Summary
FurX is a tetrameric Zn-dependent alcohol dehydrogenase (ADH) from Cupriavidus necator JMP134. The enzyme rapidly reduces furfural with NADH as the reducing power. For the first time among characterized ADHs, the high-resolution structures of all reaction steps were obtained in a time-resolved manner, thereby illustrating the complete catalytic events of NADH-dependent reduction of furfural and the dynamic Zn2+ coordination among Glu66, water, substrate and product. In the fully closed conformation of the NADH complex, the catalytic turnover proved faster than observed for the partially closed conformation due to an effective proton transfer network. The domain motion triggered by NAD(H) association/dissociation appeared to facilitate dynamic interchanges in Zn2+ coordination with substrate and product molecules, ultimately increasing the enzymatic turnover rate. NAD+ dissociation appeared to be a slow process, involving multiple steps in concert with a domain opening and reconfiguration of Glu66. This agrees with the report that the cofactor is not dissociated from FurX during ethanol-dependent reduction of furfural, in which ethanol reduces NAD+ to NADH that is subsequently used for furfural reduction.
Introduction
Furfural can be easily formed from pentose at elevated temperature especially under acidic conditions (Hoydonckx et al., 2007). One of the examples is the conversion of solid biomass into hydrolysates for biofuel production, which requires a step of heating in diluted acids (Klinke et al., 2004). Unfortunately furfural has been shown to be the key inhibitor in the hydrolysates for ethanol-producing microorganisms (Heer and Sauer, 2008).
The main toxic effect of furfural is to prolong the lag phase of growth for the microorganisms (Almeida et al., 2009; Miller et al., 2009), although it is slowly reduced to the less toxic furfuryl alcohol (Boopathy et al., 1993; Liu et al., 2004; Heer and Sauer, 2008).
In Escherichia coli, two NADPH-dependent furfural reductases, EcYqhD and EcDkgA, have been reported to deplete NADPH and decrease the growth rate (Miller et al., 2009). In yeast, a diverse group of enzymes have been reported to reduce furfural, including two NADPH-dependent cinnamyl alcohol dehydrogenases (YADH6 and YADH7) (Larroy et al., 2002a,b), an NADPH-dependent aldehyde reductase (Ari1p, ARI1, YGL157W) (Liu and Moon, 2009; Bowman et al., 2010; Jordan et al., 2011), and NADH-dependent alcohol dehydrogenase (YADH1) (Modig et al., 2002; Laadan et al., 2008). Api1p has been characterized for its reaction mechanism, and its kinetic parameters were determined (Liu and Moon, 2009; Bowman et al., 2010; Jordan et al., 2011). The contribution of those “furfural reductases” to furfural reduction in yeast is likely collective, as mutations of individual genes did not significantly affect furfural reduction (Liu et al., 2008). The YAdh1 mutant has not been tested for its effect on furfural reduction; however, kinetic data suggest that YADH1 plays a significant role in furfural reduction at high furfural concentrations (Li et al., 2011a).
Recently, we have shown that Cupriavidus necator (formerly Ralstonia eutropha) JMP134 can reduce furfural much faster than yeast and E. coli. The responsible reductase, FurX, was isolated and characterized (Li et al., 2011b). FurX is an alcohol dehydrogenase (ADH), belonging to the Zn-dependent ADH (EC 1.1.1.1) family, and it catalyses both ethanol-dependent and NADH-dependent reduction of furfural (Li et al., 2011a,b). The ethanol-dependent reduction of furfural consists of two steps: FurX first uses ethanol to reduce NAD+ to NADH that is subsequently used for furfural reduction. Kinetic data suggest that NADH remains bound to FurX during the ethanol-dependent reduction of furfural (Li et al., 2011a).
In this study, we report the time-resolved structures of FurX during furfural reduction. The results thoroughly defined the entire chemical events during the catalytic cycle, providing a comprehensive understanding of furfural reduction by FurX. Our findings are relevant to many current pressing medical and environmental problems of furfural, and thus help to design effective biotransformation of furfural not only for human health but also for industrial application such as efficient production of biofuel.
Results
Global structure and oligomeric structure
Each dimer in the tightly packed apo-form FurX tetramer was composed of individual subunits connected by continuous 12-stranded β-sheets (Fig. 1). In order to determine the exact oligomeric status of FurX in solution, a dynamic light scattering experiment was performed with a solution of purified FurX (2 mg ml−1), and its tetrameric character was verified (Supplemental Data 1). Binary complexes of FurX with NADH were crystallized in a solution containing 1 mM NADH, 2.5% isopropanol and 1 M ammonium sulphate. Each P1 unit cell of this binary crystal contained two tetramers. Since FurX was able to rapidly reduce NAD+ to NADH in the crystallization solution that contained isopropanol (Supplemental Data 2), the possibility of any NAD+ in the active site of FurX was ruled out. Among the four FurX subunits in each tetramer, only two of them contained an NADH molecule.
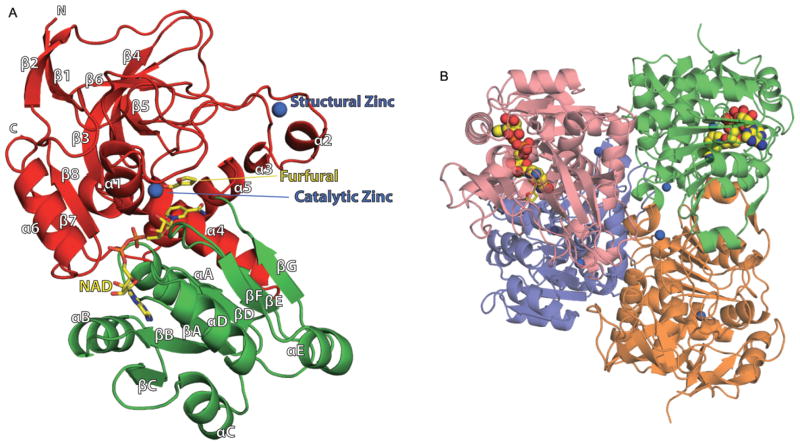
Fig. 1. Ribbon diagram representing the crystal structures of FurX and its substrate/NADH binding sites.
A. FurX monomer with secondary structural elements labelled. The catalytic and nucleotide-binding domains are coloured in red and green respectively. Secondary structural elements have been numbered sequentially as α1–α6/αA–αE and β1–β12/βA–βF following the convention. The N- and C-terminus are labelled with an N and C respectively. The catalytic and structural Zn2+ ions are depicted with blue spheres. Bound furfural and NADH in the middle of two domains are coloured in yellow.
B. Arrangements of the tetrameric FurX in a non-crystallographic D2 symmetry. Each dimer, green:orange or violet:pink, is composed of two subunits, which are connected by the continuous 12-stranded β-sheet. Only one subunit in each dimer contains NADH.
These figures were generated using Open-Source PyMOL™ (v1.2).
The furfural/NAD(H) ternary complexes were successfully made through soaking the NADH-complex crystals in a furfural containing mother-liquor solution. In addition to furfural, the substrate binding pockets of FurX showed various organic compounds that were contained in either crystallization buffer or cryoprotectant, such as isopropanol, polyethylene glycol and glycerol (Fig. 2A–C). For example, every subunit of an apo-form crystal of FurX contained penta- or hexa-ethyleneglycol, which was from the crystallization solutions, spanning from its solvent exposed surface to the substrate-binding pocket of an individual subunit (Fig. 2A). Isopropanol and glycerol molecules were identified in the open-form subunits of the NADH complex, which were in the crystallization buffer and the cryoprotectant solution respectively (Fig. 2B and C).
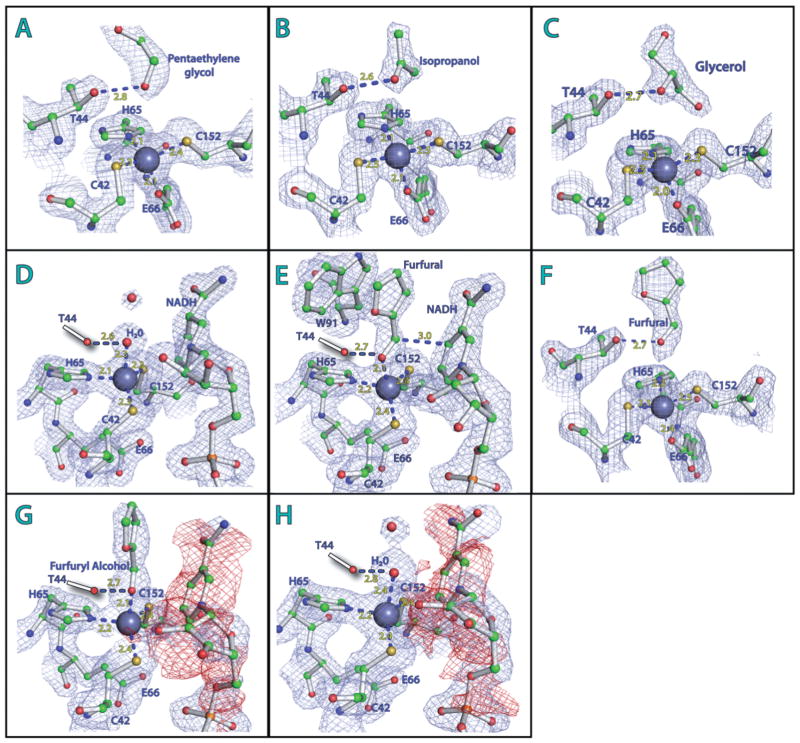
Fig. 2. Active site of FurX: Zn2+ coordination, substrate and NAD(H) binding.
A–C. Apo-form FurX: the catalytic Zn2+ ion (grey sphere) is tetrahedrally coordinated by Cys42, His65, Cys152 and Glu66. (A) Penta- or hexa-ethyleneglycol, (B) isopropanol or (C) glycerol is held by Thr44.
D. In NADH binary complex, a water molecule (red sphere) replaces the carboxyl side-chain of Glu66 (green).
E. Ternary complex of FurX with furfural and NADH. The carbonyl oxygen of the furfural participates in Zn2+ coordination instead of Glu66.
F. Without NADH, the furfural is not involved in Zn2+ coordination.
G and H. The negative density (red colour) for the nicotinamide and ribose rings of NAD+.
Figures were generated by PyMOL v1.4 (Schrödinger). The individual electron density maps were contoured at 1.5 s level.
Like other Zn-dependent ADH (Brändén et al., 1973; Eklund and Ramaswamy, 2008), each FurX subunit was composed of two distinct domains, namely a Rossmann fold forming the nucleotide-binding domain (residues 155–289) and a catalytic domain (residues 1–154 and 290–342) (Fig. 1A). The catalytic and nucleotide-binding domains of every cofactor-free subunit in both apo-form and NADH-complex crystals were arranged in the open conformation (Fig. 3). Nonetheless, the NADH-containing subunits observed in all binary and ternary complexes were in either completely closed or partially closed state (Fig. 3). Each dimer connected by 12 β-strands in a tetramer contained one open form (NADH-free subunit) and one closed form (NADH complex subunit in either fully or partially closed state).
Fig. 3.
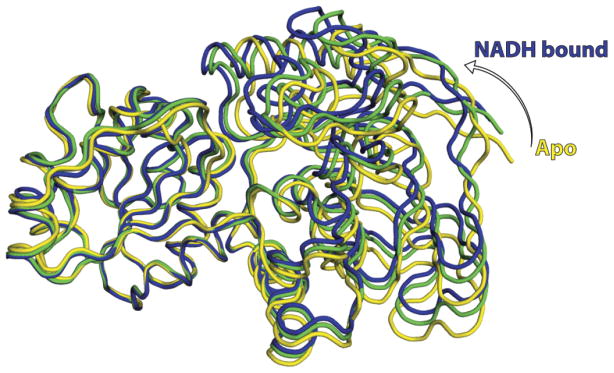
Rotational closure of FurX: superimposed views of FurX in its apo-form (yellow), its partially closed form (green) and fully closed form (blue). The Cα atoms of the open-form subunit have average root mean square deviations (rmsd) of 1.1 Å and 1.3 Å to those of partially and fully closed
NADH-containing subunits respectively.
Catalytic Zn2+ ion
The catalytic Zn2+ was located inside a cleft formed between the two domains and was positioned at the bottom of the hydrophobic substrate-binding pocket, coordinated by three residues, Cys42, His65 and Cys152 (Figs 1A and 2). The fourth coordination was different among the individual subunits locked in a unique catalytic state. In addition, a significant shift of the catalytic Zn2+ position was observed upon domain closing. When the catalytic domains of the apo-form (open state) and NADH-complex form (closed state) were superimposed, the corresponding positions of their catalytic Zn2+ ions were ~2.3 Å apart from each other. In all open-form subunits, the Oe1 atom of Glu66, located opposite to the substrate-binding pocket, was at a similar bond distance from the Zn2+ as the other three coordinating residues. However, in the NADH complexes, the oxygen atom of the substrate or water molecule replaced the coordination of Glu66. The distance between the carboxyl oxygen of Glu66 and the Zn2+ was either 4.2 Å (partially closed form) or 4.9 Å (fully closed form). Instead, the same oxygen formed a salt bridge with the nearby guanidinium side-chain of Arg335, thus being eliminated from the possibility of any direct interaction with the Zn2+. The same carboxyl group of Glu66 in the fully closed form was also within a hydrogen bond distance from the backbone amide nitrogen of Ala153, which was not observed in either open form or partially closed form.
Substrate-binding pocket and time-dependent substrate soaking
Water–NADH complex
When glycerol was used as a cryoprotectant for the NADH-complex crystal, every NADH-free subunit showed the corresponding density for glycerol with its 2-hydroxyl oxygen located within a hydrogen bond distance from Thr44 (Fig. 2C). In addition, the 1-and 3-hydroxyl groups of this bound glycerol were in hydrogen bonding interaction with neighbouring bound water molecules. The shortest distance of those hydroxyl groups from the catalytic Zn2+ was 4.25 Å. However, the NADH containing subunits in the same tetramer showed a water molecule (instead of glycerol) in a Zn2+ coordination position (2.3 Å) and the side-chains of Glu66 were not involved in Zn2+ coordination (Fig. 2D). This Zn2+-coordinated water was also within a hydrogen bond distance with the hydroxyl side-chain of Thr44 (Fig. 2D). Compared with those in the fully closed NADH-complex subunits, the corresponding densities for the Zn2+ and the side-chain of Cys42 in the partially closed NADH-complexes were somewhat delocalized indicating their multiple conformations.
Furfural–NADH complex I
When 100% furfural solution was used as a crystal cryoprotectant instead of glycerol, a clear electron density corresponding to furfural was visible. In every NADH containing subunit, the carbonyl oxygen of the bound furfural was coordinated by the Zn2+ and its furan ring was in a stacking position with the indole ring of Trp91 (Fig. 2E). The average distances of the carbonyl oxygen in furfural from the Zn2+ and C4 of nicotinamide ring were 2.1 and 3.0 Å respectively (Fig. 2E). However, the substrate-binding pocket of every apo-form subunit contained isopropanol, which was from the crystallization solution. The 2-hydroxyl oxygen of this isopropanol was within hydrogen bond distance from the side-chain of Thr44 and nearby ordered water molecules. This observed isopropanol was located at the same location as glycerol in the glycerol-cryoprotected crystal structure. Thus, in a given diffusion time, furfural could not replace the bound isopropanol in the cofactor-free subunit; however, glycerol was able to (Fig. 2C).
Furfural–NADH complex II
As a next step, furfural was added to a final concentration of 50% in the mother liquor drop containing NADH-complex crystals and incubated for 1 min. Then 100% furfural solvent was used as a crystal cryoprotectant. The resulting apo-form subunits showed the density of furfural in the position of previous isopropanol or glycerol with the carbonyl oxygen at average distances of 3.8 and 2.7 Å from the Zn2+ and the hydroxyl group of Thr44 respectively (Fig. 2F). The partially closed NADH complexes had a product molecule, furfuryl alcohol (Fig. 2G). Those furfuryl alcohols participated in Zn2+ coordination with their oxygen atoms positioned at 2.4 Å away from the Zn2+ ion, and the carboxyl side-chain of Glu66 was not at the Zn2+ coordination position (Fig. 2G). The corresponding densities for the Zn2+ and the side-chain of Cys42 of this partially close form were delocalized similarly to those observed in subunits in their water–NADH complex form. However, the corresponding electron density for the nicotinamide ring and a part of the connected ribose ring was not identifiable (Fig. 2G). At this stage, as discussed later, NADH had already reduced furfural, producing NAD+ and furfuryl alcohol. The observed delocalization could be due to weak affinity of NAD+ to FurX and poor coordination of Zn2+ to furfuryl alcohol. Similarly, the fully closed subunit had corresponding densities for only the adenine dinucleotide without any significant density for the nicotinamide and ribose rings (Fig. 2H). However, in those fully closed subunits, there was no more electron density for furfuryl alcohol. Instead, a water molecule was involved in Zn2+ coordination, which was within a hydrogen bond distance from the hydroxyl side-chain of Thr44 (Fig. 2H). The side-chains of Glu66 of both partially (Fig. 2G) and fully closed forms (Fig. 2H) were not in Zn2+ coordination position.
Furfural–NADH complex III
The furfural soaking time was further increased up to 30 min. Most of the features in this longer soaked crystal structure were very similar to those from 1 min soaked crystal data. However, the partially closed subunits did not have apparent density left for NAD+ indicating their complete dissociation, although there was furfuryl alcohol still in Zn2+ coordination distance as those of the 1 min soaked structure. In addition, the domains were further opened (~2°) from their partially closed state of 1 min soaking time. The structures of the fully closed subunits were the same as the corresponding subunits in 1 min soaked structure, still containing NAD+. Any further extended soaking experiments were not possible due to instability of resulting crystals.
Discussion
The substrate-binding pocket of FurX was predominantly hydrophobic being composed of 11 amino acids that connected the surface-exposed channel to the catalytic Zn2+. Eight residues from one subunit, Thr44, Trp53, Val55, Trp91, Asn115, Leu266, Ile289, Val290, and three residues from the other subunit, Phe276, Val279 and Leu280, constituted the substrate-binding pocket. In spite of its hydrophobic nature, the substrate-binding pocket of FurX was still hydrated by a network of ordered water molecules especially hydrogen-bonded to the side-chains of Trp91. Its indole ring was in a stacking interaction with the furan ring of furfural (Fig. 2E). Thus, Trp91 contributes to substrate binding both entropically and enthalpically. Phe93 of horse liver ADH (HLADH) (8ADH) and Phe94 of Sulfolobus solfataricus ADH (1R37), which correspond to the Trp91 of FurX, have been proposed to affect the substrate specificity in mammalian and archaeal ADHs by steric hindrance (Stone et al., 1989).
In the B-face of FurX, there were three residues, Thr156, Leu180 and Asn264, and their side-chains were in close contact with the nicotinamide ring of NADH. Those three residues are equivalent to Thr178, Val203 and Val292 in HLADH. Thus, the B-face environment of FurX is more hydrophilic than that of HLADH. Those three residues in HLADH have been proposed to facilitate hydride transfer and hydrogen tunnelling by orientation and motion (Rubach and Plapp, 2003). Considering the face of the nicotinamide in the binding pocket, its 4-re (A-face) hydrogen is abstracted for furfural reduction. This is in agreement with the stereospecificity of Zn-dependent ADHs that use the 4-re hydrogen of NADH for acetaldehyde reduction (Weinhold et al., 1991; Peretz et al., 1993). On the other hand, the yeast aldehyde reductase (Ari1p) that belongs to short-chain dehydrogenases/reductase superfamily abstracts the 4-si hydrogen of NADPH for furfural reduction (Bowman et al., 2010).
Three different open-close states of FurX
In the NADH complex of FurX, two subunits in the tetramer contained NADH molecules. However, in the crystal structure of Zn-dependent ADH from Pseudomonas aeruginosa (1LLU) (Levin et al., 2004), all four subunits in the tetramer contain NADH molecules. In the Zn-dependent ADH structures of both E. coli and yeast, the same cofactors are bound in three out of the four subunits (Ramaswamy et al., 1994; Karlsson et al., 2003). Considering those observed variations, it is likely that the individual subunits of the four subunits operate independently for cofactor-binding.
Concomitant with this cofactor binding, there occurred a change in the Zn2+ coordination of Glu66 and rotational movement between the two domains of the subunit. The observed coordination of the catalytic Zn2+ by a glutamate residue is very rare in most characterized Zn-dependent ADHs, including HLADH (Korkhin et al., 1998; Esposito et al., 2003; Karlsson et al., 2003; Kleifeld et al., 2003; Eklund and Ramaswamy, 2008). Instead, the more typical fourth ligand of the catalytic Zn2+ is a water molecule or the oxygen atom of substrate/substrate analogue (Vallee and Auld, 1990; Eklund and Ramaswamy, 2008). In FurX, Zn2+ coordination by a water molecule was observed only in the presence of bound NADH/NNAD+ (Figs 2 and 5). Therefore, it is apparent that the rotational motion between the two domains due to NADH/NAD-binding abolishes the Zn2+ coordination of Glu66. The glutamate residue at that position is one of the most highly conserved in Zn-dependent ADHs. In addition, Ala153 and Arg335, whose backbones were differentially interacting with the side-chain of Glu66 in FurX, are also completely conserved residues among compared ADHs of high Z-score (Supplemental Data 3). Supporting those observations, a replacement of the equivalent glutamate in yeast ADH resulted in a considerable reduction in overall catalytic efficiency and impaired binding of substrates (Kleifeld et al., 2003; Eklund and Ramaswamy, 2008).
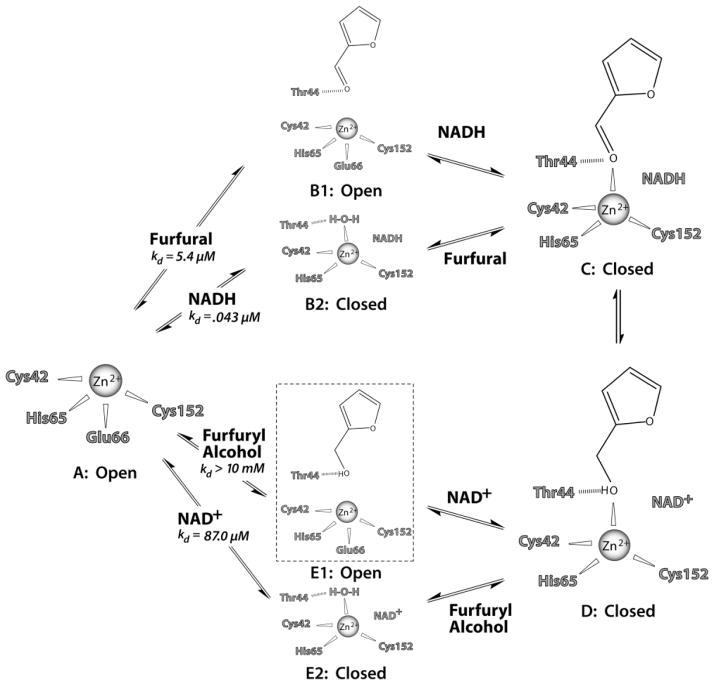
Fig. 5.
Proposed sequential reaction steps for FurX and other tetrameric ADHs. The possible hydrogen bonds are shown with a dashed line. All the steps except for the E1 step (boxed with dotted line) have been observed in the crystal structures. The corresponding step for E1 could not be observed due to the instability of FurX crystals in 100% furfuryl alcohol, thus was deduced from our observed binary complex structures of glycerol, isopropanol and furfural.
In addition, two subunits of the same FurX tetramer containing NADH displayed different levels of their domain closures. Superimposing the fully and partially closed subunits clearly showed further rotational motions of ~2° between their two domains (Fig. 3). The rotational movement between the two domains in ADH has been previously proposed to isolate the active site from the solvent, facilitating the hydride transfer reaction (Colonna-Cesari et al., 1986). However, both closed forms of FurX subunits allowed a furfural molecule to substitute a Zn2+-coordinating water molecule and to be reduced to furfuryl alcohol, which was in turn substituted by water. Thus the closed form allowed diffusion of water, substrate and product to and from the Zn2+. However, the resulting NAD+ could not be dissociated from the fully closed subunit at least within 30 min in the crystal. On the contrary, the NAD+ was able to dissociate from the partially closed subunit within a 30 min window leaving behind furfuryl alcohol in its Zn2+-coordinating position. Therefore, it is likely that domain opening with a concomitant Glu66 coordination facilitates shedding off the substrate/product, water and NAD+/NADH, increasing enzymatic turnovers. It is also likely that the partially closed form is an intermediate between the fully closed and open forms. The observed delocalized electron densities for the Zn2+ and the side-chain of Cys42 in the partially closed subunit indicate instability of coordination geometry, reflecting its intermediate nature.
The snapshots of proton transfer network
In accord with the observed time-lapse difference between fully closed and partially closed forms, two specific residues in the α1-helix, His47 and Asp52, were found in different conformations (Fig. 4). In all open-form (cofactor-free) subunits, those two residues did not show any significant interaction (Fig. 4A). In the partially closed subunits, the Ne atom of His47 was hydrogen-bonded to a water molecule, and the latter was within hydrogen bonding distance to the O3′ atom of the ribose ring of NADH (Fig. 4B). In addition, the Nd atom of the same imidazole ring was located at a distance of 3.2 Å from the Od atom of Asp52. In the fully closed conformation, the temperature factors of both His47 and Asp52 were substantially reduced and the water molecule was removed, allowing direct interaction between the Ne atom of His47 and the O3′ atom of NADH (Fig. 4C). In addition, the carboxyl oxygen of Asp52 approached more closely (2.9 Å) to the Nd of imidazole, establishing a tight hydrogen bond network among the O3′ atom of NADH, the imidazole of His47 and the carboxylate of Asp52, which allows shuttling a proton to and from the bulk solvent. Therefore, due to the apparent superiority in proton shuttling, the fully closed form could turnover water–furfural–furfuryl alcohol more quickly than the half closed form (Fig. 2G and H). The residues constituting the proton shuttling system (Eklund and Branden, 1987), Thr44, His47 and Asp52 are conserved among the compared Zn-dependent ADHs (Fig. 5), indicating that all of those ADHs probably adopt a similar system. In contrast, the alternative proton pathway, which was proposed in HLADH (Luo and Bruice, 2001; Esposito et al., 2003) from the 3′ OH of a nicotinamide-ribose to the backbone carbonyl oxygen of Ile269 (Val243 in FurX), is not possible due to the relatively long distance between the corresponding acceptor and donor groups in FurX.
Fig. 4.
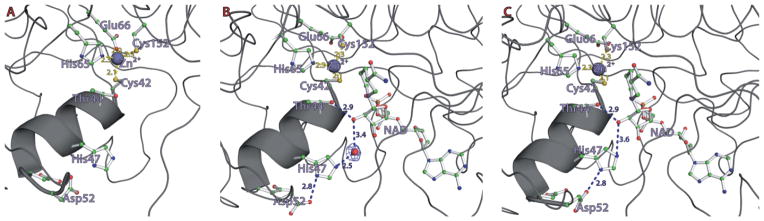
The hydrogen bond network for proton shuttling during catalysis in the active site of FurX. (A) Apo-form, (B) NAD(H) complex in its partially closed conformation, (C) NAD(H) complex in its fully closed conformation. Dotted lines in blue indicate the possible hydrogen bonds among the functional groups during catalysis.
Catalytic mechanism
Incubation of the NADH binary complex crystal with furfural allowed the observation of time resolved progress of enzymatic reaction, which clearly defined the complete catalytic events of NADH-dependent reduction of furfural.
In the absence of NADH (or NAD+), the two domains of FurX are in their open conformation and the carboxyl oxygen of Glu66 participates in the coordination of the catalytic Zn2+ (Step A and Step B1 in Fig. 5). Thus, a diffused-in substrate does not participate in Zn2+ coordination (Fig. 2A–C and F). Instead, the side-chain of Thr44 and the catalytic Zn2+ provide a hydrogen bond and electrostatic interaction, respectively, to furfural (Step B1), furfuryl alcohol (Step E1) and other potential substrates.
Upon binding of NADH to the furfural–FurX binary complex, the resulting domain closure with concomitant dissociation of the carboxyl oxygen of Glu66 from the Zn2+ brings the carbonyl oxygen of furfural into a proper position for Zn2+ coordination, establishing a productive ternary complex (Step C in Fig. 5).
As shown in our crystal structures (Fig. 2B), in the event of NADH association to the apo-form FurX, the two domains become closed and a water molecule replaces the Glu66’s Zn2+ coordination (Step B2). In the closed state, diffused-in furfural replaces this water molecule and locates its carbonyl oxygen in the Zn2+-coordinating position, establishing a productive ternary complex (Step C in Fig. 5). In this switching event of the Zn2+ coordination between furfural and a water molecule, participation of Glu66 was not observed, for which a domain opening is required. In addition, as shown in our crystal structures, alcoholic substrate such as glycerol or isopropanol was not able to replace the coordination reflecting their lower affinity than a water molecule, which was probably in its hydroxide form.
Considering both B1 and B2 steps were observed in our crystal structures, it is tempting to speculate a random-order mechanism for FurX and other tetrameric ADH. Isotope exchange and kinetic work with YADH1 has argued for a random-order mechanism for the formation of the enzyme-ethanol-NAD+ complex (Silverstein and Boyer, 1964; Dickinson and Monger, 1973; Plapp, 2010); however, the mechanism has not been widely accepted due to the lack of supporting structural evidence. In the mean time, the bound substrate in our NADH-free structures was not involved in Zn coordination and NADH binding converted this non-productively associated substrate (Step B1) into a productive ternary complex (Step C). In addition, the substrate molecule can readily diffuse into the NADH–FurX binary complex (Step B2), thus the reaction could still kinetically follow compulsory-order as HLADH and other animal liver ADHs do (Theorell, 1967; Plapp, 2010).
In both plausible routes (Step B1 or B2) of ternary complex formation (Step C), the carbonyl oxygen of furfural maintains a hydrogen bond with the hydroxyl side-chain of Thr44. Thus, the hydroxyl group of Thr44 acts as a general acid for the Zn2+-coordinated carbonyl oxygen of furfural. The closed conformation of FurX (Step C) allows a hydride to be transferred from the C4 atom of NADH to the C1 atom of furfural producing a furfuryl alcohol (Step D) through a previously proposed hydrogen-tunnelling event (Kohen et al., 1999). The fully closed conformation at Step C can establish stability and efficiency in the proton network and hydrogen tunnelling, facilitating conversion to Step E2 (Fig. 2H) via Step D (Fig. 2G). Our crystal structure of furfuryl alcohol in its Zn2+-coordinating position without cofactor should be an intermediate between Step D and Step E1, which is trapped due to the restrained domain motion under the given crystal lattice.
Upon reduction of furfural, the nicotinamide ring of a remaining NAD+ molecule becomes mobile and loses its affinity probably due to electrostatic repulsion between its positive charge and Zn2+ (Fig. 2G and H). Considering the observed progressive reduction of the electron density for NAD+, its dissociation/association could be at least a two-step process. That is, oxidized nicotinamide ring dissociates the binding pocket first followed by the adenine di-phosphoribose (ADP), which is consistent with a previous proposal (Kovaleva and Plapp, 2005). However in our observation, the ribose group attached to nicotinamide stays in the binding pocket longer than the nicotinamide ring, thus it could be a three-step process.
In our crystal structure of the fully closed subunits, a water molecule was able to replace the furfuryl alcohol while NAD+ remained with FurX (Step E2 in Fig. 5, Fig. 2H) probably due to a restricted domain motion. In solution, a rapid dissociation of NAD+ is also expected by a free relative rotation between the two domains allowing a Zn2+ coordination of the Glu66 (Step E1). Existence of this E1 form of the furfuryl alcohol complex is very possible considering the position of glycerol and isopropanol in our binary complex structure, although we have not established the binary complex for furfuryl alcohol due to an instability of FurX crystal in 100% furfuryl alcohol. It is likely that furfuryl alcohol binds to apo-form FurX through the same stacking interaction with Trp91 and a hydrogen bond interaction with the side-chain of Thr44 without any significant electrostatic interaction with the Zn2+ (Step E1). The measured affinities of FurX for both NAD+ (Kd of 87 μM) and furfuryl alcohol (> 1 mM) are lower than those of NADH (0.043 μM) and furfural (5.4 μM) (Li et al., 2011a) (Supplemental Data 4). As expected, the catalytic efficiency (kcat/Km) for furfural reduction is 650 (s−1 mM−1), superior than the value of 26.7 for furfuryl alcohol oxidation (Li et al., 2011a). The Km value for furfural (20 μM) was much lower than that (150 μM) for furfuryl alcohol (Li et al., 2011a). Thus, the binary (Step E1) and tertiary complexes (Step D) can form in the situation of high concentrations of furfuryl alcohol and/or NAD+ (counterclockwise from Step A in Fig. 5). Through the same domain closure, the substrate’s hydroxyl group approaches the Zn2+ ion and is deprotonated by Thr44 and the associated proton shuttle system, allowing the formation of a Zn2+ bound alkoxide form. A hydride from C1 is simultaneously transferred to the C4 atom of NAD+ through a hydrogen-tunnelling event (Kohen et al., 1999) forming furfural. In general, our results are in agreement with the reactions catalysed by HLADH (Plapp, 2010). However, our data provide the structural events associated with catalytic turnover that have not been obtained with HLADH due to the use of poor substrate, e.g. p-bromobenzyl alcohol, or substrate analogues, e.g. dimethyl sulphoxide (Eklund et al., 1982).
The observed slow dissociation of NAD+ is likely due to its multi-step processes in concert with a domain opening and reconfiguration of Glu66. Therefore, the time required for NAD+ dissociation is long enough for ethanol to associate and to generate NADH, which is then used to reduce another furfural without coenzyme being dissociated from FurX. This is in agreement with our previous kinetic observation that NAD+/NADH remains during ethanol-dependent reduction of furfural by FurX (Li et al., 2011a).
Experimental procedures
Expression and purification of FurX
The cloning of furX into expression vector pET30 LIC with E. coli BL21(DE3) as the host has been previously reported (Li et al., 2011a). The cells were cultured in 1 l of Luria–Bertani (LB) medium with 30 μg ml−1 kanamycin at 30°C with shaking. Protein expression was induced by addition of isopropyl β-D-thiogalactopyranoside to a final concentration of 0.2 mM at mid-log-phase growth (A600 = ~0.6). Zinc sulphate was also added to 0.2 mM, which allowed the production of more soluble FurX. After incubating for eight additional hours, the induced cells were harvested by centrifugation. The overex-pressed FurX was purified according to the reported method that was developed to purify FurX from C. necator JMP134 (Li et al., 2011a). The method consisted of a 10 min treatment at 60°C, a phenyl agarose column, and an anion-exchange column (Mono Q™ 10/100 GL GE Healthcare). The overall yields for FurX were approximately 15 mg from 1 l of culture.
Molecular mass determination
The weight-average molecular mass of FurX was measured by combined size exclusion chromatography and multi-angle laser light scattering as previously described (Youn et al., 2005).
Crystallization and data collection
Apo-form crystals of recombinant FurX were grown at 4°C using the hanging drop vapour diffusion method. For apo-form crystallization, the solution of purified FurX (10 mg ml−1) in 20 mM Tris buffer (pH 8) was mixed with an equal volume of the reservoir solution (0.2 M MgCl2, 0.1 M HEPES, 30% PEG400) and equilibrated against the reservoir. Diffraction quality crystals appeared after 3 days. The apo-form crystals of FurX belong to the P1 space group. There were four FurX molecules in the asymmetric unit. Diffraction data up to 1.7 Å resolution were collected at Berkeley Advanced Light Source (ALS, beam line 8.2.1). NADH-complex crystals were made in a solution containing 10 mg ml−1 FurX and 1 mM NADH, which were mixed with an equal volume of the reservoir solution [2 M (NH4)2SO4, 5% of isopropanol]. The space group of NADH-complex crystals was also P1 but contained eight FurX molecules in the asymmetric unit. Diffraction data were collected up to 2.0 Å resolution for the complex crystals. The ternary complex crystals were obtained by soaking the NADH-complex crystals for 1 or 30 min at 4°C in a crystallization mother liquor solution containing 50% (v/v) of furfural, then frozen in the liquid nitrogen using 100% of furfural as the cryoprotectant. The corresponding ternary complex data were collected at the Berkeley Advanced Light Source (ALS, beam line 8.2.1). All diffraction data were processed and scaled with the HKL2000 package (Otwinowski et al., 2003) and CrystalClear 1.3.6 (Rigaku/MSC). The statistics for the diffraction data are listed in Table 1. A similar approach for the ternary complex of furfuryl alcohol was unsuccessful due to the instability of FurX in furfuryl alcohol. Initial phasing of apo-form FurX diffraction data was done by the program AMoRe (Navaza, 2001) through the coordinates of ADH from Brucella melitensis (3MEQ). Iterative model building and refinement took place using the programs COOT (Emsley et al., 2010) and PHENIX (Adams et al., 2010). All FurX coordinates have been deposited in the Protein Data Bank: 3S1L (apo-form), 3S2E (Water–NADH complex), 3S2F (Furfural–NADH complex I), 3S2G (Furfural–NADH complex II, 1 min soaked crystal in 50% furfural), 3S2I (Furfural–NADH complex III, 30 min soaked crystal in 50% furfural).
Table 1.
Crystallographic data for the FurX apo, binary and ternary complex forms.
| Data | Apo | Water–NADH complex (glycerol as cryoprotectant) | Furfural–NADH complex I (furfural as cryoprotectant) | Furfural–NADH complex II (1 min soaked in 50% furfural) | Furfural–NADH complex III (30 min soaked in 50% furfural) |
|---|---|---|---|---|---|
| Wavelength (Å) | 1.03 | 1.03 | 1.54 | 1.03 | 1.03 |
| Resolution (Å) | 35.5–1.72 | 46.1–1.76 | 46.0–1.88 | 46.0–1.9 | 46.0–2.0 |
| Space group | P1 | P1 | P1 | P1 | P1 |
| Cell dimensions (Å) | a = 65.46 | a = 68.19 | a = 68.27 | a = 68.08 | a = 68.28 |
| b = 67.88 | b = 92.74 | b = 92.85 | b = 92.47 | b = 93.04 | |
| c = 84.69 | c = 117.68 | c = 117.69 | c = 116.855 | c = 117.75 | |
| α = 76.49 | α = 106.11 | α = 106.08 | α = 106.28 | α = 106.33 | |
| β = 76.96 | β = 89.95 | β = 89.97 | β = 90.00 | β = 90.07 | |
| γ = 86.30 | γ = 90.13 | γ = 89.98 | γ = 89.97 | γ = 90.00 | |
| Asymmetric unit | 4 | 8 | 8 | 8 | 8 |
| Total observations | 374 738 | 505 867 | 387 125 | 359 069 | 320 843 |
| Unique reflections | 201 668 | 262 682 | 209 479 | 196 750 | 166 248 |
| Completeness (%) | 99.9 (99.7) | 96.3 (78.2) | 93.1 (61.8) | 87.8 (31.1) | 88.8 (57.5) |
| Rsyma,b | 4.1 (13.6) | 3.8 (28.3) | 11.2 (25.4) | 11.2 (20.5) | 13.7 (27.5) |
| Refinement | |||||
| Resolution (Å) | 35.5–1.90 | 46.1–1.76 | 46–2.0 | 45.9–2.3 | 29.9–2.0 |
| Number of reflections | 104 686 (96.2%) | 251 263 (91.9%) | 166 925 (89.0%) | 114 030 (95.6%) | 154 317 (82.15%) |
| Rcrystc | 18.35 | 16.47 | 18.18 | 19.84 | 20.10 |
| Rfreed | 22.57 | 19.35 | 21.38 | 22.96 | 24.84 |
| rmsd bonds (Å) | 0.003 | 0.010 | 0.003 | 0.002 | 0.003 |
| rmsd angles (°) | 0.800 | 1.306 | 0.954 | 0.647 | 0.818 |
| Number of atoms | |||||
| Protein and ligand | 10 102 | 20 445 | 20 424 | 20 431 | 20 324 |
| Water | 905 | 2 377 | 2 225 | 1 394 | 1 501 |
Numbers in parentheses refer to the highest-resolution shell.
Rsym = Σ|Ih − <Ih>|/ΣIh, where <Ih> is the average intensity over symmetry equivalent reflections.
Rcryst = Σ|Fobs − Fcalc|/ΣFobs, where summation is over the data used for refinement.
Rfree was calculated as for Rcryst using 5% of the data that was excluded from refinement.
Supplementary Material
Acknowledgments
Original research was supported by NSF (MCB 1021148, DBI 0959778) and M.J. Murdock Charitable Trust. We thank C Ralston (Berkeley Advanced Light Source, beamline 8.2.1) for help with data collection.
Footnotes
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adams P, Afonine P, Bunkóczi G, Chen V, Davis I, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Bertilsson M, Gorwa-Grauslund M, Gorsich S, Liden G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl Microbiol Biotechnol. 2009;82:625–638. doi: 10.1007/s00253-009-1875-1. [DOI] [PubMed] [Google Scholar]
- Boopathy R, Bokang H, Daniels L. Biotrans-formation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J Ind Microbiol Biotechnol. 1993;11:147–150. [Google Scholar]
- Bowman M, Jordan D, Vermillion K, Braker J, Moon J, Liu Z. Stereochemistry of furfural reduction by a Saccharomyces cerevisiae aldehyde reductase that contributes to in situ furfural detoxification. Appl Environ Microbiol. 2010;76:4926–4932. doi: 10.1128/AEM.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändén C, Eklund H, Nordström B, Boiwe T, Söderlund G, Zeppezauer E, et al. Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci USA. 1973;70:2439–2442. doi: 10.1073/pnas.70.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna-Cesari F, Perahia D, Karplus M, Eklund H, Braden C, Tapia O. Interdomain motion in liver alcohol dehydrogenase. Structural and energetic analysis of the hinge bending mode. J Biol Chem. 1986;261:15273–15280. [PubMed] [Google Scholar]
- Dickinson F, Monger G. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973;131:261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund H, Branden C-I. Biological Macromolecules and Assemblies. New York: Wiley-Interscience; 1987. pp. 73–142. [Google Scholar]
- Eklund H, Ramaswamy S. Medium- and short-chain dehydrogenase/reductase gene and protein families: three-dimensional structures of MDR alcohol dehydrogenases. Cell Mol Life Sci. 2008;65:3907–3917. doi: 10.1007/s00018-008-8589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund H, Plapp B, Samama J, Brändén C. Binding of substrate in a ternary complex of horse liver alcohol dehydrogenase. J Biol Chem. 1982;257:14349–14358. [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott W, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito L, Bruno I, Sica F, Raia C, Giordano A, Rossi M, et al. Crystal structure of a ternary complex of the alcohol dehydrogenase from Sulfolobus solfataricus. Biochemistry. 2003;42:14397–14407. doi: 10.1021/bi035271b. [DOI] [PubMed] [Google Scholar]
- Heer D, Sauer U. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb Biotechnol. 2008;1:497–506. doi: 10.1111/j.1751-7915.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoydonckx H, Van Rhijn M, Van Rhijn W, De Vos D, Jacobs P. Furfural and Derivatives. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- Jordan D, Braker J, Bowman M, Vermillion K, Moon J, Liu Z. Kinetic mechanism of an aldehyde reductase of Saccharomyces cerevisiae that relieves toxicity of furfural and 5-hydroxymethylfurfural. Biochim Biophys Acta. 2011;1814:1686–1694. doi: 10.1016/j.bbapap.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Karlsson A, El-Ahmad M, Johansson K, Shafqat J, Jörnvall H, Eklund H, Ramaswamy S. Tetrameric NAD-dependent alcohol dehydrogenase. Chem Biol Interact. 2003:143–144. 239–245. doi: 10.1016/s0009-2797(02)00222-3. [DOI] [PubMed] [Google Scholar]
- Kleifeld O, Shi S, Zarivach R, Eisenstein M, Sagi I. The conserved Glu-60 residue in Thermoanaerobacter brockii alcohol dehydrogenase is not essential for catalysis. Protein Sci. 2003;12:468–479. doi: 10.1110/ps.0221603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke H, Thomsen A, Ahring B. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- Kohen A, Cannio R, Bartolucci S, Klinman J. Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature. 1999;399:496–499. doi: 10.1038/20981. [DOI] [PubMed] [Google Scholar]
- Korkhin Y, Kalb AJ, Peretz M, Bogin O, Burstein Y, Frolow F. NADP-dependent bacterial alcohol dehydrogenases: crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium beijerinckii and Thermoanaerobacter brockii. J Mol Biol. 1998;278:967–981. doi: 10.1006/jmbi.1998.1750. [DOI] [PubMed] [Google Scholar]
- Kovaleva E, Plapp B. Deprotonation of the horse liver alcohol dehydrogenase–NAD+ complex controls formation of the ternary complexes. Biochemistry. 2005;44:12797–12808. doi: 10.1021/bi050865v. [DOI] [PubMed] [Google Scholar]
- Laadan B, Almeida J, Rådström P, Hahn-Hägerdal B, Gorwa-Grauslund M. Identification of an NADH-dependent 5-hydroxymethylfurfural-reducing alcohol dehydrogenase in Saccharomyces cerevisiae. Yeast. 2008;25:191–198. doi: 10.1002/yea.1578. [DOI] [PubMed] [Google Scholar]
- Larroy C, Fernandez M, Gonzalez E, Pares X, Biosca J. Characterization of the Saccharomyces cerevisiae YMR318C (ADH6) gene product as a broad specificity NADPH-dependent alcohol dehydrogenase: relevance in aldehyde reduction. Biochem J. 2002a;361:163–172. doi: 10.1042/0264-6021:3610163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroy C, Pares X, Biosca J. Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur J Biochem. 2002b;269:5738–5745. doi: 10.1046/j.1432-1033.2002.03296.x. [DOI] [PubMed] [Google Scholar]
- Levin I, Meiri G, Peretz M, Burstein Y, Frolow F. The ternary complex of Pseudomonas aeruginosa alcohol dehydrogenase with NADH and ethylene glycol. Protein Sci. 2004;13:1547–1556. doi: 10.1110/ps.03531404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lam L, Xun L. Biochemical characterization of ethanol-dependent reduction of furfural by alcohol dehydrogenases. Biodegradation. 2011a;22:1227–1237. doi: 10.1007/s10532-011-9477-x. [DOI] [PubMed] [Google Scholar]
- Li Q, Lam L, Xun L. Cupriavidus necator JMP 134 rapidly reduces furfural through a Zn-dependent alcohol dehydrogenase. Biodegradation. 2011b;22:1215–1225. doi: 10.1007/s10532-011-9476-y. [DOI] [PubMed] [Google Scholar]
- Liu Z, Moon J. A novel NADPH-dependent aldehyde reductase gene from Saccharomyces cerevisiae NRRL Y-12632 involved in the detoxification of aldehyde inhibitors derived from lignocellulosic biomass conversion. Gene. 2009;446:1–10. doi: 10.1016/j.gene.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Liu Z, Slininger P, Dien B, Berhow M, Kurtzman C, Gorsich S. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol. 2004;31:345–352. doi: 10.1007/s10295-004-0148-3. [DOI] [PubMed] [Google Scholar]
- Liu Z, Moon J, Andersh A, Slininger P, Weber S. Multiple gene mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and HMF by ethanologenic yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2008;81:743–753. doi: 10.1007/s00253-008-1702-0. [DOI] [PubMed] [Google Scholar]
- Luo J, Bruice T. Dynamic structures of horse liver alcohol dehydrogenase (HLADH): results of molecular dynamics simulations of HLADH-NAD(+)-PhCH(2)OH, HLADH-NAD(+)-PhCH(2)O(−), and HLADH-NADH-PhCHO. J Am Chem Soc. 2001;123:11952–11959. doi: 10.1021/ja0109747. [DOI] [PubMed] [Google Scholar]
- Miller E, Jarboe L, Yomano L, York S, Shanmugam K, Ingram L. Silencing of NADPH-dependent oxidoreductase genes (yqhD and dkgA) in furfural-resistant ethanologenic Escherichia coli. Appl Environ Microbiol. 2009;75:4315–4323. doi: 10.1128/AEM.00567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modig T, Lidén GG, Taherzadeh M. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem J. 2002;363:769–776. doi: 10.1042/0264-6021:3630769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaza J. Implementation of molecular replacement in AMoRe. Acta Crystallogr D Biol Crystallogr. 2001;57:1367–1372. doi: 10.1107/s0907444901012422. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Borek D, Majewski W, Minor W. Multiparametric scaling of diffraction intensities. Acta Crystallogr A. 2003;59:228–234. doi: 10.1107/s0108767303005488. [DOI] [PubMed] [Google Scholar]
- Peretz M, Bogin O, Keinan E, Burstein Y. Stereospecificity of hydrogen transfer by the NADP-linked alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Int J Pept Res. 1993;42:490–495. doi: 10.1111/j.1399-3011.1993.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Plapp B. Conformational changes and catalysis by alcohol dehydrogenase. Arch Biochem Biophys. 2010;493:3–12. doi: 10.1016/j.abb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Kratzer D, Hershey A, Rogers P, Arnone A, Eklund H, Plapp B. Crystallization and preliminary crystallographic studies of Saccharomyces cerevisiae alcohol dehydrogenase I. J Mol Biol. 1994;235:777–779. doi: 10.1006/jmbi.1994.1031. [DOI] [PubMed] [Google Scholar]
- Rubach J, Plapp B. Amino acid residues in the nicotinamide binding site contribute to catalysis by horse liver alcohol dehydrogenase. Biochemistry. 2003;42:2907–2915. doi: 10.1021/bi0272656. [DOI] [PubMed] [Google Scholar]
- Silverstein E, Boyer P. Equilibrium reaction rates and the mechanisms of live and yeast alcohol dehydrogenase. J Biol Chem. 1964;239:3908–3914. [PubMed] [Google Scholar]
- Stone C, Li T, Bosron W. Stereospecific oxidation of secondary alcohols by human alcohol dehydrogenases. J Biol Chem. 1989;264:11112–11116. [PubMed] [Google Scholar]
- Theorell H. Function and structure of liver alcohol dehydrogenase. Harvey Lect. 1967;61:17–41. [PubMed] [Google Scholar]
- Vallee B, Auld D. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci USA. 1990;87:220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold E, Glasfeld A, Ellington A, Benner S. Structural determinants of stereospecificity in yeast alcohol dehydrogenase. Proc Natl Acad Sci USA. 1991;88:8420–8424. doi: 10.1073/pnas.88.19.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn B, Moinuddin S, Davin L, Lewis N, Kang C. Crystal structures of apo-form and binary/ternary complexes of Podophyllum secoisolariciresinol dehydrogenase, an enzyme involved in formation of health-protecting and plant defense lignans. J Biol Chem. 2005;280:12917–12926. doi: 10.1074/jbc.M413266200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.