Full expression of congenital heart block (CHB), a pathologic readout of autoimmunity in pregnancy with significant morbidity and mortality rates, represents the sum of preclinical intrauterine variables and a morbid triggering event. While exposure to maternal anti-SSA/Ro and anti-SSB/La antibodies likely represents the trigger of disease, characterization of variables involved in generating a vulnerable fetal heart remains elusive. In this issue of Arthritis & Rheumatism, Reed et al (1) describe a novel interaction between a major candidate target of the maternal immune response in CHB, the 60-kd component of SSA/Ro (Ro 60), and β2-glycoprotein I (β2GPI), another autoantigen generally thought of in the context of increased thrombophilia (2). This novel interaction generates the hypothesis that a specific biochemical form of Ro 60 may be an important risk variable that is silenced by a putative protective pathway involving β2GPI. Review of the molecular structure and function of Ro 60, its translocation to the surface of apoptotic cells, and its subsequent role in physiologic clearance provides the contextual framework for placing an emphasis on β2GPI in the pathogenesis of CHB.
In the last decade, there have been several advances in understanding the structure and function of Ro 60. Ro 60 exists in an intracellular as well as in a surface-exposed form. Regarding the former, Ro 60 was found to play the role of central quality control clearing house for misfolded noncoding RNA (3). The structure of the complexed (to RNA) crystal form was solved (4). Ro 60 consists of 2 domains. One is a large α-helical HEAT repeat that forms a ring with a central hole. Adjacent to it is the other domain, a von Willebrand factor A domain. In addition, structural and biochemical analyses have begun to address how Ro recognizes its RNA substrates. There is an extensive platform within the α-helical HEAT repeat that binds to hYRNA (a polymerase III transcript) with a stoichiometry of Ro 60:RNA of 1:2. Moreover, the RNA binding site displays a plasticity which includes native and denatured pre5S ribosomal RNA (5). Ro is capable of binding several other noncoding RNAs that contain a 3′ single-stranded extension (a single-stranded 3′ end of >5 nucleotides, which is a feature of many noncoding RNAs). Furthermore, this interaction between Ro 60 and RNA may provide protection during times of cellular stress. Specifically, Ro 60 is important for the survival of mammalian cells after ultraviolet irradiation (6).
Ro 60 is dramatically altered during apoptosis into a distinct apoptotic form (Figure 1). During apoptosis, Ro 60 is surface translocated (7,8). Following translocation to the apoptotic cell membrane, Ro 60 undergoes a conformational change, with exposure of a neoepitope (referred to as an apotope). The mechanism of anchorage to the cell membrane may involve the utilization of “cryptic” transcellular domains located within the von Willebrand domains and/or an interaction with the chaperone protein calreticulin. Among the SSA/Ro and SSB/La antigens, Ro 60 appears to be the sole component of “early” apoptotic structures, while both antigens, including the Ro 52 component, are present on “late” apoptotic cells (9).
Figure 1.
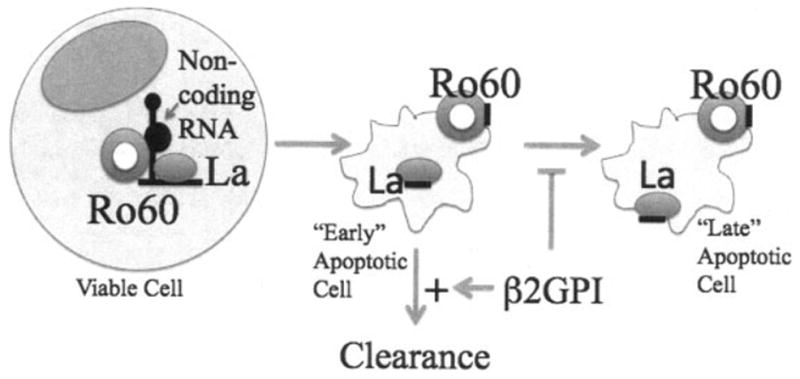
Novel role of β2-glycoprotein I (β2GPI) to protect against congenital heart block. The intracellular role of Ro 60 includes the binding of hYRNA (a polymerase III transcript) as well as many other noncoding RNAs (viable cell) (left). At the induction of apoptosis, Ro 60 and its bound RNA translocates to a surface site (early apoptotic cell) (center). The translocation of La 48 does not occur until later (late apoptotic cell) (right). β2GPI may exert beneficial effects, including its actions to promote the clearance of early apoptotic cells as well as to block the formation of late apoptotic cells.
It was recently shown in an in vitro coculturing system that human fetal cardiac myocytes participate in physiologic clearance of apoptotic cardiocytes but that clearance is inhibited by opsonization via maternal autoantibodies (10). Specifically, anti–Ro 60 IgG block the uptake of apoptotic cardiocytes by live cardiocytes, suggesting that this autoantigen may also function as a natural ligand for apoptotic cell clearance. Diversion of opsonized apoptotic cardiocytes to Fcγ receptor–mediated uptake by macrophages may then herald ligation of intracellular Toll-like receptors as they engage the newly delivered single-stranded RNA via a classic immune complex. There is strong evidence that clearance of dying cells is defective, as highlighted by immunohistologic findings in available cardiac sections obtained from several patients with CHB and/or myocarditis with varying degrees of disease (11).
Beta2-glycoprotein I is a 65-kd protein composed of 5 protein modules termed domain I through domain V (12). In addition to limiting coagulation and promoting fibrinolysis, another physiologic role of β2GPI may be to serve as an “opsonin” for promoting the clearance of apoptotic cells. Specifically, β2GPI has been shown to bind via domain V to phosphatidylserine exposed on the apoptotic cell surface and to simultaneously bind via domain I to low-density lipoprotein receptor–related protein on phagocytes.
In the context of Ro 60 as a critical component of a pathologic immune complex on the surface of fetal cardiocytes, the findings of Reed et al (1) may open a new avenue of research in CHB. The authors provide solid experimental evidence that β2GPI binds to an exposed region of Ro 60 on apoptotic cells and thereby masks the Ro 60 apotope. Using an in vitro reconstitution assay to measure the formation of heterodimers, it was demonstrated that a domain of Ro 60 (recombinant Ro 60 amino acids 82–244) binds to immobilized β2GPI and that this interaction is restricted to domain V. Late apoptotic Jurkat cells were bound by β2GPI, an interaction that was inhibited by coincubation of fluid-phase Ro 60, which inhibited the binding of β2GPI to the surface of apoptotic cells in a dose-dependent manner. These findings are consistent with the hypothesis that the Ro 60 autoantigen functions as a receptor for extracellular β2 GPI following translocation to the surface of apoptotic cells.
That Ro 60 is a newly described receptor for β2 GPI on an apoptotic cell surface raises several implications with regard to current thinking on the pathogenesis of CHB. Perhaps β2GPI represents one fetal variable that protects against antibody-triggered injury by blocking a Ro 60 apotope on cardiocytes undergoing physiologic cell death during embryogenesis and fetal remodeling. Prevention of immune complex formation would attenuate the downstream sequelae that yield inflammation and fibrosis.
Beta2-glycoprotein I can be likened to a finger pressed into a hole in a levee. Holding back destructive river waters, it staves off the tide of full-scale inflammation and fibrosis. How long can this “levee” hold? Moreover, any condition effectively lowering available levels of β2GPI would constitute a risk factor. Transplacentally derived maternal anti-β2GPI antibodies might reduce available levels of β2GPI. However, anti-β2GPI antibodies predominantly bind domain I (13) and not domain V, rendering this possibility less likely. Perhaps one important genetic fetal factor relates to gene mutations in β2GPI that alter binding to apoptotic cells. Additional issues, such as levee weakening or forceful river waters, which would be represented in our analogy by pathologic antibodies and noncoding RNAs, respectively, may prevent β2GPI from saving the town. The recruitment of additional pathogenic antibodies, such as anti-La and anti–Ro 52, may occur, as evidenced by immunohistologic findings in autopsy specimens, which show extensive apoptosis, implying onset of “late” apoptotic bodies. Also, a high demand for quality control of misfolded noncoding RNAs in fetal tissue or an environmental factor (hypoxia) may push the RNA binding to Ro 60 as well as its contribution to disease via Toll-like receptors to a maximum. Further research on β2GPI to evaluate its impact on the binding of anti–Ro 60 antibodies and its cognate antigen is clearly indicated.
References
- 1.Reed JH, Giannakopoulos B, Jackson MW, Krilis S, Gordon TP. Ro 60 functions as a receptor for β2-glycoprotein I on apoptotic cells. Arthritis Rheum. 2009;60:860–9. doi: 10.1002/art.24361. [DOI] [PubMed] [Google Scholar]
- 2.Lockshin MD. Antiphospholipid antibody. Babies, blood clots, biology. JAMA. 1997;277:1549–51. doi: 10.1001/jama.277.19.1549. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 4.Stein AJ, Fuchs G, Fu C, Wolin SL, Reinisch KM. Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity. Cell. 2005;121:529–39. doi: 10.1016/j.cell.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs G, Stein AJ, Fu C, Reinisch KM, Wolin SL. Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat Struct Mol Biol. 2006;13:1002–9. doi: 10.1038/nsmb1156. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Smith JD, Shi H, Yang DD, Flavell RA, Wolin SL. The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr Biol. 2003;13:2206–11. doi: 10.1016/j.cub.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda ME, Tseng CE, Rashbaum W, Ochs RL, Casiano CA, Di Donato F, et al. Accessibility of SSA/Ro and SSB/La antigens to maternal autoantibodies in apoptotic human fetal cardiac myocytes. J Immunol. 1998;161:5061–9. [PubMed] [Google Scholar]
- 9.Reed JH, Neufing PJ, Jackson MW, Clancy RM, Macardle PJ, Buyon JP, et al. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin Exp Immunol. 2007;148:153–60. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–22. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 12.Wang MX, Kandiah DA, Ichikawa K, Khamashta M, Hughes G, Koike T, et al. Epitope specificity of monoclonal anti-beta 2-glycoprotein I antibodies derived from patients with the antiphospholipid syndrome. J Immunol. 1995;155:1629–36. [PubMed] [Google Scholar]
- 13.De Laat B, Derksen RH, Urbanus RT, de Groot PG. IgG antibodies that recognize epitope Gly40-Arg43 in domain I of β2–glycoprotein I cause LAC, and their presence correlates strongly with thrombosis. Blood. 2005;105:1540–5. doi: 10.1182/blood-2004-09-3387. [DOI] [PubMed] [Google Scholar]


