Abstract
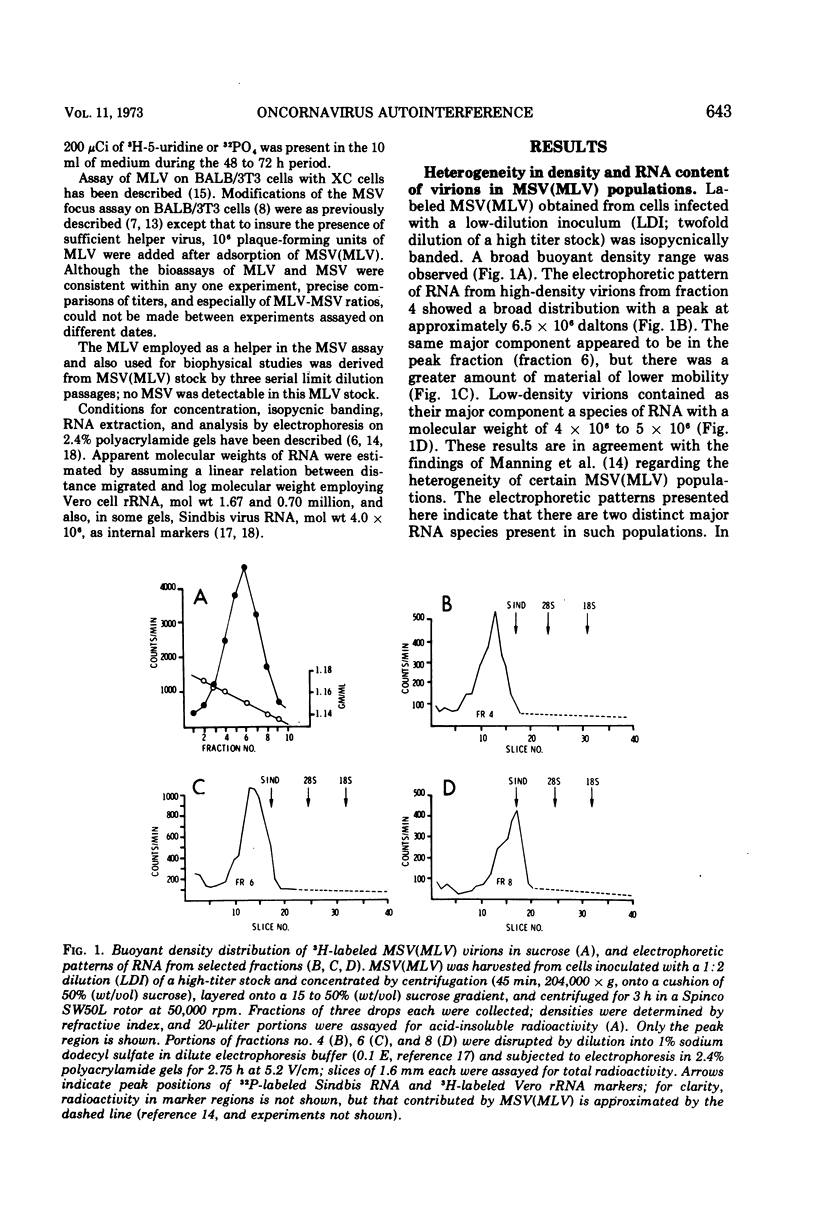
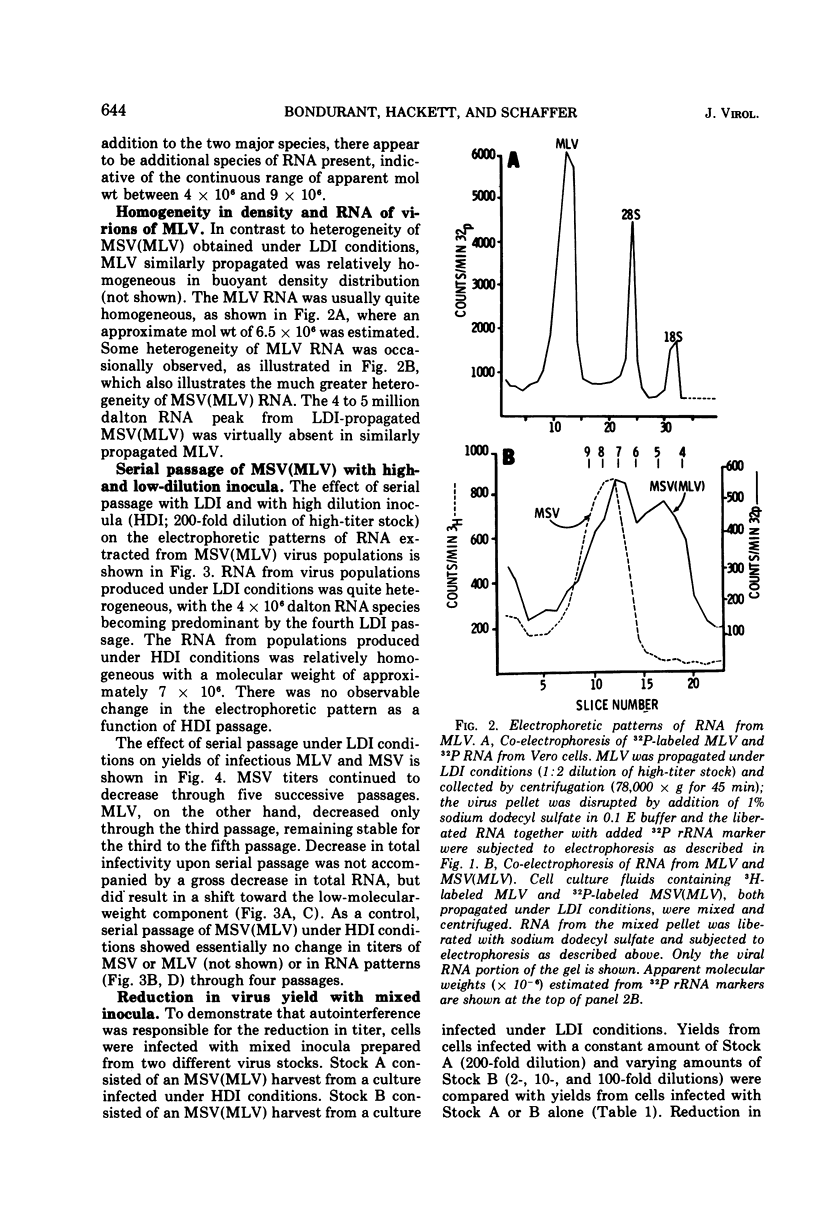
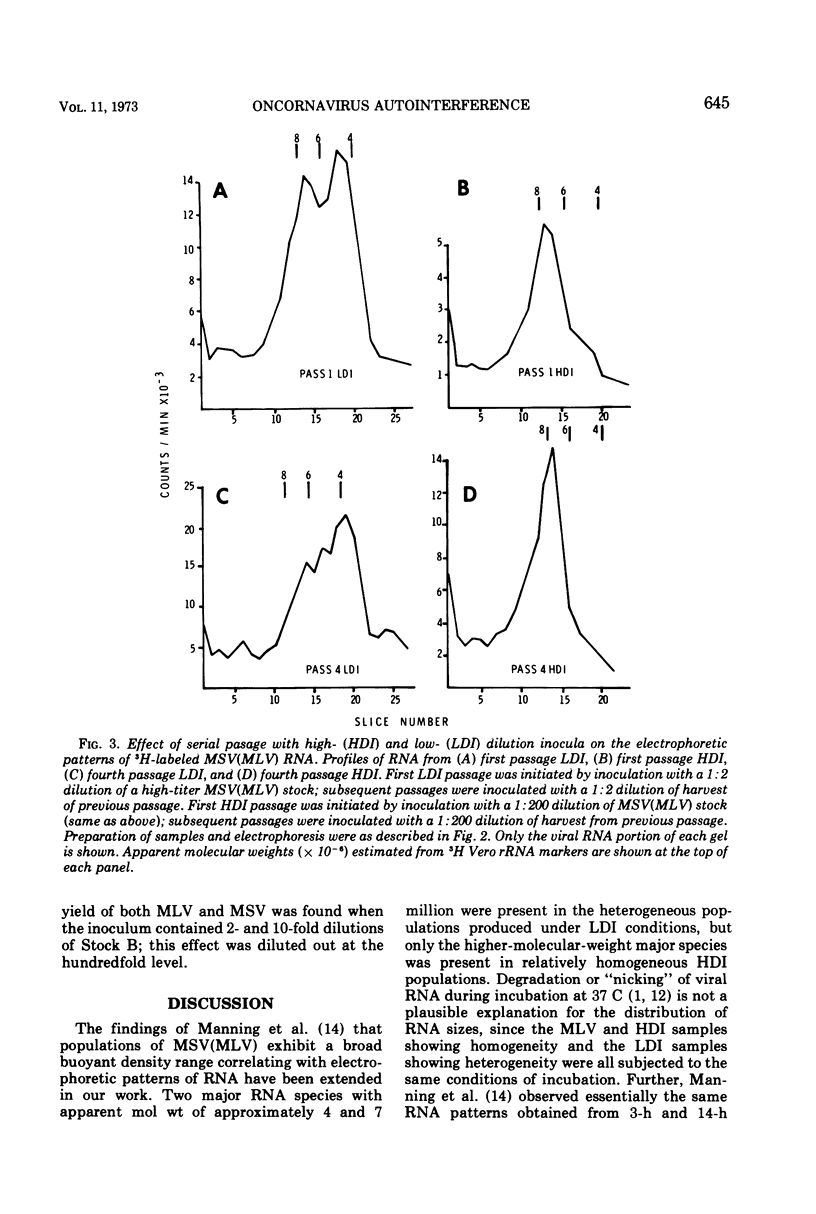
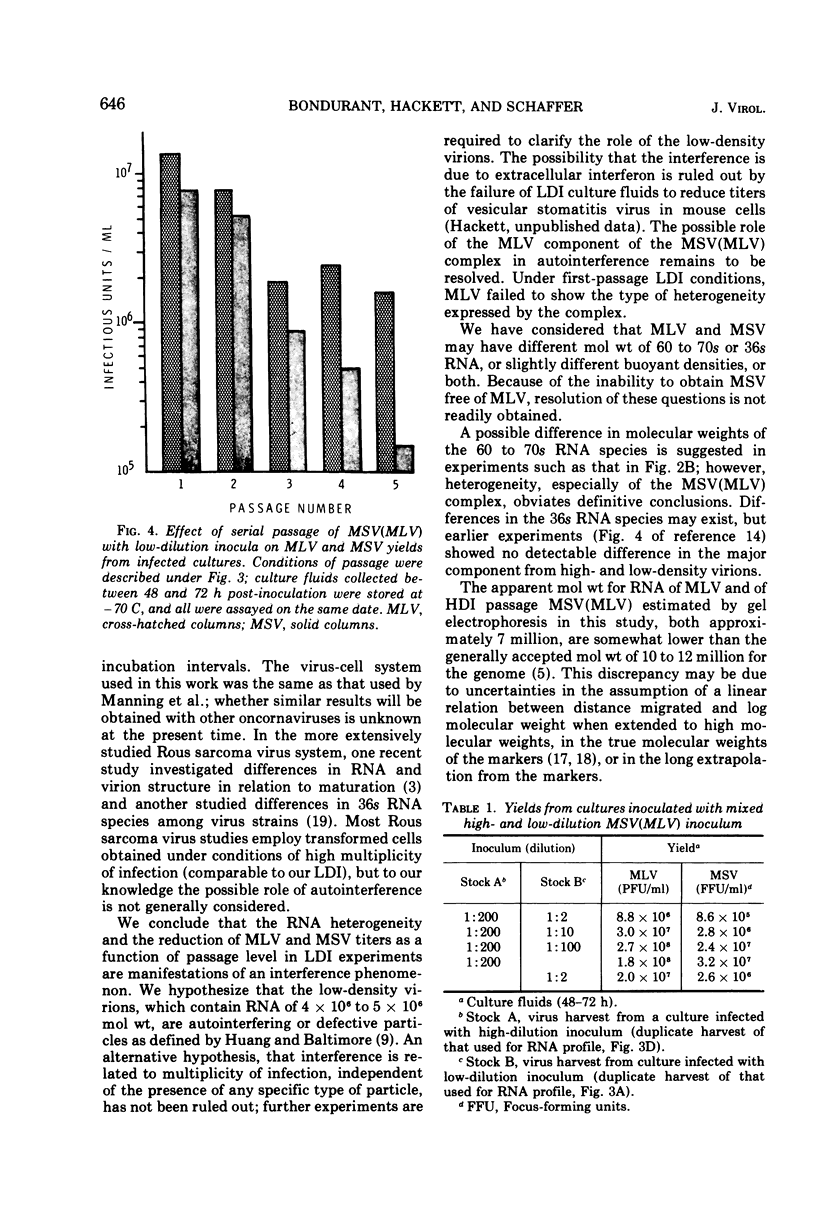
Heterogeneity of buoyant density and RNA content of virions of Moloney murine leukemia-sarcoma complex [MSV (MLV)] was the result of passage at low dilution. Heterogeneous stocks revealed two major RNA components in the population, with the smaller component, apparent mol wt 4 × 106 to 5 × 106, becoming predominant upon serial passage at low dilution. Concomitantly, infectivity titers of both MLV and MSV decreased upon serial passage at low dilution. MSV (MLV) passaged at high dilution retained high titers and a rather homogeneous high-molecular-weight RNA population characteristic of high-buoyant-density virions. Interference of both MLV and MSV replication was demonstrated by employing mixed inocula containing both low- and high-dilution passage stocks of MSV (MLV). In contrast to results with MSV (MLV), MLV freed of MSV by limit dilution did not show heterogeneity of buoyant density or of RNA when propagated at low dilution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstein M. E., Stanners C. P., Farmilo A. J. Heterogeneity of polyoma virus DNA: isolation and characterization of non-infectious small supercoiled molecules. J Mol Biol. 1969 Jun 14;42(2):301–313. doi: 10.1016/0022-2836(69)90045-x. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Manning J. S., Owens R. B. In vitro production of murine leukemia virus by cells differing in a single allele. J Natl Cancer Inst. 1971 Jun;46(6):1335–1342. [PubMed] [Google Scholar]
- Hackett A. J., Sylvester S. S. Cell line derived from Balb-3T3 that is transformed by murine leukaemia virus: a focus assay for leukaemia virus. Nat New Biol. 1972 Oct 11;239(93):164–166. doi: 10.1038/newbio239164a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Manning J. S., Hackett A. J., Darby N. B., Jr Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl Microbiol. 1971 Dec;22(6):1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. S., Schaffer F. L., Soergel M. E. Correlation between murine sarcoma virus buoyant density, infectivity, and viral RNA electrophoretic mobility. Virology. 1972 Sep;49(3):804–807. doi: 10.1016/0042-6822(72)90537-5. [DOI] [PubMed] [Google Scholar]
- McClain K., Kirsten W. H. Electrophoretic analysis of the RNA from a mouse leukemia virus. Cancer Res. 1972 Jul;32(7):1470–1475. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus autointerference. Fed Proc. 1969 Nov-Dec;28(6):1867–1874. [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E. Molecular weight estimates of vesicular stomatitis virus ribonucleic acids from virions, defective particles, and infected cells. Arch Gesamte Virusforsch. 1972;39(1):203–222. doi: 10.1007/BF01241543. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Soergel M. E., Straube D. C. Electrophoretic analysis of ribosomal and viral ribonucleic acids with a simple technique for slicing low-concentration polyacrylamide gels. Appl Microbiol. 1971 Oct;22(4):538–545. doi: 10.1128/am.22.4.538-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Electrophoretic analysis of the RNA of avian tumor viruses. Virology. 1972 Dec;50(3):753–764. doi: 10.1016/0042-6822(72)90429-1. [DOI] [PubMed] [Google Scholar]



