Abstract
Purpose
To study the expression and function of a novel cell-cycle regulatory protein, human ecdysoneless (Ecd), during pancreatic cancer pathogenesis.
Experimental Design
Immunohistochemical expression profiling of Ecd was done in nonneoplastic normal pancreatic tissues and pancreatic ductal adenocarcinoma lesions (from tissue microarray and Rapid Autopsy program) as well as precancerous PanIN lesions and metastatic organs. To analyze the biological significance of Ecd in pancreatic cancer progression, Ecd was stably knocked down in pancreatic cancer cell line followed by in vitro and in vivo functional assays.
Results
Normal pancreatic ducts showed very weak to no Ecd expression compared to significant positive expression in pancreatic cancer tissues (mean ± SE composite score: 0.3 ± 0.2 and 3.8 ± 0.2 respectively, P < 0.0001) as well as in PanIN precursor lesions with a progressive increase in Ecd expression with increasing dysplasia (PanIN-1–PanIN-3). Analysis of matched primary tumors and metastases from patients with pancreatic cancer revealed that Ecd is highly expressed in both primary pancreatic tumor and in distant metastatic sites. Furthermore, knockdown of Ecd suppressed cell proliferation in vitro and tumorigenicity of pancreatic cancer cells in mice orthotopic tumors. Microarray study revealed that Ecd regulates expression of glucose transporter GLUT4 in pancreatic cancer cells and was subsequently shown to modulate glucose uptake, lactate production, and ATP generation by pancreatic cancer cells. Finally, knockdown of Ecd also reduced level of pAkt, key signaling molecule known to regulate aerobic glycolysis in cancer cells.
Conclusion
Ecd is a novel tumor-promoting factor that is differentially expressed in pancreatic cancer and potentially regulates glucose metabolism within cancer cells.
Introduction
Pancreatic cancer is one of the most lethal malignancies characterized by late clinical presentation and an extremely poor prognosis (median survival of patients with pancreatic cancer is about 3–6 months; ref. 1). It is estimated that in 2012, about 43,920 individuals will be diagnosed with pancreatic cancer, and nearly 37,390 will die from it (2). Its aggressive nature together with the poor response to chemo and radiotherapy and a tendency for recurrence makes it one of the few cancers with a nearly 100% post-diagnosis mortality. The identification of biomarkers for the early detection of pancreatic cancer is thus an urgent clinical need (1).
Human ecdysoneless protein (Ecd) is the human ortho-log of the fruit fly (Drosophila melanogaster) ecd protein. The ecd protein is named after its role in regulating the synthesis of the steroid hormone ecdysone, which is required for molting in insects (3, 4). Ecd is required for both development and oogenesis in Drosophila (5). However, the molecular function of its mammalian orthologue (Ecd) is not yet defined. The human Ecd gene was first identified in a complementation assay conducted to rescue yeast mutants defective in glycolytic gene expression (6). Initial studies reported role of Ecd in stabilizing p53 and preventing its turnover by mdm-2 (7). Recently, it was reported that Ecd plays a role in cell-cycle regulation (8). The conditional deletion of Ecd (the mouse homologue of Ecd) in murine embryonic fibroblasts led to an arrest of cells in the G1–S phase of the cell cycle and a marked downregulation of genes involved in cell-cycle progression, whereas a whole body knockout of the protein was embryonically lethal (8). Studies done with Ecd null mouse embryonic fibroblasts (MEF) also showed that Ecd indirectly regulates E2F target gene expression during cell cycle through competitive binding to Rb protein (8). Furthermore, a recent study shows that even in the absence of a DNA binding domain, Ecd possesses transactivation property, which is enhanced by its interaction with the histone acetyltransferase p300 (9).
On the basis of the role of Ecd in cell-cycle regulation, which is often dysregulated in pancreatic cancer, we investigated the expression and functional significance of Ecd in pancreatic cancer. Here, we report that although the normal pancreatic ducts have little or no Ecd expression, it is significantly upregulated in pancreatic cancer tissues including premalignant lesions that are known to precede the development of invasive ductal carcinoma (termed as pancreatic intraepithelial neoplasia or PanINs) as well as in the metastatic organs. Functional analysis shows that knockdown of Ecd reduces pancreatic cancer cell growth and tumorigenicity. Ecd depletion also downregulates GLUT4 mRNA and protein levels with consequent decrease in glucose uptake, ATP, and lactate levels. In conclusion, our results show that Ecd is aberrantly expressed during the progression of pancreatic cancer and regulates pancreatic cancer cell growth through modulation of glucose metabolism.
Materials and Methods
Tissues, cell lines, and reagents
Tissue microarrays (TMA) made from formalin-fixed, paraffin-embedded (FFPE) tissues were purchased from US Biomax comprising normal and pancreatic cancer tissue sections (catalog number PA481 and PA804). Ecd monoclonal antibody has been described previously (8). Furthermore, matched tissues from primary pancreatic cancer and metastases were obtained from the University of Nebraska Medical Center (UNMC, Omaha, NE) Pancreatic Rapid Autopsy Program (IRB number 091–01). In addition, FFPE-archived tissue sections comprising adjacent areas of normal, PanIN, and pancreatic cancer were provided by our collaborator Dr. S. Lele (pathologist). Thirteen pancreatic cancer cell lines were grown in complete medium [Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 1% pencillin–streptomycin) at 37°C and 5% CO2. Ecd scrambled and knockdown (KD) cells were generated by retroviral transfection of vector control or 2 different Ecd-specific shRNA vectors (7) into phoenix cells (package cells) and viral supernatant was used to infect CD18/HPAF, Capan1, and MiaPaCa pancreatic cancer cells followed by selection with puromycin (5 μg/mL). Selected cells were maintained in 10% DMEM containing puromycin (3 μg/mL).
Western blotting analysis
The cell lysates preparation and Western blotting was done as per the standard procedures (10). For all Western blots, 20 μg of protein lysate was resolved by 10% SDS-PAGE conducted under reducing conditions. Primary antibodies used for immunodetection were anti-Ecd mouse monoclonal antibody (Dr. V. Band), GLUT4 (H-61, Santa Cruz), p-Akt (Ser-473), and Akt antibodies from Cell Signaling Technology Inc. Resolved proteins were transferred on to the polyvinylidene difluoride (PVDF) membrane and immunoblot assay was conducted.
Immunohistochemistry
The immunostaining technique was conducted as described previously (8, 11, 12). All tissue sections were observed under a Nikon light microscope and photographs of representative areas taken with the Q-capture Micropublisher 5.0 camera (Leeds Precision Instruments) by using the Q-capture suite software package (QImaging). The intensity of Ecd expression was graded on a scale of 0 to 3 (0: no staining, 1+: weakly positive, 2+: moderately positive, 3+: strongly positive). The percentage of cells stained for Ecd in a given section was graded on a scale of 1 to 4 (1: <25%; 2: 25%–50%; 3: 50%–75%; and 4: 75%–100%). The overall staining was then represented by a composite score (product of the above 2 scores, ranging from 0–12).
Statistical analysis
The data was analyzed using the Medcalc software for Windows version 9·6·4·0 (MedCalc Software). The intensities of Ecd expression were considered as continuous variables, whereas the stage and grade of pancreatic cancer and gender of the patient were considered as categorical variables. Continuous variables were compared using the Student’s 2-tailed t test assuming unequal variance, whereas the categorical variables were compared using the 2-way ANOVA. A P value of less than 0.05 was considered significant.
RNA isolation from pancreatic tissues, cDNA preparation, and real-time PCR analysis
12 pancreatic cancer patient tissue samples were obtained after approval of the protocol by the IRB (IRB-491-97) at the University of Nebraska medical Center. RNA was isolated from the flash frozen tissues using the mirVana kit from Ambion using the manufacturer’s protocol. The mRNA was converted to cDNA using oligo dT primers. The primers used to determine Ecd expression are: Ecd (forward primer; FP) 5′-ACT TTG AAA CAC ACG AAC CTG GCG-3′and Ecd (reverse primer; RP) 5′-TGA TGC AGG TGT GTG CTA GTT CCT-3′.
Confocal microscopy
The Ecd scrambled and KD cells were seeded at 80% confluency on sterile coverslips in 12-well plates. Following overnight serum starvation and subsequent insulin stimulation with 100 nmol/L insulin for the mentioned time period, the cells were fixed in ice-cold methanol for 2 minutes. The cells were then washed with 1× PBS, blocked with 10% goat serum, and incubated with primary antibody against GLUT4 (Santa Cruz) for 1 hour. After washing, the cells were incubated further with Texas-red–labeled anti-rabbit secondary antibody and then washed and mounted with Vectashield containing 4′ 6′-diamidino-2-phenylin-dole (DAPI) stain.
Glucose uptake assay
For in vitro glucose uptake assay, cells were seeded at a density of 50,000 cells per well in a 12-well plate and then serum starved for 24 hours. Following starvation, cells were stimulated with 100 nmol/L insulin for half hour. Cells were then washed with 1× PBS and incubated with 1 μCi of (3H)-2DG for 15 minutes at 37°C. After washing with 1× PBS, cells were permeabilized using 1% SDS solution, and the radioactivity was measured with a liquid scintillation counter. The total radioactive counts were normalized for the cell count in each well. For in vivo glucose uptake assay, 0.5 × 106 cells of each type (Ecd KD and SCR, 10 mice/group) were injected orthotopically into the pancreas of athymic female mice. 20 days post-implantation, the mice were intraperitonially injected with 100 μL of 10 nmol/L IR800 dye coupled 2-deox-yglucose (IRDye 800CW 2DG, from LI-COR Biosciences). The mice were imaged 24 hours after injection by using PEARL IMPULSE, a near-infrared in vivo imager from LI-COR. The total intensity and mean fluorescence intensity was measured using Pearl Cam software and compared between the 2 groups.
Lactate assay
For lactate measurement, 50,000 cells were seeded per well in a 12-well plate. After 24 hours, the supernatant was collected and lactate production was measured using the Lactate Assay Kit (Biovision) following the manufacturer’s protocol.
ATP measurement
The total ATP content of the cells was determined using a luminescence-based assay (Cell titer Glo, Promega). 10,000 cells were seeded per well of a 96-well plate (provided by the manufacturer) and after 24 hours, 100 μL of the reagent mixture was added to each well and the luminescence was measured using luminometer. The luminescence per well was normalized with the amount of protein in each well.
Microarray analysis
The scrambled and Ecd KD HPAF/CD18 cells were processed for RNA isolation as previously described in this section. The purity of the RNA was determined by Nano-Drop Spectrophotometer followed by verification with Bioanalyzer. The RNA samples in 3 biologic replicates were submitted for microarray using the Phalanx Biotech Spotted Microarray (HOA_005). The microarray results were analyzed by biostatistician for top differentially expressed genes using LOWESS Normalization protocol using BRB Array-Tools. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE39988 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE39988).
Results
Ecd is overexpressed in pancreatic cancer tissues
Given the role of Ecd as a cell-cycle regulator, we wished to examine if Ecd expression is altered in pancreatic cancers. Commercial TMAs containing pancreatic tissues from 201 patients, representing normal pancreatic tissue, cancer adjacent normal and ductal adenocarcinoma, were immunostained using the mouse anti-Ecd monoclonal antibody. In 3 of 25 (12%) normal pancreatic tissues, the nonneoplastic ducts showed weak focal expression of Ecd. A strong Ecd staining was, however, noted in the islets and weak to moderate staining was seen in the acinar cells of normal pancreas tissue sections (Fig. 1A). In comparison, 127 of 144 (88%) cases of ductal adenocarcinoma showed some staining for Ecd protein (i.e., composite score >0). The mean (±SE) composite score for Ecd expression in pancreatic cancer was significantly higher than that in the non-neoplastic ducts (3.8 ± 0.2 and 0.3 ± 0.2 respectively, P < 0.00001). Among other histologic subtypes of pancreatic cancer, Ecd staining was strongest in pancreatic neuroendocrine tumors (panNETs) followed by intraductal papillary mucinous neoplasm (IPMN), mucinous carcinoma (either mucinous cystadenomacarcinoma derived from MCNs or noncystic mucinous carcinoma derived from IPMNs), acinus cell carcinoma, and squamous cell carcinoma. The results are summarized in Supplementary Table S1.
Figure 1.
Ecd expression in normal pancreas and pancreatic ductal adenocarcinoma. A, expression of Ecd in different grades of PDAC and nonneoplastic pancreas. I, normal pancreatic tissue with non-neoplastic pancreatic ducts (black arrowhead) shows no significant Ecd expression, whereas islet cells (red arrowhead) shows strong and adjacent normal acini (star) shows weak Ecd expression. II–IV, Ecd expression was significantly upregulated in invasive ductal adenocarcinoma showing decreasing expression with advancing grade of pancreatic cancer: II–IV represents well, moderate, and poorly differentiated pancreatic cancer, respectively. B, expression of Ecd during initiation and development of pancreatic cancer. I, Ecd was not expressed in any of the nonneoplastic pancreatic ducts. II, normal pancreatic islet cells on the other hand showed a strong expression of Ecd. III, PanIN-1 lesion showing a weak, predominantly granular cytoplasmic Ecd staining in the dysplastic columnar epithelial cells, occasional strong focal staining is noted in the apical portion of the cells (inset). IV, PanIN-2 lesion showing moderately strong Ecd staining, predominantly cytoplasmic in distribution. Ecd expression was significantly stronger towards the apical region of the cells compared with the basal region (inset). V, PanIN-3 lesions also showing strong Ecd expression with cytoplasmic and apical membrane staining similar to that for PanIN-2 (inset). VI, well-differentiated adenocarcinoma showing moderate Ecd expression. Notably, the expression was cytoplasmic with an apical membrane accentuation (inset). Original magnification × 100.
Notably, Ecd expression showed an inverse correlation with the degree of differentiation being highest in well differentiated and lowest in poorly differentiated pancreatic cancer (Fig. 1A). Although the percentage of Ecd-positive cases increased with increasing stage of pancreatic cancer (87%, 92%, 100%, and 100% in stage 1, 2, 3 and 4, respectively), the intensity (measured by the composite score) was not significantly different between different stages (Supplementary Table S2)
We further analyzed variation in Ecd expression during the initiation and progression of pancreatic cancer. In recent years, a progression model that describes the transformation of normal ducts into invasive adenocarcinoma through a series of premalignant lesions has been proposed. These lesions termed as PanINs; ref. 13) are characterized by specific cytologic, molecular, and genetic changes (1, 8). We analyzed Ecd expression in tissues containing pancreatic cancer, PanIN lesions of various grades, and normal adjacent pancreatic tissue in terms of the composite score. There was a trend towards increase in the percentage of Ecd-positive ducts (composite score >0) with increasing grade of dysplasia (0%, 55%, 80%, and 74% of nonneoplastic, PanIN-1, PanIN-2, and PanIN-3 lesions were positive, respectively). Furthermore, there was a significant increase in Ecd expression with increasing dysplasia as evidenced by the increase in composite score. Overall, the immunohistochemical staining results indicate that Ecd is overexpressed during pancreatic cancer initiation and progression as compared with nonneoplastic normal tissue, thereby suggesting its potential contribution in pancreatic cancer × pathogenesis.
Positive correlation of Ecd expression in primary pancreatic cancer and metastatic sites
Many cell-cycle regulatory proteins are known to undergo an alteration in either their level of expression or subcellular localization as the malignancy progresses from a localized to a metastatic stage. Given the fact that Ecd is differentially upregulated in the malignant ducts, we next sought to investigate whether there was a variation in Ecd expression during the metastasis of pancreatic cancer. For this, tissue samples collected from patients with metastatic pancreatic cancer as part of the rapid autopsy program (at UNMC) were stained for Ecd protein. Samples were obtained from 32 patients that consisted of primary pancreatic tumors (n = 29 cases) and metastases occurring in distant organs including the lungs (n = 14 cases), liver (n = 18), lymph nodes (n = 16), and the omentum (n = 14). In 14 of 29 (48%) patients, expression of Ecd was noticed in primary pancreatic tumor (composite score = 4.3 ± 1, Fig. 2A; III, IV). There was also Ecd expression in metastatic deposits from all organs at levels similar to that of the primary tumor (Supplementary Table S3). An analysis of Ecd positivity at sites of distant metastasis revealed that cases where the primary tumor was positive for Ecd expression were more likely to be also positive for Ecd at the site of metastasis (Supplementary Table S4). Specifically, the percentage positivity for Ecd was higher in the lungs (80% vs. 56%), lymph nodes (100% vs. 44%), and omentum (75% vs. 50%) metastasis when comparing primary tumor specimens that were positive (top) to those which were negative (bottom) for Ecd expression. The intensity of Ecd expression (as measured by the composite score) was higher in the omentum and lymph nodes, but not in the lungs for cases where the primary pancreatic cancer was positive for Ecd (Fig. 2A and B; III–2XII). Interestingly, primary tumors negative for Ecd also had corresponding meta-static lesions without Ecd expression (Fig. 2B; I–X). In case of liver metastases, however, 100% of the metastatic tumors expressed Ecd irrespective of the positivity of the primary pancreatic tumor. Overall, the intensity of Ecd expression was significantly higher in metastatic sites for cases where the primary pancreatic cancer was positive (8.6 ± 1.1) compared with cases where the primary pancreatic cancer was negative (4.3 ± 0.9, P = 0.017). Therefore, the above results show that Ecd expression is maintained in the metastatic stage of pancreatic ductal adenocarcinoma (PDAC) in different distant organs and is positively correlated to its expression within the primary tumor.
Figure 2.
Ecd expression in primary versus metastatic pancreatic cancer specimens from Rapid Autopsy A.I and II, immunostaining of cancer adjacent normal pancreatic ducts from a patient with metastatic pancreatic cancer shows no Ecd expression (black arrow head). III and IV, moderate to poorly differentiated primary pancreatic cancer tissue section from the same patient showing high Ecd expression. V–X, metastatic pancreatic cancer from the same patient reveals strong Ecd expression in the malignant cells at all sites (liver, lung, lymph node, and diaphragm, respectively). Ecd expression was primarily cytoplasmic in both primary and metastatic cancer cells. III, V, VII, IX, and XI = 3,3′-diaminobenzidine (DAB) stained; brown areas indicate Ecd expression. IV, VI, VIII, X, and XII = corresponding hematoxylin and eosin (H&E)–stained sections. Ecd expression in metastatic organs shows strong correlation with that in the primary tumor. B, immunostaining of Ecd in poorly differentiated pancreatic cancer tissue and corresponding metastatic lesions from liver, lung, lymph, omentum, respectively from the same patient. Primary tumor as well as corresponding metastatic tissues show no Ecd expression. (II, IV, VI, VIII, X) represent H&E staining for each of PDAC, liver, lung, lymph node, and omentum metastasis tissue sections.
Ecd knockdown inhibits proliferation and tumorigenicity of pancreatic cancer cells
Our previous observation that Ecd is a cell-cycle regulator overexpressed in pancreatic ductal adenocarcinoma as compared with normal pancreatic ducts prompted us to investigate the functional role of Ecd in pancreatic cancer pathogenesis. For this, Ecd expression was analyzed in a panel of pancreatic cancer cell lines, all of which showed moderate to high levels of Ecd expression both at protein and mRNA levels (Supplementary Fig. S1). Following this, stable knockdown clones of Ecd were established in CD18/HPAF, Capan1, and MiaPaca pancreatic cancer cells using 2 different shRNA (KD1 and KD2) constructs. Growth kinetics assay conducted on the Ecd shRNA (Ecd KD) and scrambled shRNA-transfected cells (Scr) revealed that downregulation of Ecd leads to reduced proliferation of HPAF/CD18 (Fig. 3A I) and MiaPaca pancreatic cancer cells. Cell-cycle analysis of Scr and Ecd KD CD18 pancreatic cancer cells using flow cytometry analysis further revealed that decrease in Ecd induces cell-cycle arrest of pancreatic cancer cells at the G1–S checkpoint (Fig 3A). Ecd KD cells showed increased cellular population at G1 phase and decreased cell population at the S phase. This data is in corroboration with our earlier observation that Ecd depletion causes G1–S block in breast cancer cells (7). Annexin V-staining analysis on Scr versus Ecd KD cells did not show any varitation in cell death (data not shown), thereby indicating that difference in cell proliferation could be primarily attributed to inhibition of cell growth. In addition, there was no significant effect of Ecd knockdown on cell motility or invasive properties (data not shown) of pancreatic cancer cells. To investigate the effect of Ecd knockdown on pancreatic cancer cell tumorigenicity, CD18/HPAF Ecd KD or Scr cells were injected orthotopically into the pancreas of nude mice and tumor growth was evaluated after 21 days. In vivo tumorogenesis data revealed that decrease in Ecd protein level leads to reduced tumor weight (Fig. 3B; I) as well as lesser number of metastatic foci at distant organs (Supplementary Table S5A). Immunohistochemical analysis of the tumors resected from Scr and Ecd KD cell injected mice confirmed that knockdown of Ecd was maintained within the tumors (Fig. 3B II). Although the exact mechanism of decreased tumor weight and less metastatic foci upon Ecd deletion needs further evaluation, these results are consistent with cell-cycle regulatory function of Ecd.
Figure 3.
Knockdown of Ecd reduces cell growth (in vitro) and tumorigenicity (in vivo) of pancreatic cancer cells. A, Ecd depletion reduces pancreatic cancer cell proliferation (I). Ecd KD pancreatic cancer cells exhibit reduced rate of proliferation compared with Ecd scrambled (Scr) cells. (*, P < 0.01). Experiments were carried out in triplicates (n = 3; II). Bar diagram representing the distribution of Ecd scr and KD cells in different phases of cell cycle analyzed by Annexin V staining and fluorescence-activated cell sorting (FACS). Ecd KD cells exhibit G1–S block having significantly higher population of cells in the G1 phase and lower in the S1 phase compared with the control cells; n = 3. B, Ecd knockdown inhibits pancreatic cancer cell tumorigenicity. I, boxed-plot representation of the distribution of tumor weight isolated from mice injected with Ecd KD pancreatic cancer cells versus control cells. Error bars represent SE for n = 10 mice. II, immunohistochemistry of Ecd in tumor sections (left) obtained from mice injected with Ecd KD pancreatic cancer cells (bottom) and control cells (top) along with corresponding H&E sections (right).
Ecd regulates expression of insulin-dependent glucose transporter GLUT4
To elucidate the mechanism underlying the role of Ecd in pancreatic cancer pathogenesis, microarray analysis was conducted by using the mRNA isolated from Scr and Ecd KD HPAF/CD18 pancreatic cancer cells to identify differentially regulated genes (GEO Series accession number GSE39988). There were 227 genes that showed more than 2-fold change in expression due to Ecd downregulation. Clustering of the differentially expressed genes indicated that many of the genes were involved in the cholesterol/fatty acid synthetic pathway (Supplementary Table S5B), consistent with yeast Ecd KD data (14). However, given the previously established role of Ecd in regulation of glycolytic pathway, we focussed on the insulin-dependent glucose transporter GLUT4 gene, which was one of the differentially expressed genes in Ecd KD versus Scr pancreatic cancer cells. Both quantitative real-time PCR (Fig. 4A left) as well as Western blotting confirmed that GLUT4 mRNA and protein (Fig. 4A right) were downregulated in pancreatic cancer cells upon Ecd knockdown. Similar changes in GLUT4 protein and transcript levels were observed in MiaPaCa and Capan1 Scr and Ecd KD pancreatic cancer cells (Supplementary Fig S2A). These findings although contradictory to the initial microarray data showing upregulation of the GLUT4 gene, were validated by multiple experiments and reanalysis of microarray data further revealed downregulation of critical GLUT4 transcriptional regulator GEF (GLUT4 Enhancer Factor) in Ecd KD cells. These results suggest that human ecdysoneless protein regulates GLUT4 expression in pancreatic cancer cells. GLUT4 is a facilitative glucose transporter, which resides within cytoplasmic vesicles that are translocated to the plasma membrane for glucose uptake upon insulin stimulation. Because we observed an effect of Ecd downregulation on GLUT4 level, we then investigated if Ecd regulated GLUT4 membrane translocation. Membrane fractionation of MiaPaca pancreatic cancer cells showed reduced GLUT4 protein level in the membrane fraction of Ecd KD cells as compared with the SCR cells (Supplementary Fig S2B). Further confocal microscopy of GLUT4 under basal condition and insulin stimulation in HPAF/CD18 cells showed that there is delayed transport of GLUT4-containing vesicles to the membrane upon insulin stimulation in Ecd KD cells compared with the scrambled cells (Fig 4B). Overall, these results indicate that Ecd regulates expression as well as trafficking of the glucose transporter GLUT4 in pancreatic cancer cells.
Figure 4.
Ecd regulates expression of insulin-dependent glucose transporter GLUT4. A, the graph represents quantitative real-time PCR analysis of Ecd and GLUT4 genes in RNA isolated from Ecd scr and KD cells. The Ecd KD cells show a significant decrease in the level of Ecd as well as GLUT4 mRNA compared with the Ecd scrambled cells (left); n = 3. Western blot analysis also shows decrease in the total level of GLUT4 protein in Ecd-depleted pancreatic cancer cells (right). B, distribution of GLUT4 molecule (red) in Ecd scr versus KD cells, before and 10 and 20 minutes after insulin stimulation (100 nmol/L) were studied by confocal microscopy. Ecd KD cells show delayed transport of GLUT4 to the plasma membrane on insulin stimulation compared with the scrambled cells.
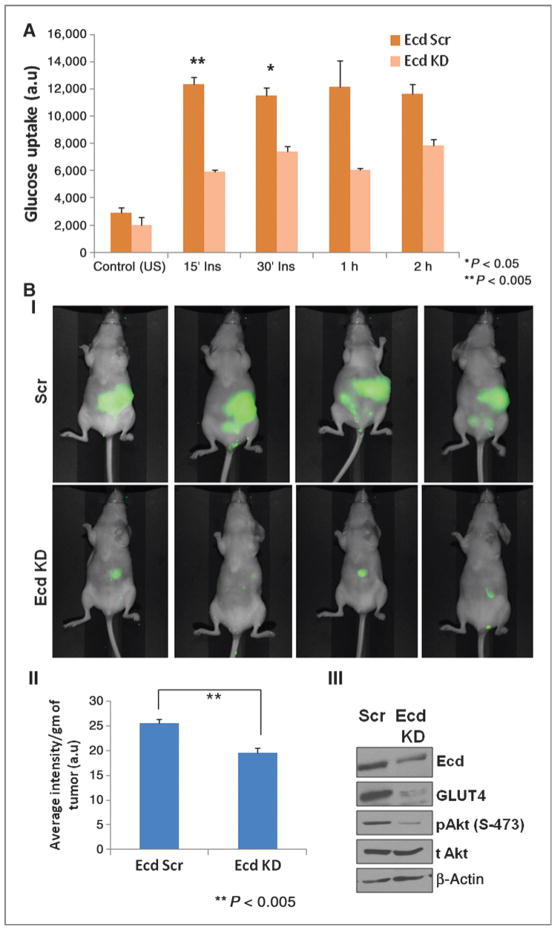
Ecd depletion leads to reduced glucose uptake in vitro and in vivo
Glucose, the chief energy metabolite of cancer cells, is mainly used by the aerobic glycolytic pathway following glucose uptake through the transmembrane glucose transporters. Because we showed that Ecd regulates expression and localization of the glucose transporter GLUT4 in pancreatic cancer cells, we then sought to investigate the effect of Ecd on glucose uptake by pancreatic cancer cells, which simultaneously also represents the rate of glycolysis within the cells. Because GLUT4 is an insulin-responsive glucose transporter, radiolabeled glucose was used to study glucose uptake in HPAF/CD18 Scr and Ecd KD cells either in the presence or absence of insulin (100 nmol/L) at different time intervals. The Ecd KD cells showed decreased level of glucose uptake compared with the scrambled cells both at basal level and upon insulin stimulation (Fig. 5A). The effect was observed to be more significant on insulin stimulation at the initial time points. We extended the study to investigate glucose uptake by the tumor mass formed from HPAF/CD18 scr and Ecd KD pancreatic cancer cells after orthotopic injection into nude mice. 20 days following implantation, IRDye 800CW 2DG was injected intraperitoneally into each mouse. After 24 hours, the mice were imaged by using PEARL IMPULSE IR imager, which showed that the glucose uptake by tumors formed in mice injected with Scr pancreatic cancer cells was significantly higher than that of the Ecd KD cells (Fig. 5B). Similar differences in glucose uptake were also obtained in mice orthotopic tumors developed from Capan1 Scr and Ecd KD pancreatic cancer cells (Supplementary Fig. S3). Comparison of protein level of Ecd and GLUT4 isolated from mice orthotopic tumors also show decreased expression in the Ecd KD cell injected group versus the Scr cells (Fig 5B III). Interestingly, decrease in level of pAkt level was also observed in the tumor lysates from Ecd KD–injected mice. Akt pathway is known to be a critical regulator of GLUT4 expression and translocation (15, 16) as well as the cancer cell glycolytic pathway (17–19). Therefore, our observation suggests a critical role of Ecd in modulation of glucose uptake and consequently glycolysis by pancreatic cancer cells potentially through regulation of GLUT4 protein and modulation of key signaling pathways.
Figure 5.
Ecd depletion affects glucose uptake by pancreatic cancer cells both in vitro and in vivo. A, the graph represents the rate of radiolabeled glucose uptake by Ecd scr and KD pancreatic cancer cells under basal condition as well as under insulin (100 nmol/L) stimulation. Ecd KD cells show a significant decrease in glucose uptake rate under insulin stimulation compared with the control cells; n = 3. B in vivo glucose uptake was measured in mice orthotopic tumors by intraperitoneal injection of IR800-labeled 2-deoxy glucose; (n = 5 mice/group). I, the images obtained through PEARL IR imager reflect glucose uptake in representative animals from Ecd scr (top) and Ecd KD (bottom) tumor-bearing groups. II, the images show markedly reduced glucose uptake in tumor mass with Ecd KD compared with the control tumors, also reflected by a significant decrease (P < 0.005) in the calculated average intensity per gram of tumor. III, The protein lysates prepared from Ecd scr and KD tumors also show reduced expression of Ecd and GLUT4 in the Ecd KD tumors.
Ecd promotes aerobic glycolysis in pancreatic cancer cells
According to the Warburg’s hypothesis, cancer cells use glucose primarily through glycolysis, whereas the mitochondrial oxidative respiration pathway is inhibited. As a result of this, the final product of glycolysis, pyruvate, is converted to lactate instead of entering the tricarboxylic acid (TCA) cycle. The results of the current study have shown that Ecd promotes pancreatic cancer cell growth as well as glucose uptake. Hence, we examined whether Ecd favors pancreatic cancer cell aerobic glycolysis by estimating lactate production. The HPAF/CD18 Ecd KD cells showed decrease in lactate production as compared with the Scr cells (Figure 6A). The chief energy quotient of the cells from any metabolic pathway including glycolysis is ATP. Quantification of ATP production by the CD18 pancreatic cancer cells showed that Ecd depletion leads to reduction in total ATP content in cells (Fig. 6B). Thus, our data shows that Ecd affects the glucose metabolism pathway in pancreatic cancer cells and that Ecd may be a key player in the energy production network.
Figure 6.

Reduced ATP and Lactate production in Ecd KD pancreatic cancer cells. A, lactate production by Ecd scr and KD pancreatic cancer cells was measured under basal and insulin-stimulated conditions. Under both the conditions, Ecd KD cells exhibited significantly reduced lactate production compared with the Ecd scr cells. B, graph represents decrease in ATP production by the Ecd KD pancreatic cancer cells with respect to the control cells. The ATP content was normalized to the protein content of the cells. Error bars represent SE for n = 3. C, schematic representation depicting the interconnected network of the possible role of Ecd in modulating glucose metabolism and cell cycle through p53, Rb and Akt pathways.
Discussion
With an incidence rate almost equal to the mortality rate, pancreatic cancer is the 4th leading cause of cancer-related deaths in the United States (1). In this report, we observe that while the nonneoplastic pancreatic ducts had a weak to no expression of Ecd, nearly 88% of ductal carcinomas have high expression of this protein. In addition, Ecd expression was higher in well and moderately differentiated pancreatic cancer compared with poorly differentiated pancreatic cancer indicating that Ecd expression was altered during the progression of invasive adenocarcinoma. This aberrant expression pattern of Ecd underlines its potential role in pancreatic cancer pathogenesis. Interestingly, upregulation of Ecd occurs as early as in the PDAC precursor lesions or PanINs, increasing gradually from the low-grade PanINs reaching the highest expression in the high-grade PanIN-3 lesions. While PanIN-1 and 2 lesions may be discovered incidentally and could represent age-related changes, PanIN-3 lesions have been shown to be at the highest risk for the development of invasive adenocarcinoma (1). Our observations suggest that Ecd immunostaining might be potentially useful for the early detection of pancreatic cancer in high-risk patients and future use of this novel molecule as an early biomarker for pancreatic cancer.
The increased Ecd expression in pancreatic ductal adenocarcinoma as compared with its minimal expression in the nonneoplastic pancreatic ducts, suggests its potential role as a growth promoting factor that facilitates the process of pancreatic cancer pathogenesis. The in vivo study with Ecd KD in pancreatic cancer cells (Fig 4) in our current report supports the above hypothesis, as it shows that Ecd knockdown reduces the tumorigenicity of pancreatic cancer cells when orthotopically injected into nude mice. Previous studies showing that knockdown of Ecd in breast cancer cells leads to decrease in cell proliferation and G1–S block in cell cycle, also corroborate our observations (8). Therefore, role of Ecd in cell cycle might define the underlying mechanism related to its potential role in pancreatic cancer initiation, development, and metastasis.
Otto Warburg had described cancer metabolism early in the year 1924, mentioning that tumor cells characteristically thrive on glycolysis rather than oxidative phosphorylation for their energy source. In this phenomenon defined as “aerobic glycolysis”, pyruvate, the end product of glycolysis is converted to lactate along with ATP generation instead of entering the TCA cycle (20, 21). In this study, we show that Ecd is a novel regulator of pancreatic cancer cell metabolism as depletion of Ecd affects the expression of glucose transporter GLUT4 and subsequently glucose uptake by the pancreatic cancer cells. Because glucose is the chief source of energy for the cancer cells, hence Ecd might be a critical player in pancreatic cancer cell survival and growth by modulating the glucose transporters. Furthermore, decrease in lactate and ATP concentration in Ecd knockdown cells reflects that energy production by the pancreatic cancer cells is compromised in the absence of Ecd. Also, key signaling pathways, like PI3K/Akt, which regulate glycolysis and cell survival (22), are affected by Ecd downregulation, indicating multidimensional involvement of this protein in controlling pancreatic cancer cell glucose metabolism. However, the mechanism of regulation needs further exploration. In an earlier study, the human Ecd gene was found to complement for the yeast gene GCR2, which is a transcriptional coactivator for glycolytic genes, indicating its role in regulation of glucose metabolism (6). Hence, it is also known as hSGT1 (human suppressor of GCR 2). Another study in yeast illustrated the role of Ecd in modulating carbohydrate and amino acid metabolic pathways (14). Consistent with yeast data, our current results indicate a conserved function of Ecd from yeast to mammals in controlling glucose metabolism, potentially by regulation of genes involved in the metabolic network through its transactivation function. Interestingly, the cell-cycle regulatory function and metabolic function of Ecd seems highly interdependent, placing it at the crossroad of these 2 fundamental cell survival pathways (Figure 6C). Earlier studies with Ecd-null MEFs show that a decrease in Ecd levels leads to G1–S block with reduced levels of cyclin E. It is important to note that cyclin E is known to serve as a critical “sensor” for any imbalance in cellular ATP/AMP ratio, ensuring G1–S block until the cellular energy pool is restored (23, 24). p53, another binding partner for Ecd, also has direct effects on metabolic fate of the cells through downstream effectors such as TIGAR (TP53-induced glycolysis and apoptosis regulator; ref. 25). Another Ecdinteracting cell cycle regulatory protein Rb (Retinoblastoma) has been shown to regulate the expression of PDK4 (Pyruvate Dehydrogenase Kinase), which inhibits PDH (Pyruvate Dehydrogenase) thereby preventing the entry of pyruvate into the TCA (Tri Carboxylic Acid) cycle, redirecting it towards lactate production (26). Therefore, Ecd emerges as a critical molecule relaying the signals between cell cycle and metabolic pathways of the cell.
In conclusion, our study suggests that Ecd, a protein highly conserved across species, is differentially expressed during the transformation of the normal exocrine pancreatic ducts into invasive ductal adenocarcinoma. The expression of Ecd in PanIN lesions suggests a potential role for Ecd as a novel early diagnostic biomarker in pancreatic cancer tissues. In addition, role of Ecd in modulating glucose metabolism pathways in pancreatic cancer cells might underlie its growth promoting potential during pancreatic cancer pathogenesis. The current results have identified Ecd as a novel protein, overexpression of which might provide the rapidly proliferating cancer cells with a growth advantage due to increased potential for aerobic glycolysis. Future studies should delineate the role of Ecd for early detection, prognosis, and/or therapy target in pancreatic adenocarcinoma.
Supplementary Material
Table S1. Ecd expression in normal pancreas and various histological types of pancreas cancer
Table S2. Variation in Ecd expression with grade and stage of Pancreatic Cancer
Table S3. Expression of Ecd in primary and metastatic pancreatic cancer
Table S4. Expression of Ecd in metastasis from Ecd positive vs. negative pancreatic tumors
Translational Relevance.
Pancreatic cancer is one of the deadliest forms of cancer owing to its cryptic nature and late clinical manifestation. In this study, we analyze the expression and function of a novel cell-cycle regulatory protein, called human ecdysoneless (Ecd), in pancreatic cancer. Our study shows that Ecd is overexpressed in preneoplastic pancreatic cancer precursor lesions as well as in primary and metastatic pancreatic cancer tissues as compared to normal pancreas. Knockdown of Ecd in pancreatic cancer cells reduces cancer cell proliferation and tumorigenicity. Functional analysis shows role of Ecd in regulating pancreatic cancer cell glucose uptake through regulation of glucose transporter GLUT4 and thereby affect lactate and ATP production. Together, our results suggest that Ecd, which is overexpressed in pancreatic cancer, plays an important role in pancreatic cancer cell growth and metabolism and hence represent a novel prognostic biomarker and therapeutic target for the disease.
Acknowledgments
The authors thank the invaluable technical support from Erik Moore and Kavita Mallya. We also thank Janice A. Tayor and James R. Talaska of the confocal laser scanning microscope core facility at the UNMC, and Lynette Smith from the department of Biostatistics for their support.
Grant Support
This research is supported by the NIH grant SPORE P50CA127297, U54163120, and UO1CA11294 (to S.K. Batra and M.A. Hollingsworth); CA96844 and CA144027, and Department of Defense grants W81XWH-07-1-0351 and W81XWH-11-1-0171 (to V. Band).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: P. Dey, S. Chakraborty, P.K. Singh, M.A. Hollingsworth, V. Band, S.K. Batra Development of methodology: P. Dey, P.K. Singh, X. Zhao, V. Band
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): P. Dey, P.K. Singh, J.M. Anderson, S. Lele
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): P. Dey, S. Chakraborty, P.K. Singh, V. Band, S.K. Batra
Writing, review, and/or revision of the manuscript: P. Dey, S. Chakraborty, P.K. Singh, C.B. Gurumurthy, S. Lele, M.A. Hollingsworth, V. Band, S.K. Batra
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): P. Dey, X. Zhao, J.M. Anderson
Study supervision: V. Band, S.K. Batra
Animal experiments like orthotopic implantation, glucose uptake, and tumorigenecity metastasis: S. Rachagani
References
- 1.Chakraborty S, Baine MJ, Sasson AR, Batra SK. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815:44–64. doi: 10.1016/j.bbcan.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Henrich VC, Livingston L, Gilbert LI. Developmental requirements for the ecdysoneless (ecd) locus in Drosophila melanogaster. Dev Genet. 1993;14:369–77. doi: 10.1002/dvg.1020140506. [DOI] [PubMed] [Google Scholar]
- 4.Henrich VC, Tucker RL, Maroni G, Gilbert LI. The ecdysoneless (ecd1ts) mutation disrupts ecdysteroid synthesis autonomously in the ring gland of Drosophila melanogaster. Dev Biol. 1987;120:50–5. doi: 10.1016/0012-1606(87)90102-3. [DOI] [PubMed] [Google Scholar]
- 5.Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131:2715–25. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Jigami Y, Suzuki T, Uemura H. A human gene, hSGT1, can substitute for GCR2, which encodes a general regulatory factor of glycolytic gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1999;260:535–40. doi: 10.1007/s004380050926. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen J, Gurumurthy CB, Kim J, Bhat I, Gao Q, et al. The human orthologue of Drosophila ecdysoneless protein interacts with p53 and regulates its function. Cancer Res. 2006;66:7167–75. doi: 10.1158/0008-5472.CAN-06-0722. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Gurumurthy CB, Naramura M, Zhang Y, Dudley AT, Doglio L, et al. Role of mammalian Ecdysoneless in cell cycle regulation. J Biol Chem. 2009;284:26402–10. doi: 10.1074/jbc.M109.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Gurumurthy CB, Band H, Band V. Biochemical characterization of human Ecdysoneless reveals a role in transcriptional regulation. Biol Chem. 2010;391:9–19. doi: 10.1515/BC.2010.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty S, Swanson BJ, Bonthu N, Batra SK. Aberrant upregulation of MUC4 mucin expression in cutaneous condyloma acuminatum and squamous cell carcinoma suggests a potential role in the diagnosis and therapy of skin diseases. J Clin Pathol. 2010;63:579–84. doi: 10.1136/jcp.2010.076125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540–7. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224–32. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kainou T, Shinzato T, Sasaki K, Mitsui Y, Giga-Hama Y, Kumagai H, et al. Spsgt1, a new essential gene of Schizosaccharomyces pombe, is involved in carbohydrate metabolism. Yeast. 2006;23:35–53. doi: 10.1002/yea.1336. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez R, Teruel T, Lorenzo M. Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett. 2001;494:225–31. doi: 10.1016/s0014-5793(01)02353-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–18. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 18.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–28. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–23. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WARBURG O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 21.WARBURG O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 22.Coloff JL, Macintyre AN, Nichols AG, Liu T, Gallo CA, Plas DR, et al. Akt-dependent glucose metabolism promotes Mcl-1 synthesis to maintain cell survival and resistance to Bcl-2 inhibition. Cancer Res. 2011;71:5204–13. doi: 10.1158/0008-5472.CAN-10-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol. 2010;11:715–27. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 24.Mandal S, Freije WA, Guptan P, Banerjee U. Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J Cell Biol. 2010;188:473–9. doi: 10.1083/jcb.200912024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh MC, Das D, Sambandam N, Zhang MQ, Nahle Z. Regulation of the PDK4 isozyme by the Rb-E2F1 complex. J Biol Chem. 2008;283:27410–7. doi: 10.1074/jbc.M802418200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Ecd expression in normal pancreas and various histological types of pancreas cancer
Table S2. Variation in Ecd expression with grade and stage of Pancreatic Cancer
Table S3. Expression of Ecd in primary and metastatic pancreatic cancer
Table S4. Expression of Ecd in metastasis from Ecd positive vs. negative pancreatic tumors