressive [3, 4]. Mutations in genes involving BMP signaling associated with skeletal abnormalities in humans are summarized in Table 1 [5-12].
Table 1.
Skeletal abnormalities associated with the BMP signaling
| Gene | Disease | Ref. |
|---|---|---|
| BMP2 regulatory element | brachydactyly type A2 | 5 |
| BMP4 | poly/syndactyly | 6 |
| CDMP1/GDF5 | acromesomelic chondrodysplasia brachydactyly type C | 3, 7 |
| GDF6 | hemi-vertebrae, Polydactyly, Klippel-Feil, rib malformation, spondylothoracic dysostosis | 8 |
| GDF3 | scoliosis, Klippel–Feil, vertebral fusion | 9 |
| BMPR1B | brachydactyly type A2 acromesomelic chondrodysplasia | 10, 11 |
| ALK2 | fibrodysplasia ossificans progressiva | 4 |
| NOGGIN | brachydactyly type B | 12 |
These facts indicate that BMP signaling is involved in the proper development of many components of the skeletomuscular system including bone, cartilage, and soft tissues such as muscle, fat, and tendons. Among them, bone and cartilage are the major components in the skeletal system, and the osteoblast and chondrocyte are the responsible cell types for formation and functions of these tissues, respectively. The osteogenic function of BMPs and BMP signaling has been further investigated over the last decade using a gene targeting technology. This article focuses on the physiological effects of BMP signaling on bone formation, bone resorption, and bone mass, specifically via its action on osteoblasts or chondrocytes by reviewing mouse genetic studies of skeletal development and bone remodeling.
2. BMP signaling and kinase
BMPs belong to the transforming growth factor-β (TGF-β) gene superfamily [13, 14], and this family of BMPs comprises approximately 30 structurally related members. Similar to TGF-β, BMPs signal through transmembrane serine/threonine kinase receptors such as BMP type I and type II receptors. Upon ligand binding, type I and II receptors form hetero-multimers [15], and a type II receptor phosphorylates a short stretch of amino acids called a GS box (the glycine- and serine-rich domain between the transmembrane and kinase domains) in the type I receptor to activate its kinase activity. Activated BMP type I receptors relay the signal to the cytoplasm by phosphorylating their immediate downstream targets, Smad1, Smad5, and Smad8 proteins, which then interact with Smad4 and translocate into the nucleus [16]. There are three type I receptors (BMPRIA, BMPRIB and ACVRI) and three type II receptors (BMPRII, ActRIIA and ActRIIB) that bind to BMP ligands to signal. The type I receptor ACVRI was originally found as an activin receptor, but it is now believed to be a receptor for BMPs. The specificity of signaling is primarily determined by type I receptors [17]; however, the specificity of ligand binding is altered by the combination of type I and II receptors [18].
3. Genetic approaches to uncover functions of BMP signaling in mice
Along with the huge advancement in technologies involving mouse genetics over the last decade, many of the BMP signaling related genes have been knocked out in mice. BMP2, BMP4, BMP6 and BMP7 and their receptor BMPRIA and ACVRI are abundantly expressed in bone. It has been reported that BMPRIA is a potent receptor of BMP2 and BMP4 [19, 20], and ACVR1 is a receptor of BMP7 [21]. However, conventional knockout mice for these genes result in an early embryonic lethality and thus, it is not possible to investigate bone development and remodeling using these models [22-28]. To avoid the embryonic lethality, a strategy of conditional knockout mice using a Cre-loxP system has been employed. A bone-specific conditional deletion of Bmpr1a using an Og2-Cre mouse, in which a Cre recombination is restricted in differentiated osteoblasts under the osteocalcin promoter, was first reported in 2004 [29]. Interestingly, this study demonstrated that the response of osteoblasts to BMP signaling is age-dependent; in the mutant mice, bone volume decreased in young mice but increased in aged mice. In addition, the activity of osteoclasts was reduced in the aged osteoblast-specific Bmpr1a-deficient mice, which may have lead to the complex skeletal phenotype. These facts suggest that the BMP signaling in differentiated osteoblasts can control the balance between bone formation by osteoblasts and resorption by osteoclasts, thereby affecting the final outcome of the amount of bone mass in an age-dependent manner. The increased bone mass in the Bmpr1a-deficient mice appeared to be in opposition to the general concept of BMPs as osteogenic inducers. This leads to a possibility that osteogenic targets of BMPs would be mesenchymal cells or chondrocytes, rather than osteoblasts. It is reasonable to speculate that different cell types exhibit differing responses to BMPs as evidenced by their multifaceted functions in vivo [14, 30]. The “opposite” outcome in the Bmpr1a-deficient mice will be discussed later at a cellular mechanistic point of view in section 4 and at a molecular mechanistic point of view in sections 5, 6, and 7.
4. BMP signaling and chondrocytes
During skeletogenesis, bones are formed via two distinct processes: intramembranous and endochondral bone formation [31]. Intramembranous bone formation occurs primarily in flat bones (e.g., calvarial bones) where mesenchymal cells differentiate directly into osteoblasts [32]. Endochondral bone formation occurs primarily in long bones where condensed mesenchymal cells differentiate into chondrocytes to form cartilage templates, and then chondrocytes are replaced by osteoblasts [33]. Recently many studies have been designed to investigate the difference in the molecular mechanism by which BMP signaling regulates these cell types. A variety of Cre mouse lines have been used to target different cell types including osteoblast, chondrocyte, and mesenchymal cells as summarized in Table 2.
Table 2.
Bone mass observed in genetically engineered mutant mice of BMP signaling
| Cell type/Targeted gene | Promoter Cre-mouse | BMP signal | Stage | Bone mass | Ref. |
|---|---|---|---|---|---|
| Mesenchymal cell | |||||
| Double knockout of BMP2 and BMP4 | Prx1-Cre | down | E10.5-newborn 3w | Reduced | 34 |
| Bmp2 cKO | Prx1-Cre | down | 5M | Reduced | 35 |
| Bmp7 cKO | Prx1-Cre | down | E10.5-13M | No change | 36 |
| Chondrocyte | |||||
| Bmpr1a cKO | Gdf5-Cre | down | E12.5-E16.5, 7w, 9M | Reduced | 37 |
| Double knockout of Bmpr1a and Bmpr1b | Col2-Cre | down | E12.5-E16.5, | Reduced | 38 |
| Bmp4 overexpression | Col11a2 | up | E18.5 | Increased | 39 |
| Noggin overexpression | Col11a2 | down | E18.5 | Reduced | 39 |
| Double knockout of Smad1 and Smad5 | Col2-Cre | down | E12.5-newborn | Reduced | 40 |
| Osteoblast | |||||
| Bmpr1a cKO | Og2-Cre | down | 3M 10M | Reduced Increased | 29 |
| Bmp4 overexpression | 2.3 kb Col1 | up | E18.5 | Reduced | 41 |
| Noggin overexpression | 2.3 kb Col1 | down | E17.5, 3w | Increased | 41 |
| Bmpr1a cKO | 3.2 kb Col1-CreER | down | E18.5, 3 w, 5M | Increased | 47,48, 68 |
There are several lines of evidence that show that BMP signaling in chondrocytes is required for bone size and the amount of bone mass. BMP signaling through BMPRIA is essential for postnatal maintenance of articular cartilage, using a Gdf5-Cre mouse line specific for chondrocytes in joints [34]. Similarly, the critical role of Bmpr1a together with Bmpr1b in chondrocytes during endochondral bone formation using a Col2-Cre mouse line was reported.[35]. Moreover, in chondrocytes a simultaneous deficiency in Smad 1 and Smad 5, which are BMPs’ downstream target molecules, reduces bone mass [36]. In parallel, studies focusing on BMP ligands and their antagonists provide further evidence that BMPs are critical for normal development of cartilage. A transgenic mouse line to overexpress Bmp4 in mesenchymal cells/chondrocytes using a type XI collagen promoter (Col11a2) was generated, and bone mass was increased in the mutant mice [37]. Another transgenic mouse line in which Noggin was overexpressed in the same cells (Col11a2-Noggin) demonstrated a decreased bone mass. As Noggin is an antagonist for BMPs (BMP2, BMP4, BMP5, BMP6, and BMP7) with various degrees of affinity [38], these results suggest that BMP signaling positively controls proliferation and differentiation of chondrocytes.
Similar to chondrocytes, a few studies demonstrated a requirement of BMP signaling in mesenchymal cells for proper bone development and remodeling. In a Prx1-Cre mouse line, Cre is active in mesenchymal cells as early as embryonic day 9.5 [39]. Using the Prx1-Cre mouse, the simultaneously conditional deletions of Bmp2 and Bmp4 in mesenchymal cells resulted in an impairment of osteogenesis during late embryogenesis [40, 41]. In contrast, the conditional deletion of Bmp2 in mesenchymal cells does not show overt developmental abnormalities, suggesting a compensation of BMP2 function by other BMPs such as BMP4. Interestingly, the Bmp2-deficient mice lack an initiation of fracture healing [40, 41]. Interestingly, Bmp7-deficiency in mesenchymal cells did not affect bone mass probably due to the compensation by Bmp4 [42]. Taken together, it is possible that the defects in the BMP signaling in chondrocytes largely contribute to the phenotypes described above because chondrocytes are derived from mesenchymal cells and play an important role in the process of fracture repair.
Recent histological findings suggest that endochondral bone formation plays a critical role in the process of ectopic bone formation [43]. The origin of precursor cells for the ectopic bone is under investigation [44, 45]; however, it is possible that formation of ectopic bones by BMPs [1] is largely due to the stimulation of chondrocytes or mesenchymal cells in soft tissue, which results in an expansion of ectopic cartilage subsequently replaced by osteoblasts. There is another possibility that the BMP signaling directly affects osteoblasts to form ectopic bone. However, this possibility is less likely based on recent evidence that reduced BMP signaling in osteoblasts results in an increase in bone mass.
5. BMP signaling and osteoblasts
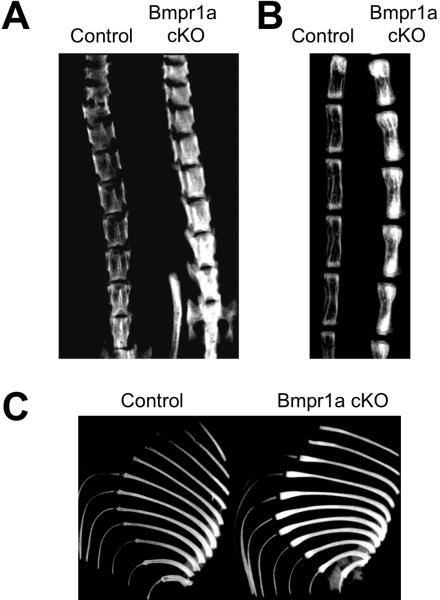
As aforementioned, a differentiated osteoblast-specific deletion of Bmpr1a caused an increase in bone mass in aged mice [29]. Similar to this finding, an overexpression of a BMP antagonist, Noggin, in osteoblasts increases bone volume with a reduced osteoclast number and osteoclastogenesis both at embryonic day 17.5 (E17.5) and at 3 weeks [46]. In parallel, the overexpression of Bmp4 in osteoblasts reduced bone mass presumably due to the increase in the osteoclast number at E18.5 [46]. Recently, Bmpr1a was conditionally disrupted in immature osteoblasts using a tamoxifen inducible Cre driven by a 3.2-kb alpha1(I) collagen chain gene (Col1a1) promoter. In the mutant mice, bone mass was dramatically increased during the bone remodeling stage at 22 weeks as well as the bone developmental stages at E18.5 and 3 weeks (Fig. 1) [47, 48]. This result is an interesting contrast to previous works that disruption of Bmpr1a in differentiated osteoblasts results in decrease of bone mass in young adult stages (3-4 weeks). The increased bone mass in the Bmpr1a-deficient mice resulted from severely suppressed bone resorption due to reduced osteoclastogenesis, despite a simultaneous small reduction in the rate of bone formation [48]. Levels of RANKL and OPG (see chapter 6 for details) are changed in the Bmpr1a-deficient osteoblasts and fail to support osteoclastogenesis [47, 48]. In addition, the conditional disruption of Acvr1 in osteoblasts also demonstrated a dramatic increase in bone mass, similar to the bone phenotype of Bmpr1a-deficient mice (unpublished data). These findings suggest that BMP signaling has dual roles in osteoblasts; to stimulate both bone formation by osteoblasts and bone resorption supporting osteoclastogenesis. Disruption of BMP signaling in immature osteoblasts alters the balance of bone turn over to increase the bone mass, which is opposite to what people have expected for the past 4 decades.
Figure 1.
Increased bone mass in the osteoblast-specific Bmpr1a conditional knockout (cKO) mouse at the adult stage. Bmpr1a cKO mouse was generated by crossing a floxed Bmpr1a mouse line with a transgenic mouse line harboring a tamoxifen–inducible Cre driven by a 3.2 kb mouse procollagen α1(I) promoter. The Cre recombination was induced specifically in the osteoblasts by 10 weeks of tamoxifen administration from 10 weeks after birth, and bones were removed at 22 weeks. Radiodensity of the spine (A), tail (B) and rib bones (C) was dramatically increased in the Bmpr1a cKO mice (Cre+, Bmpr1afx/fx) compared with controls (Cre–, Bmpr1afx/fx) when assessed by X-ray imaging.
6. BMP signaling in osteoblasts that regulates osteoclastogenesis
Bone mass is determined by the balance between bone formation and bone resorption. Osteoclasts are multinuclear cells derived from hematopoietic stem cells to secrete enzymes for bone resorption [49]. It is expected that BMPs play roles in osteoclastogenesis and their functions, because receptors for BMPs are expressed in these cells [50]. Additionally, osteoblasts also play critical roles in bone resorption by regulating osteoclastogenesis because they produce RANK ligand (RANKL), essential to promote osteoclastogenesis, and its decoy receptor, osteoprotegerin (OPG) [51, 52]. A balance between RANKL and OPG is important to determine the degree of osteoclastogenesis, i.e. more RANKL production by osteoblasts leads to more osteoclasts; thus more bone resorption is expected. As RANKL is an osteoblastic product and BMPs induce osteoblast maturation, BMPs indirectly stimulate osteoclastogenesis and thus, osteoclastogenesis is impaired when osteoblastogenesis is blocked with BMP antagonists in culture [53]. The physiological effects of BMP signaling in osteoblasts on osteoclastogenesis were determined later using an osteoblast-specific gain-of-function or loss-of-function mouse model. For the cases of the osteoblast-specific deletion of Bmpr1a and osteoblast-specific over expression of Noggin, osteoclastogenesis is highly compromised leading to an increase of bone mass [29, 46]. In contrast, osteoblast-specific overexpression of Bmp4 increased osteoclastogenesis [46]. The regulation of RANKL by BMPs was suggested based on an in vitro study [54]. This concept was recently proven in mouse studies, as Bmpr1a-deficient osteoblasts were not able to support osteoclastogenesis due to an imbalance between RANKL and OPG [47, 48].
There is accumulating evidence that Wnt signaling also plays a critical role in osteoclastogenesis regulated by osteoblasts through the RANKL-OPG pathway. As discussed in chapter 7, how BMP and Wnt signaling interact with each other is an interesting topic. Recently, two in vivo studies have suggested that the canonical Wnt signaling is important in the regulation of osteoclastogenesis by osteoblasts. One study provided evidence that the Wnt pathway positively regulates the expression of Opg in osteoblasts [55]. Overexpression of stabilized β-catenin in osteoblasts, which results in an increase of canonical Wnt signaling level, decreases osteoclast differentiation leading to increased bone volume in mice [55]. Another study showed that an osteoblast-specific deletion of β-catenin leads to an impaired maturation and mineralization of bones in mice due to the elevated expression of Rankl and diminished Opg [56]. These facts suggest that the canonical Wnt pathway negatively regulates osteoblasts in their supporting function in osteoclastogenesis, and thus upregulation of Wnt signaling in osteoblasts can suppress osteoclast-mediated bone resorption [56].
7. Interplays between BMP and Wnt signaling in bone
Both BMP and Wnt signaling regulate development and remodeling of many tissues and organs. Numerous studies have reported functions of each signaling [57-62]. Results from these studies suggest that these two signals regulate one another synergistically or antagonistically in context-dependent and age-dependent manners. In bone, experiments using pluripotent mesenchymal cell lines to test the interaction between BMP and Wnt signaling in osteoblasts have yielded both synergistic and antagonistic results: BMP2 induces both Wnt3a and Wnt/β-catenin signaling [63, 64], while Wnt3a in turn enhances the BMP4 expression [65], suggesting a positive autocrine loop [66, 67]. However, inhibition of BMP signaling by treatment of osteoblasts with dorsomorphin, a selective inhibitor for BMP type I receptors, increases the canonical Wnt signaling [68]. Further, Wnt3a is reported to repress BMP2-dependent Id1 expression [69], suggesting a negative feedback loop. In vivo, the BMP signaling in osteoblasts downregulates the canonical Wnt signaling during embryonic and postnatal bone development [47, 68]. This is due to the fact that Wnt inhibitors Sost (sclerostin) and Dkk1 are direct targets of the BMP signaling (Fig. 2). It is noted that the Sost was the most downregulated gene in the Bmpr1a-deficient bone as assessed by microarray analysis [47]. Interestingly, both Smad-dependent and Smad-independent pathways appear to contribute to the Dkk1 expression, whereas Sost expression requires only Smad-dependent signaling, suggesting a differential regulation of these genes by the BMP signaling via BMPRIA (Fig. 2) [68].
Figure 2.
A proposed model of the relationship between the BMP signaling via BMPRIA and the canonical Wnt signaling in osteoblasts. Both Dkk1 and sclerostin/Sost are downstream targets of the BMP signaling. The BMP signaling upregulates the Sost expression primarily through the Smad-dependent signaling while it upregulates the Dkk1 expression through both the Smad and non-Smad signaling (p38 MAPK). As Dkk1 and sclerostin/Sost act as Wnt signaling inhibitors, BMP signaling in osteoblasts, in turn, leads to a decrease in bone mass through regulating expressions of RANKL and OPG to suppress osteoclastogenesis. Dkk1 and sclerostin/Sost play an important role in regulating bone mass as downstream effectors of BMPRIA signaling in bone taking balances between BMP signaling and Wnt signaling, as well as bone formation and bone resorption.
Both Dkk1 and Sost are expressed by osteoblasts as secreted proteins which inhibit Wnt/β-catenin signaling by binding to co-receptors, low density lipoprotein receptor-related protein 5 and 6 (LRP5 and LRP6) [70]. Conventional knockouts of Dkk1 die in utero from defective head induction and limb formation [71]. Mice heterozygous for Dkk1 (Dkk1+/– mice) exhibit a high bone mass (HBM) phenotype [72], whereas overexpression of Dkk1 in osteoblasts causes osteopenia [73]. In addition, the increased DKK1 expression in bone marrow has also been associated with lytic bone lesions in patients with multiple myeloma [74]. Collectively, these results support the hypothesis that Dkk1 functions as a potent negative regulator of bone mass. Conventional knockouts of Sost are viable and exhibit increased bone mass [75], similar to Dkk1+/– mice. In humans, loss-of-function and hypomorphic mutations in SOST cause sclerosteosis [76, 77] and Van Buchem disease [78, 79], respectively, with a HBM phenotype. Consistent with these observations, conditional knockouts of Bmpr1a, which are deficient in the Dkk1 and Sost expression, show a HBM phenotype [48]. Furthermore, an increased expression of Dkk1 and Sost in osteoblasts by constitutively activated BMPRIA signaling is associated with partial rescue of the bone phenotype of Bmpr1a-deficient mice [68]. Therefore, it is possible that Dkk1 and Sost (sclerostin) act physiologically as downstream molecules of BMP signaling to inhibit canonical Wnt signaling and therefore negatively regulate bone mass, at least, in mice as shown in Figure 2.
8. Clinical application of BMPs
The FDA has approved BMP2 and BMP7 for clinical use in long bone open-fractures, non-union fractures and spinal fusion, and BMPs’ treatment has shown a clear benefit for patients. However, despite significant evidence of their abilities for bone regeneration in animal and preclinical studies, some clinical data is unconvincing to support the effectiveness of BMP treatment on fracture healing and spine surgery [80-83]. This is partly because of BMPs’ numerous functions by cell type. It is important to understand that BMPs have variable and context-sensitive effects on diverse cell types in bone including chondrocytes, osteoblasts, and osteoclasts. Studies focusing on BMP receptors in chondrocytes including mesenchymal cells suggest that these cells can respond to BMP signaling by increasing bone mass during the endochondral formation process as discussed earlier. In contrast, when the function of osteoblast-dependent BMP signaling is examined with respect to bone mass determination, BMP signals can consistently inhibit Wnt signaling and bone mass while exerting concordant effects on Dkk1 and Sost. The function of the BMP signaling in osteoclasts remains largely unknown and merits future study, although the BMP signaling regulates osteoblast-dependent osteoclastogenesis via the RANKL-OPG pathway. This revision of traditional understanding of the BMP signaling pathway in clinical therapeutics might suggest that in some circumstances, BMP inhibition would be desirable for promoting bone mass.
9. Conclusion
Understanding the complex roles of the BMP signaling pathway in a variety of cell-types in bone including chondrocytes, osteoblasts and osteoclasts, which contribute to bone development, homeostasis, and remodeling will not only help to improve current knowledge of the dynamic processes which are perturbed in the settings of bone fracture, mechanical loading, and congenital and aging-related bone diseases but may provide novel therapeutically useful strategies.
Acknowledgments
We gratefully acknowledge Tatsuya Kobayashi and Henry M. Kronenberg for generation of the Col1-CreERTM mouse line and Mitsuo Yamauchi, Jian Q. Feng and Harry K. W. Kim for long-term collaborations. This work was supported by the Intramural Research Program of the NIEHS/NIH ES071003-11 and DE020843 (Y. M) and the Lilly Fellowship Foundation supported (N. K) and done by intensive collaboration with the Knockout Core at the NIEHS/NIH.
References
- 1.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Simpson AH, Mills L, Noble B. The role of growth factors and related agents in accelerating fracture healing. J. Bone Joint Surg. Br. 2006;88:701–705. doi: 10.1302/0301-620X.88B6.17524. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat. Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- 4.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 5.Dathe K, Kjaer KW, Brehm A, Meinecke P, Nurnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, Seemann P, Mundlos S. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am. J. Hum. Genet. 2009;84:483–492. doi: 10.1016/j.ajhg.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakrania P, Efthymiou M, Klein JC, Salt A, Bunyan DJ, Wyatt A, Ponting CP, Martin A, Williams S, Lindley V, Gilmore J, Restori M, Robson AG, Neveu MM, Holder GE, Collin JR, Robinson DO, Farndon P, Johansen-Berg H, Gerrelli D, Ragge NK. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am. J. Hum. Genet. 2008;82:304–319. doi: 10.1016/j.ajhg.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polinkovsky A, Robin NH, Thomas JT, Irons M, Lynn A, Goodman FR, Reardon W, Kant SG, Brunner HG, van der Burgt I, Chitayat D, McGaughran J, Donnai D, Luyten FP, Warman ML. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat. Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- 8.Asai-Coakwell M, French CR, Ye M, Garcha K, Bigot K, Perera AG, Staehling-Hampton K, Mema SC, Chanda B, Mushegian A, Bamforth S, Doschak MR, Li G, Dobbs MB, Giampietro PF, Brooks BP, Vijayalakshmi P, Sauve Y, Abitbol M, Sundaresan P, van Heyningen V, Pourquie O, Underhill TM, Waskiewicz AJ, Lehmann OJ. Incomplete penetrance and phenotypic variability characterize Gdf6-attributable oculo-skeletal phenotypes. Hum. Mol. Genet. 2009;18:1110–1121. doi: 10.1093/hmg/ddp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye M, Berry-Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, French CR, Abitbol M, Fleisch VC, Corbett N, Allison WT, Drummond G, Walter MA, Underhill TM, Waskiewicz AJ, Lehmann OJ. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum. Mol. Genet. 2010;19:287–298. doi: 10.1093/hmg/ddp496. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Suring K, Majewski F, Tinschert S, Grzeschik KH, Muller D, Knaus P, Nurnberg P, Mundlos S. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12277–12282. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirhan O, Turkmen S, Schwabe GC, Soyupak S, Akgul E, Tastemir D, Karahan D, Mundlos S, Lehmann K. A homozygous BMPR1B mutation causes a new subtype of acromesomelic chondrodysplasia with genital anomalies. J. Med. Genet. 2005;42:314–317. doi: 10.1136/jmg.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann K, Seemann P, Silan F, Goecke TO, Irgang S, Kjaer KW, Kjaergaard S, Mahoney MJ, Morlot S, Reissner C, Kerr B, Wilkie AO, Mundlos S. A new subtype of brachydactyly type B caused by point mutations in the bone morphogenetic protein antagonist NOGGIN. Am. J. Hum. Genet. 2007;81:388–396. doi: 10.1086/519697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J. Receptors for the TGF-beta family. Cell. 1992;69:1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- 14.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 17.Carcamo J, Weis FM, Ventura F, Wieser R, Wrana JL, Attisano L, Massague J. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor beta and activin. Mol. Cell. Biol. 1994;14:3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massague J. TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 19.Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD. Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 2004;11:481–488. doi: 10.1038/nsmb756. [DOI] [PubMed] [Google Scholar]
- 20.Hatta T, Konishi H, Katoh E, Natsume T, Ueno N, Kobayashi Y, Yamazaki T. Identification of the ligand-binding site of the BMP type IA receptor for BMP-4. Biopolymers. 2000;55:399–406. doi: 10.1002/1097-0282(2000)55:5<399::AID-BIP1014>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 23.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 24.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 25.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev. Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 26.Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 27.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 28.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 29.Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J. Biol. Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 30.Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front. Biosci. 2003;8:d855–869. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 33.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J. Bone Miner. Res. 2002;17:898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 39.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 40.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic Analysis of the Roles of BMP2, BMP4, and BMP7 in Limb Patterning and Skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji K, Cox K, Gamer L, Graf D, Economides A, Rosen V. Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair. J. Orthop. Res. 2010;28:384–389. doi: 10.1002/jor.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J. Bone Miner. Res. 2006;21:1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- 47.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J. Bone Miner. Res. 2008;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz MC, Lorenzo JA. The origins of osteoclasts. Curr. Opin. Rheumatol. 2004;16:464–468. doi: 10.1097/01.bor.0000127825.05580.eb. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27:479–486. doi: 10.1016/s8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 51.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 52.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 53.Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Miner. Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 54.Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, Higashio K, Quinn JM, Gillespie MT, Martin TJ, Suda T, Takahashi N. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 55.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J. Biol. Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 57.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 58.Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 60.Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, 3rd, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miura S, Singh AP, Mishina Y. Bmpr1a is required for proper migration of the AVE through regulation of Dkk1 expression in the pre-streak mouse embryo. Dev. Biol. 341:246–254. doi: 10.1016/j.ydbio.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- 63.Bain G, Muller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem. Biophys. Res. Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 64.Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng SL, Chen D, Civitelli R. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell. Biochem. 2005;94:403–418. doi: 10.1002/jcb.20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkler DG, Sutherland MS, Ojala E, Turcott E, Geoghegan JC, Shpektor D, Skonier JE, Yu C, Latham JA. Sclerostin inhibition of Wnt- 3a-induced C3H10T1/2 cell differentiation is indirect and mediated by bone morphogenetic proteins. J. Biol. Chem. 2005;280:2498–2502. doi: 10.1074/jbc.M400524200. [DOI] [PubMed] [Google Scholar]
- 66.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Whetstone HC, Youn A, Nadesan P, Chow EC, Lin AC, Alman BA. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 2007;282:526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 68.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt Inhibitors Dkk1 and Sost are Downstream Targets of BMP Signaling Through the Type IA Receptor (BMPRIA) in Osteoblasts. J. Bone Miner. Res. 2010;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakashima A, Katagiri T, Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J. Biol. Chem. 2005;280:37660–37668. doi: 10.1074/jbc.M504612200. [DOI] [PubMed] [Google Scholar]
- 70.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 71.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 72.Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 74.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 75.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 76.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 77.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am. J. Hum. Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, Papapoulos S, Hamersma H, Brunkow ME. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 80.Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J. Bone Joint. Surg. Am. 2010;92:411–426. doi: 10.2106/JBJS.H.01732. [DOI] [PubMed] [Google Scholar]
- 81.Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2006;31:E277–284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 82.Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J. Bone Joint. Surg. Br. 2007;89:342–345. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 83.Laursen M, Hoy K, Hansen ES, Gelineck J, Christensen FB, Bunger CE. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur. Spine. J. 1999;8:485–490. doi: 10.1007/s005860050210. [DOI] [PMC free article] [PubMed] [Google Scholar]