Antagonism between stem cells is not new. Competitive mechanisms are known to be critical for the modulation of organ homeostasis and regeneration. Competitive interaction within the niches results in survival of the fittest stem cells and death of the more vulnerable cells. An upregulation of c-myc transforms cells into “supercompetitors” capable of clonal expansion. The cluster of supercompetitors influences the behavior of the weakest surrounding cells, which are at a growth disadvantage.1 The presence of supercompetitors within niches regulates niche function, and the absence of supercompetitors may alter the preservation of stem cell self-renewal, leading to the generation of old dysfunctional niches.2 However, supercompetitor stem cells may fail to trigger apoptosis in the neighboring aging cells, promoting uncontrolled growth arrest and cellular senescence. This process may become excessive, favoring the formation of crowded niches where old stem cells predominate, opposing the activation of young functionally-competent stem cells. Thus, cooperative cell-to-cell communication may regulate more effectively the fate of stem cells within the niches, since the supporting cells transmit growth signals to stem cells according to the need of the organ and organism.
The concept of cooperation between stem cells and cardiomyocytes, functioning as supporting cells within the myocardial niches, has previously been documented3 but the study by Williams et al. in this issue of Circulation reports the cooperation between human mesenchymal stromal cells (MSCs) and human c-kit-positive cardiac stem cells (CSCs) following myocardial infarction in the swine heart.4 This clever approach has combined the regenerative potential of CSCs with the powerful secretory phenotype of MSCs. The low efficiency of transdifferentiation of MSCs has been overcome by the ability of the delivered CSCs to expand locally and create a large myocyte progeny, together with the required coronary microcirculation.
Despite the significant growth reserve of the human heart provided by the pool of resident CSCs,5 spontaneous cardiac repair does not occur after infarction and the necrotic tissue is not restored by intact myocardium.6 Myocyte and vessel formation, mediated by activation and lineage specification of resident CSCs, is restricted to the surviving myocardium, while apoptosis of CSCs distributed within the ischemic region of the ventricular wall prevents tissue reconstitution allowing the evolution of the healing process and the generation of a thick, nonfunctional scar. This inevitable progression of myocardial infarction establishes the basis for cell therapy and the search for the most appropriate cell for the replacement of a non-contracting scar with mechanically efficient cardiomyocytes, and arterioles and capillaries integrated with the main coronary circulation. The lack of endogenous regeneration after infarction is not restricted to the human heart but is present in solid and non-solid organs including the skin, liver, intestine, kidney, and bone marrow. In all cases, occlusion of a supplying artery leads to scar formation mimicking cardiac pathology.7 The stem cell compartment is properly equipped to regulate tissue homeostasis8 but does not respond effectively to ischemic injury, or late in life to aging and senescence of the organ and organism.8,9
Shortly after the experimental evidence that hematopoietic stem cells (HSCs) induce myocardial regeneration after infarction, unfractionated mononuclear bone marrow cells and CD34 positive cells were administered to patients affected by acute and chronic myocardial infarction, dilated cardiomyopathy, and refractory angina. Although the individual outcomes have been inconsistent and variability exists among trials, meta-analyses of pooled data indicate that bone marrow cell therapy results in a 3–4% increase in ejection fraction, reduction in infarct size and left ventricular end-systolic volume, together with decreases in left ventricular end-diastolic volume, and improved survival.10 The positive consequences of bone marrow cells have consistently been documented regardless of the differences in the type of cells injected, choice of clinical endpoints, methods for the evaluation of cardiac function, and the interval between the onset of the cardiac disease and bone marrow cell infusion.10 A critical question, however, persists and will have to be answered; none of these trials has employed c-kit-positive HSCs, which provided the initial experimental evidence of significant myocardial regeneration after infarction, resulting in a dramatic improvement of ventricular performance and a significantly decreased mortality in animal models of the human disease.
Recently, allogeneic and autologous bone marrow-derived MSCs have been employed in small clinical trials, and encouraging results have been published.11–13 Although the benefits may seem modest, these initial data highlight the need for larger randomized trials designed to critically evaluate the long-term effects of MSCs on a broader patient population. However, the mechanisms involved in the positive impact of MSCs on human beings remains to be identified. The impossibility to permanently label the cells to be delivered and the difficulty to obtain cardiac biopsies to assess parameters consistent with myocardial regeneration leaves uncertain our understanding of the cellular processes mediating partial myocardial recovery. Measurements of coronary blood flow suggest that vasculogenesis may be operative, while the contribution of de novo myocyte formation is uncertain. Reductions in infarct scar size speak in favor of myocardial regeneration, but unequivocal data have not been obtained yet. The most popular hypotheses include development of coronary vessels and enhanced blood supply to areas of hibernating myocardium, vasculogenesis and cardiomyogenesis, and growth activation and differentiation of resident CSCs via a paracrine effect, mediated by the release of a multiplicity of cytokines by the administered MSCs.14 The ability of MSCs to interfere with the relevant inflammatory response associated with ischemic myocardial injury adds value to the therapeutic efficacy of this class of primitive bone marrow cells.
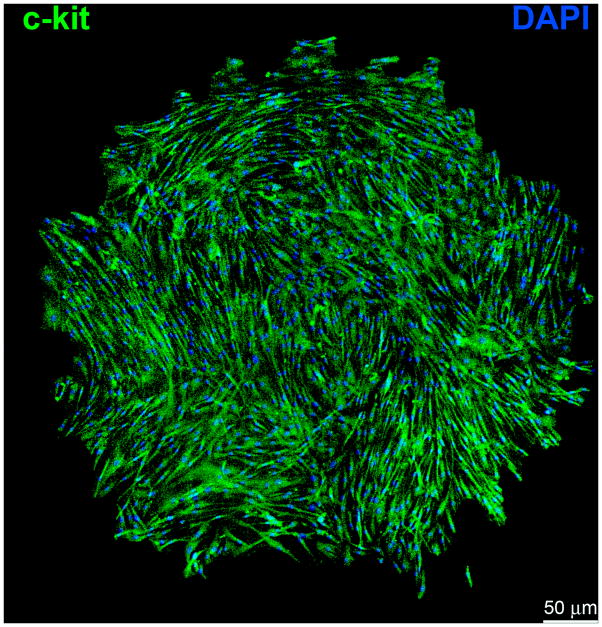
Importantly, the identification of c-kit-positive CSCs (Figure 1) has shifted the attention to endogenous cell processes as novel targets of cell-based therapy for the failing heart.5,15 The appreciation that the heart is a dynamic organ constantly renewing its cell populations8 has generated great enthusiasm in the scientific and medical community. Understanding the mechanisms of cardiac homeostasis would offer the extraordinary opportunity to potentiate this naturally occurring process and promote myocardial regeneration following injury. Currently, a phase 1 clinical trial utilizing autologous c-kit-positive CSCs is being completed with encouraging results.16
Figure 1.
Clonal c-kit-positive human cardiac stem cells (CSCs). Single cell-derived clone in Terasaki plate. Human CSCs are self-renewing and clonogenic, fundamental properties of tissue specific adult stem cells. c-kit, green; nuclei, DAPI, blue.
An attempt to mimic in humans the strategy implemented by Williams et al. in large animals4 has been made by delivering to patients with myocardial infarction cardiosphere-derived cells.17 Cardiospheres contain a core of c-kit-positive primitive cells, several layers of differentiating cells expressing myocyte proteins and connexin 43, and an outer sheet composed of cells positive for CD105, a classic epitope of MSCs. Biologically, cardiospheres can be regarded as a simplified in vitro system of cardiac differentiation.18 But whether the utilization of cells already committed to the myocyte, endothelial cell and smooth muscle cell lineages is preferable to the use of a pure population of undifferentiated human CSCs remains to be determined.
The protocol introduced experimentally in Dr. Hare’s laboratory aims at the combination of an established clinically-relevant MSC with an equally documented effective undifferentiated, extensively characterized human c-kit-positive CSC. Conversely, the clinical use of cardiospheres, which are a partially defined heterogeneous cell preparation, may result in a greater array of unpredictable effects than a uniform population of identical cells with well-defined biological characteristics. In analogy with pharmacological approaches, the combination of different drugs in the same pill is coupled with ease of administration but may not allow dosage flexibility and personalized therapy. This is not the case in the approach utilized by Williams et al. in which the relative proportion of MSCs and CSCs was carefully controlled. In their study, 10 intramyocardial injections, each consisting of 20 × 106 MSCs and 10 × 104 CSCs, were employed to rescue the infarcted myocardium in a large animal model.4
Whether adjustments in the ratio between MSCs and CSCs are required, and whether repeated injections are needed to sustain over time the functional benefit remains to be determined. If different classes of progenitor cells have to be tested, Dr. Hare’s strategy offers a sophisticated carefully planned protocol that may not be initially perfect, but can be properly modified to reach the maximum effect in animals and, ultimately, in humans. The striking difference in the number of injected MSCs and CSCs makes a direct comparison between these two cell classes essentially impossible, but this has to be seen as the beginning of a more complex and demanding approach for the management of human ischemic cardiomyopathy. Clearly there is the need for more work in order to properly advance the field of cell therapy which is currently in its infancy.
The remarkable recovery in the anatomical and functional integrity of the infarcted myocardium following combined cell therapy4 raises important questions concerning the mechanisms involved. The heart typically shows randomly oriented ellipsoid compartments where CSCs are clustered together forming myocardial niches. These pockets of CSCs are connected structurally and functionally to myocytes and fibroblasts that constitute the supporting cells within the niches; gap and adherens junctions made, respectively, by connexins and cadherins ensure the “cooperative” interaction regulating stem cell function and niche homeostasis.3,19 Whether the administration of MSCs and CSCs leads to the formation of temporary and/or permanent niches within the host swine myocardium has not yet been resolved and constitutes a major challenge for future research. The actual transfer of molecules between MSCs and CSCs remains to be shown to uncover the fundamental cellular processes involved in the extensive repair of the damaged heart.
Cardiac niches create the necessary, permissive milieu for the long-term residence and growth of CSCs.20 Similarly, the survival of exogenously administered stem cells requires their engraftment within the recipient myocardium. However, the region bordering the infarct is exposed to increased wall stress and functional demand, which promotes myocyte hypertrophy, activation of interstitial fibroblasts and collagen accumulation. These variables may modify transiently the environment of the preexisting niches and prevent, at least in part, the formation of new temporary niches. The combination therapy applied by Dr. Hare and his team may attenuate this problem; the delivered MSCs may provide an environment favoring the engraftment of CSCs and the acquisition of the cardiogenic fate.
The border zone is characterized by an intense regenerative response that promotes, in part, the recovery of the lost muscle mass following myocardial infarction. This adaptation requires the sustained activation of CSCs, which give rise to dynamic sites of cardiomyogenesis. The presence of MSCs may enhance the symmetric division of exogenous and endogenous CSCs into two daughter committed cells potentiating cardiac repair shortly after injury. However, the layers of MSCs organized in niche-like structures may prevent the cross-talk between CSCs and their supporting cells, the cardiomyocytes (Figure 2). The lack of physiological communication may fail to preserve the asymmetric modality of stem cell division. In the absence of this pattern of cell growth, depletion of resident CSCs may ensue, affecting ventricular performance chronically. MSCs may represent a temporary protective shield in which the survival of CSCs is favored but the long-term proliferation and self-renewal ability of exogenous and endogenous CSCs may be hampered, possibly attenuating the stability and durability of cell-based therapy in its various forms. These issues are consistent with the questions posed by Dr. Hare and his team, and collaborative efforts between laboratories with extensive experience in MSCs and CSCs are warranted in the search for the most powerful form of cell therapy for the injured human heart.
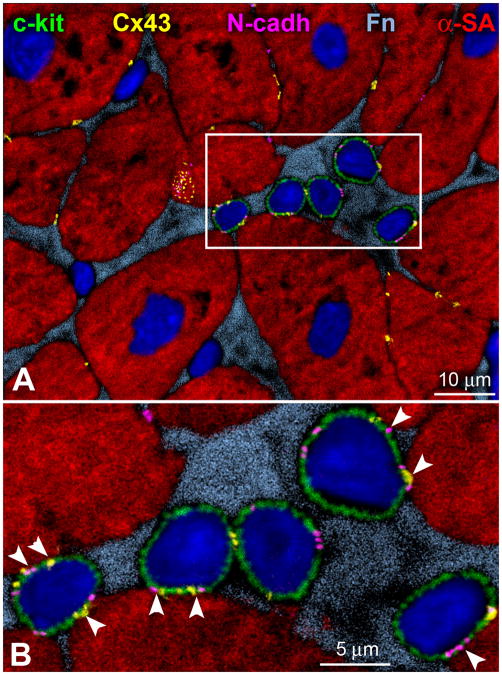
Figure 2.
Human CSC niche. A, c-kit-positive (green) human CSCs are nested together in the myocardial interstitium and are surounded by fibronectin (Fn, bright blue). These human CSCs are connected by gap junctions represented by connexin 43 (Cx43, yellow dots), and adherens junctions shown by N-cadherin (N-cadh, magenta) to cardiomyocytes (α-sarcomeric actin, α-SA, red). B, The area included in the rectangle in panel A is illustrated at higher magnification in panel B where these cell-to-cell communications between CSCs and cardiomyocytes are indicated by arrowheads. Additionally, Cx43 is detected between CSCs.
Acknowledgments
Funding Sources: This work was supported by NIH grants.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Anversa P, Leri A, Kajstura J. Biased DNA segregation during stem cell division. Circ Res. 2012;110:1403–1407. doi: 10.1161/CIRCRESAHA.112.268961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivarelli S, Wagstaff L, Piddini E. Cell wars: regulation of cell survival and proliferation by cell competition. Essays Biochem. 2012;53:69–82. doi: 10.1042/bse0530069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogórek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation. 2013;127:XX–XXX. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajstura J, Bai Y, Cappetta D, Kim J, Arranto C, Sanada F, D’Amario D, Matsuda A, Bardelli S, Ferreira-Martins J, Hosoda T, Leri A, Rota M, Loscalzo J, Anversa P. Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium. Circ Res. 2012;111:894–906. doi: 10.1161/CIRCRESAHA.112.273649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–712. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kajstura J, Rota M, Cappetta D, Ogórek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D’Alessandro DA, Michler RE, Del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of Allogeneic vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA. 2012:1–11. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2012;113:1460–1469. doi: 10.1002/jcb.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundararaman B, Avitabile D, Konstandin MH, Cottage CT, Gude N, Sussman MA. Asymmetric chromatid segregation in cardiac progenitor cells is enhanced by Pim-1 kinase. Circ Res. 2012;110:1169–1173. doi: 10.1161/CIRCRESAHA.112.267716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anversa P, Kajstura J, Leri A. If I can stop one heart from breaking. Circulation. 2007;115:829–832. doi: 10.1161/CIRCULATIONAHA.106.682195. [DOI] [PubMed] [Google Scholar]
- 19.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]