SUMMARY
The precise connectivity of inputs and outputs is critical for cerebral cortex function; however, the cellular mechanisms that establish these connections are poorly understood. Here, we show that the secreted molecule Sonic Hedgehog (Shh) is involved in synapse formation of a specific cortical circuit. Shh is expressed in layer V corticofugal projection neurons and the Shh receptor, Brother of CDO (Boc), is expressed in local and callosal projection neurons of layer II/III that synapse onto the subcortical projection neurons. Layer V neurons of mice lacking functional Shh exhibit decreased synapses. Conversely, the loss of functional Boc leads to a reduction in the strength of synaptic connections onto layer Vb, but not layer II/III, pyramidal neurons. These results demonstrate that Shh is expressed in postsynaptic target cells while Boc is expressed in a complementary population of presynaptic input neurons, and they function to guide the formation of cortical microcircuitry.
INTRODUCTION
Over the course of development, numerous molecules are repurposed to function in distinct cellular contexts (Charron and Tessier-Lavigne, 2007). During the earliest phases of neural development the Hedgehog signaling pathway plays an important role establishing patterning of the central nervous system. (Ericson et al., 1995; Roelink et al., 1995; Xu et al., 2005; Xu et al., 2010). The secreted protein Sonic Hedgehog (Shh) is expressed in the notochord and floor plate of the neural tube and, cells adopt specific fates based upon their level of exposure to the established Shh gradient. At later stages, during development of the telencephalon, Sonic Hedgehog adopts a similar function where it is expressed in the ventral telencephalon and functions to maintain ventral identity through its regulation of expression of the transcription factor Nkx2.1 (Xu et al., 2010). Shh is also expressed in adult neural stem cell niches where it helps maintain adult neural stem cell identity (Machold et al., 2003; Palma et al., 2005). Cell fate specification by Shh is regulated through the canonical Shh signaling pathway whereby binding the Shh receptor Patched (Ptc) relieves inhibition of the transmembrane protein Smoothened (Smo) (Rohatgi et al., 2007). Smoothened signaling leads to the activation of the Gli family of transcription factors, which mediates the cell fate specification functions of Shh (Ahn and Joyner, 2005; Palma et al., 2005). Later in development, after the tissues have been specified, Shh expressed from the floor plate functions to guide spinal cord commissural axons across the ventral midline (Charron et al., 2003), and Shh expressed at the chiasm functions as a regulator of retinal ganglion cell growth cone extension (Trousse et al., 2001). The Shh-dependent guidance of commissural axons in the spinal cord appears to require the Shh coreceptor Boc (Okada et al., 2006), but does not require Gli transcriptional activation (Yam et al., 2009). Shh expression has also been observed in both the juvenile and adult cerebral cortex (Charytoniuk et al., 2002) outside of known progenitor zones. Recently Shh expression has also been identified in cortical pyramidal neurons (Garcia et al., 2010). However, the function Shh in cortical neurons and the type of neurons expressing Shh remained unknown.
Here we demonstrate that Shh is expressed in specific subpopulations of corticofugal projection neurons during periods of peak cortical synaptic development, and that the cortex-specific loss of Shh function leads to cell autonomous deficits in dendritic growth and synapse formation. Furthermore, we show that the Shh receptor Boc is expressed in a complementary, but distinct subpopulation of local and callosal projection neurons, and that the loss of Boc leads to specific deficits in synapse formation onto deep-layer corticofugal projection neurons.
RESULTS
Sonic Hedgehog Is Expressed by Deep-Layer Cortical Projection Neurons
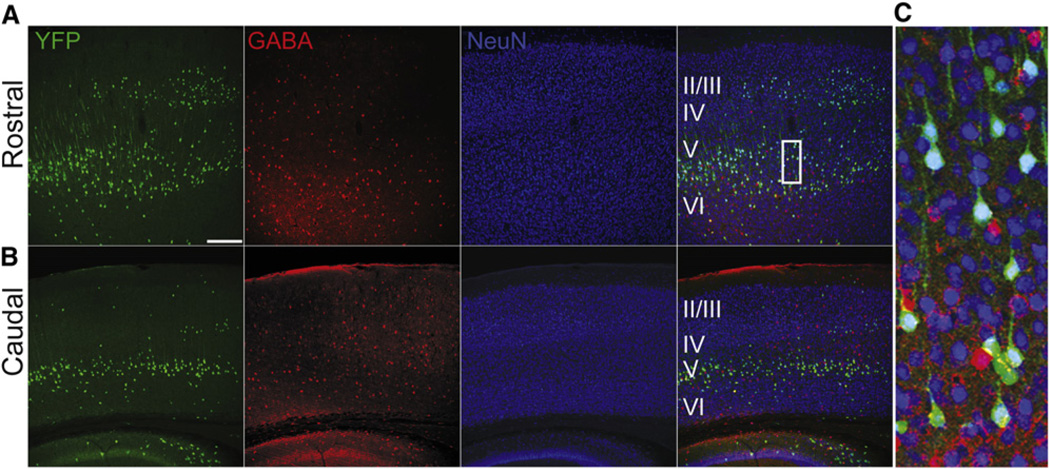
In order to characterize the spatial and cell type expression pattern of Shh in the cortex we crossed a ShhGFPCre knockin mouse (Harfe et al., 2004) to a ROSA-YFP (yellow fluorescent protein) (Srinivas et al., 2001) reporter mouse to fate map Shh-expressing neurons. We found YFP positive fate mapped cells throughout the rostrocaudal axis of the cortex, nearly all of which were positive for NeuN. The vast majority of Shh lineage cells in the cortex were GABA negative pyramidal neurons located primarily in layers III, Vb, and VI of the postnatal cortex (Figures 1A–1C). Subsequent staining for Shh in postnatal cortex also labeled a small population of glial cells (Figure S1A available online), while the majority of cells expressing Shh protein appeared to be pyramidal neurons (Figures S1B–S1D). This was surprising due to the high level of expression and large number of Shh lineage cells found in the mantle zone of the medial ganglionic eminence, the source of tangentially migrating cortical interneurons. However, this observation is consistent with previous Shh fate mapping studies from other groups (Flandin et al., 2010).
Figure 1. Sonic Hedgehog Is Expressed Primarily in Deep-Layer Cortical Pyramidal Neurons.
(A–C) Immunohistochemistry of coronal sections of adult (8 weeks) ShhCre; RYFP brains, located in both the (A) rostral and (B) caudal axis of the brain. Ninety-six percent YFP+ cells (green) are NeuN positive (blue), while only 4% of cells are also positive for GABA (red), indicating that the vast majority of Shh expressing cells in the cortex are cortical pyramidal neurons. n = 3 animals, 812 cells. Scale bars represent 250 µm (A and B).
Cortical Sonic Hedgehog Expression Is Specific to Corticofugal Projection Neurons
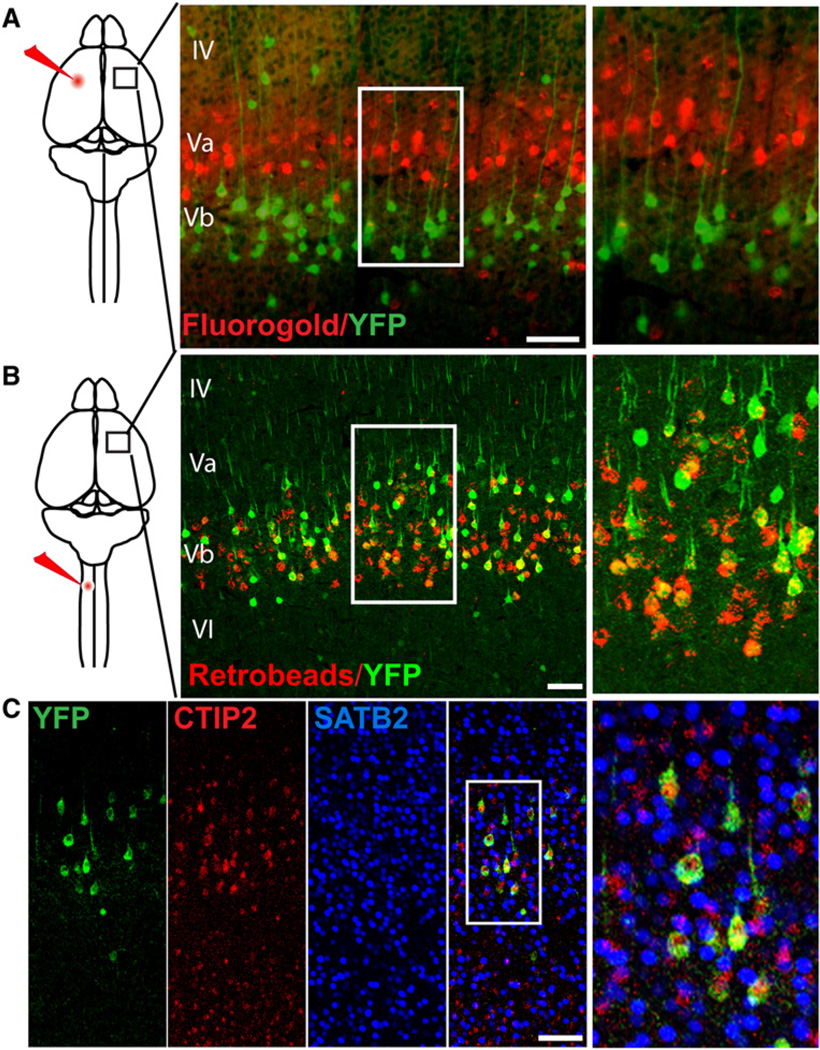
Projection neurons in the cerebral cortex can be separated into two broad classes consisting of callosal or local projection neurons that primarily reside in the more superficial layers of the cortex, and subcerebral or corticofugal projection neurons that are primarily located in the deeper layers of the cortex. Because the ShhGFPCre fate-mapped cells reside primarily in the deep cortical layers, which contain corticofugal projection neurons (Molyneaux et al., 2007), we wanted to test the possibility that Shh expression is specific to corticofugal projection neurons. To label callosal projection neurons we injected the retrograde tracer fluorogold into the somatosensory/motor area of one cortical hemisphere in ShhCre;RYFP mice (Figure 2A). We then examined the contralateral hemisphere of the injected animals and found no overlap between Shh lineage YFP positive cells and callosal neurons labeled with fluorogold. Shh lineage YFP positive neurons were spatially segregated from fluorogold labeled projection neurons within layer V, residing primarily in the Vb sublayer, while fluorogold callosal projections were found in the Va sublayer. Because of the particularly large fraction of YFP labeled neurons were observed in layer V of the motor cortex, we decided to retrogradely label corticospinal projection neurons by injecting fluorescent retrobeads into the cervical spinal cord of the ShhGFPCre;RYFP mice. We observed that 60% of retrobead labeled corticospinal neurons were YFP positive (Figure 2B). To further address whether ShhGFPCre;RYFP labeled other subcortical projection subtypes, we performed immunohistochemical labeling using CTIP2, a transcription factor expressed broadly by corticofugal projection neurons (Arlotta et al., 2005). We found that 92% of all YFP positive cells located in layer V of the cortex colabeled with CTIP2 while there was no colabeling with the transcription factor SATB2, which is expressed exclusively by callosal projection neurons in the cortex (Alcamo et al., 2008; Britanova et al., 2008) (Figure 2C). Collectively these findings suggest that Shh is expressed by a significant portion of subcortical projection neuron subtypes.
Figure 2. Sonic Hedgehog Is Expressed Exclusively in Corticofugal Projection Neurons.
(A) Schematic of fluorogold (red) injection into the cortex of ShhGFPCre; RYFP mice. A coronal section showing no overlap between fluorogold labeled callosal projections (red) and YFP+ (green) Shh lineage neurons.
(B) In contrast, when fluorescent retrobeads (red) were injected into the corticospinal tract, 60% (n = 2 animals, 612 cells) of bead positive cells were YFP+ (green).
(C) P14 ShhGFPCre; RYFP mice were immunostained for the corticofugal projection marker CTIP2 (red) and 92% (n = 2 animals, 200 cells) YFP+ cells were CTIP2+, while none of the cells were labeled with the callosal projection marker SATB2 (blue). Scale bars represent 150 µm.
Cortical Sonic Hedgehog Is Required for Normal Dendritic Growth and Spine Formation of Layer Vb Neurons
Previous studies have shown that cortical Shh expression peaks approximately at the second postnatal week of development and is downregulated and maintained at a lower expression level in the adult cortex (Charytoniuk et al., 2002). This pattern coincides with the period of peak dendritogenesis and synaptogenesis in the mouse cerebral cortex (Micheva and Beaulieu, 1996). To assess Shh function in the developing cortex, we utilized a conditional loss of function approach by specifically removing Shh from cortical pyramidal neurons without affecting patterning and specification in the early developing nervous system (Ericson et al., 1995; Roelink et al., 1995; Xu et al., 2005; Xu et al., 2010), by crossing animals with an Emx1-ires-Cre knocked into the Emx1 locus (Gorski et al., 2002) with animals carrying a conditional null allele of Shh (Dassule et al., 2000) (ShhcKO). ShhcKO mice are viable with no gross defects in the patterning or morphology of the brain. While the gross morphology of the brain is indicative of normal patterns of proliferation, we chose to investigate the possibility of a more subtle phenotype. While cortical neurogenesis is nearly complete before birth, gliogenesis continues on through postnatal development (Ivanova et al., 2003). To assess whether cortical Shh had any role in the production or survival of glial cells during early postnatal development, we administered a pulse of BrdU between postnatal day 1 to postnatal day 3 (P1–P3) and examined the number of labeled cells in both the cortex and spinal cord (Figures S2C–S2E). We observed no change in cell death or proliferation in the postnatal brain and spinal cord of ShhcKO animals. We also analyzed whether loss of cortical Shh had a cell autonomous effect on the formation or maintenance of corticospinal axonal projections and found no differences between the conditional mutants and control animals (Figure S2A), indicating that cortical Shh did not play a significant role in the maintenance or survival of neurons or glia in these regions during this window of neural development.
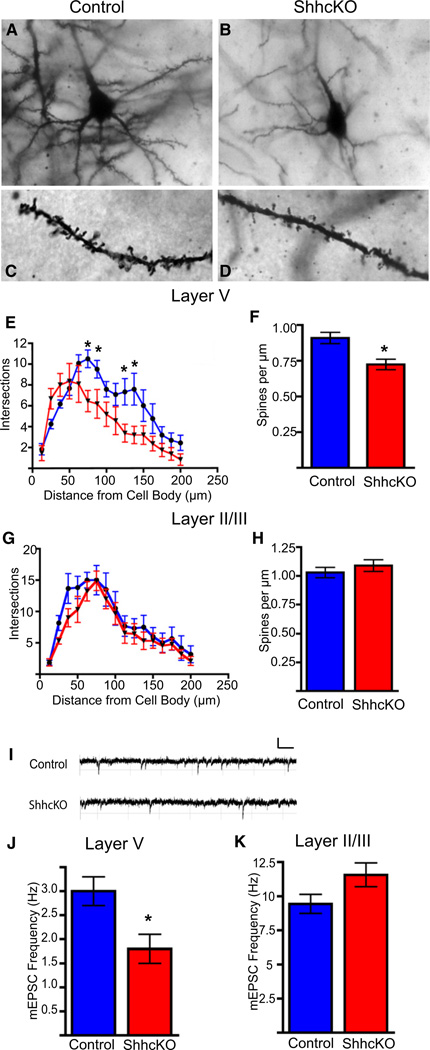
To assess the involvement of Shh in the regulation of neuronal growth and synaptogenesis, we performed Golgi analysis on P21–P28 brains of ShhcKO mice and wild-type control littermates (Figures 3A–3D). We observed significant reductions in the growth and complexity of basal dendrites of layer V neurons in the somatosensory and motor area of Shh conditional null animals (Figure 3E). We also observed a reduction in basal dendritic spine density of layer V neurons in the conditional null mutants (Figure 3F). To address whether this phenotype was specific to neurons of the deep cortical layers, we examined dendritic growth and complexity and spine density in neurons located in the superficial layers of the cortex (Figures 3G and 3H), and did not observe any significant differences between ShhcKO and control animals. To evaluate the functional consequences of these anatomical changes in Shh conditional null animals, we performed whole-cell voltage clamp recordings and examined spontaneous miniature excitatory postsynaptic currents (mEPSCs) in layer V and layer II/III pyramidal neurons in acute brain slices from P21–P28 ShhcKO mice and wild-type littermates (Figure 3I).We found that spontaneous mEPSC frequency of layer V neurons was decreased in the Shh conditional null animals, while mEPSC frequency in layer II/III (Figures 3J and 3K) was not significantly different. We also observed a 1.6-fold increase in the input resistance of conditional mutants, but no change in mEPSC amplitude between the groups, consistent with a decrease in membrane surface area likely attributable to the decrease in dendritic growth observed in the Golgi analysis (Figures S3A–S3E). Together, these results suggest a requirement for cortical Shh for proper neuron growth and cortical circuit development in vivo.
Figure 3. Sonic Hedgehog Is Required for Proper Synaptic Development of Deep-Layer Cortical Neurons In Vivo.
(A–D) Golgi stained control and ShhcKO (Emx1;Shhfl/fl) brains show decreased branching and dendritic complexity and spine density of layer V neurons in Shh conditional knockouts compared to controls.
(E) Sholl analysis of layer V neurons reveals significantly fewer intersections of dendritic processes at 75, 87.5, 125, and 137.5 µm from the cell soma.
(F) There is also significant reduction in basal dendritic spine density in the ShhcKO animals when compared to the controls (0.91 ± 0.04 versus 0.72 ± 0.04).
(G and H) There is no significant change in dendritic branching or spine density in neurons of the superficial cortical layers.
(I–K) Recordings of mEPSCs from layer V neurons reveals a significant decrease in mEPSC frequency in Shh conditional knockouts (1.8 ± 0.3 Hz) when compared to littermate controls (3.0 ± 0.3 Hz). There is no significant change in mini frequency observed in superficial layer neurons (5.34 Hz versus 5.36 Hz), n = 3 animals per group and 9 cells per group. *p < 0.01, t test.
Expression of the Sonic Hedgehog Receptor Boc Is Specific to Callosal Projection Neurons
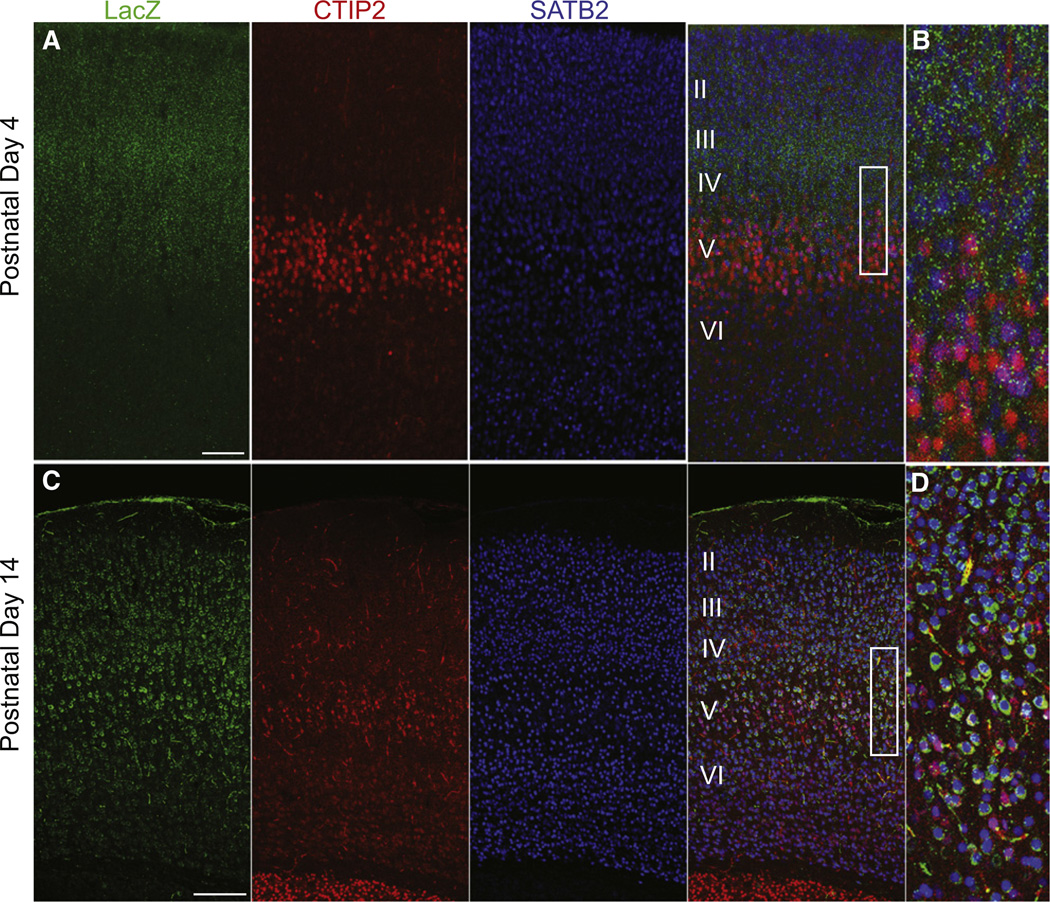
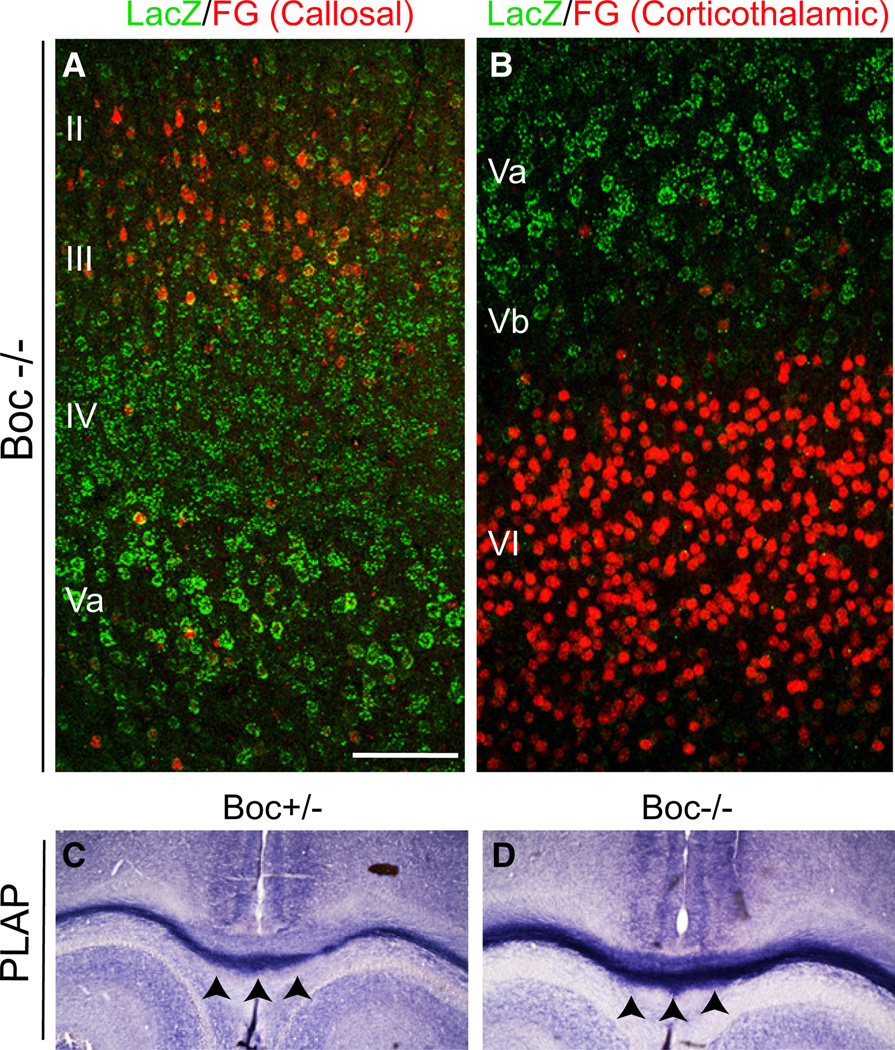
We reasoned that if cortical Shh is expressed by layer V corticofugal projection neurons, and a loss of Shh results in a reduction in the number of postsynaptic contacts, then the Shh receptor would be expressed by the presynaptic partners of those layer V neurons. A potential candidate receptor for cortical Shh function is the receptor Boc, a Robo-related Ig/fibronectin superfamily member that binds directly and with high affinity to Hedgehog family members (Kavran et al., 2010; Okada et al., 2006; Tenzen et al., 2006; Yao et al., 2006). Boc is expressed in the developing and adult nervous system and has been shown to be necessary for Shh-mediated guidance of axon projections (Connor et al., 2005; Fabre et al., 2010; Okada et al., 2006). To determine if Boc could be a candidate to mediate Shh function in cortical circuitry, we visualized Boc expression by RNA in situ hybridization and by reporter activity (Figure S4A). We used tissue harvested from a Boc heterozygous mutant mouse with the Boc locus targeted with a cassette encoding a β-galactosidase-neomycin fusion (b-geo) and human placental alkaline phosphatase (hPLAP) reporter genes using a gene trap method (Okada et al., 2006). We found that Boc is expressed strongly in the cortex in a population of neurons in layers II/III, IV, and Va in adult mice (Figure S4C), whereas a close homolog of Boc, Cdon, is not expressed in these cells (A.O., data not shown). We further examined the cell type and temporal specificity of Boc expression by performing immunofluorescent staining in P4 and P14 Boc heterozygous mutant mice. We found LacZ expression at both P4 and P14, with the level of expression markedly increased at P14 (Figures 4A and 4C). We also looked at coexpression with the neuron projection subtype markers CTIP2 and SATB2. We found that the majority of LacZ positive cells were also positive for the callosal projection subtype marker SATB2, while very few neurons colabelled with the corticofugal projection marker CTIP2. We also stained sagittal brain sections for placental alkaline phosphatase (PLAP) activity, which preferentially labels axonal projections in the Boc heterozygous mutant mice (Friedel et al., 2005; Leighton et al., 2001; Okada et al., 2006). We found that there was an absence of PLAP labeled descending axonal projections in the internal capsule, where corticofugal projections normally exit the cortex (Figure S5B). Fluorogold labeling of ipsilateral local projection neurons in layer III and Va also colocalized with LacZ positive cells (Figure S5A). Taken together these findings reveal that Boc is expressed predominantly by callosal and local projection neurons, many of which are known to form synaptic connections onto deep-layer corticofugal projection neurons (Petreanu et al., 2007; Wise and Jones, 1976).
Figure 4. The Sonic Hedgehog Receptor Boc Is Expressed Primarily by SATB2-Positive Callosal Projection Neuron Subtypes.
(A) Postnatal day 3 coronal sections from Boc+/− LacZ-ires-PLAP mice showing LacZ expression primarily in SATB2 positive neurons located in layers II, III, IV, and Va.
(B) Higher, magnification view of the box area in (A).
(C) Postnatal day 14 coronal sections from Boc+/− LacZ-ires-PLAP mice showing LacZ expression primarily in SATB2 positive neurons located in layers II, III, IV, and Va.
(D) Higher magnification of the boxed area in (C). Note the increased intensity of LacZ staining in at P14. Scale bars represent 100 µm (A); 250 µm (B).
Boc Is Not Required for Neuron Migration or Callosal Axon Guidance
Boc is known to be highly expressed in the nervous system both in embryonic and adult tissues (Mulieri et al., 2002), and has previously described roles in attracting commissural axons across the midline in the developing spinal cord (Okada et al., 2006), and in repulsion of ipsilateral retinal ganglion neurons to prevent aberrant crossing of the optic chiasm (Fabre et al., 2010). Thus Boc expression in the embryonic telencephalon, or early postnatal expression in callosal projection neurons, may regulate aspects of cortical development that precede synaptogenesis, such as neuron migration and/or long-range axon guidance across the corpus callosum. To explore other possible functions of Boc in the development of the cortex, we examined the brains of homozygous Boc mutant mice (BocKO). The brains of BocKO mice appeared grossly normal with no obvious differences in size or cortical thickness. Fluorogold labeling of callosal projection neurons in layer II/III colocalized with LacZ positive cells in the null mutant (Figure 5A). Examination of the layer Va boundary of LacZ expression with fluorogold labeling of layer VI corticothalamic projections suggested that neuronal migration and layer formation is also normal in Boc null mutants (Figure 5B). Direct examination of PLAP staining of callosal projections in heterozygous and BocKO mice also did not reveal any change in the number or pattern of callosal axons projecting across the midline (Figures 5C and 5D). Together these findings suggest that Boc function is not required for neuronal migration or cortical axon guidance.
Figure 5. Boc Is Not Required for Long-Range Callosal Neuron Axon Guidance.
(A) Fluorogold (FG) retrograde labeling of callosal projection neurons located in layers II/III in homozygous Boc mutant mice showing that axons still reach the contralateral cortex.
(B) Fluorogold retrograde labeling of corticothalamic projection neurons located primarily in cortical layer VI in homozygous Boc mutant mice, showing that the boundary of expression and maintenance of projection identity is maintained in the mutants.
(C and D) PLAP staining of callosal axon projections in (C) heterozygous and (D) homozygous Boc mutants. Black arrowheads indicate the corpus callosum. Note the darker PLAP staining in the homozygote due to the additional copy of the PLAP gene.
Boc Function Is Required for Circuit-Specific Synapse Formation
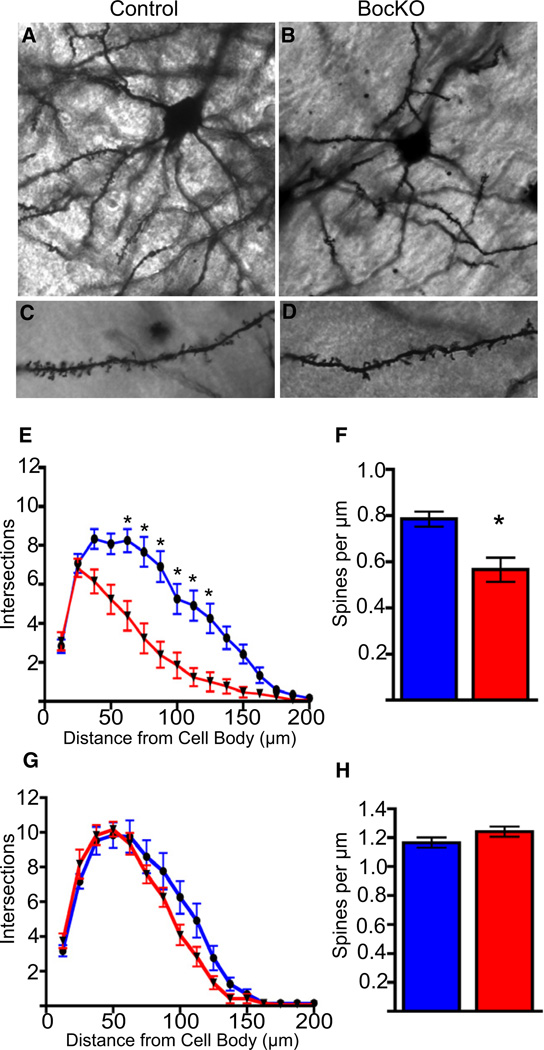
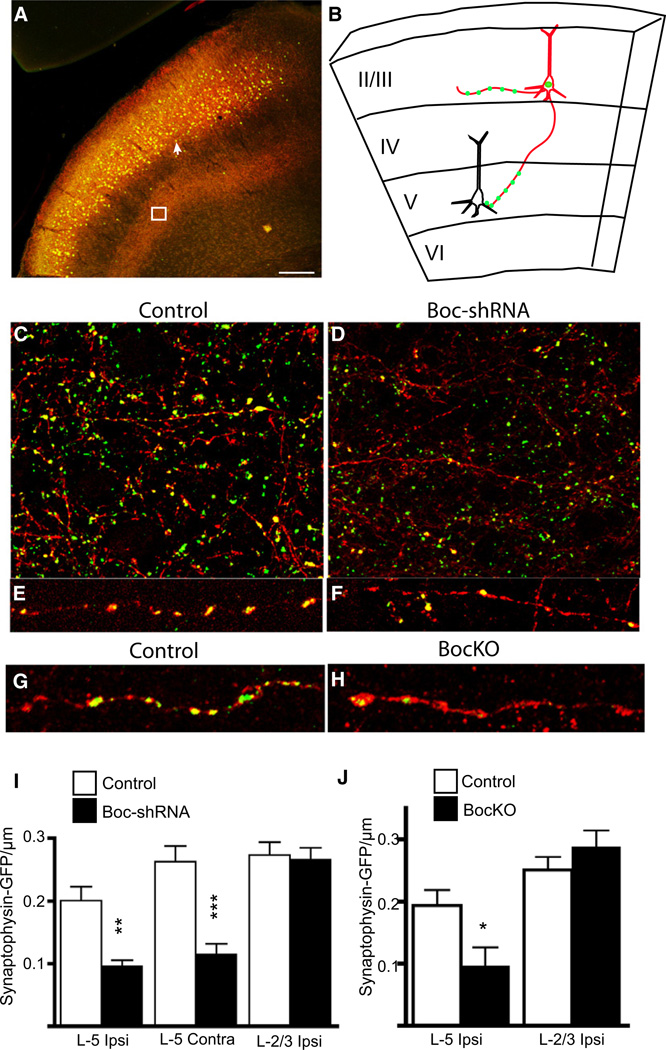
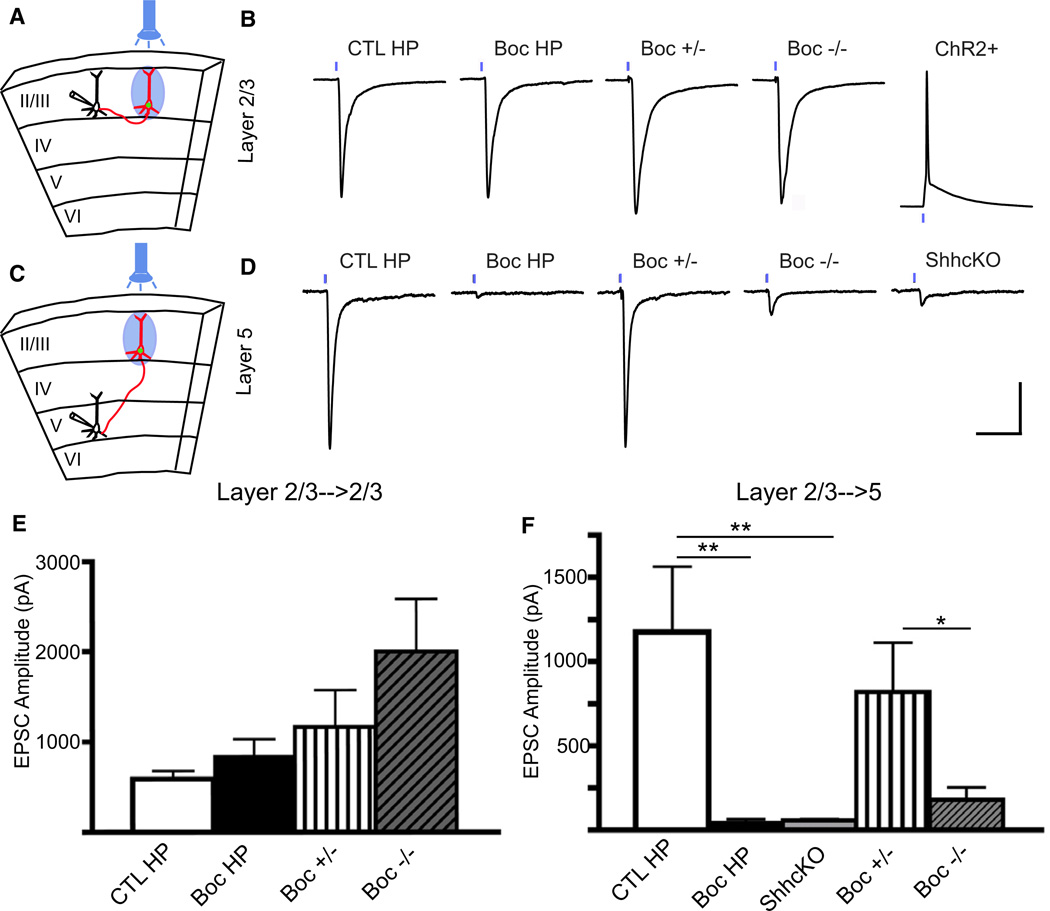
The expression of Shh and its receptor Boc by two complementary nonoverlapping populations of neurons during synaptogenesis suggests a mechanism for achieving specificity of circuitry, where the target cell expresses the ligand (Shh) and the presynaptic cell expresses the corresponding receptor (Boc) (Sanes and Yamagata, 2009; Williams et al., 2010). To examine whether the Boc mutant animals have a similar cortical phenotype to ShhcKO mutants, we performed Golgi analysis on P20 brains of BocKO mice and wild-type littermate controls (Figures 6A–6D). We observed significant reductions in spine density, and growth and complexity of basal dendrites located in layer V neurons of BocKO animals (Figures 6E and 6F), while there was no difference in branch growth and spine density in layer II/III (Figures 6G and 6H). These findings suggest that the non-cell-autonomous decrease in dendritic growth of layer V neurons may be due to a loss of synaptic activity from presynaptic Boc expressing neurons (McAllister et al., 1996). To test for the loss of presynaptic input from Boc expressing neurons we utilized in utero electroporation to introduce synaptophysin-GFP, a marker for active presynaptic terminals (Kelsch et al., 2008; Li and Murthy, 2001; Meyer and Smith, 2006; Nakata et al., 1998), into lower layer II/III cortical neurons at embryonic day 14 (E14) (Figures S6A–S6C). We targeted neurons in lower layer II/III because of the extensive number of these cells exhibiting LacZ reporter gene expression observed in the Boc gene trap reporter mice, and also because of the preference for neurons located in this layer to make synaptic connections onto layer V pyramidal neurons (Anderson et al., 2010; Petreanu et al., 2007). In addition to synaptophysin-GFP, we coelectroporated a plasmid for a pCAG-mTdTom-2A-H2BGFP plasmid (Trichas et al., 2008) that labels electroporated cells with a nuclear GFP, and a membrane TdTomato, in order to label axonal projections (Figures 7A–7C). In BocKO mice we coelectroporated the synaptophysin-GFP along with the mTdTomato axonal marker. We also coelectroporated a short hairpin RNA targeted against Boc (Boc-shRNA) into the brains of wild-type non mutant mice that should have normal levels of Boc and Shh, except for the population of electroporated cells. When we compared the density of synaptophysin-GFP puncta located on the axons of Boc-shRNA expressing versus control-shRNA expressing cells, we found a significant reduction in the density of puncta on axons located in layer V (Figures 7C–7F). Notably, this reduction was observed in both ipsilateral and contralateral layer V axons, while there was no significant difference in the puncta density in layers II/III (Figure 7J). We found a similar pattern of reduced puncta when we compared BocKO and control animals (Figure 7K). Together these findings suggest a cell autonomous requirement for Boc in the development of the proper number of layer II/III to layer V presynaptic connections, and that loss of these connections leads to a reduction in dendritic growth of the target cells. While it is presumed that the dense collection of axons located in layer V is primarily targeting projection neurons located within this layer, the target of the lost presynaptic terminals cannot be known for certain, and the reduction in synaptophysin-GFP puncta may not be specific to any particular postsynaptic partner. In order to evaluate the functional consequences of reduced Boc expression and its effect on connectivity to postsynaptic partners located in either layer II/III or layer V, we used an optogenetic approach. We coelectroporated Channelrhodopsin-2 (ChR2) (Nagel et al., 2003) into layer II/III cortical neurons, thus allowing us to photoactivate the cells and identify their postsynaptic target cells (Petreanu et al., 2007) (Figures 8A and 8C). We examined the strength of layer II/III to layer V connectivity by flashing the cells with blue light, causing the ChR2-electroporated neurons to fire action potentials. We found a significant reduction in the excitatory postsynaptic current (EPSC) amplitude in layer V neurons in which functional Boc had been disrupted when compared to controls (Figures 8E and 8F). We also tested for a similar deficit in Shh conditional knockouts and found an 8-fold reduction in EPSC amplitude, suggesting that loss of either ligand in the target cell or receptor from the input neuron yields similar deficits in layer V connectivity (Figure 8F). In contrast, when we compared the strength of layer II/III to layer II/III connections, we did not find a significant difference in EPSC amplitude between conditions where Boc function is perturbed and control conditions (Figure 8E). Taken together, these findings suggest that the reduction in presynaptic terminal formation observed when Boc function is disrupted is specific to layer II/III to layer V cortical circuits, while the strength of connectivity of layer II/III to layer II/III cells remains unchanged.
Figure 6. Loss of Boc Function Reduces Dendritic Growth and Spine Density of Deep-Layer Cortical Neurons In Vivo.
(A–D) P21 Golgi stained control and BocKO brains show that there is decreased branching, dendritic complexity, and spine density of layer V neurons when compared to control animals.
(E) Sholl analysis of layer V neurons reveals significantly fewer intersections of dendritic processes between 72 to 120 µm from the cell soma.
(F) There is also a significant reduction in the density of basal dendritic spines of layer V neurons (0.78 ± 0.03 versus 0.57 ± 0.05).
(G and H) Sholl analysis and spine counts in layer II/III did not reveal significant differences between groups.
Figure 7. Layer II/III Boc Knockdown Leads to Loss of Synaptophysin Puncta in Layer V.
(A) Coronal section of a P28 brain coelectroporated at E14 with a control or BocshRNA vector, pCAG-H2B-GFP-2A-myr-Tdtomato and a synaptophysin-GFP plasmid. The white arrow indicates H2B-GFP nuclei of electroporated cells while membrane Tdtomato/Synaptophysin-GFP labeled axons concentrated in layer V are highlighted inside the white box.
(B) Schematic of an electroporated cell with red axons in layers II/III and V, and dots of synaptophysin-GFP puncta along the axon.
(C–F) Images of axons (red) and synaptophysin-GFP puncta (green) in layer V of control and Boc-shRNA electroporated cells.
(G and H) Images of layer V axons from P14 control and BocKO animals.
(I) There is a significant reduction in the density of GFP puncta from axons located in both ipsilateral and contralateral layer V (0.1 ± 0.01 and 0.11 ± 0.02, respectively, n = 80 axon segments) when compared with control hairpin electroporated (0.2 ± 0.02 and 0.26 ± 0.03 ±, n = 63 axon segments) cells. There is no significant difference in density of axons located in layer II/III (0.27 ± 0.02 and 0.26 ± 0.02, n = 37 and n = 29 axon segments).
(J) There is a similar pattern of reduced synaptophysin-GFP puncta in layer V of BocKO animals when compared with controls (0.10 ± 0.03 and 0.19 ± 0.02, n = 15 axon segments for each group), while layer II/III axons were not significantly different (0.29 ± 0.03 and 0.25 ± 0.02, n = 15 axon segments for each group). *p < 0.05, **p < 0.01, ***p < 0.001, t test. Scale bars represent 200 µm.
Figure 8. Boc Is Required for Proper Layer III to V Cortical Circuit Formation.
(A) Schematic of coelectroporation of hairpin plasmids with a channelrhodopsin-2 (ChR2) vector where (red) electroporated cells in layer II/III are activated with blue light while responses of (black) unelectroporated cells in layer II/III are recorded.
(B) Voltage clamp recordings of the responses of unelectroporated cells located in layers II/III, and a recording from ChR2 electroporated cells showing normal spiking when flashed with blue light.
(C) Schematic of coelectroporation of hairpin plasmids with a channelrhodopsin-2 (ChR2) vector where (red) electroporated cells in layer II/III are activated with blue light while responses of (black) unelectroporated cells in layer V are recorded.
(D) Voltage clamp recordings of the responses of unelectroporated cells located in layer V.
(E) There is no significant change in the response amplitude of layer II/III neurons of control (587.7 ± 90.1 pA) and Boc-shRNA (852.2 ± 180.4 pA) or BocHet (1,165.7 ± 412.8 pA) and BocKO (2,002.2 ± 585.3 pA). n = 4 animals, and 4–10 cells per group (see Experimental Procedures for more details). **p < 0.01, *p < 0.05 one-way ANOVA. (CTL HP, ShhcKO, BocHP) unpaired t test (BocKO and BocHet) Scale bars represent 200 pA and 40 ms.
(F) EPSC amplitude is significantly reduced in layer V cells of Boc-shRNA, BocKO, and ShhcKO (51.3 ± 14.3 and 180.4 ± 73.0 pA and 58.3 ± 6.5.0 pA, respectively) when compared to the control condition (1,176.0 ± 386.0 pA) or Boc heterozygous animals (820 ± 291.0 pA). Scale bars represent 100 pA and 40 ms.
DISCUSSION
Using a combination of genetics, anatomy, and electrophysiology, we have shown that Shh and its receptor Boc are required for the formation of specific cortical microcircuits. A common theme in developmental biology is the multitude of cellular functions a signaling molecule will regulate depending upon the cellular context in which it is expressed. Shh is most well known and best characterized for its functions in regulating unspecified populations of stem and progenitor cells to regulate the fate of their progeny. However, as development proceeds, so too does the context of expression, where Shh function shifts from specifying the cell types within the spinal cord to guiding axons to their targets. Our finding that Shh expression is specific to a particular cortical projection neuron subtype, during a period when cell fates have been specified and long-range axonal projections have been made, indicates that Shh serves a role in cortical circuit development. Additionally, we find that the Shh receptor Boc is expressed exclusively in a complementary nonoverlapping population of callosal and local projection neurons in the cortex that are known to preferentially form connections onto deep-layer subcortical projection neurons. This pattern of expression where Shh is expressed by layer V corticofugal “target” neurons, and Boc is expressed by layer II/III callosal inputs is consistent with the model of known connection preferences in cortical microcircuitry (Figure 9). While the peak expression of both Boc and Shh appears to coincide with peak periods of cortical synaptogenesis, both genes continue to be expressed in the cortex through adulthood. It remains possible that in addition to its role in the initial formation of cortical circuits, Shh function may continue to play an important role in the adult brain in regulating synaptic plasticity of these circuits.
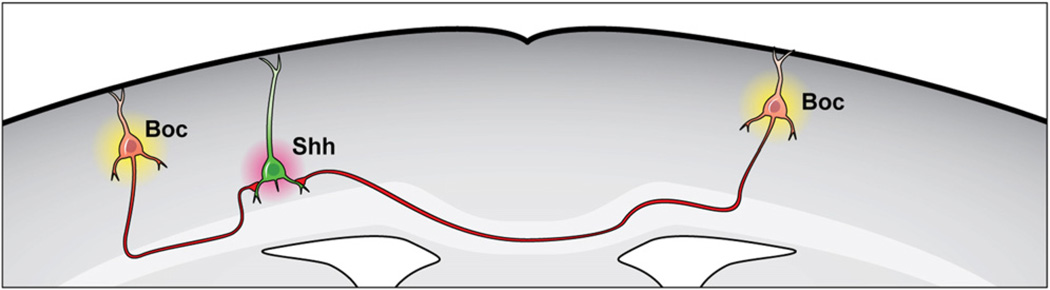
Figure 9. Complementary Expression of Sonic Hedgehog and Boc Directs Synapse Formation.
Sonic Hedgehog expression by corticofugal neurons directs cortical circuit formation with local/callosal Boc expressing cells. Model illustrates the cell type-specific expression of the ligand Sonic Hedgehog and its receptor Boc into distinct nonoverlapping populations of projection neurons. Sonic Hedgehog is expressed by corticofugal neurons, while Boc is expressed by local and callosal projection neurons. Sonic Hedgehog released by corticofugal neurons functions to guide the formation of cortical microcircuitry with Boc expressing neurons.
Previous studies of Shh function have largely focused on its regulation through the canonical Hh pathway in which Shh binds to Patched and disinhibits Smoothened to promote activation of Gli family transcription factors. Many studies use Gli activation as a measure of Shh activity within target tissues. However, recent work has shown that Shh function during axon guidance is mediated through a noncanonical pathway that requires the Boc-dependent activation of Src family kinase members, and may not require Gli family transcription (Yam et al., 2009). Considering that Gli1 activation is not found in cortical neurons (Garcia et al., 2010), a similar pathway involving Boc receptor mediated activation of Src family kinases could be responsible for Shh function during cortical circuit development.
While Gli activity is not found in postnatal cortical neurons, recent work has shown that Gli1 activation is found in cortical astrocytes. In light of our finding of a population of Shh expressing glial cells in the cortex, this raises an additional intriguing possibility that Shh could be signaling to two different cell populations through two distinct signaling pathways. Astrocytes appear to have numerous roles in maintaining normal brain function, including roles regulating synapse formation and even synaptic plasticity (Eroglu and Barres, 2010). Thus Shh expression could provide a mechanism for coordinating the formation of specific circuits by differentially regulating the activities of both neurons and astrocytes. Neurons could be regulated through the noncanonical Src family kinase-dependent Shh pathway, and astrocytes through the canonical Gli-dependent pathway.
Shh is most well known for its role in the patterning of the nervous system, and mutations in the human Shh gene are known to cause holoprosencephaly. However clinical studies have shown that patients with Shh mutations present with a wide spectrum of intrafamilial phenotypic variability, with clinical signs ranging from the severe classic holoprosencephaly to milder learning disabilities and delays in speech acquisition (Hehr et al., 2004). Perhaps Shh function is critical not only for the development of ventral structures in these patients, but it may also have roles in human cortical circuit formation as well. It will be interesting to determine if changes in behavior or learning and memory result as a consequence of the loss of Shh or Boc in the cerebral cortex of mutant mice.
Precision and specificity of synaptic connectivity is essential for normal brain function. In the cerebral cortex axons must traverse both short and long distances in order to make connections with their proper synaptic partners. Identifying the molecular mediators of synaptic specificity will be key to understanding mechanisms regulating the construction of cortical circuits. Here we have shown that the secreted protein, Sonic Hedgehog, functioning through its receptor Boc, has a role in conferring synaptic specificity to neurons that are part of a stereotypical cortical microcircuit.
EXPERIMENTAL PROCEDURES
Golgi Staining
Freshly dissected P21 and 8-week-old mouse brains were incubated in the dark in Golgi solution A+B (FD Rapid Golgi Stain Kit, PK401, FD NeuroTechnologies) for 2–3 weeks. After incubation, all brains were washed thoroughly with Solution C for 2 days at room temperature, and then mouse brains were blocked and embedded in OCT embedding medium (Tissue-Tek). Coronal sections (150 µm) through the medial somatosensory cortex were cut with a Leica CM3050 cryostat and mounted on 3% gelatin-coated slides. Staining procedures were followed as described (FD NeuroTechnologies), and slides were dehydrated in ethanol and mounted with Permount (Fisher Scientific) for microscopy. Pyramidal neurons from layers II, III, and V of the primary motor and medial somatosensory cortex were used for analysis.
In Utero Electroporations
Animals were maintained according to protocols approved by the Institutional Animal Care and Use Committee at UCSF. Plasmids were introduced into the developing cortex in vivo by intraventricular injection and electroporation. Intraventricular injections were carried out in E14 timed-pregnant mice, where the morning of the plug is designated embryonic day 1 (E1). Electroporations were performed using an Electro Square Porator ECM830 (Genetronics) (5 pulses, 50V, 100 ms, 1 s interval).DNAwas prepared in endotoxin-free conditions and 1 µl was injected per brain at the following ratios 3:1:1.5:0.5 (3 Boc/ control-shRNA: 1 CAG-H2BGFP-2A-MyrTdTomato: 1 AAV-CAG-DIO-Synaptophysin-GFP; 0.5 CAG-Cre), 2:1:1 (2Boc/control-shRNA: 1 CAG-H2BGFP-2A-MyrTdTomato: 1 CAG-ChR2). Boc hairpin RNA vectors were purchased from Open Biosystems (SM2446B12, SM2438G6).
Spine Density
Spine density (spines per µm) along Golgi-stained neurons in P21 and adult brains were viewed in coronal sections of pyramidal neurons in layer II/III and V on the basal dendrite between 100–200 µm from the soma. Three control animals and four conditional knockouts were used with 25 layer V neurons and 25 layer II/III neurons for each genotype. All counts were performed blinded to the genotype of the animals
Sholl Analysis
Golgi impregnated neurons were visualized in brightfield with a 403 objective and traced using Neurolucida software (MBF Bioscience). Neuronal tracings were subjected to Sholl analysis using Neuroexplorer software. The center of all concentric circles is defined as the center of the soma. The starting radius was 12.5 µm, and the ending radius was 200 µm from the center with an interval of 12.5 µm between radii.
Immunohistochemistry and Imaging
Mice were perfused with 4% PFA and postfixed overnight and vibratome sectioned at 70–100 µm. Sections were permeabilized and blocked for 2 hr in PBS plus 0.1% Triton X-100, 10% serum, 0.2% gelatin. Sections were incubated 48 hr in primary antibodies: chicken anti-GFP (1:500, Aves Labs), rabbit anti-GABA (1:2,000, Sigma A2052,), rat anti-CTIP2 (1:500, Abcam ab18465), rabbit anti-SATB2 (1:1,000, Abcam ab34735), mouse anti-NeuN (1:500, Millipore MAB377), rabbit anti-RFP (1:500, MBL PM005), and rabbit anti-Shh (1:200, a kind gift from S. Scales, Genentech).
Image Analysis
Images were acquired using a Leica SP5 laser scanning confocal microscope. For synaptophysin-GFP puncta counts images were analyzed using Imaris (Bitplane). Only axon segments with a minimum length of 20 µm and a least one GFP puncta were included in the analysis. All counts were performed blind to the treatment.
Retrograde Labeling
Corticospinal projections were retrogradely labeled with red fluorescent microspheres (Lumafluor) injected into the spinal cord at the C2–C3 level at P21–P28. Callosal projections were labeled by injecting fluorgold (Fluorochrome, LLC) into the contralateral sensorimotor cortex. Brains were collected for processing 24–48 hr after injections.
Electrophysiology
Mutant mice and their wild-type littermates ages P21–P28 were anesthetized with Avertin and decapitated. Brains were quickly dissected in ice-cold “sucrose-ACSF” buffer containing 252 mM sucrose, 126 mM NaCL, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 26 mM NaHCO3, and 10 mM D-glucose. Brains were vibratome sectioned in the same solution at 300 µm and transferred to ACSF without sucrose. Slices were recovered at 35°C for 30 min and then maintained at room temperature. Neurons were targeted for whole-cell patch clamp recording with borosilicate glass electrodes having a resistance of 2–6 MΩ. The electrode internal solutions was composed of 130 mM potassium gluconate, 10 mM KCl, 10 mM HEPES, 1 mM MgCl2, 16 mM sucrose, 5 mM EGTA, 4 mM Na2ATP, and 1 mM NaGTP, titrated to pH 7.3 with KOH for recording mEPSCs. Channelrhodopsin recordings were done with an internal solution composed of (in mM) 120 CsMeSO3, 15 CsCl, 8 NaCl, 0.5 EGTA, 10 HEPES, 5 QX-314, 10 TEA-Cl, 2 Mg-ATP, 0.3 Na-GTP; pH adjusted to 7.3 with CsOH, 290 mOsm. During collection of miniature EPSCs external solution was supplemented with 1 µM tetrodotoxin and 10 µM bicuculline. Data were collected with a MultiClamp 700B amplifier (Molecular Devices) and ITC-18 A/D board (HEKA) using Igor Pro software (Wavemetrics) and custom acquisition routines (mafPC, courtesy of M.A. Xu-Friedman). Current-clamp recordings were filtered at 10 kHz and digitized at 40 kHz; voltage-clamp recordings were filtered at 2 kHz and digitized at 10 kHz. Whole-cell recordings were performed in layers II, III, and V of somatosensory cortex. The number of cells recorded for each condition was as follows: Ctl-hp, n = 8 L2/3, 8 L5; Boc-hp, n = 9 L2/3, 8 L5; ShhcKO, n = 4 L5; BocHet, n = 7 L2/3, 10 L5; and BocKO, n = 8 L2/3, 8 L5 cells. Using an LED system (Thorlabs) mounted onto the microscope (Olympus BX51WI), 1 ms 470 nm light pulses were delivered full-field through the microscope objective. Data were analyzed in Igor Pro.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Kriegstein lab for critical reading of the manuscript. We thank A. Alvarez-Buylla, D. Rowitch, S. Anderson, M.E. Ross and Kriegstein lab members for ideas arising from numerous critical discussions. We thank P. O’Hara for training and access to Neurolucida and Neuroexplorer software. J. Agudelo and B. Wang for assistance with histology, and H.H. Tsai, S. Fancy for technical advice. UCSF Pediatric Neuropathology Research Laboratory for additional access to Neurolucida and Neuroexplorer software. We thank S. Srinivas for the CAG2A plasmid and L. Wilbrecht for the CAG-ChR2 plasmids. We thank J.S. Espinosa, M. Stryker and S. Arber for the kind gift of the DIO-Synaptophysin-GFP. This work was funded by grants from the National Institute of Health (5P01NS048120) and (2R37N5035710). C.C.H. was supported by a UNCF-Merck Postdoctoral Fellowship, and a UC Presidents Postdoctoral Fellowship.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and can be found with this article online at doi:10.1016/j.neuron.2012.02.009.
REFERENCES
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, Shepherd GMG. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat. Neurosci. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. The Hedgehog, TGF-beta/BMP and Wnt families of morphogens in axon guidance. Adv. Exp. Med. Biol. 2007;621:116–133. doi: 10.1007/978-0-387-76715-4_9. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Charytoniuk D, Porcel B, Rodríguez Gomez J, Faure H, Ruat M, Traiffort E. Sonic Hedgehog signalling in the developing and adult brain. J. Physiol. Paris. 2002;96:9–16. doi: 10.1016/s0928-4257(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Connor RM, Allen CL, Devine CA, Claxton C, Key B. BOC, brother of CDO, is a dorsoventral axon-guidance molecule in the embryonic vertebrate brain. J. Comp. Neurol. 2005;485:32–42. doi: 10.1002/cne.20503. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre PJ, Shimogori T, Charron F. Segregation of ipsilateral retinal ganglion cell axons at the optic chiasm requires the Shh receptor Boc. J. Neurosci. 2010;30:266–275. doi: 10.1523/JNEUROSCI.3778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JLR. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel RH, Plump A, Lu X, Spilker K, Jolicoeur C, Wong K, Venkatesh TR, Yaron A, Hynes M, Chen B, et al. Gene targeting using a promoterless gene trap vector (“targeted trapping”) is an efficient method to mutate a large fraction of genes. Proc. Natl. Acad. Sci. USA. 2005;102:13188–13193. doi: 10.1073/pnas.0505474102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ADR, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J. Neurosci. 2010;30:13597–13608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JLR, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hehr U, Gross C, Diebold U, Wahl D, Beudt U, Heidemann P, Hehr A, Mueller D. Wide phenotypic variability in families with holoprosencephaly and a sonic hedgehog mutation. Eur. J. Pediatr. 2004;163:347–352. doi: 10.1007/s00431-004-1459-0. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Nakahira E, Kagawa T, Oba A, Wada T, Takebayashi H, Spassky N, Levine J, Zalc B, Ikenaka K. Evidence for a second wave of oligodendrogenesis in the postnatal cerebral cortex of the mouse. J. Neurosci. Res. 2003;73:581–592. doi: 10.1002/jnr.10717. [DOI] [PubMed] [Google Scholar]
- Kavran JM, Ward MD, Oladosu OO, Mulepati S, Leahy DJ. All mammalian Hedgehog proteins interact with cell adhesion molecule, down-regulated by oncogenes (CDO) and brother of CDO (BOC) in a conserved manner. J. Biol. Chem. 2010;285:24584–24590. doi: 10.1074/jbc.M110.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Lin C-W, Lois C. Sequential development of synapses in dendritic domains during adult neurogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:16803–16808. doi: 10.1073/pnas.0807970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Li Z, Murthy VN. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J. Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mulieri PJ, Kang J-S, Sassoon DA, Krauss RS. Expression of the boc gene during murine embryogenesis. Dev. Dyn. 2002;223:379–388. doi: 10.1002/dvdy.10063. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J. Cell Biol. 1998;140:659–674. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang J-S, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev. Cell. 2006;10:647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousse F, Martí E, Gruss P, Torres M, Bovolenta P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development. 2001;128:3927–3936. doi: 10.1242/dev.128.20.3927. [DOI] [PubMed] [Google Scholar]
- Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J. Comp. Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.