Abstract
Background
MA17 showed improved outcomes in postmenopausal women given extended letrozole (LET) after completing 5 years of adjuvant tamoxifen.
Patients and methods
Exploratory subgroup analyses of disease-free survival (DFS), distant DFS (DDFS), overall survival (OS), toxic effects and quality of life (QOL) in MA17 were performed based on menopausal status at breast cancer diagnosis.
Results
At diagnosis, 877 women were premenopausal and 4289 were postmenopausal. Extended LET was significantly better than placebo (PLAC) in DFS for premenopausal [hazard ratio (HR) = 0.26, 95% confidence interval (CI) 0.13–0.55; P = 0.0003] and postmenopausal women (HR = 0.67; 95% CI 0.51–0.89; P = 0.006), with greater DFS benefit in those premenopausal (interaction P = 0.03). In adjusted post-unblinding analysis, those who switched from PLAC to LET improved DDFS in premenopausal (HR = 0.15; 95% CI 0.03–0.79; P = 0.02) and postmenopausal women (HR = 0.45; 95% CI 0.22–0.94; P = 0.03).
Conclusions
Extended LET after 5 years of tamoxifen was effective in pre- and postmenopausal women at diagnosis, and significantly better in those premenopausal. Women premenopausal at diagnosis should be considered for extended adjuvant therapy with LET if menopausal after completing tamoxifen.
Keywords: adjuvant therapy, aromatase inhibitors, breast cancer, extended therapy, letrozole, menopausal status
introduction
Ovarian function suppression, tamoxifen, and aromatase inhibitors (AI) antagonize estrogen in hormone-dependent breast cancer [1]. Adjuvant therapy for women postmenopausal at diagnosis with ER+ tumors includes 5 years of tamoxifen or AI, or tamoxifen for 2–3 years followed by 2–3 years of AI, or 5 years of tamoxifen followed by 5 years of AI [2]. In women premenopausal at diagnosis, standard adjuvant therapy is 5 years of tamoxifen which decreases the risk of recurrence and improves survival [3, 4]. Longer durations afford no additional benefit [3, 5, 6].
MA17 is one of few adjuvant AI trials that included women who were premenopausal at initial diagnosis [7]. The NCIC Clinical Trials Group (NCIC CTG) MA17 trial randomized 5187 women who were within 3 months of completing 4.5–6 years of tamoxifen and postmenopausal after tamoxifen, to receive letrozole (LET) or placebo (PLAC) [8]. At median follow-up of 2.5 years on extended LET, disease-free survival (DFS) was improved [hazard ratio (HR) 0.58, 95% confidence interval (CI) 0.45–0.76); P < 0.001] as was survival in node-positive patients (N = 2594) in the initial analysis (HR 0.61, 95% CI 0.38–0.98; P = 0.04), and a more recent analysis showed OS advantage in the overall study population [9, 10]. Analysis after unblinding and allowing late cross-over from PLAC to LET improved DFS (adjusted HR 0.37; 95% CI 0.23–0.61; P < 0.0001) and distant DFS (DDFS; HR 0.39; 95% CI 0.20–0.74; P = 0.004) at median follow-up of 5.3 years from randomization on MA17 [11]. Despite 66% cross-over to LET after unblinding, an intent-to-treat analysis of women originally assigned to LET in MA17 showed improvement in DFS (HR 0.68, 95% CI 0.55–0.83, P = 0.0001) in both node-positive and -negative patients, as well in the risk of contralateral breast cancer (CLBC; HR 0.61, 95% CI 0.39–0.97, P = 0.033) [12].
All women on MA17 were postmenopausal at randomization after completing tamoxifen, and although the majority had also been postmenopausal at the time of primary diagnosis, a minority had been premenopausal. In light of known benefit of adjuvant ovarian function suppression in premenopausal endocrine receptor-positive disease [1, 13], we hypothesized differences in outcomes between women on MA17 who were premenopausal at original diagnosis and became postmenopausal by the time they completed 5 years of tamoxifen, versus those who had been postmenopausal from the beginning. Here, we report analyses from the MA17 trial based on menopausal status at the time of first diagnosis. In addition to our primary analysis, we report outcomes among women who at unblinding (at times 2–7 years after tamoxifen) were found to be on placebo and were offered the choice of taking letrozole or remaining on observation.
methods
study design
Details of the design and inclusion criteria for MA17 have been reported previously [8, 9]. In brief, MA17 was a phase III, randomized, double-blinded, PLAC-controlled trial investigating extended LET in women with ER+ breast cancer who were postmenopausal after 5 years of initial tamoxifen. Patients were randomized to LET 2.5 mg or PLAC daily for 5 years stratifying for hormone receptor status (positive or unknown), lymph node involvement (positive, negative, or unknown), and prior chemotherapy (yes or no). All patients were defined as postmenopausal after tamoxifen and at the time of randomization by the following criteria: ≥50 years at the start of prior tamoxifen therapy; <50 years but postmenopausal at the initiation of tamoxifen therapy; <50 years at the start of tamoxifen but had undergone bilateral oophorectomy; premenopausal and younger than 50 years at the start of tamoxifen therapy but became amenorrhoeic during chemotherapy or treatment with tamoxifen and had postmenopausal levels of luteinizing hormone (LH) or follicle-stimulating hormone (FSH) (the minimum period of amenorrhea was ≥1 year). After reaching the primary end point, MA17 was unblinded in October 2003 after the first interim analysis demonstrated a statistically highly significant improvement in DFS and an overall survival trend in patients receiving LET compared with those receiving PLAC [8, 9]. At unblinding, participants receiving PLAC were offered a choice of LET for a planned period of 5 years.
All women randomized on MA17 continued follow-up annually until death. Two databases were available for analyses: one comprising data observed until unblinding October 2003 and one data up to July 2006. The median follow-up for all patients in these two databases was 2.5 and 5.3 years with 4923 (95%) and 2383 (97%) women censored for DFS outcome, respectively. For this analysis, all women randomized to MA17 were divided into two groups: (i) premenopausal (<50 years of age with menses but underwent bilateral oophorectomy at time when tamoxifen treatment started, or <50 years of age with menses but became permanently amenorrhoeic (≥12 months) from systemic chemotherapy, or on tamoxifen and (ii) postmenopausal (≥50 years of age without menses at diagnosis or, <50 years of age without menses and considered postmenopausal at diagnosis, or considered postmenopausal by the virtue of menopausal LH/FSH). Four patients missing menopausal status data were excluded. Details of quality of life (QOL) measures were previously published [14].
statistical analysis
As in MA17, DFS was defined as the time from randomization to the time of disease recurrence (in the breast, chest wall, nodal or metastatic sites), or development of new CLBC. Deaths without recurrence, or without CLBC, or secondary malignancy were not considered events. DDFS was defined as time from randomization to time of distance recurrence. OS was defined from randomization to death from any cause [8]. Because of potential imbalances in baseline characteristics between women in the pre- and postmenopausal groups, the chi-square test was used to perform univariate analyses for the association between menopausal status and baseline characteristics, and the logistic regression model was used to perform multivariate analyses to identify independent baseline characteristics associated with menopausal status. For time-to-event end points observed before unblinding, the hazard ratios and associated 95% CI's and P values between menopausal or treatment groups were obtained from a Cox regression model adjusting for other potential prognostic factors including LET or PLAC treatment, duration of prior tamoxifen, nodal status, and prior chemotherapy. In the analyses of outcomes after unblinding, patients who recurred or died before unblinding were excluded from the analyses. In these analyses, hazard ratios of women randomized originally to PLAC but who switched to LET after unblinding (PLAC–LET) were compared with those for women who remained on observation (PLAC–PLAC), and the associated 95% CI's and P values were obtained from the Cox model, adjusting for ethnicity (white versus nonwhite), age (<70 versus ≥70 years), performance status (0 versus 1 or 2), time from initial diagnosis to random assignment (<5 versus ≥5 years), pathologic N stage (0 versus other), hormone receptor status (positive versus others), prior chemotherapy (yes versus no), and axillary node dissection (yes versus no), all of which were found to be significantly associated with the choice to switch to LET or not. The Wilcoxon test was used to compare change scores of QOL between the groups from baseline to 24 months after initial randomization.
results
patient population
Baseline patient and disease characteristics are shown in Table 1. About 877 patients were identified as premenopausal and 4289 as postmenopausal at diagnosis. A significantly higher proportion of premenopausal than postmenopausal women had ER+ PR+ versus ER+PR− tumors at diagnosis (89.7% versus 84.9%; P = 0.0006). Univariate analyses showed age, race, nodal status, prior adjuvant chemotherapy, and mastectomy to be associated with menopausal status. By multivariate analysis treatment, race, duration of tamoxifen, prior adjuvant chemotherapy, and mastectomy were independent factors associated with menopausal status.
Table 1.
Baseline patient and disease characteristics
| Characteristics | Premenopausal (N = 877) No. (%) | Postmenopausal (N = 4289) No. (%) | P (univariate) | P (multivariate) |
|---|---|---|---|---|
| Treatment | 0.14 | 0.04 | ||
| Letrozole | 418 (48) | 2163 (50) | ||
| Placebo | 459 (52) | 2126 (50) | ||
| Age at randomization (years) | ||||
| <60 | 877 (100) | 1271 (30) | <0.001 | 0.99 |
| 60–70 | 0 (0) | 1694 (40) | ||
| ≥70 | 0 (0) | 1323 (31) | ||
| Missing | 0 (0) | 1 (0) | ||
| Race | ||||
| White | 779 (89) | 3925 (92) | 0.005 | 0.002 |
| Black | 33 (4) | 146 (3) | ||
| Other | 54 (6) | 150 (4) | ||
| Unknown | 5 (1) | 39 (1) | ||
| Missing | 6 (1) | 29 (1) | ||
| Axillary lymph node status | ||||
| Negative | 369 (42) | 2198 (51) | <0.001 | 0.11 |
| Positive | 495 (56) | 1863 (43) | ||
| Unknown | 12 (1) | 214 (5) | ||
| Missing | 1 (0) | 14 (0) | ||
| Hormone receptor status | ||||
| Positive | 860 (98) | 4172 (97) | 0.94 | 0.70 |
| Negative | 1 (0) | 7 (0) | ||
| Unknown | 10 (1) | 81 (2) | ||
| Missing | 6 (1) | 29 (1) | ||
| Duration of prior tamoxifen | ||||
| Treatment years | ||||
| ≤5 | 379 (43) | 1988 (46) | 0.09 | 0.01 |
| >5 | 497 (57) | 2294 (54) | ||
| Missing | 1 (0) | 7 (0) | ||
| Median (years) | 5.01 | 5.01 | ||
| Prior adjuvant chemotherapy | ||||
| No | 170 (19) | 2650 (62) | <0.001 | <0.0001 |
| Yes | 705 (80) | 1634 (38) | ||
| Missing | 2 (0) | 5 (0) | ||
| Prior surgery | ||||
| Lumpectomy or segmental mastectomy | 496 (57) | 2436 (57) | 0.90 | 0.37 |
| Mastectomy | 480 (55) | 2119 (49) | 0.004 | 0.07 |
patient outcomes after randomization
Table 2 shows DFS, DDFS, and OS, by menopausal status, prior treatment at randomization, and nodal status based on data before unblinding. For women randomized to LET, postmenopausal women had the higher 4-year DFS rates (90.5% versus 86.8%) and DDFS rates (93.9% versus 92.8%) than premenopausal women but none was significant (DFS HR 0.72, 95% CI 0.48–1.48; P = 0.11, and DDFS HR 0.98, 95% CI 0.57–1.69; P = 0.95). As expected, 4-year OS was lower in postmenopausal women but not statistically significantly so (94.5% versus 96.8%; HR 2.33, 95% CI 0.80–6.76; P = 0.12). LET was significantly better than PLAC in DFS for both menopausal groups and in DDFS for postmenopausal women with borderline significance for premenopausal women. No difference in OS was found between the two treatments in either of the menopausal groups. The interaction between treatment and menopausal status was statistically significant for DFS (P = 0.03) (Figure 1), which indicates that women premenopausal at diagnosis experienced significantly greater benefit (HR 0.26, 95% CI 0.13–0.55; P = 0.0003) with LET in terms of DFS than those postmenopausal at diagnosis (HR 0.67, 95% CI 0.51–0.89; P = 0.006). The analyses by nodal status showed a significant difference between LET and PLAC in DFS for postmenopausal women with either node-negative or -positive breast cancer and for premenopausal women with positive nodes (premenopausal women with negative nodes cannot be analyzed because no event was observed among those who received LET); and in DDFS for postmenopausal women with positive nodes.
Table 2.
Outcomes before unblinding by menopausal status, treatment and nodal status for disease-free survival, distant disease-free survival and overall survival
| Group | Letrozole |
Placebo |
Hazard ratioa (95% CI) | Pa | ||
|---|---|---|---|---|---|---|
| N | Four-year DFS (%) | N | Four-year DFS (%) | |||
| Disease-free survival | ||||||
| Premenopausal | 418 | 96.5 | 459 | 86.8 | 0.26 (0.13–0.55) | 0.0003 |
| N+ | 231 | 93.8 | 264 | 85.0 | 0.40 (0.18–0.85) | 0.02 |
| N− | 182 | 100.0 | 187 | 88.5 | NAb (NA–NA) | NA |
| Postmenopausal | 2163 | 93.9 | 2126 | 90.5 | 0.67 (0.51–0.89) | 0.006 |
| N+ | 939 | 91.9 | 924 | 84.7 | 0.67 (0.47–0.95) | 0.02 |
| N− | 1110 | 95.6 | 1088 | 94.5 | 0.58 (0.35–0.98) | 0.04 |
| Distant disease-free survival | ||||||
| Premenopausal | 418 | 97.4 | 459 | 92.8 | 0.45 (0.20–1.02) | 0.055 |
| N+ | 231 | 95.4 | 264 | 90.4 | 0.61 (0.26–1.46) | 0.27 |
| N− | 182 | 100.0 | 187 | 95.6 | NA (NA–NA) | NA |
| Postmenopausal | 2163 | 96.4 | 2126 | 93.9 | 0.64 (0.45–0.92) | 0.02 |
| N+ | 939 | 94.8 | 924 | 88.2 | 0.51 (0.33–0.78) | 0.002 |
| N− | 1110 | 97.9 | 1088 | 97.9 | 0.84 (0.40–1.76) | 0.63 |
| Overall survival | ||||||
| Premenopausal | 418 | 99.3 | 459 | 96.8 | 0.43 (0.08–2.22) | 0.31 |
| N+ | 231 | 98.8 | 264 | 94.4 | 0.51 (0.09–2.81) | 0.44 |
| N− | 182 | 100.0 | 187 | 100.0 | NA (NA–NA) | NA |
| Postmenopausal | 2163 | 94.6 | 2126 | 94.5 | 0.83 (0.57–1.22) | 0.35 |
| N+ | 939 | 93.0 | 924 | 90.4 | 0.61 (0.38–1.00) | 0.051 |
| N− | 1110 | 95.8 | 1088 | 97.7 | 1.49 (0.74–2.99) | 0.26 |
aFrom multivariate Cox model for the comparison between letrozole and placebo.
bNo event was observed in one or both treatment arms.
Figure 1.
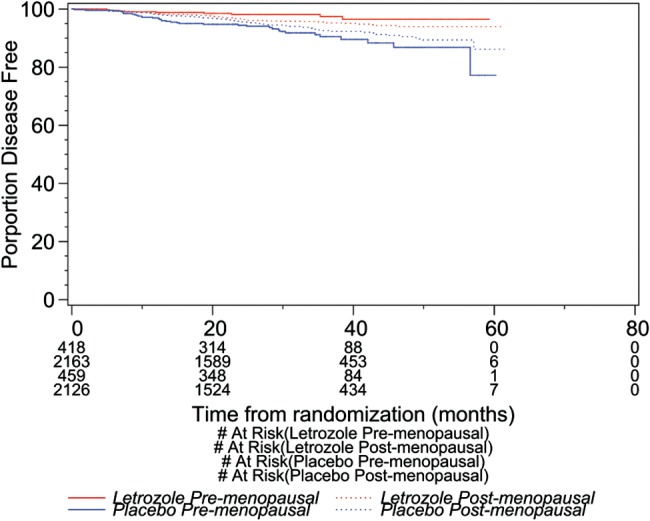
Kaplan–Meier curves for DFS by treatment and menopausal status
patient outcomes after unblinding
The outcomes including data observed after unblinding for women randomized to PLAC are presented in Table 3 by menopausal status and whether women switched to LET. Both pre- and postmenopausal women who switched to LET were found to have higher 5-year DFS and DDFS rates than those who remained on observation, which were statistically significant except no significance in DFS was observed for premenopausal women. Postmenopausal women who switched to LET also had a significantly longer OS than those remaining on PLAC.
Table 3.
Outcomes for patients randomized to placebo by menopausal status and nodal status according to whether they elected to receive letrozole after unblinding
| Group | PLAC–LET |
PLAC–PLAC |
Hazard ratioa (95% CI) | Pa | ||
|---|---|---|---|---|---|---|
| N | Five-year outcome (%) | N | Five-year outcome (%) | |||
| Disease-free survival (DFS) | ||||||
| Premenopausal | 287 | 98.8 | 134 | 90.5 | 0.39 (0.14–1.09) | 0.07 |
| Postmenopausal | 1291 | 97.0 | 670 | 94.0 | 0.36 (0.21–0.62) | 0.0003 |
| Distant disease-free survival (DDFS) | ||||||
| Premenopausal | 287 | 99.6 | 134 | 93.7 | 0.15 (0.03–0.79) | 0.02 |
| Postmenopausal | 1291 | 98.1 | 670 | 96.7 | 0.45 (0.22–0.94) | 0.033 |
| Overall survival (OS) | ||||||
| Premenopausal | 287 | 99.0 | 134 | 98.2 | 0.51 (0.06–4.11) | 0.53 |
| Postmenopausal | 1291 | 98.1 | 670 | 93.0 | 0.28 (0.15–0.49) | <0.0001 |
aFrom multivariate Cox model for the comparison between PLAC–LET and PLAC–PLAC.
safety during blinded study
Table 4 presents subgroup analyses of toxic effects before unblinding in each of the two menopausal groups. Premenopausal women on LET had a lower incidence of intermittent vaginal bleeding (10% versus 16%; P = 0.01) and higher incidence of arthralgia (24% versus 16%; P = 0.004) compared with those on PLAC. In contrast postmenopausal women on LET had a higher incidence of hot flashes/flashes (55% versus 50%; P = 0.001), arthralgia (25% versus 21%; P = 0.002), myalgia (15% versus 12%; P = 0.007), and alopecia (5% versus 3%; P = 0.003) than those on PLAC.
Table 4.
Patient self reported acute toxic effects (grade 1–4) by menopausal status and treatment
| Toxicity | Premenopausal |
Postmenopausal |
||||
|---|---|---|---|---|---|---|
| Letrozole (N = 416) | Placebo (N = 416) | P | Letrozole (N = 2161) | Placebo (N = 2128) | P | |
| Edema | 20 | 19 | 0.57 | 22 | 21 | 0.40 |
| Hypertension | 2 | 5 | 0.06 | 6 | 5 | 0.44 |
| Hot flushes | 69 | 68 | 0.84 | 55 | 50 | 0.001 |
| Fatigue | 37 | 33 | 0.16 | 39 | 40 | 0.60 |
| Sweating | 40 | 35 | 0.14 | 28 | 28 | 0.77 |
| Anorexia | 3 | 2 | 0.65 | 6 | 5 | 0.05 |
| Constipation | 12 | 12 | 0.88 | 14 | 15 | 0.42 |
| Diarrhea | 5 | 5 | 0.68 | 7 | 7 | 0.75 |
| Nausea | 10 | 12 | 0.28 | 12 | 12 | 0.82 |
| Vaginal bleeding | 10 | 16 | 0.01 | 5 | 6 | 0.16 |
| Infection w/o neutropenia | 4 | 5 | 0.20 | 5 | 4 | 0.13 |
| Arthritis | 4 | 2 | 0.29 | 7 | 6 | 0.14 |
| High cholesterola | 16 | 13 | 0.27 | 16 | 16 | 0.86 |
| Dizziness | 14 | 16 | 0.62 | 18 | 17 | 0.36 |
| Insomnia | 8 | 6 | 0.23 | 6 | 5 | 0.12 |
| Depression | 7 | 6 | 0.50 | 5 | 5 | 0.56 |
| Headache | 31 | 31 | 0.94 | 27 | 25 | 0.36 |
| Arthralgia | 24 | 16 | 0.004 | 25 | 21 | 0.002 |
| Myalgia | 16 | 12 | 0.16 | 15 | 12 | 0.007 |
| Bone pain | 6 | 5 | 0.95 | 5 | 6 | 0.60 |
| Dyspnea | 3 | 5 | 0.17 | 7 | 7 | 0.72 |
| Alopecia | 5 | 5 | 0.90 | 5 | 3 | 0.003 |
| Vaginal dryness | 11 | 8 | 0.29 | 5 | 4 | 0.40 |
aChange from normal to high.
From chi-square test for the comparison in the incidence of toxicity between letrozole and placebo.
quality of life during blinded study
Supplementary Table S1, available at Annals of Oncology online shows SF-36 and menopause-specific quality of life (MENQOL) questionnaire measures by menopausal status and treatment at 24 months. Higher scores in SF-36 domains indicate better QOL whereas in MENQOL domains indicate worse QOL. Change scores from baseline at 24 months from randomization indicated that premenopausal women treated with LET had significantly worse QOL than those treated with PLAC in SF-36 role function-physical (−6.9 versus −1.5; P = 0.02), general health (−3.1 versus −0.05; P = 0.04), and vitality (−7.0 versus 0.4; P = 0.001) domains and physical overall scale (−1.8 versus 0.1; P = 0.04), while postmenopausal women treated with LET had significantly worse QOL than those treated by PLAC in MENQOL vasomotor (−0.2 versus −0.5; P = 0.004) and sexual (0.2 versus −0.05; P = 0.01) domains at 24 months after randomization.
discussion
Five years of tamoxifen remains standard adjuvant endocrine therapy for premenopausal women with low to moderate risk early ER+ breast cancer [13].
Guidelines for premenopausal patients with higher risk of recurrence differ, with ovarian function suppression often used in combination with tamoxifen [15–17]. This difference is reflected in the control arms of the ongoing ‘Suppression of Ovarian Function Trial’ and the ‘Tamoxifen and Exemestane Trial’, respectively [18, 19]. Longer than 5 years of tamoxifen has not shown added benefit but increases toxicity [20], and guidelines currently advise restricting use to 5 years.
The chances of permanent menopause from adjuvant chemotherapy vary by age. Older premenopausal women and those receiving more regimens that are dose-intensive regimens or contain alkylating agents are more likely to become menopausal [20]. Either way, women presenting premenopausally with ER+ breast cancer frequently become amenorrhoeic during the course of their adjuvant tamoxifen. Deciding whether an amenorrhoeic woman is still premenopausal by virtue of resumption of menses or biochemical measurements can be inaccurate and make decision whether AI monotherapy is appropriate and to avoid unwanted pregnancy is difficult. In the MA17 clinical trial, criteria of menopause after completion of tamoxifen and before randomization to AI monotherapy were employed. Fewer than 5 of 5187 women resumed regular menses on the trial suggesting that the definition of menopause by these criteria was very accurate and clinically useful. Our subgroup analysis is interesting although only 877 of 5187 women randomized to MA17 were premenopausal at initial diagnosis of breast cancer. Not surprisingly premenopausal women in our trial had higher breast cancer risk factors at baseline than those postmenopausal as reflected by younger age, ethnicity, and more frequent node-positive disease. Eighty percent of the ‘premenopausal’ cohort had received prior adjuvant chemotherapy, likely contributing to their menopause.
The 4-year relapse rate in PLAC-treated women premenopausal at diagnosis was 13.2% compared with 9.5% in those postmenopausal. Among the premenopausal improvement in DFS at 4 years was 9.7% and 3.4% in those postmenopausal. The improvement in DFS was more significant in premenopausal women than those postmenopausal (P = 0.03 for interaction between treatment and menopausal status). Premenopausal patients in general presented more frequently with ER+ PR− rather than ER+ PR+ primary tumors, with greater recurrence rates early in follow-up. In contrast among our cohort of premenopausal women ‘disease free at 5 years’, their primary tumors were more commonly ER+ PR+ rather than ER+ PR− reinforcing the notion that those with ER+PR− primary tumors have disproportionately recurrence rates before completing 5 years of tamoxifen. We previously published an analysis of all women on MA17 showing that women with ER+ PR+ tumors had superior outcomes on LET and the findings of a significantly higher proportion of such tumors among our pre- versus postmenopausal women is in keeping with these previous findings [21]. Taken together, our results support the notion that women with ER+ disease late in follow-up who remain vulnerable to recurrence are more likely to have micrometastatic disease that is estrogen sensitive and responsive to reductions in circulating and tumor estrogen levels with aromatase inhibition. We also previously published results from MA17 showing that late administration of LET confers a reduction in the risk of recurrence [10], and this was also true in this analysis of women premenopausal at diagnosis. This finding is clinically important in women who have completed 5 years of tamoxifen some time before and did not receive extended therapy and remain free of recurrence in follow-up.
Finally, analysis of outcomes by nodal status in our patients showed benefit from extended therapy with LET for both subsets and in both pre- and postmenopausal women. A remarkable finding in this analysis is that premenopausal women with node-negative disease experienced 100% DFS on extended LET. This is of great potential clinical significance and merits follow-up.
Importantly, all MA17 participants regardless of age and menopausal status had been able to tolerate 4.5–6 years of prior tamoxifen, possibly making tolerability of further therapy and compliance in this extended endocrine therapy setting relatively better than among those receiving antiestrogen therapy for the first time. Ultimately, the relative and absolute benefits of taking extended LET immediately or delayed after completing tamoxifen have to take into consideration several factors, some of which are best ascertained by a ‘trial of therapy’ as symptoms and QOL changes are not easy to predict in any given patient. These include absolute and relative risks of recurrent breast cancer or new CLBC, baseline symptoms of menopause, and QOL. Regarding QOL in women who took LET, our results showed that premenopausal women were negatively affected in more domains than postmenopausal women; however, this should be carefully interpreted since the number of premenopausal women was smaller, and our analysis was not corrected for multiple testing. Of importance menopausal status should be carefully ascertained before offering extended therapy with LET particularly as the administration of AI as monotherapy to women can be both ineffective and possibly induce unwanted pregnancies [22]. On the basis of initial MA17 results, using the criteria employed in MA17 is a reliable way to determine menopausal status after tamoxifen, and combined with biochemical evaluation, should enable confirmation of menopause. In the absence of a prospective randomized clinical trial confined to women who meet the criteria for ‘premenopausal’ status as defined here, the findings from this analysis merit discussion with patients in the clinic. This and other analyses we have published from MA17 including the post-unblinding results, suggest that the introduction of antiestrogen therapy at any time during follow-up is likely to benefit patients in terms of breast cancer outcomes, and this also merits discussion with patients.
funding
This work was supported by the Canadian Cancer Society through National Cancer Institute of Canada grant (10362) and grants from the National Cancer Institute in the United States (CA31946, CA21115, CA25224, CA38926, and CA32102). PEG and YC-G are supported by the Avon Foundation in New York.
disclosures
We have the following conflicts of interest:
PEG: Speakers Honoraria: Pfizer, Novartis.
NJR: Consultant or advisory role: Novartis; Honoraria: Novartis.
HBM: Consultant or advisory role: Wyeth/Pfizer, Amgen, Roche, Bristol Myers Squibb, Boehringer-Ingelheim, Sandoz, Abraxis and Eisai.
EAP: Research funding: Genentech and Sanofi.
DAC: Consultant or advisory role: Novartis, Astra-Zeneca and Pfizer; Honoraria: Novartis, Astra-Zeneca and Pfizer; Research Funding: Novartis, Astra-Zeneca and Pfizer.
KIP: Consultant or advisory role: Novartis; Honoraria: Novartis; Research Funding: Novartis: Expert Testimony: Novartis.
TW: Honoraria: Novartis.
All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgments
The authors are very grateful to the 5187 extraordinary women who agreed to participate in this study. The authors also acknowledge the trial committee; the many hundreds of investigators, pharmacists, and clinical research associates from the National Cancer Institute of Canada Clinical Trials Group, the Southwest Oncology Group, the Eastern Cooperative Oncology Group, the Cancer and Leukemia Group B, the North Central Cancer Treatment Group, the European Organization for Research and Treatment of Cancer, the International Breast Cancer Study Group, and Centers in England, including the Royal Marsden, St. George's, and Withington Hospitals; the members of the Data Safety Monitoring Committee: the central office staff of the NCIC Clinical Trials Group who contributed to the conduct of the trial; and to Novartis Pharmaceuticals for their support and provision of LET and PLAC. The authors thank Pedro Liedke for his review of the manuscript.
references
- 1.Goss P, von Eichel L. Summary of aromatase inhibitor trials: the past and future. J Steroid Biochem Mol Biol. 2007;106:40–48. doi: 10.1016/j.jsbmb.2007.05.023. doi:10.1016/j.jsbmb.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. doi:10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. doi:10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. doi:10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. doi:10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 6.Stewart HJ, Forrest AP, Everington D, et al. Randomized comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. The Scottish Cancer Trials Breast Group. Br J Cancer. 1996;74:297–299. doi: 10.1038/bjc.1996.356. doi:10.1038/bjc.1996.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zolendronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. doi:10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Ingle JN, Martino S, et al. A randomized trial of LET in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. doi:10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Martino S, et al. Randomized trial of LET following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. doi:10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 10.Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol. 2011;30:718–721. doi: 10.1200/JCO.2010.34.4010. doi:10.1200/JCO.2010.34.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. doi:10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 12.Ingle JN, Tu D, Pater JL, et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCIC CTG MA.17. Ann Oncol. 2008;19:877–882. doi: 10.1093/annonc/mdm566. doi:10.1093/annonc/mdm566. [DOI] [PubMed] [Google Scholar]
- 13.Puhalla S, Brufsky A, Davidson N. Adjuvant endocrine therapy for premenopausal women with breast cancer. Breast. 2009;18(Suppl 3):S122–S130. doi: 10.1016/S0960-9776(09)70286-3. doi:10.1016/S0960-9776(09)70286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23:6931–6940. doi: 10.1200/JCO.2005.11.181. doi:10.1200/JCO.2005.11.181. [DOI] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. doi:10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson RW, Allred DC, Anderson BO, et al. NCCN Breast Cancer Clinical Practice Guidelines Panel . Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 17.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology . American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. doi:10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ClinicalTrials.gov NCT00917969. http://clinicaltrials.gov/ct2/show/NCT00917969?term=NCT00917969&rank=1. (19 January 2012, date last accessed).
- 19. ClinicalTrials.gov NCT00066703. http://clinicaltrials.gov/ct2/show/NCT00066703?term=NCT0066703&rank=1. (19 January 2012, date last accessed).
- 20.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. doi:10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss PE, Ingle JN, Martino S, et al. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol. 2007;25:2006–2011. doi: 10.1200/JCO.2006.09.4482. doi:10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 22.Polyzos NP, Tzioras S, Badawy AM, et al. Aromatase inhibitors for female infertility: a systematic review of the literature. Reprod Biomed Online. 2009;19:456–471. doi: 10.1016/j.rbmo.2009.06.008. doi:10.1016/j.rbmo.2009.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


