Abstract
Background
Non-Hodgkin lymphoma (NHL) subtypes, diffuse large B-cell (DLBCL) and follicular lymphoma (FL) have different sex ratios and are diagnosed at ages over 60 years; DLBCL is more common in men and diagnosed at older ages than FL, which occurs more among women. This analysis of postmenopausal women examines the relationship between postmenopausal hormone therapy and NHL.
Design
Self-reported use of postmenopausal hormone therapy from 2094 postmenopausal women with NHL and 2731 without were pooled across nine case–control studies (1983–2005) from North America, Europe and Japan. Study-specific odds ratios (OR) and 95% confidence intervals (CI) estimated using logistic regression were pooled using random-effects meta-analyses.
Results
Postmenopausal women who used hormone therapy were at decreased risk of NHL (pooled OR = 0.79, 95% CI 0.69–0.90). Risks were reduced when the age of starting was 50 years or older. There was no clear trend with number of years of use. Current users were at decreased risk while those stopping over 2 years before diagnosis were not. Having a hysterectomy or not did not affect the risk. Favourable effects were present for DLBCL (pooled OR = 0.66, 95% CI 0.54–0.80) and FL (pooled OR = 0.82, 95% CI 0.66–1.01).
Conclusion
Postmenopausal hormone therapy, particularly used close to menopause, is associated with a decreased risk of NHL.
Keywords: case–control studies, diffuse large B-cell lymphoma, follicular lymphoma, menopausal hormone therapy, non-Hodgkin lymphoma
introduction
Among non-Hodgkin lymphomas (NHL), different sex ratios are seen for the two most common subtypes, with diffuse large B-cell lymphoma (DLBCL) occurring among more men and follicular lymphoma (FL) among more women [1]. NHLs are also typically diagnosed at older ages and the median age at diagnosis is 70 years for DLBCL and 64 years for FL. Little is known of the causes of NHL, but factors which alter immune function such as sex hormones are thought to be involved [2, 3].
Hormone therapy for menopause has been widely studied with respect to women's health [4]. For postmenopausal women, there has been some suggestion that the current use of hormone therapy may be associated with a reduced risk of NHL [5, 6], although on the whole, no association either way has been reported and findings to date are inconclusive [5–17]. Because of the known associations with breast and endometrial cancers and cardiovascular disease, postmenopausal hormone therapy should not be used to prevent disease, nevertheless studying its relationship with NHL could inform NHL aetiology. To investigate whether there is an association between menopausal hormone therapy and NHL, we conducted a pooled analysis of case–control studies which are involved in the International Lymphoma Epidemiology Consortium (InterLymph).
materials and methods
Information on postmenopausal hormonal therapy was collected in nine case–control studies involved in the InterLymph Consortium. Study design details have been published [8, 10, 13, 15, 18–22] (supplementary Table S1, available at Annals of Oncology online) and for five studies, postmenopausal hormonal therapy data have been described [8, 10, 13, 15, 18]. Cases were identified using rapid ascertainment techniques and lymphoma diagnoses confirmed using pathologic review of reports or samples by pathologists. Controls were randomly selected from population registers or hospital or clinic patients, and were matched to cases on age and ethnicity. Each study was approved by the appropriate ethical committees and participants gave their informed consent. The studies were conducted in parts of USA, Canada, England, Italy and Japan. Six studies collected data in the late 1990s to early 2000s and three during the 1980s to early 1990s.
Lymphoma diagnoses were classified according to the International Classification of Diseases for Oncology third edition (ICD-O-3) (Mayo, British Columbia, UK and HERPACC2 studies), or the Working Formulation (Connecticut, UCSF, Los Angeles and Northern Italy studies). Methods to translate other coding schemes to ICD-O-3 have been described, and NHL subtypes were consistent with other InterLymph pooled analyses [23]. B-cell subtypes were DLBCL (ICD-O-3 codes 9679/3, 9680/3, 9684/3), FL (9690/3, 9691/3, 9695/3, 9698/3), chronic lymphocytic leukaemia/small lymphocytic lymphoma (9670/3, 9823/3), marginal zone lymphoma (9689/3, 9699/3), mantle cell lymphoma (9673/3), Burkitt lymphoma (9687/3, 9826/3) and other unspecified B-cell lymphoma (9671/3, 9728/3). T-cell lymphomas were grouped as a whole (9700/3, 9701/3, 9702/3, 9705/3, 9708/3, 9709/3, 9714/3, 9716/3, 9717/3, 9718/3, 9719/3, 9729/3, 9827/3). NHL was defined by the above ICD-O-3 codes as well as 9591/3, 9675/3 and 9727/3. Cases with HIV-associated lymphoma or multiple myeloma were excluded from the pooled dataset because most studies did not recruit these cases.
Women were asked about their use of postmenopausal hormonal therapies through in-person or telephone interviews, or self-completed questionnaires. Women were asked whether they had used postmenopausal hormone therapy, the age at which they started and stopped using it, and the number of years therapy was taken. Analyses were restricted to postmenopausal women, defined as women who had reported that their periods had stopped, or if this information was missing, cases who were aged 55 or older at diagnosis or interview date for controls. For most studies, it is not known whether menopause was surgically induced, as information on bilateral oopherectomy was only collected in two studies (Mayo, Los Angeles). Data on hormone therapy was censored at 1 year before diagnosis or interview date. Since hormone therapy formulation was only collected in the UCSF study [10], hysterectomy was used as a surrogate. Women who had undergone a hysterectomy from the late 1980s onwards were more likely to have been prescribed oestrogen-only preparations, while women with an intact uterus were more likely to have been prescribed combined oestrogen–progestogen therapy [4]. Potential confounders included body mass index (BMI) and socioeconomic status. BMI was calculated from self-reported height and weight, where weight was before (Mayo, British Columbia, North Italy, Italy, UK) or at (HERPACC2) diagnosis/interview, or was the usual adult weight (Connecticut, UCSF). Socioeconomic status was derived from educational attainment (Mayo, UCSF, Los Angeles, North Italy and Italy), income level (British Columbia) or census data (UK). Four studies had no data on hysterectomy (Connecticut, British Columbia, North Italy and Italy), one had no BMI data (Los Angeles) and two no socioeconomic status information (Connecticut and HERPACC2).
An anonymized dataset was supplied for each study and was checked for inconsistencies before harmonizing variables across studies. Analysis was conducted using a two-step process using Stata/IC 11.1 for Windows (StataCorp LP, Texas, 2010). First, study-specific odds ratios (OR) and confidence intervals (CI) were estimated by logistic regression adjusted for age as a continuous variable and ethnicity grouped as Caucasian or other as potential confounders. Where cell frequencies were five or less, risk estimates were computed by exact methods, with a half added to all cell frequencies if there were no cases or controls. The second step was to pool the study-specific ORs in a meta-analysis. Fixed-effects meta-analysis was used where study-specific risk estimates were homogeneous, while a random-effects model was used when heterogeneity was present. Heterogeneity was tested using Cochrane's Q test, significant at Pheterogeneity < 0.10, and the amount of heterogeneity was described by the I2 statistic. Pooled risk estimates for trend were estimated by pooling the study-specific ORs for trend and were based on categorical data. To examine whether findings were consistent, sensitivity analyses were conducted stratified by factors such as study design and participation rates, and excluding ORs that were computed using exact methods.
results
Among the nine InterLymph studies, 2094 case and 2731 control women were considered postmenopausal (Table 1). The most common diagnoses were lymphomas of B-cell origin (81%), notably DLBCL (32%) and FL (25%) (Table 1). T-cell lymphoma and other B-cell subtypes such as chronic lymphocytic leukaemia/small lymphocytic lymphoma and marginal zone lymphoma accounted for <10% of cases, and NHL subtype was not known for 16%. Most women were Caucasian (90%), had gone through the menopause around the age of 47 and the median age at diagnosis was 64 years. Cases were more likely to be Caucasian, of slightly younger age, of lower socioeconomic status and to be overweight or more obese than controls; similar proportions of cases and controls had had a hysterectomy. Across studies, 37% of cases and 41% of controls reported using hormone therapy for their menopausal symptoms.
Table 1.
Characteristics of postmenopausal women included in the pooled analysis
| Cases |
Controls |
|
|---|---|---|
| N (%) | N (%) | |
| NHL subtype | 2094 (100) | – |
| Diffuse large B-cell lymphoma | 675 (32) | – |
| Follicular lymphoma | 531 (25) | – |
| Other B-cell lymphoma | 480 (23) | – |
| T-cell lymphoma | 83 (4) | – |
| Unclassified | 325 (16) | – |
| Age (years) | 2094 (100) | 2731 (100) |
| ≤55 | 358 (17) | 405 (15) |
| 56–65 | 791 (38) | 1036 (38) |
| >65 | 945 (45) | 1290 (47) |
| Socioeconomic status | 1463 (100) | 1956 (100) |
| High | 328 (22) | 501 (26) |
| Medium | 475 (32) | 691 (35) |
| Low | 655 (45) | 758 (39) |
| Not known | 5 (0.3) | 6 (0.3) |
| Body mass index | 1984 (100) | 2627 (100) |
| Underweight (<18.5 kg m−2) | 36 (2) | 86 (3) |
| Normal (18.5–24.99 kg m−2) | 1014 (51) | 1420 (54) |
| Overweight (25–29.99 kg m−2) | 597 (30) | 751 (29) |
| Obese (≥30 kg m−2) | 312 (16) | 340 (13) |
| Not known | 25 (1) | 30 (1) |
| Hysterectomy | 1098 (100) | 1512 (100) |
| No | 712 (65) | 1016 (67) |
| Yes | 373 (34) | 477 (32) |
| Not known | 13 (1) | 19 (1) |
Socioeconomic status data were collected in seven studies (Mayo, UCSF, Los Angeles, British Columbia, UK, North Italy and Italy), body mass index data in eight studies (Connecticut, Mayo, UCSF, British Columbia, UK, North Italy, Italy and HERPACC2) and hysterectomy data in five studies (Mayo, UCSF, Los Angeles, UK and HERPACC2). NHL, non-Hodgkin lymphoma.
Postmenopausal women who had used hormone therapy were at decreased risk of NHL overall (pooled risk estimate = 0.79, 95% CI 0.69–0.90) compared with never users (Table 2). Inverse associations were strongest when use started at older ages (ages 50 and 54 years: pooled risk estimate = 0.74, 95% CI 0.61–0.91; aged ≥55 years: pooled risk estimate = 0.78, 95% CI 0.62–0.98). Regardless of the years hormonal therapy was used for, risks were below one, but there was no clear trend in risk. Current users were at decreased risk (pooled risk estimate = 0.70, 95% CI 0.54–0.91) while women who stopped in the 5 years before diagnosis or longer ago were not. Heterogeneity in study-specific risks among current users was moderate (I2 = 40%) and influence analyses found that the pooled risk remained below one when each study was excluded in turn (pooled risk estimate ranged from 0.62, 95% CI 0.51–0.75, I2 = 0%, Pheterogeneity = 0.64 to 0.77, 95% CI 0.57–1.03, I2 = 31%, Pheterogeneity = 0.21). Postmenopausal hormone therapy reduced the risks of DLBCL and FL (pooled OR = 0.66, 95% CI 0.54–0.80; pooled OR = 0.82, 95% CI 0.66–1.01, respectively), but not of chronic lymphocytic leukaemia, marginal zone lymphoma, mantle cell lymphoma or T-cell lymphoma (pooled OR = 1.24, 95% CI 0.80–1.93; pooled OR = 0.88, 95% CI 0.56–1.40; pooled OR = 1.28, 95% CI 0.66–2.48; pooled OR = 0.86, 95% CI 0.52–1.41 respectively). Associations between hormone therapy, NHL, DLBCL and FL were consistent across studies and study-specific ORs tended to be below one (supplementary Figure S1, available at Annals of Oncology online).
Table 2.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and postmenopausal hormonal therapy among postmenopausal women
| Hormone therapya | Controls | NHL | Pooled ORb | 95% CI | I2 (%) | Pheterogeneity | DLBCL | Pooled ORb | 95% CI | I2 (%) | Pheterogeneity | FL | Pooled ORb | 95% CI | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone therapy | No. of studies = 9 | |||||||||||||||
| Totalc | 2731 | 2094 | 675 | 531 | ||||||||||||
| Never used | 1584 | 1275 | 1 | ref | 439 | 1 | ref | 299 | 1 | ref | ||||||
| Ever used | 1109 | 772 | 0.79 | 0.69–0.90 | 0 | 0.50 | 217 | 0.66 | 0.54–0.80 | 0 | 0.45 | 218 | 0.82 | 0.66–1.01 | 0 | 0.55 |
| Age first used (years) | No. of studies = 8d | |||||||||||||||
| Totalc | 2519 | 1987 | 637 | 501 | ||||||||||||
| Never used | 1395 | 1176 | 1 | ref | 405 | 1 | ref | 273 | 1 | ref | ||||||
| <45 | 244 | 188 | 0.88 | 0.71–1.10 | 0 | 0.64 | 51 | 0.72 | 0.51–1.02 | 0 | 0.60 | 57 | 1.01 | 0.72–1.43 | 0 | 0.49 |
| 45–49 | 259 | 193 | 0.84 | 0.68–1.05 | 0 | 0.57 | 59 | 0.77 | 0.55–1.06 | 0 | 0.85 | 54 | 0.86 | 0.62–1.21 | 0 | 0.91 |
| 50–54 | 322 | 209 | 0.74 | 0.61–0.91 | 0 | 0.59 | 54 | 0.58 | 0.42–0.80 | 0 | 0.93 | 63 | 0.82 | 0.60–1.12 | 0 | 0.70 |
| ≥55 | 235 | 156 | 0.78 | 0.62–0.98 | 0 | 0.65 | 43 | 0.70 | 0.48–1.01 | 0 | 0.62 | 35 | 0.73 | 0.45–1.18 | 22 | 0.25 |
| Trend | 0.92 | 0.88–0.96 | 0 | 0.44 | 0.87 | 0.81–0.94 | 0 | 0.50 | 0.92 | 0.85–0.99 | 8 | 0.37 | ||||
| Years used | No. of studies = 9 | |||||||||||||||
| Totalc | 2731 | 2094 | 675 | 531 | ||||||||||||
| Never used | 1584 | 1275 | 1 | ref | 439 | 1 | ref | 299 | 1 | ref | ||||||
| <2 | 242 | 160 | 0.78 | 0.62–0.98 | 0 | 0.81 | 53 | 0.73 | 0.52–1.02 | 0 | 0.46 | 39 | 0.78 | 0.52–1.16 | 4 | 0.40 |
| 2 to <5 | 231 | 180 | 0.90 | 0.72–1.13 | 0 | 0.69 | 51 | 0.81 | 0.57–1.14 | 0 | 0.72 | 52 | 0.97 | 0.69–1.37 | 0 | 0.87 |
| 5 to <10 | 238 | 152 | 0.72 | 0.57–0.91 | 0 | 0.80 | 37 | 0.59 | 0.40–0.86 | 0 | 0.44 | 41 | 0.73 | 0.51–1.06 | 0 | 0.61 |
| ≥10 | 363 | 258 | 0.86 | 0.65–1.13 | 35 | 0.14 | 72 | 0.79 | 0.52–1.20 | 32 | 0.16 | 79 | 0.99 | 0.73–1.34 | 0 | 0.53 |
| Trend | 0.94 | 0.90–0.98 | 7 | 0.38 | 0.91 | 0.83–1.00 | 35 | 0.14 | 0.98 | 0.90–1.06 | 24 | 0.23 | ||||
| Years since last used | No. of studies = 6d | |||||||||||||||
| Totalc | 2035 | 1647 | 552 | 418 | ||||||||||||
| Never used | 1237 | 1050 | 1 | ref | 368 | 1 | ref | 246 | 1 | ref | ||||||
| >5 | 153 | 128 | 1.00 | 0.77–1.30 | 0 | 0.47 | 40 | 0.88 | 0.60–1.30 | 0 | 0.67 | 26 | 0.76 | 0.48–1.19 | 0 | 0.99 |
| ≤5 | 89 | 80 | 0.97 | 0.69–1.35 | 0 | 0.58 | 23 | 0.84 | 0.50–1.40 | 0 | 0.47 | 25 | 1.05 | 0.64–1.71 | 0 | 0.85 |
| Current | 504 | 331 | 0.70 | 0.54–0.90 | 40 | 0.14 | 100 | 0.57 | 0.44–0.74 | 0 | 0.54 | 103 | 0.76 | 0.58–0.99 | 0 | 0.46 |
| Trend | 0.89 | 0.82–0.97 | 44 | 0.11 | 0.83 | 0.77–0.91 | 0 | 0.49 | 0.92 | 0.84–1.00 | 0 | 0.46 |
aHormone therapy among postmenopausal women where menopause was defined as the reporting of periods that stopped naturally or due to a bilateral oopherectomy, or, in the absence of these data, women aged ≥55 years were considered postmenopausal.
bPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
cFrequencies do not sum to the total due to missing values.
dHERPACC2 did not collect age when hormone therapy was first used, and Mayo, Los Angeles and HERPACC2 did not collect age when hormone therapy was last used.
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma.
Five studies asked women whether they had had a hysterectomy and the pooled risk estimate for using postmenopausal hormone therapy in these five studies was similar to that for all nine studies (Table 3). For NHL overall, there was no difference in the risk associated with hormone therapy by whether women had had a hysterectomy (pooled risk estimate = 0.78, 95% CI 0.55–1.10) or not (pooled risk estimate = 0.78, 95% CI 0.58–1.03). Statistically significant decreased risks were found for DLBCL among women who had had a hysterectomy (pooled risk estimate = 0.48, 95% CI 0.30–0.77) and for FL among women who had not undergone this surgery (pooled risk estimate = 0.66, 95% CI 0.47–0.93). However, confidence limits for these subtype-specific risks overlapped with those in the other stratum.
Table 3.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and postmenopausal hormonal therapy among postmenopausal women by hysterectomy status
| Hormone therapya | Controls | NHL | Pooled ORb | 95% CI | I2 | Pheterogeneity | DLBCL | Pooled ORb | 95% CI | I2 | Pheterogeneity | FL | Pooled ORb | 95% CI | I2 | Pheterogeneity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies with hysterectomy datac | ||||||||||||||||
| Totald | 1512 | 1098 | 378 | 307 | ||||||||||||
| Never used | 696 | 539 | 1 | ref | 205 | 1 | ref | 150 | 1 | ref | ||||||
| Ever used | 782 | 525 | 0.82 | 0.69–0.97 | 0 | 0.50 | 157 | 0.72 | 0.51–1.02 | 36 | 0.18 | 146 | 0.80 | 0.61–1.05 | 2 | 0.39 |
| Missing | 34 | 34 | 16 | 11 | ||||||||||||
| No hysterectomye | ||||||||||||||||
| Totald | 1016 | 712 | 245 | 203 | ||||||||||||
| Never used | 579 | 434 | 1 | ref | 155 | 1 | ref | 129 | 1 | ref | ||||||
| Ever used | 416 | 262 | 0.78 | 0.58–1.03 | 38 | 0.17 | 80 | 0.72 | 0.49–1.07 | 27 | 0.24 | 68 | 0.66 | 0.47–0.93 | 0 | 0.45 |
| Missing | 21 | 16 | 10 | 6 | ||||||||||||
| Hysterectomye | ||||||||||||||||
| Totald | 477 | 373 | 130 | 101 | ||||||||||||
| Never used | 117 | 102 | 1 | ref | 50 | 1 | ref | 20 | 1 | ref | ||||||
| Ever used | 353 | 255 | 0.78 | 0.55–1.10 | 0 | 0.80 | 74 | 0.48 | 0.30–0.77 | 0 | 0.87 | 77 | 1.28 | 0.45–3.63 | 50 | 0.09 |
| Missing | 7 | 16 | 6 | 4 | ||||||||||||
aHormone therapy among postmenopausal women where menopause was defined as the reporting of periods that stopped naturally or due to a bilateral oopherectomy, or, in the absence of these data, women aged ≥55 years were considered postmenopausal.
bPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
cHysterectomy data were collected in five studies (Mayo, UCSF, Los Angeles, UK and HERPACC2).
dFrequencies do not sum to the total due to missing values.
eTests for heterogeneity between risks among women who had had a hysterectomy and those who had not were not statistically significant (NHL: χ2 = 0.00, P = 0.99; DLBCL: χ2 = 1.90, P = 0.17; FL: χ2 = 1.56, P = 0.21).
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma.
NHL risk associated with postmenopausal hormone therapy was reduced among women of normal weight (pooled risk estimate = 0.77, 95% CI 0.62–0.96) (Table 4). Similarly for DLBCL, but not FL, risks were decreased in women who were of normal weight (pooled risk estimate = 0.57, 95% CI 0.43–0.75) and not among women who were overweight (pooled risk estimate = 0.87, 95% CI 0.63–1.20).
Table 4.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and postmenopausal hormonal therapy among postmenopausal women by normal and over- weight
| Hormone therapya | Controls | NHL | Pooled ORb | 95% CI | I2 | Pheterogeneity | DLBCL | Pooled ORb | 95% CI | I2 | Pheterogeneity | FL | Pooled ORb | 95% CI | I2 | Pheterogeneity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies with BMI datac | ||||||||||||||||
| Totald | 2627 | 1984 | 632 | 515 | ||||||||||||
| Never used | 1540 | 1218 | 1 | ref | 413 | 1 | ref | 290 | 1 | ref | ||||||
| Ever used | 1049 | 719 | 0.80 | 0.70–0.91 | 0 | 0.45 | 200 | 0.68 | 0.55–0.83 | 1 | 0.42 | 211 | 0.83 | 0.67–1.03 | 0 | 0.55 |
| Missing | 38 | 47 | 19 | 14 | ||||||||||||
| Normal weighte | ||||||||||||||||
| Totald | 1506 | 1050 | 341 | 276 | ||||||||||||
| Never used | 849 | 629 | 1 | ref | 223 | 1 | ref | 142 | 1 | ref | ||||||
| Ever used | 634 | 399 | 0.77 | 0.62–0.96 | 20 | 0.27 | 109 | 0.57 | 0.43–0.75 | 0 | 0.63 | 128 | 0.94 | 0.68–1.30 | 12 | 0.34 |
| Missing | 23 | 22 | 9 | 6 | ||||||||||||
| Overweight/obesee | ||||||||||||||||
| Totald | 1091 | 909 | 281 | 233 | ||||||||||||
| Never used | 671 | 571 | 1 | ref | 184 | 1 | ref | 143 | 1 | ref | ||||||
| Ever used | 407 | 315 | 0.86 | 0.70–1.06 | 0 | 0.91 | 88 | 0.87 | 0.63–1.20 | 0 | 0.80 | 82 | 0.74 | 0.53–1.03 | 0 | 0.99 |
| Missing | 13 | 23 | 9 | 8 | ||||||||||||
aHormone therapy among postmenopausal women where menopause was defined as the reporting of periods that stopped naturally or due to a bilateral oopherectomy, or, in the absence of these data, women aged ≥55 years were considered postmenopausal.
bPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
cBody mass index data were collected in eight studies (Connecticut, Mayo, UCSF, British Columbia, UK, North Italy, Italy and HERPACC2).
dFrequencies do not sum to the total due to missing values.
eTests for heterogeneity between risks among women who were normal weight (BMI < 25 kg m–2) and those who were overweight or obese (BMI ≥ 25 kg m–2) were statistically significant for DLBCL (χ2 = 3.79, P = 0.05) but not for NHL or FL (NHL: χ2 = 0.78, P = 0.38; FL: χ2 = 0.97, P = 0.32).
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma.
discussion
The nine studies from parts of North America, Europe and Japan suggested that postmenopausal women who used hormone therapy had a lower risk of NHL overall than those who had never used it. Current users were at decreased risk, but past users had risks comparable to those who had never taken hormonal therapy. Risks below one were found in all categories of duration of use and age when hormonal treatment started; no clear trends were found for either variable. Findings were similar when these analyses were restricted to current users. When hormones were started between the ages of 50–54, the decreased risk reached statistical significance. For the two most common subtypes, DLBCL and FL, findings were similar to NHL overall. Findings were consistent among population-, hospital- and clinic-based studies, in studies where case and control participation was over 70%, and when risk estimates were adjusted for socioeconomic status.
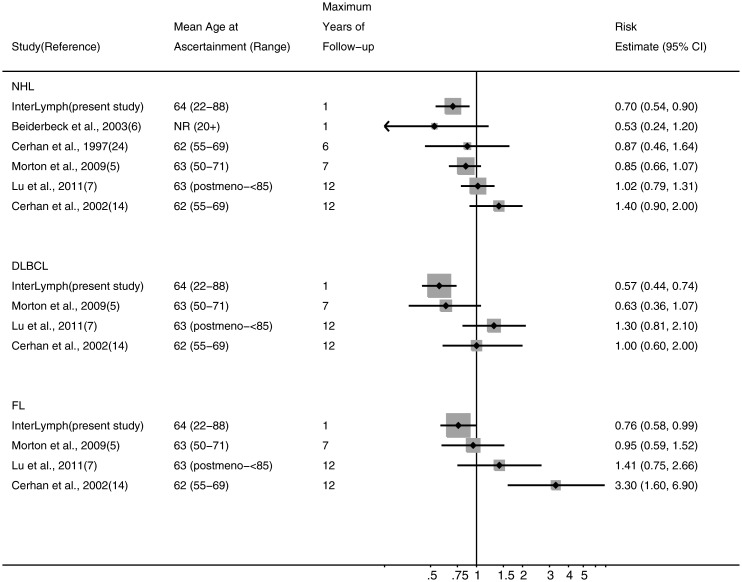
Other evidence for postmenopausal hormone therapy's role in NHL aetiology comes from five cohort [5, 7, 9, 11, 14] and two case–control studies [6, 17]. Among these further studies, one case–control study reported that hormonal therapy reduced the risk of NHL overall [6] but on the whole, cohort studies found risks to be around one. For DLBCL, two cohorts reported risks below one [5, 11] while two others found no association [7, 14]. As for FL, there has been less consistency, with one study reporting a risk below one [11], another close to one [5] and two raised [7], one of which was statistically important [14]. When we conducted a meta-analysis of cohort data, the results suggested no association with ever use for NHL, DLBCL or FL (pooled risk estimate = 1.01, 95% CI 0.89–1.14, I2 = 0%, Pheterogeneity = 0.49; pooled risk estimate = 0.87, 95% CI 0.67–1.14, I2 = 21%, Pheterogeneity = 0.28; pooled risk estimate = 1.31, 95% CI 0.70–2.45, I2 = 76%, Pheterogeneity = 0.01, respectively). However, our protective finding was among women who were using hormonal therapy 1 year before diagnosis. Current users were also at reduced risks in one cohort [5] and a case–control study [6], but not two other cohort studies [7, 14] (Figure 1). Cohort studies would have collected information on hormonal therapy many years before diagnosis. Interestingly, studies with follow-up periods that were shorter and closer to menopausal age found risks for current users more similar to ours while for longer follow-up and further from menopause, risks were closer to one (Figure 1). One cohort reported a lower risk after 7 [24] than after 13 years of follow-up [14]. In cohort studies, information may not have been available as to whether women had stopped using hormone therapy since recruitment into the cohort. One possible explanation for differences between our study and the cohorts' findings is that in cohorts, women may have been more likely to have stopped where follow-up continued further from menopausal age.
Figure 1.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and current use of postmenopausal hormonal therapy reported in published studies. Current use of hormone therapy was reported at recruitment in cohort studies and a year before diagnosis/reference date in case–control studies. Risk estimates are ordered by the maximum length of follow-up for cohort studies and latency period for case–control studies. NR, not reported; postmeno, postmenopausal. Cohort studies are Cerhan et al. [24], Morton et al. [5], Lu et al. [7] and Cerhan et al. [14]. Case–control studies are InterLymph and Beiderbeck et al. [6].
In our data, whether oestrogen only or combined oestrogen and progestin therapy contributed to the reduced risk could not be examined directly as only one study collected this information. As an alternative, we investigated whether risks were different among women who had or not had a hysterectomy since from the late 1980s, unopposed oestrogen tended to be prescribed following a hysterectomy and combined therapy to menopausal women with an intact uterus [4]. Although we found no difference for NHL overall, decreased risks of DLBCL for hormonal therapy were found among women who had had a hysterectomy in particular, but also in those who had not. For FL, risks were reduced among women with an intact uterus and not among those who did not. Findings for the two formulations have been reported in two cohorts [5, 7] and one case–control study included here [10]. No associations with either therapy were found in the cohort studies [5, 7] while reduced risks were found for both therapies in the case–control study [10]. However, some decreased risks were found in the cohort studies when hysterectomy data were considered in conjunction with treatment types [5, 7]. In the NIH-AARP cohort, women who had had a hysterectomy and were treated with unopposed oestrogen were at decreased risk of DLBCL while women with an intact uterus and on oestrogen plus progestin were not [5]. The California Teachers Study found that removal of both ovaries increased the risk of B-cell NHL possibly due to the absence of circulating hormones of ovarian origin, and when oestrogen only therapy was given to ovariectomized women, the treatment mediated the increased risk [7]. Hence, there appears to be some consistency between these findings [5, 7] and ours, but further investigation on treatment type is needed.
The mechanisms by which hormone therapy may act to reduce NHL risk among postmenopausal women are not known but may involve pro-inflammatory cytokines such as interleukins and tumour necrosis factor. As age increases, changes in immune function occur [3]. These alterations, which include increased production of pro-inflammatory cytokines, may be increased further in postmenopausal women due to oestrogen deprivation, at least in the years soon after menopause [25]. Taking hormone therapy may normalize the immune response and decrease production of tumour necrosis factor and interleukin-6 [2, 3]. Such mechanisms may explain the lower NHL risk in postmenopausal women who take hormone therapy compared with those who are untreated, although the exact processes by which hormone therapy acts on the immune system and lymphomagenesis are unknown. We also found a reduced risk of DLBCL among hormone therapy users of normal weight, a finding that has not been reported before. Although adipose tissue is a major source of oestrogen in the postmenopausal period, being overweight may increase DLBCL risk [26, 27] and so other obesity-related alterations to immune function are likely to be involved.
Our studies, like others of similar design, have several limitations. First, the use of postmenopausal hormone therapy was self-reported, although evidence suggests that there is reasonable agreement with medical records [8, 28–31]. Secondly, the controls' hormone therapy use may not be typical of postmenopausal women in general. Data for comparison are lacking, but across case–control studies, it seems unlikely that there is systematic selection bias given that hormone therapy has also been associated with disease excess, most notably breast cancer [32]. Thirdly, we could not assess risks associated with the type of postmenopausal hormone therapy, route of administration and dose as most studies did not collect this information. Fourthly, our finding of a decreased risk with current use could be a consequence of cases stopping use as lymphoma ensues. However, 75% of cases who were using hormone therapy at 1 year before diagnosis were still using it at the time their lymphoma was diagnosed. Fifthly, some cases would have died soon after diagnosis or been too ill to be interviewed, while a minority of interviewed cases may have had another cancer before their NHL diagnosis. Whether these cases were more (or less) likely to have used hormone therapy than those who were interviewed or who did not have a previous cancer is uncertain. Sixthly, it is also not known whether cases were less likely than controls to have had menopausal symptoms or conditions such as osteoporosis which are treated with hormone therapy. Lastly, our findings may be a consequence of selection bias since our controls tended to be of higher socioeconomic status than cases. Women of higher socioeconomic status are more likely to take hormones to treat menopausal symptoms than those of lower, but nevertheless, our protective effect remained when risks were adjusted for socioeconomic status.
In this pooled analysis of individual data, we found protective associations between postmenopausal hormone therapy and NHL and its two most common subtypes, DLBCL and FL. Our findings suggest that current use may reduce NHL risk. An advantage of our data, as well as the large number of subjects, was the ability to study hormone therapy use up to the time of diagnosis. The implications of our findings are not the prevention of NHL by taking postmenopausal hormone therapy since its use can lead to the development of breast and endometrial cancers and cardiovascular disease. Rather, our study gives insight into the possible role of exogenous sex hormones on NHL aetiology.
funding
This work was supported by National Cancer Institute grant CA62006 (Connecticut); National Cancer Institute grants R01 CA92153 and P50 CA97274 (Mayo); National Institute of Health grants CA45614, CA89745, CA87014, CA143947 and CA150037 (UCSF); National Cancer Institute grant CA50850 (Los Angeles); the Canadian Cancer Society through the National Cancer Institute of Canada, the Canadian Institutes for Health Research and the Chan Sisters Foundation (British Columbia); Leukaemia and Lymphoma Research (UK); the National Research Council (CNR) Applied Project ‘Clinical Applications of Oncological Research’ and the Italian Association for Cancer Research (Northern Italy); the European Community (Europe Against Cancer Programme), the Lega Italiana per la Lotta Contro i Tumori and Italian Association for Research on Cancer (Italy); Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan (HERPACC2). The study sponsors had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the paper for publication. EK is supported by the Leukaemia and Lymphoma Research, UK.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Smith A, Roman E, Howell D, et al. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010;148:739–753. doi: 10.1111/j.1365-2141.2009.08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 3.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system—a review. Maturitas. 2010;67:316–320. doi: 10.1016/j.maturitas.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Combined Estrogen−Progestogen Contraceptives and Combined Estrogen−Progestogen Menopausal Therapy (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans), Vol. 91. Lyon, France: International Agency for Research on Cancer. World Health Org and International Agency for Research on Cancer; 2007. . , : [PMC free article] [PubMed] [Google Scholar]
- 5.Morton LM, Wang SS, Richesson DA, et al. Reproductive factors, exogenous hormone use and risk of lymphoid neoplasms among women in the National Institutes of Health-AARP Diet and Health Study Cohort. Int J Cancer. 2009;124:2737–2743. doi: 10.1002/ijc.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beiderbeck AB, Holly EA, Sturkenboom MC, et al. No increased risk of non-Hodgkin's lymphoma with steroids, estrogens and psychotropics (Netherlands) Cancer Causes Control. 2003;14:639–644. doi: 10.1023/a:1025698109991. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Wang SS, Sullivan-Halley J, et al. Oral contraceptives, menopausal hormone therapy use and risk of B-cell non-Hodgkin lymphoma in the California Teachers Study. Int J Cancer. 2011;129:974–982. doi: 10.1002/ijc.25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mildon KH, Ansell P, Roman E, et al. Reproductive factors, menopausal hormone therapy, and risk of non-Hodgkin, diffuse large B-cell and follicular lymphomas: a UK case-control study. Cancer Causes Control. 2010;21:2079–2083. doi: 10.1007/s10552-010-9626-2. [DOI] [PubMed] [Google Scholar]
- 9.Corrao G, Zambon A, Conti V, et al. Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol. 2008;19:150–155. doi: 10.1093/annonc/mdm404. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Bracci PM, Holly EA. Non-Hodgkin lymphoma in women: reproductive factors and exogenous hormone use. Am J Epidemiol. 2008;168:278–288. doi: 10.1093/aje/kwn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norgaard M, Poulsen AH, Pedersen L, et al. Use of postmenopausal hormone replacement therapy and risk of non-Hodgkin's lymphoma: a Danish population-based cohort study. Br J Cancer. 2006;94:1339–1341. doi: 10.1038/sj.bjc.6603123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altieri A, Gallus S, Franceschi S, et al. Hormone replacement therapy and risk of lymphomas and myelomas. Eur J Cancer Prev. 2004;13:349–351. doi: 10.1097/01.cej.0000136573.16740.9c. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Holford TR, Leaderer B, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15:419–428. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 14.Cerhan JR, Vachon CM, Habermann TM, et al. Hormone replacement therapy and risk of non-hodgkin lymphoma and chronic lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev. 2002;11:1466–1471. [PubMed] [Google Scholar]
- 15.Nelson RA, Levine AM, Bernstein L. Reproductive factors and risk of intermediate- or high-grade B-Cell non-Hodgkin's lymphoma in women. J Clin Oncol. 2001;19:1381–1387. doi: 10.1200/JCO.2001.19.5.1381. [DOI] [PubMed] [Google Scholar]
- 16.Schiff D, Suman VJ, Yang P, et al. Risk factors for primary central nervous system lymphoma: a case-control study. Cancer. 1998;82:975–982. [PubMed] [Google Scholar]
- 17.Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. 1992;52:5510s–5515s. [PubMed] [Google Scholar]
- 18.Tavani A, Pregnolato A, La Vecchia C, et al. A case-control study of reproductive factors and risk of lymphomas and myelomas. Leuk Res. 1997;21:885–888. doi: 10.1016/s0145-2126(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 19.Cerhan JR, Ansell SM, Fredericksen ZS, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110:4455–4463. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spinelli JJ, Ng C, Weber JP, et al. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121:2767–2775. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- 21.Talamini R, Montella M, Crovatto M, et al. Non-Hodgkin's lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380–385. doi: 10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Matsuo K, Ito H, et al. A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis. 2006;27:1391–1397. doi: 10.1093/carcin/bgi334. [DOI] [PubMed] [Google Scholar]
- 23.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerhan JR, Wallace RB, Folsom AR, et al. Medical history risk factors for non-Hodgkin's lymphoma in older women. J Natl Cancer Inst. 1997;89:314–318. doi: 10.1093/jnci/89.4.314. [DOI] [PubMed] [Google Scholar]
- 25.Pfeilschifter J, Koditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 26.Willett EV, Morton LM, Hartge P, et al. Non-Hodgkin lymphoma and obesity: a pooled analysis from the InterLymph Consortium. Int J Cancer. 2008;122:2062–2070. doi: 10.1002/ijc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin's and Hodgkin's lymphoma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:2422–2430. doi: 10.1016/j.ejca.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Banks E, Beral V, Cameron R, et al. Agreement between general practice prescription data and self-reported use of hormone replacement therapy and treatment for various illnesses. J Epidemiol Biostat. 2001;6:357–363. doi: 10.1080/13595220152601837. [DOI] [PubMed] [Google Scholar]
- 29.Kropp S, Terboven T, Hedicke J, et al. Good agreement between physician and self-reported hormone therapy data in a case-control study. J Clin Epidemiol. 2007;60:1280–1287. doi: 10.1016/j.jclinepi.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Lokkegaard EL, Johnsen SP, Heitmann BL, et al. The validity of self-reported use of hormone replacement therapy among Danish nurses. Acta Obstet Gynecol Scand. 2004;83:476–481. doi: 10.1111/j.0001-6349.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 31.Paganini-Hill A, Clark LJ. Comparison of patient recall of hormone therapy with physician records. Menopause. 2007;14:230–234. doi: 10.1097/01.gme.0000235364.50028.b5. [DOI] [PubMed] [Google Scholar]
- 32.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.