Abstract
Objectives
To determine if adherence to the recommended well-child care (WCC) visit schedule, independent of continuity of care (COC), is associated with lower risk for Ambulatory Care Sensitive Hospitalizations (ACSH) and whether this association varies by chronic disease status.
Design
Population-based, retrospective cohort study
Setting
Hawaii’s largest health plan from 1999 to 2006
Patients/Participants
36,944 children ≤ 3.5 years-old who were eligible if they were enrolled prior to 2 months-old, had ≥ 4 outpatient visits during the study period, and had an enrollment period that overlapped with ≥ 1 WCC visit interval.
Main Exposure(s)
WCC visit adherence and COC Index
Main Outcome Measure(s)
Risk for ACSH (Hazard Ratio [HR])
Results
Overall, 8,921 (24%) children had ≥ 1 chronic disease. The proportions of ACSH among healthy children versus those with ≥ 1 chronic disease were 3% (n= 751) and 7% (n= 645), respectively. For children with chronic disease, those with the lowest WCC visit adherence (0–25%) had 1.9 times (HR: 1.9, 95% Confidence Interval [CI]: 1.5–2.5) the risk of ACSH compared to those in the highest category (75–100%). The risk of ACSH for children with chronic disease who fell into the lowest COC category (0–0.25) was 2.4 times (HR 2.4, 95% CI: 1.7–3.5) higher than for those who fell into the highest category (0.75–1.0).
Conclusions
For children with chronic disease, both low WCC visit adherence and COC are independently associated with an increased risk of ACSH. Providing access to a consistent source of primary care appears important for this vulnerable population.
INTRODUCTION
Regularly scheduled well-child care (WCC) visits are a key component of health care for young children. The American Academy of Pediatrics (AAP) guideline recommends attending 14 WCC visits within the first 5 years of life and then annual visits thereafter until age 21.1 Between 2000 and 2002, children less than 5 years-old missed between 20% to 30% of their recommended WCC visits.2
Educating parents during WCC visits about what to do for their otherwise healthy children during acute illnesses (e.g., calling the physician’s office for advice) as well as providing guidance on optimal management for children with chronic diseases (e.g., review of steps to follow in an asthma action plan) may decrease the risk for poor outcomes such as ambulatory care sensitive hospitalizations (ACSH). Receipt of recommended WCC content (e.g., immunizations) may also prevent such hospitalizations. However, the evidence supporting WCC visit adherence is limited and inconsistent for a wide range of outcomes,3–8 including hospitalizations.4, 6, 7 Only one prior study found a protective association between high WCC visit adherence and preventable hospitalizations.4 These studies were limited in that they did not account for continuity of care (COC), a measure of how often a child saw the same provider for these WCC visits. In contrast to WCC visit adherence, high COC levels for both adults and children have consistently been associated with improved outcomes,9–25 including hospitalizations.13, 17, 19, 26, 27
In this population-based study, we examined whether high WCC visit adherence is associated with decreased risk for ACSH above and beyond the known decrease in risk associated with high COC. We also examined whether these relationships differ for children with chronic diseases. Understanding these relationships may assist providers, insurers, and policymakers in evaluating the degree to which additional economic and health care resources should be devoted towards greater access to WCC services in addition to improving COC.
METHODS
Design and Setting
This was a population-based, retrospective cohort study of Hawaii’s largest single health insurer which captures nearly 70% of Hawaii’s civilian adult and child population (n= 700,000), and contracts with ~95% of Hawaii’s physicians. This study was approved by the University of Hawaii Institutional Review Board.
Patient Population
We focused on younger children and those with chronic disease as these children are at the highest risk for hospitalizations.28, 29 We used administrative data to identify all children enrolled prior to 2 months-old in one of the insurer’s two commercial plans between January 1, 1999 and December 31, 2006. Children entered the study on either January 1, 1999 if they were already a plan member or on their first day of enrollment during the study period. Children exited the study when they had an ACSH, reached the end of the study (December 31, 2006), disenrolled from the insurer’s commercial plans, or turned 3 ½ years-old, whichever came first. We chose 3 ½ years-old because the number and frequency of required WCC visits is highest for children younger than this age. Continuous enrollment was also required which meant a child could have no gaps in coverage of > 45 days.30 Only the first continuous enrollment period after January 1, 1999 was included for children with multiple eligible enrollment periods between 1999 and 2006.
To calculate WCC visit adherence and COC, further eligibility criteria were required. First, a child needed to be enrolled prior to a recommended WCC visit and through at least one of the subsequent recommended WCC visits. Second, children were required to have at least four outpatient visits prior to exiting the study to allow an adequate number of visits to calculate COC.
Variables and Measures
Well-Child Care Visit Adherence
A WCC visit was identified from outpatient claims with the standard WCC visit International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (e.g. V20.2) in any of the diagnosis fields. Only WCC visits billed by primary care providers were included. These providers included pediatricians, family physicians, general practitioners, osteopaths, nurse practitioners, or physician assistants.
WCC visits were included in the adherence calculation if they were “timely” based on the age intervals (i.e. WCC intervals) recommended in the AAP’s 2000 guidelines. For example, a “4-month WCC visit” needed to occur between 4 and 6 months-old.31 The AAP’s schedule was modified for visits after 2 years-old so that a “2 year-old WCC visit” could occur between 2 and 2 ½ years-old while a “3 year-old WCC visit” could occur between 2 ½ and 3 ½ years-old. Only the first WCC visit within each age interval was counted if there was more than one WCC visit. Duplicate visits occurred in 10% of all eligible WCC intervals with 47% of duplicate visits occurring during the birth to 2 month-old WCC interval. For WCC visits within an interval to be counted, children had to be continuously enrolled during the entire interval.
WCC visit adherence (range 0% to 100%) was a time-varying variable whose value was only updated at the end of each age-specific WCC interval. For all WCC intervals that a child’s enrollment overlapped completely, WCC visit adherence was calculated by dividing a child’s total number of “eligible WCC visits” by the total number of recommended WCC visits from start of enrollment through the end of each age-specific WCC interval. WCC visit adherence was examined as both a continuous and categorical variable. However, it was modeled categorically to facilitate interpretation as follows: 0% to 25%, 26% to 50%, 51% to 74%, and 75% to 100% (referent).
Continuity of Care Index
We used Bice and Boxerman’s COC index32 (range 0 to 1) to quantify the number of times a child saw the same provider. We modeled COC index as a time-varying variable using the same methodology described previously for WCC visit adherence. COC index was based on all outpatient claims to clinical health care providers that contained at least one Evaluation and Management Service code for a sick (e.g. 99213) or preventative care (e.g. 99391) visit and/or had a WCC visit ICD-9-CM code in any of the diagnosis fields. For the COC index calculation, if two WCC visits occurred within 7 days of each other, the second visit was excluded to improve capturing only “true” WCC visits. This eliminated 2,640 WCC visits from the eligible sample of 285,223.
The COC index is non-linear13 and varies depending on the number of different providers seen, the number of visits to each provider, and the total number of visits. An index of 0 represents seeing a different provider for all visits while an index of 1 represents seeing the same provider for all visits. An index of 0.30 corresponds to seeing three different providers for 6 visits each while an index of 0.80 corresponds to seeing the same provider for 16 of 18 visits. The COC index was examined as both a continuous and categorical variable. However, it was modeled categorically to facilitate interpretation as follows: 0 to 0.25, 0.26 to 0.50, 0.51 to 0.74, and 0.75 to 1.0 (referent).
Ambulatory Care Sensitive Hospitalizations
ACSH was the main outcome measure for all analyses. Birth hospitalizations and hospitalizations prior to 7 days-old were excluded. A hospitalization was classified as an ACSH if the primary or secondary discharge diagnosis matched one of the ACSH conditions as defined by the Agency for Healthcare Research and Quality.33 The standard list of ACSH conditions for adults was modified by excluding any “Adult” conditions (e.g., angina) similar to other investigators34 as well as “congenital syphilis”. The following additional ACSH diagnoses were included since they are highly applicable to children less than 3 ½ years-old and/or vaccine preventable: “acute respiratory tract infections” (ICD-9-CM 464, 466),4 “pneumococcal meningitis” (ICD-9-CM 320.1), “streptococcal meningitis” (ICD-9-CM 320.2), and “septicemia due to H. influenza” (ICD-9-CM 038.41).
Chronic Disease Status
Based on previous literature,7, 28, 35 we classified children as having no chronic disease (i.e. healthy) or as having ≥ one chronic disease. Children were classified as having ≥ one chronic disease if they had one or more claims prior to exiting the study with a diagnosis included in a validated list of ICD-9-CM chronic disease codes for children.36 Since diagnosing asthma in children less than 4 years-old can be challenging,37 two or more claims for asthma (ICD-9-CM 493)13 were required for a child to be classified as having asthma.
Statistical Analysis
Univariate and bivariate analyses were performed to understand the non-time dependent relationships between all independent variables and ACSH (a dichotomous variable). The Student’s t-test was used for comparisons of continuous variables and the Pearson’s Chi-squared test was used for comparisons of categorical variables. Our choice of covariates to adjust for in multivariate analyses were selected a priori based on the existing literature.6, 28, 34, 35, 38–46 Similar to other studies, patient age at start of enrollment28, 34, 35, 38–46 and gender6, 34, 35, 39–43, 45, 46 were adjusted for in all multivariate models. Geographical location, based on the child’s billing address at the time of study entrance (Oahu versus other islands) was also included because of better access to care on Oahu resulting from a higher physician per capita.47
A Cox proportional hazards regression model was used to determine the association between WCC visit adherence, COC index, and time to first ACSH from birth. WCC visit adherence and COC index were modeled as time-varying categorical variables as previously described. Chronic disease was initially included as any chronic disease versus none. The proportional hazard assumption was tested for all models.
To assess for the presence of interactions, we compared models with the following interaction terms to models without them using the likelihood ratio test: WCC visit adherence (categorical) and chronic disease status (dichotomous), COC Index (categorical) and chronic disease status (dichotomous), and WCC visit adherence (categorical) and COC Index (categorical). We decided to stratify for any relationship that was statistically significant at P<0.05.
We also performed a sensitivity analysis using propensity scores in order to attempt to control for self-selection bias (e.g., children at greatest risk for ACSH may also be less likely to be compliant with WCC visits).48, 49 We predicted WCC visit adherence (categorical) propensity score probabilities with multinomial logistic regression using age at start of enrollment (continuous), chronic disease status (dichotomous), and island of residence based on billing address (dichotomous).
SAS 9.1 was used to create the data sets and STATA10 was used to analyze the data. Statistical significance was determined at the p < 0.05 level.
RESULTS
Of the 43,510 children that enrolled prior to 2 months old, 37,811 (87%) had both an enrollment period that overlapped completely with at least one WCC visit interval and had at least four outpatient claims. Of these children, 867 (2%) children were excluded due to missing geographical location or a location outside of Hawaii. Thus, 36,944 (85%) children, with 35,078 (95%) followed from birth, met the final eligibility requirements (Figure 1).
Figure 1. Patient Eligibility.
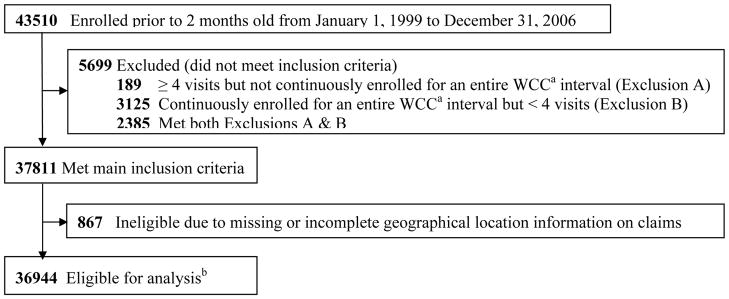
aWCC, Well-Child Care
bChildren were “eligible for analysis” if they had at least 4 outpatient visits prior to censoring, their enrollment period completely overlapped with at least one of the recommended well-child care visit age intervals, and did not have incomplete claims information
Participant demographics
Healthy children and children with ≥ one chronic disease comprised 76% (n= 28,023) and 24% (n= 8,921) of the study population, respectively (Table 1). Among children with chronic disease, 47% were classified as having asthma (Table 2). The top 10 chronic disease diagnoses were present in 84% of children with ≥ one chronic disease.
Table 1.
Patient Characteristicsa
| All Children | Diagnosed with ≥ 1 Chronic Diseased
|
||
|---|---|---|---|
| No | Yes | ||
|
| |||
| N=36,944 | N=28,023 | N=8,921 | |
| WCC Visit Adherenceb | |||
| 0–25% | 2,854 (8) | 2,271 (8) | 583 (7) |
| 26–50% | 2,200 (6) | 1,578 (6) | 622 (7) |
| 51–74% | 4,849 (13) | 3,413 (12) | 1,436 (16) |
| 75–100% | 27,041 (73) | 20,761 (74) | 6,280 (70) |
|
| |||
| COC Indexb | |||
| 0–0.25 | 925 (3) | 627 (2) | 298 (3) |
| 0.26–0.50 | 6,291 (17) | 4,271 (15) | 2,020 (23) |
| 0.51–0.74 | 8,284 (22) | 5,960 (22) | 2,324 (26) |
| 0.75–1.0 | 21,444 (58) | 17,165 (61) | 4,279 (48) |
|
| |||
| Geographic Location | |||
| Oahu | 25,856 (70) | 19,740 (70) | 6,116 (69) |
|
| |||
| Non-Oahu | 11,088 (30) | 8,283 (30) | 2,805 (31) |
|
| |||
| Gender | |||
| Female | 17,726 (48) | 13,823 (49) | 3,903 (44) |
| Male | 19,218 (52) | 14,200 (51) | 5,018 (56) |
|
| |||
| Enrollment, monthsc | |||
| Time in study | 31 [14–42] | 28 [12–42] | 41 [23–42] |
|
| |||
| Age at start | 0 [0–0] | 0 [0–0] | 0 [0–0] |
|
| |||
| ACSH | 1,396 (4) | 751 (3) | 645 (7) |
Abbreviations: ACSH, Ambulatory Care Sensitive Hospitalizations, WCC, Well-Child Care, COC, Continuity of Care
No. (%) of eligible children
WCC visit adherence and COC index for this table are based on values at exit from analysis
Median [Interquartile Range]
P<0.001 for all comparisons between children with ≥ one chronic disease and healthy children except for “age at start of enrollment” (P>0.05)
Table 2.
Top 10 Chronic Disease Classifications
| Chronic Disease Classification | ICD9 Code(s) | N (%) |
|---|---|---|
| (Total = 10930)a | ||
| Asthma | 493 | 5142 (47) |
| Failure To Thrive | 783.4 | 1232 (11) |
| Congenital Heart Disease | 745–747.9, 424.1–424.3 | 1172 (11) |
| Hereditary and Acquired Hemolytic Anemia | 282–283.9 | 514 (5) |
| Diseases of White Blood Cells | 288–288.9 | 265 (2) |
| Epilepsy | 345–345.9 | 239 (2) |
| Other Congenital Anomalies of Nervous System | 742–742.9 | 205 (2) |
| Tuberculosis | 010–018 | 202 (2) |
| Inborn Errors of Metabolism | 270–273.9 | 187 (2) |
| Remaining Classifications | N/A | 1772 (16) |
Total is larger than number of children with at least one chronic disease since 16% of children had more than 1 chronic disease
The two groups of children were similar (Table 1). However, children with ≥ one chronic disease were in the study longer than healthy children (median 41 vs. 28 months, P<0.001).
WCC Visit Adherence and COC Index
Overall, children were recommended to have a median of 9 WCC visits (Interquartile Range [IQR] 5–10). For 85% of the children, WCC visit adherence was calculated based on at least four recommended WCC visits. A majority of children fell into the highest WCC visit adherence category (Table 1). This was similar for healthy children (74%) and children with ≥ one chronic disease (70%).
For COC index calculation, a median of 18 claims was used (IQR 11–26). Compared to healthy children, children with ≥ one chronic disease had 10 more total outpatient claims, visited 1 more different provider, and had 9 more claims by a primary care physician. The majority of children (58%) fell into the highest COC index category (0.75 to 1). However, compared to healthy children, a lower percentage of children with ≥ one chronic disease fell into the highest COC index category (48% vs. 61%, P<0.001; Table 1).
ACSH
Of the 36,944 children eligible for study inclusion (Figure 1), 1,396 (4%) had an ACSH. The median age of children with an ACSH was 14 months (IQR 8–23). The proportion of children with an ACSH was 2.7 times greater for children with ≥ one chronic disease compared to healthy children (3% vs. 7%, P<0.001; Table 1).
More than three-quarters of all ACSH were accounted for by the following five conditions: dehydration (24%), acute respiratory tract infections (18%), bacterial pneumonia (17%), seizures (13%), and asthma (12%). While the top five conditions were similar for all children, the most common ACSH condition differed by chronic disease status with asthma being the most common for children with ≥ one chronic disease (20%) and dehydration being the most common for healthy children (28%).
Multivariate, Time-Varying Analyses
The adjusted HR for all children together revealed that both high WCC visit adherence and COC index were associated with decreased risk of an ACSH (Table 3). The relationship between WCC visit adherence and risk of ACSH as well as COC index and risk of ACSH differed significantly by chronic disease status (Table 3). Our exploratory analysis revealed no statistically significant results when testing for interactions between WCC visit adherence and COC index. The results from the sensitivity analysis using propensity scores to determine whether self-selection bias was occurring were similar to the original model and did not change our conclusions (data not shown).
Table 3.
Adjusted Hazard Ratios for rate of ACSH, Hazard Ratio (95% Confidence Interval)a
| All Children | Diagnosed with ≥ 1 Chronic Disease
|
||
|---|---|---|---|
| No | Yes | ||
|
| |||
| (N = 36944) | (N = 28023) | (N = 8921) | |
| WCC Visit Adherence | |||
| 0–25% | 1.5 (1.2–1.7) | 1.2 (0.9–1.5) | 1.9 (1.5–2.5) |
| 26–50% | 1.3 (1.0–1.6) | 1.1 (0.8–1.5) | 1.5 (1.1–2.0) |
| 51–74% | 1.1 (0.9–1.3) | 0.9 (0.7–1.2) | 1.2 (1.0–1.6) |
| 75–100% | 1 (Reference) | 1 (Reference) | 1 (Reference) |
|
| |||
| COC Index | |||
| 0–0.25 | 2.1 (1.6–2.8) | 1.9 (1.2–2.9) | 2.4 (1.7–3.5) |
| 0.26–0.50 | 1.5 (1.3–1.8) | 1.3 (1.0–1.6) | 1.8 (1.5–2.2) |
| 0.51–0.74 | 1.4 (1.3–1.6) | 1.5 (1.3–1.8) | 1.4 (1.2–1.7) |
| 0.75–1.0 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
|
| |||
| Chronic Disease | 2.1 (1.9–2.4) | N/A | N/A |
|
| |||
| Oahu | 1.1 (0.9–1.2) | 1.1 (0.9–1.3) | 1.1 (0.9–1.2) |
|
| |||
| Female | 0.8 (0.7–0.9) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) |
|
| |||
| Age at Start | 0.9 (0.7–1.1) | 1.0 (0.7–1.3) | 0.8 (0.5–1.1) |
Abbreviations: ACSH, Ambulatory Care Sensitive Hospitalizations, WCC, Well-Child Care, COC, Continuity of Care
Reference category (if applicable): No Chronic Disease, non-Oahu, and Male. Age at start of enrollment (months) was modeled as a continuous variable
For children with ≥ one chronic disease, those in the lowest WCC visit adherence category had nearly 2 times (HR 1.9, 95% CI: 1.5–2.5) the risk of an ACSH compared to those in the highest category. The HR increased as WCC visit adherence decreased (Table 3). Similarly, those in the lowest COC index category had 2.4 times (HR 2.4, 95% CI: 1.7–3.5) the risk of an ACSH compared to those in the highest COC index category. The HR also increased as COC index decreased (Table 3).
For healthy children, there was no significant association between WCC visit adherence and ACSH. In contrast, similar to children with ≥ one chronic disease, healthy children in the lowest COC index category had nearly 2 times (HR 1.9, 95% CI: 1.2–2.9) the risk of an ACSH compared to those in the highest category. The HR increased as COC index decreased (Table 3).
COMMENT
For children with chronic disease, we found that high WCC visit adherence and COC were independently associated with decreased risk of ACSH. High COC was also associated with decreased risk of ACSH for healthy children. Our study is unique because unlike prior studies evaluating the benefits of WCC visit adherence3–7 we adjusted for COC (a well established factor in reducing hospitalizations10, 13, 16, 19, 25, 27, 50) and we present our results separately by chronic disease status making policy implications more clear. This study suggests the need for efforts aimed at improving COC for all children as well as improving WCC visit adherence for children with chronic disease.
Regular WCC visits provide opportunities to help parents of children with chronic disease understand how to proactively manage their child’s medical conditions, a key aspect of the Chronic Care Model51 and a top priority for these parents52. A child whose disease is poorly controlled often requires higher levels of medical care, such as a hospitalization. Similar to a prior study of COC,13 our study lends support to the idea that when children are sick, seeing their primary care provider increases the likelihood that medical decisions are made by somebody who is knowledgeable about and comfortable with the child’s medical needs which may prevent poor outcomes such as hospitalizations. Thus, ensuring that children with chronic disease have access to continuous, comprehensive, and coordinated care with a personal primary care physician, all aspects of the AAP’s medical home,53 appears to be of key importance. Finding unique solutions that facilitate establishment of a medical home for these children may improve their COC, adherence to WCC visits, and health outcomes.
For young, healthy children, preventable hospitalizations may be due to poor access to outpatient medical care or parents not knowing who to call when their child is sick due to low COC. Our results examining the relationship between COC and ACSH were similar to previous studies that did not stratify by a child’s chronic disease status.10, 13, 19 Although the content of WCC visits, such as immunizations, may prevent hospitalizations, WCC visit adherence in our study was likely a proxy for timely access to health care for young children. Our findings suggest that healthy children in the study population likely have adequate access to their primary care provider. Other WCC visit content (e.g., providing age appropriate injury prevention or monitoring a child’s development) are more likely to affect outcomes not measured by ACSH such as decreasing emergency room visits or visits for injuries,54 or providing appropriate referrals for evaluation of potential developmental delays.55
Limitations
This study has several limitations. The study population included children enrolled in a single health plan in one state whose pediatric patients had high COC.8, 11–15 Thus these results may not be generalizable to other populations with more variability in COC. Future studies should be more representative and include both Medicaid and uninsured populations. As this study was observational, our findings represent associations rather than causal relationships. We could not adjust for all potential confounders due to the limits of administrative data. Although previous authors identified “acute respiratory tract infections” as potentially avoidable hospitalizations,4 many of these hospitalizations may be due to factors (e.g. hypoxia) unaffected by adequate outpatient care. In sensitivity analysis, we found similar results when excluding these hospitalizations (data not shown).
Self-selection bias may have resulted in children who are less adherent to the WCC visit schedule also being less adherent to other aspects of their health care (e.g., less likely to take their medications or follow other treatment regiments) thus resulting in overestimation of the associations we have reported. However, children who fell into each WCC visit adherence category had a similar number of total visits (WCC and other visits) per year in the study (median 8–9; data not shown). Some children may have been misclassified into the chronic disease category which would bias our results toward the null. However, the percentage of children with asthma (11%) in our study was similar to recent prevalence estimates for Hawaii (over 9.8%).56
We were unable to account for severity of chronic disease. If children with chronic disease with low WCC visit adherence were sicker than those with high WCC visit adherence, this would cause an overestimation of the association between WCC visit adherence and ACSH. However, we found that the top 10 chronic disease classifications and number of non-WCC visits for these children were similar within each WCC visit adherence category (data not shown). We were also unable to fully characterize the children in our sample with poor WCC visit adherence due to the limits of the available data. Future research should determine who these children are as well as the specific mechanism by which WCC visits may prevent ACSH.
We excluded 6,566 children (15%) who had incomplete data for geographic location or who did not meet the eligibility requirements for WCC visit adherence or COC index calculations. These ineligible children may be a higher risk population since they were enrolled for shorter periods of time and had a higher percentage (6%) of ACSH (n= 392) than the eligible population. Therefore our findings may underestimate the strength of associations between WCC visit adherence, COC, and ACSH.
Conclusions/Policy Implications
The complexity of caring for children with chronic disease can make prioritizing and attending all recommended WCC and sub-specialty visits difficult for parents. Finding ways to facilitate this process may improve timely WCC visits. These children are also at increased risk for breakdowns in communication57 between their multiple health care providers and between their providers and parents. This can result in fragmentation of medical care and therefore improving communication and COC are also important. Health information technology (IT) solutions, such as personal health records and shared medical records, have the potential to improve timely WCC visits through automated appointment reminders and convenient appointment scheduling, improve communication through secured electronic messaging, and improve COC by substituting “provider COC” with “informational COC”. Although health IT is not the only way to potentially improve outcomes for children with chronic disease, it has been identified by the Institute of Medicine as a key component of achieving high quality care.58 Future directions aimed at preventing ACSH should focus on finding unique solutions that help children with chronic disease obtain a medical home.
Acknowledgments
Funding/Support: This study was supported by the following Health Resources and Services Administration (D55HP05143 and T32HP10002-21) and National Institute of Health (R25RR019321 and P20RR11091) grants.
Role of the Sponsors: The Health Resources and Services Administration and the National Institute of Health had no role in the design of the study, analysis and interpretation of data, or the preparation and approval of the manuscript. Dr. Davis works for the health insurer that provided the data but the health insurer had no role in the design of the study, analysis and interpretation of data, or the preparation and approval of the manuscript.
Footnotes
Author Contributions: Dr. Tom had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tom, Tseng, Mangione-Smith
Acquisition of data: Davis
Analysis and interpretation of data: Tom, Tseng, Davis, Solomon, Zhou, Mangione-Smith
Drafting of the manuscript: Tom, Tseng, Mangione-Smith
Critical revision of the manuscript: Tom, Tseng, Solomon, Zhou, Mangione-Smith
Statistical analysis: Tom, Tseng, Davis, Solomon, Zhou
Supervision: Tom, Tseng, Mangione-Smith
Additional Contributions: The authors would like to thank James M. Perrin, MD (MassGeneral Hospital for Children Center for Child and Adolescent Health Policy) for use of the Center’s chronic disease list and the University of Hawaii Department of Pediatrics’ Koko’okolu Community Pediatrics Fellowship Program (Chris Derauf, MD).
References
- 1.Recommendations for Preventive Pediatric Health Care: Committee on Practice and Ambulatory Medicine. Pediatrics. 2007 Dec;120(6):1376. [PubMed] [Google Scholar]
- 2.Selden TM. Compliance With Well-Child Visit Recommendations: Evidence From the Medical Expenditure Panel Survey, 2000–2002. Pediatrics. 2006 Dec;118(6):1766–1778. doi: 10.1542/peds.2006-0286. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert JR, Feldman W, Siegel LS, Mills DA, Dunnett C, Stoddart G. How many well-baby visits are necessary in the first 2 years of life? Can Med Assoc J. 1984 Apr 1;130(7):857–861. [PMC free article] [PubMed] [Google Scholar]
- 4.Hakim RB, Bye BV. Effectiveness of Compliance With Pediatric Preventive Care Guidelines Among Medicaid Beneficiaries. Pediatrics. 2001 Jul;108(1):90–97. doi: 10.1542/peds.108.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Hoekelman RA. What constitutes adequate well-baby care? Pediatrics. 1975 Mar;55(3):313–326. [PubMed] [Google Scholar]
- 6.Pittard WB, 3rd, Laditka JN, Laditka SB. Early and periodic screening, diagnosis, and treatment and infant health outcomes in Medicaid-insured infants in South Carolina. J Pediatr. 2007 Oct;151(4):414–418. doi: 10.1016/j.jpeds.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Steiner JF, Braun PA, Melinkovich P, et al. Primary-care visits and hospitalizations for ambulatory-care-sensitive conditions in an inner-city health care system. Ambul Pediatr. 2003 Nov-Dec;3(6):324–328. doi: 10.1367/1539-4409(2003)003<0324:pvahfa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Flores AI, Bilker WB, Alessandrini EA. Effects of continuity of care in infancy on receipt of lead, anemia, and tuberculosis screening. Pediatrics. 2008 Mar;121(3):e399–406. doi: 10.1542/peds.2007-1497. [DOI] [PubMed] [Google Scholar]
- 9.Brousseau DC, Meurer JR, Isenberg ML, Kuhn EM, Gorelick MH. Association Between Continuity of Care and Pediatric Emergency Department Utilization. Pediatrics. 2004 Apr;113(4):738–741. doi: 10.1542/peds.113.4.738. [DOI] [PubMed] [Google Scholar]
- 10.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004 Dec;53(12):974–980. [PubMed] [Google Scholar]
- 11.Chirstakis DA, Wright JA, Koepsell TD, Emerson S, Connell FA. Is Greater Continuity of Care Associated With Less Emergency Department Utilization? Pediatrics. 1999 Apr;103(4):738–742. doi: 10.1542/peds.103.4.738. [DOI] [PubMed] [Google Scholar]
- 12.Christakis DA, Feudtner C, Pihoker C, Connell FA. Continuity and quality of care for children with diabetes who are covered by medicaid. Ambul Pediatr. 2001 Mar-Apr;1(2):99–103. doi: 10.1367/1539-4409(2001)001<0099:caqocf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Christakis DA, Mell L, Koepsell TD, Zimmerman FJ, Connell FA. Association of lower continuity of care with greater risk of emergency department use and hospitalization in children. Pediatrics. 2001 Mar;107(3):524–529. doi: 10.1542/peds.107.3.524. [DOI] [PubMed] [Google Scholar]
- 14.Christakis DA, Wright JA, Zimmerman FJ, Bassett AL, Connell FA. Continuity of care is associated with high-quality care by parental report. Pediatrics. 2002 Apr;109(4):e54. doi: 10.1542/peds.109.4.e54. [DOI] [PubMed] [Google Scholar]
- 15.Christakis DA, Wright JA, Zimmerman FJ, Bassett AL, Connell FA. Continuity of care is associated with well-coordinated care. Ambul Pediatr. 2003 Mar-Apr;3(2):82–86. doi: 10.1367/1539-4409(2003)003<0082:cociaw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Cree M, Bell NR, Johnson D, Carriere KC. Increased continuity of care associated with decreased hospital care and emergency department visits for patients with asthma. Dis Manag. 2006 Feb;9(1):63–71. doi: 10.1089/dis.2006.9.63. [DOI] [PubMed] [Google Scholar]
- 17.Cyr MC, Martens AC, Berbiche D, Perreault S, Blais L. Continuity of care in the ambulatory treatment of adolescents with asthma. J Adolesc Health. 2006 Dec;39(6):926, e911–927. doi: 10.1016/j.jadohealth.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 18.DiGiuseppe DL, Christakis DA. Continuity of care for children in foster care. Pediatrics. 2003 Mar;111(3):e208–213. doi: 10.1542/peds.111.3.e208. [DOI] [PubMed] [Google Scholar]
- 19.Gill JM, Mainous AG., 3rd The role of provider continuity in preventing hospitalizations. Archives of Family Medicine. 1998 Jul-Aug;7(4):352–357. doi: 10.1001/archfami.7.4.352. [DOI] [PubMed] [Google Scholar]
- 20.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ. 2003 Nov 22;327(7425):1219–1221. doi: 10.1136/bmj.327.7425.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inkelas M, Schuster MA, Olson LM, Park CH, Halfon N. Continuity of primary care clinician in early childhood. Pediatrics. 2004 Jun;113(6 Suppl):1917–1925. [PubMed] [Google Scholar]
- 22.Irigoyen M, Findley SE, Chen S, et al. Early continuity of care and immunization coverage. Ambul Pediatr. 2004 May-Jun;4(3):199–203. doi: 10.1367/A03-138R1.1. [DOI] [PubMed] [Google Scholar]
- 23.O’Malley AS, Forrest CB. Continuity of care and delivery of ambulatory services to children in community health clinics. J Community Health. 1996 Jun;21(3):159–173. doi: 10.1007/BF01557996. [DOI] [PubMed] [Google Scholar]
- 24.Starfield BH, Simborg DW, Horn SD, Yourtee SA. Continuity and coordination in primary care: their achievement and utility. Medical Care. 1976 Jul;14(7):625–636. doi: 10.1097/00005650-197607000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sudhakar-Krishnan V, Rudolf MC. How important is continuity of care? Arch Dis Child. 2007 May;92(5):381–383. doi: 10.1136/adc.2006.099853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill JM. Can Hospitalizations Be Avoided by Having a Regular Source of Care? Family Medicine. 1997 Mar;29(3):166–171. [PubMed] [Google Scholar]
- 27.Mainous AG, 3rd, Gill JM. The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician? American Journal of Public Health. 1998 Oct;88(10):1539–1541. doi: 10.2105/ajph.88.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falik M, Needleman J, Wells BL, Korb J. Ambulatory care sensitive hospitalizations and emergency visits: experiences of Medicaid patients using federally qualified health centers. Med Care. 2001 Jun;39(6):551–561. doi: 10.1097/00005650-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Newacheck PW, Kim SE. A national profile of health care utilization and expenditures for children with special health care needs. Arch Pediatr Adolesc Med. 2005 Jan;159(1):10–17. doi: 10.1001/archpedi.159.1.10. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed January 16, 2009];Child Health Care Quality Toolbox: Established Child Health Care Quality Measures (HEDIS) http://www.ahrq.gov/CHToolBx/measure4.htm.
- 31.Recommendations for Preventive Pediatric Health Care: Committee on Practice and Ambulatory Medicine. Pediatrics. 2000 Mar 1;105(3):645–646. [PubMed] [Google Scholar]
- 32.Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care. 1977 Apr;15(4):347–349. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed January 27, 2009];Using Administrative Data To Monitor Access, Identify Disparities, and Assess Performance of the Safety Net. http://www.ahrq.gov/data/safetynet/billings.htm.
- 34.Gadomski A, Jenkins P, Nichols M. Impact of a Medicaid primary care provider and preventive care on pediatric hospitalization. Pediatrics. 1998 Mar;101(3):E1. doi: 10.1542/peds.101.3.e1. [DOI] [PubMed] [Google Scholar]
- 35.Gaskin DJ, Hoffman C. Racial and ethnic differences in preventable hospitalizations across 10 states. Med Care Res Rev. 2000;57( Suppl 1):85–107. doi: 10.1177/1077558700057001S05. [DOI] [PubMed] [Google Scholar]
- 36.Kuhlthau KA, Beal AC, Ferris TG, Perrin JM. Comparing a diagnosis list with a survey method to identify children with chronic conditions in an urban health center. Ambul Pediatr. 2002 Jan-Feb;2(1):58–62. doi: 10.1367/1539-4409(2002)002<0058:cadlwa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed January 18, 2009];NHLBI Guidelines for the Diagnosis and Treatment of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/
- 38.Falik M, Needleman J, Herbert R, Wells B, Politzer R, Benedict MB. Comparative effectiveness of health centers as regular source of care: application of sentinel ACSC events as performance measures. J Ambul Care Manage. 2006 Jan-Mar;29(1):24–35. doi: 10.1097/00004479-200601000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Friedman B, Basu J. Health insurance, primary care, and preventable hospitalization of children in a large state. Am J Manag Care. 2001 May;7(5):473–481. [PubMed] [Google Scholar]
- 40.Garg A, Probst JC, Sease T, Samuels ME. Potentially preventable care: ambulatory care-sensitive pediatric hospitalizations in South Carolina in 1998. South Med J. 2003 Sep;96(9):850–858. doi: 10.1097/01.SMJ.0000083853.30256.0A. [DOI] [PubMed] [Google Scholar]
- 41.Kaestner R, Joyce T, Racine A. Medicaid eligibility and the incidence of ambulatory care sensitive hospitalizations for children. Soc Sci Med. 2001 Jan;52(2):305–313. doi: 10.1016/s0277-9536(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 42.Laditka SB, Johnston JM. Preventable hospitalization and avoidable maternity outcomes: implications for access to health services for Medicaid recipients. J Health Soc Policy. 1999;11(2):41–56. doi: 10.1300/j045v11n02_04. [DOI] [PubMed] [Google Scholar]
- 43.Pappas G, Hadden WC, Kozak LJ, Fisher GF. Potentially avoidable hospitalizations: inequalities in rates between US socioeconomic groups. Am J Public Health. 1997 May;87(5):811–816. doi: 10.2105/ajph.87.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker JD, Schoendorf KC. Variation in hospital discharges for ambulatory care-sensitive conditions among children. Pediatrics. 2000 Oct;106(4 Suppl):942–948. [PubMed] [Google Scholar]
- 45.Shi L, Lu N. Individual sociodemographic characteristics associated with hospitalization for pediatric ambulatory care sensitive conditions. J Health Care Poor Underserved. 2000 Nov;11(4):373–384. doi: 10.1353/hpu.2010.0732. [DOI] [PubMed] [Google Scholar]
- 46.Shi L, Samuels ME, Pease M, Bailey WP, Corley EH. Patient characteristics associated with hospitalizations for ambulatory care sensitive conditions in South Carolina. South Med J. 1999 Oct;92(10):989–998. doi: 10.1097/00007611-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Health Trends. Hawaii: Health Resources; [Accessed August 21, 2009]. http://www.healthtrends.org/resources_overview.aspx. [Google Scholar]
- 48.Frick KD, Lantz PM. Selection Bias in Prenatal Care Utilization: An Interdisciplinary Framework and Review of the Literature. Med Care Res Rev. 1996 Dec;53(4):371–396. doi: 10.1177/107755879605300401. [DOI] [PubMed] [Google Scholar]
- 49.Spreeuwenberg MD, Bartak A, Croon MA, et al. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Medical Care. Feb;48(2):166–174. doi: 10.1097/MLR.0b013e3181c1328f. [DOI] [PubMed] [Google Scholar]
- 50.O’Malley AS. Current evidence on the impact of continuity of care. Curr Opin Pediatr. 2004 Dec;16(6):693–699. doi: 10.1097/01.mop.0000142488.67171.02. [DOI] [PubMed] [Google Scholar]
- 51.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998 Aug-Sep;1(1):2–4. [PubMed] [Google Scholar]
- 52.Van Cleave J, Heisler M, Devries JM, Joiner TA, Davis MM. Discussion of illness during well-child care visits with parents of children with and without special health care needs. Arch Pediatr Adolesc Med. 2007 Dec;161(12):1170–1175. doi: 10.1001/archpedi.161.12.1170. [DOI] [PubMed] [Google Scholar]
- 53. [Accessed January 13, 2009];AAP National Center of Medical Home Initiatives for Children with Special Needs. http://www.medicalhomeinfo.org/
- 54.Simon TD, Phibbs S, Dickinson LM, et al. Less anticipatory guidance is associated with more subsequent injury visits among infants. Ambul Pediatr. 2006 Nov-Dec;6(6):318–325. doi: 10.1016/j.ambp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Earls MF, Hay SS. Setting the stage for success: implementation of developmental and behavioral screening and surveillance in primary care practice--the North Carolina Assuring Better Child Health and Development (ABCD) Project. Pediatrics. 2006 Jul;118(1):e183–188. doi: 10.1542/peds.2006-0475. [DOI] [PubMed] [Google Scholar]
- 56.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006 Dec 12;381:1–24. [PubMed] [Google Scholar]
- 57.Stille CJ, Primack WA, McLaughlin TJ, Wasserman RC. Parents as information intermediaries between primary care and specialty physicians. Pediatrics. 2007 Dec;120(6):1238–1246. doi: 10.1542/peds.2007-1112. [DOI] [PubMed] [Google Scholar]
- 58.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C: National Academy Press; 2001. [PubMed] [Google Scholar]


