Abstract
Background
Activation of the c-Met pathway occurs in a range of malignancies, including papillary renal cell carcinoma (RCC). Its activity in clear cell RCC is less clear. We investigated c-Met expression and inhibition in a large cohort of RCC tumors and cell lines.
Methods
c-Met protein expression was determined by automated quantitative analysis (AQUA) on a tissue microarray (TMA) constructed from 330 RCC tumors paired with adjacent normal renal tissue. c-Met expression and selective inhibition with SU11274 and ARQ 197 were studied in clear cell RCC cell lines.
Results
Higher c-Met expression was detected in all RCC subtypes than in the adjacent normal renal tissue (P < 0.0001). Expression was highest in papillary and sarcomatoid subtypes, and high-grade and stage tumors. Higher c-Met expression correlated with worse disease-specific survival [risk ratio = 1.36; 95% confidence interval (CI) 1.08–1.74; P = 0.0091] and was an independent predictor of survival, maintained in clear cell subset analyses. c-Met protein was activated in all cell lines, and proliferation (and colony formation) was blocked by SU11274 and ARQ 197.
Conclusions
c-Met is associated with poor pathologic features and prognosis in RCC. c-Met inhibition demonstrates in vitro activity against clear cell RCC. Further study of ARQ 197 with appropriate biomarker studies in RCC is warranted.
Keywords: c-Met, clear cell carcinoma, hepatocyte growth factor, renal cell carcinoma, tissue microarray
introduction
In the United States, an estimated 64 770 renal cell carcinoma (RCC) cases and 13 570 RCC-related deaths are expected in 2012 [1]. Until recently, immunotherapeutic regimens (interferon-α and interleukin-2) had been the primary treatment strategies, despite low response rates and significant toxic effects [2–5]. The identification of dysregulated molecular pathways in RCC has led to a new treatment paradigm in targeted therapeutic approaches [6]. Much focus has been put on the vascular endothelial growth factor (VEGFR), platelet-derived growth factor, and PI3K pathways, leading to the development and Food and Drug Administration (FDA) approval of multiple targeted agents for RCC. As these drugs generally slow the progression of disease with only modest objective response rates, it is necessary to identify new molecular targets in RCC for the development of more effective therapeutic strategies.
The receptor tyrosine kinase, c-Met, is involved in cell growth/differentiation, neovascularization and tissue repair in normal tissues, but also has been identified as a proto-oncogene. Dysregulation of c-Met and its ligand, hepatocyte growth factor (HGF), has been implicated in tumor development, invasion, and angiogenesis for a range of malignancies [7, 8]. The direct activation of c-Met through mutations in the c-Met gene has been identified in hereditary and sporadic papillary RCC [9]. Furthermore, in vitro studies have shown that loss of von Hippel–Lindau (VHL) expression and hypoxia lead to upregulation of c-Met expression in clear cell RCC [10, 11]. Also, a small study of 26 primary clear cell RCC tumors demonstrated an association between VHL mutation/loss of heterozygosity and increased c-Met expression (and HGF levels) [12]. Based on these findings and the frequent loss of VHL expression in clear cell RCC, further investigation of c-Met in this disease is of great interest.
Limited data exist on the relationship between c-Met expression in RCC tumors and outcomes. Miyata et al. showed high c-Met expression by immunohistochemistry in 73 out of 114 RCC tumor specimens, with 40% of tumors exhibiting greater phosphorylated c-Met expression than normal adjunct tubular cells [13]. Phosphorylated c-Met, but not total c-Met, was correlated with greater proliferation index, greater tumor diameter, and worse cause-specific survival. In another study of 66 resected primary clear cell RCC tumors (11% stage III and IV patients), higher c-Met mRNA expression occurred in tumor compared with adjacent normal renal tissue [14]. Additionally, a higher c-Met mRNA tumor to normal renal tissue ratio was associated with worse overall survival. A similar finding was also observed with the HGF mRNA expression ratio. While these studies suggest that c-Met and HGF may be prognostic markers in RCC, they are limited in sample size, number of advanced stage patients, and confirmatory analyses.
The goal of this investigation was to provide the preclinical rationale for targeting c-Met in all subtypes of RCC, including clear cell. We demonstrated the quantitative expression of c-Met protein in primary RCC tumors from a large cohort of patients with local and advanced disease. Subsequently, in vitro studies were carried out in clear cell RCC cell lines to demonstrate c-Met expression and inhibition with the well characterized c-Met inhibitor SU11274. Selective c-Met and growth inhibition was then confirmed with the novel non-ATP-competitive c-Met inhibitor, ARQ 197, which is now in clinical development.
materials and methods
RCC tissue microarray (TMA)
To quantify c-Met protein expression in a large cohort of RCC patients, primary RCC tumor samples and clinical data were analyzed from 330 patients treated at Yale New Haven Hospital as previously described, with approval of the Yale University institutional review board as previously described [15]. Briefly, tissue microarrays (TMAs) contained two core tumor specimens paired with adjacent normal renal tissue from nephrectomies carried out between 1987 and 1999. TMA slides were deparaffinized and processed for antigen-retrieval. Endogenous peroxidase activity and non-specific background staining were blocked before overnight incubation with anti-c-Met antibody (MET4, mouse species, 1:7500 dilution; a gift from Dr George Vande Woude, Van Andel Institute®, Grand Rapids, MI) and then anti-mouse secondary antibody (Envision, Dako North America, Inc., Carpinteria, CA) with cyanine-5-tyramide (Cy5; Perkin Elmer, Inc, Waltham, MA) for signal amplification. Cytokeratin was identified with rabbit anti-cytokeratin antibody (1:100 dilution; Cat. No. M5315, Dako) plus streptavidin-horseradish peroxidase (1:50 dilution; Cat. No. S2438, Sigma-Aldrich Co., LLC, St Louis, MO), followed by anti-rabbit secondary antibody (Envision, Dako) with cyanine-2-tyramide (Cy2; Perkin Elmer). Slides were then processed with 4′, 6-diamidino-2-phenylindole (DAPI) (1:500) for nuclear staining and mounted with ProLong® Gold antifade medium (Cat. No. P36931, Invitrogen/Life Technologies™, Grand Island, NY).
Automated quantitative analysis (AQUA) images were acquired and analyzed as previously described [16]. Monochromatic, high-resolution (1280 × 1024 pixel) images were obtained of each histospot. A tumor mask (and normal kidney tissue mask) was created from the Cy2 signal; cytoplasmic versus nuclear compartments were distinguished by Cy2 and DAPI stains, respectively. The target signal (c-Met) was compartmentalized and expressed as the average signal intensity within the assayed component (AQUA score), with scores on a scale of 0–255.
cell lines
Six human clear cell carcinoma cell lines (A498, ACHN, Caki-1, Caki-2, 769P, and 786-O) were utilized based on the availability from the American Type Culture Collection and maintained according to guidelines (http://www.atcc.org). Medium was supplemented with 10% fetal bovine serum and maintained at 37°C in a humidified atmosphere at 5% CO2.
western blot assays
c-Met and phosphorylated c-Met expression in six RCC cell lines were assayed. The cell lines were not stimulated with HGF in this initial assay. Additional studies were carried out to confirm selective inhibition of the c-Met pathway. A498 and 769P cell lines were chosen for mechanistic assays due to VHL mutation and VHL silencing by methylation, respectively [17]. Cell lines were treated with increasing concentrations of SU11274 (Cat. No. 448101 Calbiochem/EMD Chemicals, Inc, Philadelphia, PA), ARQ 197 (gift from ArQule®, Woburn, MA), or control medium. After 24-h exposure to the drug, the medium was removed and the cells were incubated with 100 nM recombinant HGF (Cat. No. PHG0254; Gibco®/Invitrogen, Carlsbad, CA) for 10 min and then collected.
Western blots were carried out using the standard methods. Membranes were blocked with 5% milk and incubated overnight at 4°C in the following primary rabbit anti-human antibodies: anti-c-Met (Cat. No. SC-10, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-phosphorylated-c-Met Y1349 and Y1234/Y1235 (Cat. No. 44-892G and 44887, respectively, Invitrogen), anti-phosphorylated-ERK1/2 (Cat. No. 4370, Cell Signaling Technology, Inc., Beverly, MA), anti-phosphorylated AKT (Cat. No. 3787, Cell Signaling Technology, Inc.), and anti-phosphorylated-p70S6kinase (Cat. No. 9205, Cell Signaling Technology, Inc.). Detection of proteins was done with peroxidase-conjugated anti-rabbit IgG secondary antibody (Cat. No. 711035152, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). β-Actin levels were measured to ensure consistent protein loading. Specific protein bands were visualized using a ChemiDoc XRS/BioRad system.
cell proliferation assays
Cell lines were plated on 96-well plates, grown for 24 h, and exposed to either SU11274 or ARQ 197 at increasing concentrations for 72 h. The relative number of viable cells was assessed by the luminometric Cell-Titer Glo® assay (Cat. No. G7573, Promega, Corporation, Madison, WI) and quantified using a Victor plate reader (Perkin Elmer). Concentrations that inhibited cell proliferation by 50% (IC50 values) were generated with logistic regression modeling using GraphPad Prism 3.0 Software.
colony formation assays
Cell lines were plated in six well plates, treated with medium (control) or ARQ 197 (0.05, 0.5, or 5 μM), and maintained in a humidified incubator. After 14 days, colonies were fixed with 25% glutaraldehyde and 1% methanol, stained with 0.5% crystal violet solution (Cat. No. HT90132-1L, Sigma-Aldrich Co., LLC) and counted. Experiments were carried out in triplicate.
statistical analyses
JMP 5.0 software (SAS Institute, Cary, NC), SPSS 19 (IBM Corporation, Armonk, NY) and GraphPad Prism 3.0 (La Jolla, CA) were used for all data analyses. The TMA AQUA scores for replicate cores were averaged after normalization. Prognostic significance of parameters was assessed using the Cox proportional hazards model with RCC-specific survival as an endpoint. Associations between clinical/pathological parameters and AQUA scores were assessed using unpaired t-test. For survival analyses, continuous c-Met AQUA scores were divided into quartiles for Kaplan-Meier survival curves, with significance evaluated using the Mantel-Cox log-rank test.
results
c-Met expression in RCC tumors
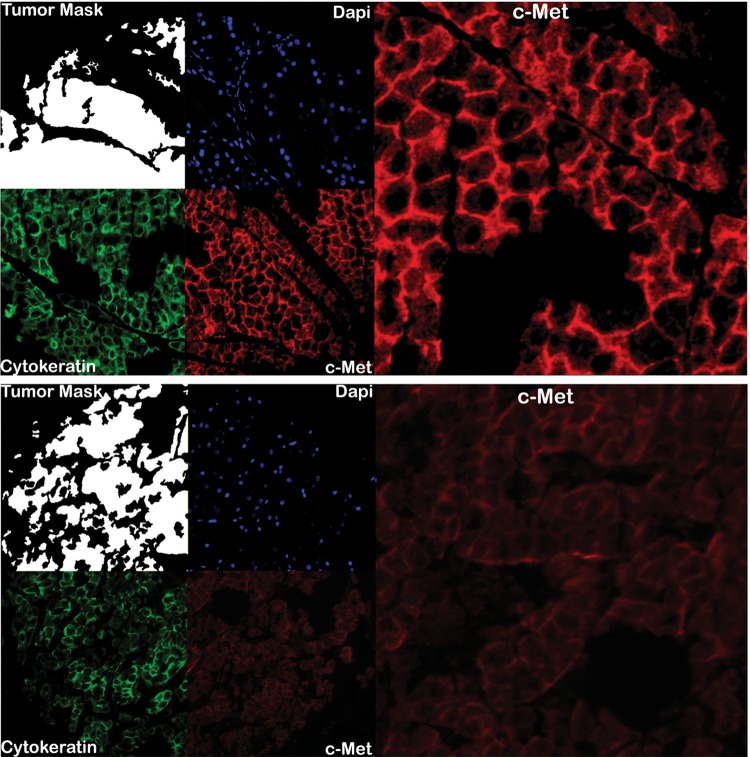
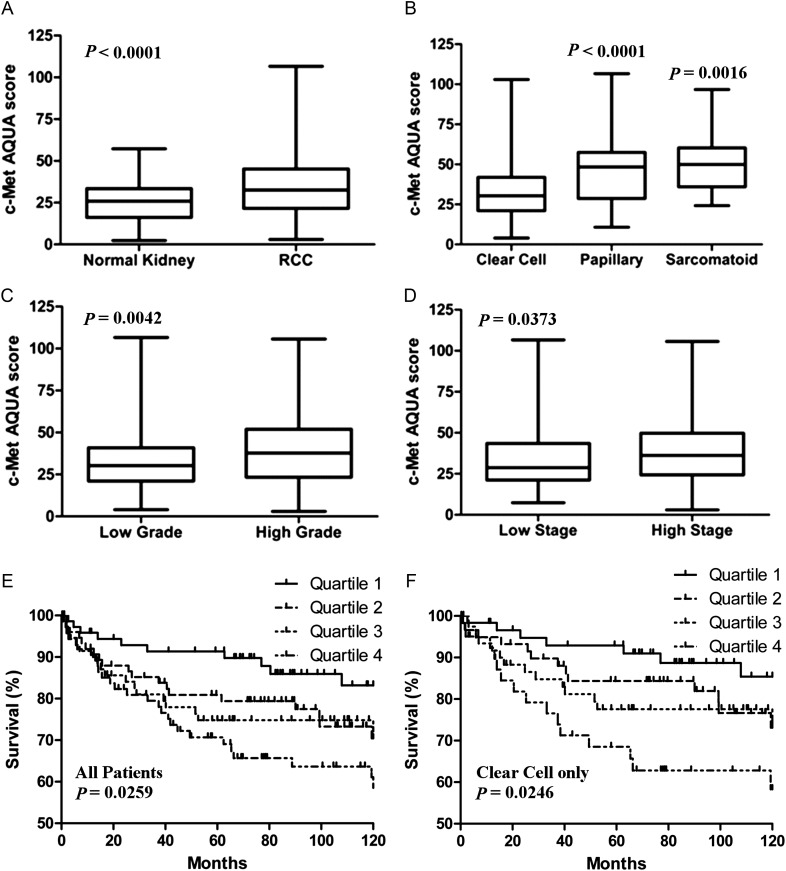
The TMA consisted of resected primary RCC tumors from patients of different age range, tumor subtypes, nuclear grades, and clinical stages (Table 1). Complete data were available for 317 patients. c-Met protein expression was quantified on RCC histospots and paired adjacent normal renal tissue. c-Met was localized to the cytoplasmic compartment and present in all histologic RCC subtypes. Examples of strong and weak c-Met expressing histospots are shown in Figure 1. Although c-Met expression was identified in oncocytomas as well, this subgroup was removed from further analyses due to its low malignant potential [18]. The mean AQUA score for c-Met expression was significantly greater in RCC tissue than in adjacent paired normal renal tissue (Figure 2A; mean expression of 36 versus 25, respectively, P < 0.0001). Expression of c-Met was significantly higher in papillary and sarcomatoid than in clear cell subtypes (P < 0.0001 and P = 0.0016, respectively; Figure 2B). Additionally, greater mean c-Met levels were seen with higher Fuhrman grade (Figure 2C, grades 3/4 = 39 versus grades 1/2 = 33, P = 0.0042) and more advanced disease stage (Figure 2D, stages III/IV = 38 versus stages I/II 34, P = 0.0373).
Table 1.
Clinical and pathologic data for the RCC tissue microarray
| Characteristic | Number (N = 317) |
|---|---|
| Median patient age | 64 years (range 25–87 years) |
| Male gender | 197 (62%) |
| RCC tumor subtype, n (%) | |
| Clear cell | 222 (70.0) |
| Papillary | 45 (14.2) |
| Oncocytoma | 20 (6.3) |
| Mixed histology | 12 (3.8) |
| Sarcomatoid | 11 (3.5) |
| Chromophobe | 7 (2.2) |
| Nuclear grade, n (%) | |
| I | 41 (12.9) |
| II | 161 (50.8) |
| III | 84 (26.5) |
| IV | 31 (9.5) |
| Stage, n (%) | |
| I | 179 (56.5) |
| II | 26 (8.2) |
| III | 88 (27.8) |
| IV | 24 (7.6) |
RCC, renal cell carcinoma.
Figure 1.
Automated, quantitative analysis (AQUA) of histospots from high c-Met (upper) and low c-Met (lower) renal cell carcinoma (RCC) tumors. Anti-cytokeratin conjugated to Cy2 identified the cytoplasmic areas and was used to create the tumor mask (white). DAPI stained the nuclear component. Anti-c-Met antibody conjugated to Cy5 demonstrated cytoplasmic c-Met expression.
Figure 2.
Box plots and Kaplan–Meier survival curves of c-Met expression by AQUA score in renal cell carcinoma (RCC) tumors. (A) c-Met level was significantly greater in RCC compared with paired normal renal tissue. (B) c-Met was present in clear cell, papillary, and sarcomatoid RCC subtypes, with greater levels in papillary and sarcomatoid when compared with clear cell RCC. (C and D) c-Met level was significantly greater in tumors with higher grade and higher stage. (E and F) Kaplan–Meier analyses demonstrated worse RCC-specific survival for patients with the highest quartile of c-Met expressing tumors based on the AQUA score in both the entire cohort and the clear cell subset. Tick marks represent censored events. P values represent log-rank (Mantel-Cox) overall comparison for survival by c-Met quartile.
RCC-cause specific survival data were censored at 120 months from surgery. There were 73 confirmed events (deaths) during this period (stage I = 18/163 patients, stage II = 1/22 patients, stage III = 35/88 patients, and stage IV = 19/24 patients). Univariate Cox proportional analysis for continuous AQUA scores demonstrated a likelihood-ratio chi-square value of 13.5 (P = 0.0002). Multivariate Cox proportional analysis showed that high c-Met expression correlated to worse survival independent of grade and stage (P = 0.015; Table 2). Kaplan–Meier survival curves demonstrated significantly worse survival in patients with higher c-Met expression (Figure 2E). Analysis of survival dichotomized by high versus low c-Met expression (median AQUA score = 32.5; High ≥ 32.5 or Low < 32.5) yielded a risk ratio of 1.36 [95% confidence interval (CI) 1.08–1.74; P = 0.0091]. The median AQUA score was arbitrarily selected as there was no well justified biological cut-off point to otherwise use. When the data were analyzed for the clear cell histologic subtype only, high c-Met expression remained associated with worse survival (Figure 2F) and was an independent predictor of survival by Multivariate Cox proportional analysis (Table 2).
Table 2.
Multivariate Cox proportional hazards model for renal cell carcinoma-specific survival
| Variable | Risk ratio | 95% confidence interval (CI) | P-value | |
|---|---|---|---|---|
| All RCC | c-Met AQUA score | 1.013 | 1.002–1.023 | 0.015 |
| Fuhrman grade III/IV versus I/II | 3.202 | 1.904–5.385 | <0.001 | |
| Stage III/IV versus I/II | 5.304 | 3.083–9.126 | <0.001 | |
| Clear cell | c-Met AQUA score | 1.017 | 1.003–1.032 | 0.018 |
| Fuhrman grade III/IV versus I/II | 2.337 | 1.284–4.256 | 0.005 | |
| Stage III/IV versus I/II | 4.321 | 2.260–8.264 | <0.001 |
c-Met and phosphorylated-c-Met expression in RCC cell lines
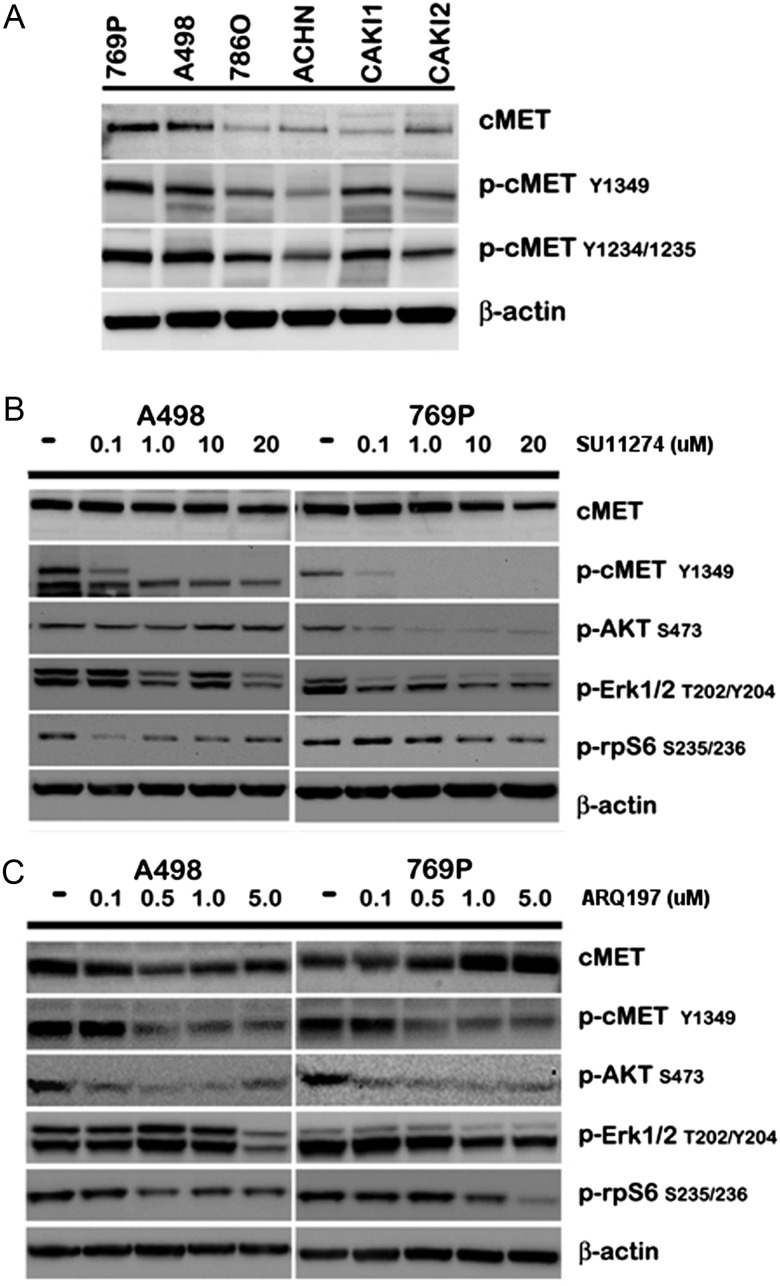
Western blot analysis showed that c-Met was expressed in all six RCC cell lines analyzed in this study. As shown in Figure 3A, antibodies to phosphorylated c-Met Y1234/Y1235 (kinase domain) and Y1349 (carboxy-terminal docking site) demonstrated varying degrees of activated c-Met among the cell lines. A498 and 769P cell lines demonstrated the highest c-Met and phosphorylated c-Met expression.
Figure 3.
Western blotting for c-Met, phosphorylated c-Met and selected downstream mediators in renal cell carcinoma (RCC) cell lines. (A) c-Met and phosphorylated c-Met (Y1234/Y1235 kinase and Y1349 carboxy-terminal docking site domains) were present in all cell lines with varying expression levels without supplemental HGF stimulation. (B and C) A498 and 769P cell lines were treated with increasing concentrations of SU11274 or ARQ 197 for 24 h. Total c-Met expression remained relatively stable with drug treatment. Phosphorylated c-Met expression was highest in control (untreated cells) and was blocked with increasing concentrations of SU11274 or ARQ 197. Downstream changes in the c-Met pathway were seen predominantly in phosphorylated AKT, while decreased phosphorylated ERK1/2 and phosphorylated rpS6 (P70S6Kinase) occurred at higher c-Met inhibitor concentrations.
c-Met inhibition with SU11274 in RCC cell lines
The activity of SU11274 was studied in five RCC cell lines. The IC50 values after 72 h treatment with SU11274 ranged from 5.81 to 8.39 μM in ACHN and 769P cell lines, respectively (Table 3). Western blot analyses carried out on A498 and 769P cell lines demonstrated diminished phosphorylated c-Met signal and stable total c-Met levels with increasing concentrations of SU11274 (Figure 3B). Changes in downstream effectors of the c-Met pathway were also observed, including decreased phosphorylated AKT, ERK1/2, and pS6K at higher SU11274 concentrations.
Table 3.
IC50 values for renal cell carcinoma (RCC) cell lines with two selective c-Met inhibitors
| Cell line | SU11274 (μM) | ARQ 197 (μM) |
|---|---|---|
| 769P | 8.39 | 0.68 |
| A498 | 6.11 | 0.58 |
| 786-O | 7.56 | 0.67 |
| ACHN | 5.81 | 0.53 |
| Caki-1 | 8.22 | 0.35 |
c-Met inhibition with ARQ 197 in RCC cell lines
Since SU11274 is not currently being developed for clinical use, we evaluated ARQ 197, which is currently in clinical trials. Under the same experimental conditions, ARQ 197 demonstrated IC50 values ranging from 0.35 to 0.68 μM in Caki-1 and 769P cell lines, respectively (Table 3). Western blot analyses of A498 and 769P cell lines confirmed inhibition of c-Met and decreased phosphorylated AKT, ERK1/2, and pS6K, with increasing concentrations of ARQ 197 (Figure 3C).
ARQ 197 inhibits colony formation in RCC cell lines
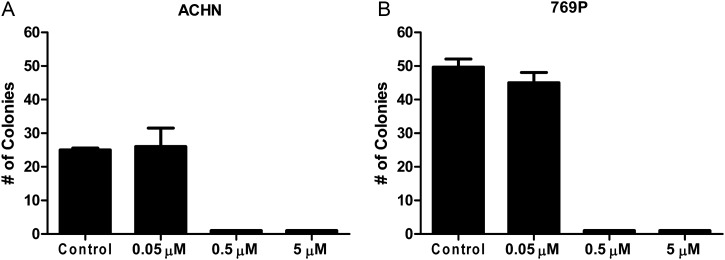
To confirm the in vitro activity of ARQ 197, colony formation assays were carried out (Figure 4). ACHN and 769P cell lines were chosen as other cell lines did not readily form colonies. At a low concentration of ARQ 197 (0.05 μM), minimal inhibition of colony formation was seen. Higher concentrations of ARQ 197 (0.5 and 5 μM) abolished colony formation in both the cell lines.
Figure 4.
Colony formation assays. Cells were treated with medium (control) or increasing concentrations of ARQ 197. Cells were maintained in a humidified incubator for 14 days before colony counting. Experiments were carried out in triplicate. Colony formation was strongly inhibited with ARQ 197.
discussion
A preclinical study of the c-Met pathway has become increasingly important for the successful application of c-Met-targeted therapy in solid tumors, as exemplified by recent clinical investigations in non-small cell lung cancer patients [19, 20]. To date, there has been limited data implicating the importance of the c-Met pathway in advanced RCC. To the best of our knowledge, this is the largest cohort studied for c-Met expression in RCC tumors of different histologic subtypes. Similar to data generated by Betsunoh et al., where c-Met mRNA levels were higher in RCC tumor compared with paired normal tissue, we observed that total c-Met protein expression is higher in RCC tumor compared with normal renal tissue [14].
Total c-Met protein expression correlated to more aggressive disease and worse survival for both the entire cohort and the clear cell subset of patients. This is consistent with previous reports of phosphorylated c-Met protein expression and c-Met mRNA levels in clear cell RCC [13, 14]. Our data also showed that c-Met expression retains its independence as a predictor of survival on multivariate analysis. However, the analyzed covariates were limited to clinical stage and tumor grade. Taken together, these results suggest that c-Met might be a valuable therapeutic target in RCC, including the clear cell histologic subtype.
To further investigate the role of the c-Met pathway in clear cell RCC, in vitro studies were carried out. c-Met and activated c-Met were identified in all six clear cell RCC cell lines analyzed. Exposure to either c-Met inhibitor, SU11274 or ARQ 197, resulted in substantial inhibition of cellular proliferation. Both of these IC50 data sets were similar to previous reports in other cell lines [21, 22]. As MAPK/ERK and PI3K/AKT pathways are known to be downstream from c-Met [7, 23, 24], we demonstrated that c-Met inhibition led to decreased activity of both pathways. Furthermore, data from phase I evaluation of ARQ 197 indicate that the concentrations used in our in vitro experiments are achieved in humans, where the peak serum concentrations of 2467 ng/ml (6.7 μM) on day 1 and 2719 ng/ml (7.4 μM) on day 22 were observed in patients treated at the maximum tolerated dose of ARQ 197 [25].
While multiple c-Met/HGF-targeted therapies are under clinical development, limited data are available on the efficacy of these agents in clear cell RCC. Modest clinical activity was seen with the anti-HGF antibody AMG 102, with only one partial response and stable disease in 43% of patients evaluated by RECIST criteria [26]. Direct targeting of c-Met might be more effective as seen in the phase II randomized discontinuation trial of XL184 (c-Met and VEGFR2 inhibitor), which showed objective partial responses (RECIST criteria) in 24% of patients and some tumor regression in at least one post-baseline scan in 86% of patients [27]. While less data are available for ARQ 197, the phase I trial demonstrated systemic drug levels significantly above in vitro IC50 values and confirmed c-Met inhibition in pre- and post-treatment tumor biopsies [25, 28]. Disease stabilization was observed in all six RCC patients (including three clear cell patients) in the dose-escalation cohort [28]. A recently published phase II study of ARQ 197 in patients with microphthalmia transcription factor-associated tumors included six patients with TFE3 translocation-associated RCC, three patients had disease stabilization, and three patients had disease progression [29].
Unlike previous reports, this study included all RCC subtypes. Our data demonstrated that c-Met expression was greatest among papillary and sarcomatoid subtypes. Whereas the presence of germline and spontaneous c-Met mutations in papillary RCC is a known occurrence [9], increased c-Met protein expression has not been well documented in either subtype. Commercially available cell lines of papillary and sarcomatoid RCCs for in vitro testing with c-Met inhibitors were not found for this study. However, given the upregulation of c-Met protein expression and possible tumor-dependency on this pathway, c-Met inhibition may prove to be therapeutically effective in papillary and sarcomatoid RCCs and warrants further investigation. Interestingly, the phase II clinical trial with foretinib (GSK1363089, XL880), a dual c-Met and VEGFR-2 inhibitor, in papillary RCC patients with either c-Met mutations or c-Met gene amplification demonstrated clinical activity [30].
In summary, our work confirms that c-Met protein expression is associated with aggressive disease and poor survival in RCC. Based on these findings and the demonstration of in vitro activity of ARQ 197 in clear cell RCC cell lines, c-Met might be a good drug target for this disease. However, several limitations of this investigation should be acknowledged. This includes the modest number of stage IV patients and the extent of clinical/pathologic variables included in the TMA analysis. For the in vitro studies, a more extensive panel of RCC cell lines, including papillary and sarcomatoid RCCs, could strengthen the broad application of c-Met-targeted therapy. Moreover, in vivo studies in RCC models may demonstrate even more robust antitumor activity due to the role of c-Met and HGF in the tumor microenvironment and angiogenesis. Further evaluation of ARQ 197 and other c-Met inhibitors in both clear cell and non-clear cell RCCs is warranted, including the incorporation of c-Met expression as a predictive biomarker. As ARQ 197 is being evaluated together with other agents, a combination therapy with c-Met inhibitors may prove more effective.
funding
This work was supported by NIH grants R0-1 CA158167 (to HMK) and by American Cancer Society Award M130572 (to H. Kluger).
disclosures
CRC is an employee of ArQule, Inc. BES is a stockholder and an employee of ArQule, Inc. RLC has part ownership and serves as a consultant for HistoRx. All the remaining authors have declared no conflicts of interest.
references
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. doi:10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. doi:10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 3.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 4.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. doi:10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 5.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. doi:10.1016/S0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 6.Sun M, Lughezzani G, Perrotte P, Karakiewicz PI. Treatment of metastatic renal cell carcinoma. Nat Rev Urol. 2010;7:327–338. doi: 10.1038/nrurol.2010.57. doi:10.1038/nrurol.2010.57. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. doi:10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 8.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. doi:10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 9.Giubellino A, Linehan WM, Bottaro DP. Targeting the Met signaling pathway in renal cancer. Expert Rev Anticancer Ther. 2009;9:785–793. doi: 10.1586/era.09.43. doi:10.1586/era.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakaigawa N, Yao M, Baba M, et al. Inactivation of von Hippel-Lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinoma. Cancer Res. 2006;66:3699–3705. doi: 10.1158/0008-5472.CAN-05-0617. doi:10.1158/0008-5472.CAN-05-0617. [DOI] [PubMed] [Google Scholar]
- 11.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. doi:10.1016/S1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 12.Oh RR, Park JY, Lee JH, et al. Expression of HGF/SF and Met protein is associated with genetic alterations of VHL gene in primary renal cell carcinomas. APMIS. 2002;110:229–238. doi: 10.1034/j.1600-0463.2002.100305.x. doi:10.1034/j.1600-0463.2002.100305.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyata Y, Kanetake H, Kanda S. Presence of phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor progression and survival in patients with conventional renal cell carcinoma. Clin Cancer Res. 2006;12:4876–4881. doi: 10.1158/1078-0432.CCR-06-0362. doi:10.1158/1078-0432.CCR-06-0362. [DOI] [PubMed] [Google Scholar]
- 14.Betsunoh H, Mukai S, Akiyama Y, et al. Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma. Cancer Sci. 2007;98:491–498. doi: 10.1111/j.1349-7006.2007.00412.x. doi:10.1111/j.1349-7006.2007.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluger HM, Siddiqui SF, Angeletti C, et al. Classification of renal cell carcinoma based on expression of VEGF and VEGF receptors in both tumor cells and endothelial cells. Lab Invest. 2008;88:962–972. doi: 10.1038/labinvest.2008.65. doi:10.1038/labinvest.2008.65. [DOI] [PubMed] [Google Scholar]
- 16.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. doi:10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 17.Shinojima T, Oya M, Takayanagi A, et al. Renal cancer cells lacking hypoxia inducible factor (HIF)-1alpha expression maintain vascular endothelial growth factor expression through HIF-2alpha. Carcinogenesis. 2007;28:529–536. doi: 10.1093/carcin/bgl143. doi:10.1093/carcin/bgl143. [DOI] [PubMed] [Google Scholar]
- 18.Van der Kwast T, Perez-Ordonez B. Renal oncocytoma, yet another tumour that does not fit in the dualistic benign/malignant paradigm? J Clin Pathol. 2007;60:585–586. doi: 10.1136/jcp.2006.044438. doi:10.1136/jcp.2006.044438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. doi:10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 20.Spigel D, Ervin T, Ramlau R, et al. Randomized multicenter double-blind placebo controlled phase II study evaluating MetMab, an antibody to MET receptor, in combination with erlotinib, in patients with advanced non-small cell lung cancer. Ann Oncol. 2010;221:LBA15. [Google Scholar]
- 21.Munshi N, Jeay S, Li Y, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010;9:1544–1553. doi: 10.1158/1535-7163.MCT-09-1173. doi:10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 22.Puri N, Ahmed S, Janamanchi V, et al. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res. 2007;13:2246–2253. doi: 10.1158/1078-0432.CCR-06-0776. doi:10.1158/1078-0432.CCR-06-0776. [DOI] [PubMed] [Google Scholar]
- 23.Christensen JG, Schreck R, Burrows J, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 24.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Mol Cancer Ther. 2010;9:1554–1561. doi: 10.1158/1535-7163.MCT-10-0359. doi:10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 25.Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–1279. doi: 10.1200/JCO.2010.31.0367. doi:10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 26.Schoffski P, Garcia JA, Stadler WM, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int. 2011;108:679–686. doi: 10.1111/j.1464-410X.2010.09947.x. [DOI] [PubMed] [Google Scholar]
- 27.Choueiri TK, Pal SK, McDermott DF, et al. Activity of cabozantinib (XL184) in patients (pts) with metastatic, refractory renal cell carcinoma (RCC) J Clin Oncol. 2012;(suppl. 5) abstract 364. [Google Scholar]
- 28.Rosen LS, Senzer N, Mekhail T, et al. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res. 2011;17:7754–7764. doi: 10.1158/1078-0432.CCR-11-1002. doi:10.1158/1078-0432.CCR-11-1002. [DOI] [PubMed] [Google Scholar]
- 29.Wagner AJ, Goldberg JM, Dubois SG, et al. Tivantinib (ARQ 197), a selective inhibitor of MET, in patients with microphthalmia transcription factor-associated tumors: results of a multicenter phase 2 trial. Cancer. 2012 doi: 10.1002/cncr.27582. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Vaishampayan UN, Rosenberg JE, et al. A phase II and biomarker study (MET111644) of the dual Met/VEGFR-2 inhibitor foretinib in patients with sporadic and hereditary papillary renal cell carcinoma: Final efficacy, safety, and PD results. J Clin Oncol. 2012;(suppl. 5) doi: 10.1200/JCO.2012.43.3383. abstract 355. [DOI] [PMC free article] [PubMed] [Google Scholar]