Abstract
The optic lobe forms a prominent compartment of the Drosophila adult brain that processes visual input from the compound eye. Neurons of the optic lobe are produced during the larval period from two neuroepithelial layers called the outer and inner optic anlage (OOA, IOA). In the early larva, the optic anlagen grow as epithelia by symmetric cell division. Subsequently, neuroepithelial cells (NE) convert into neuroblasts (NB) in a tightly regulated spatio-temporal progression that starts at the edges of the epithelia and gradually move towards its centers. Neuroblasts divide at a much faster pace in an asymmetric mode, producing lineages of neurons that populate the different parts of the optic lobe. In this paper we have reconstructed the complex morphogenesis of the optic lobe during the larval period, and established a role for the Notch and Jak/Stat signaling pathways during the NE-NB conversion. After an early phase of complete overlap in the OOA, signaling activities sort out such that Jak/Stat is active in the lateral OOA which gives rise to the lamina, and Notch remains in the medial cells that form the medulla. During the third instar, a wave front of enhanced Notch activity progressing over the OOA from medial to lateral controls the gradual NE-NB conversion. Neuroepithelial cells at the medial edge of the OOA, shortly prior to becoming neuroblasts, express high levels of Delta, which activates the Notch pathway and thereby maintains the OOA in an epithelial state. Loss of Notch signaling, as well as Jak/Stat signaling, results in a premature NE-NB conversion of the OOA, which in turn has severe effects on optic lobe patterning. Our findings present the Drosophila optic lobe as a useful model to analyze the key signaling mechanisms controlling transitions of progenitor cells from symmetric (growth) to asymmetric (differentiative) divisions.
Keywords: optic lobe, neurogenesis, neuroblast, neuroepithelium, Notch, Jak/Stat
INTRODUCTION
Neurons and most glial cells of the Drosophila brain are generated by a population of neural stem cells called neuroblasts (Yu et al., 2006; Egger et al., 2008). Neuroblasts divide asymmetrically, producing with each round of mitosis another neuroblast and a smaller daughter cell, the ganglion mother cell (GMC), which after one more round of division differentiates into neurons or glial cells. Neuroblasts of the central brain and ventral nerve cord (analog of the vertebrate spinal cord) are born in the early embryo; these neuroblasts are relatively few in number (less than 500 in all), and each one produces an invariant “lineage” of neurons/glial cells. By contrast, the optic lobe, in terms of cell number by far the largest part of the insect brain, is formed by neuroblasts that are born in the late larva from two neuroepithelial layers called the inner and outer optic anlagen (IOA, OOA; Meinertzhagen and Hanson, 1993; Fischbach and Hiesinger, 2008). The optic anlagen arise in close proximity to the eye imaginal disc in the embryonic head ectoderm (Green et al., 1993). In the early larva, both IOA and OOA grow by symmetric cell division. With the beginning of the third instar (about half way through the larval period), neuroepithelial cells (NE) convert into neuroblasts (NB) in a tightly regulated spatio-temporal progression that starts at the edges of the epithelia and gradually move towards its centers (Hofbauer and Campos-Ortega, 1990; Meinertzhagen and Hanson, 1993; Egger et al., 2007). Neuroblasts divide at a much faster pace in an asymmetric mode, producing the lineages of neurons that populate the different parts of the optic lobe. The optic anlagen, as well as the neuroblasts and lineages derived from them, together form the complex optic lobe primordium that accounts for fully half of each late larval brain hemisphere.
Neuroblasts of the central brain and ventral nerve cord are specified in a two-step process. During the first step, discrete clusters of neuroectodermal cells (“proneural clusters”) express a combination of regulatory genes, the proneural genes, which makes the cells with neural potential “competent” to form neuroblasts. Proneural genes encode DNA binding proteins that belong to the large family of basic helix-loop-helix (bHLH) transcription factors, including the Achaete-Scute complex (AS-C) and their vertebrate homologs (Campuzano and Modolell, 1992; Guillemot, 1995; Kageyama et al., 1995). In the second step of neuroblast specification, called lateral inhibition, cells of each proneural cluster “compete” with each other to become a neuroblast. On the molecular level, this competition is mediated by the Notch signaling pathway, whose members are encoded by the so-called neurogenic genes (Campos-Ortega and Knust, 1990; Artavanis-Tsakonas and Simpson, 1991; Posakony 1994; Lewis 1996; Chan and Jan, 1999). Expression of the Notch ligand Delta is upregulated within the proneural clusters by AS-C genes. Binding of Delta causes a conformational change followed by a cleavage of the Notch receptor. The released intracellular Notch fragment (NICD) moves into the nucleus and upregulates the expression of the bHLH repressor, Enhancer of split, E(spl) (consisting of the loci mδ, mγ, mβ, m3, m5, m7, m8 and groucho; Delidakis and Artavanis-Tsakonas, 1992; Knust et al., 1992; Schrons et al.,1992). The expression of these genes down-regulate the transcription of the AS-C gene complex, causing the cell to abandon its neural fate and become epidermal. In neurogenic mutants (Lehmann et al., 1983), in which lateral inhibition is perturbed, expression of the proneural genes does not become restricted to individual cells, but persists in all cells of the proneural cluster (Cabrera 1990; Martín-Bermudo et al., 1995; Ruiz-Gomez and Ghysen, 1993; Skeath and Carroll, 1992). As a result, all cells of the neurogenic region become neuroblasts, a phenotype called neural hyperplasia.
It stands to reason that the AS-C and neurogenic genes are also essential for the neuroepithelial-to-neuroblast (NE-NB) transition which occurs in the optic anlagen. Yasugi and colleagues (2008) have recently demonstrated that the proneural gene lethal of scute (l’sc) is indeed expressed in a narrow band of cells that fall within the epithelium-to-neuroblast transition zone of the OOA. L’sc expression is negatively regulated by the Janus Kinase/signal transducer and activator of transcription (Jak/Stat) signaling cascade (Yasugi et al., 2008). Loss of Jak/Stat activity in Stat92E clones resulted in excess cells expressing l’sc, and, subsequently, causing these cells to adopt a neuroblast fate. This finding matches similar results with Jak/Stat signaling in the vertebrate retina, where expression of the dominant-negative form of Stat3 promoted neurogenesis instead of astrogliogenesis (Gu et al., 2005). Furthermore, Reddy et al. (2010) show that the Hippo-Fat pathway signals a cell cycle arrest in the OOA epithelium, which in turns maybe responsible for an upregulation of the Notch ligand Delta, and concomitant activation of the Notch pathway.
In this paper we have reconstructed the NE-NB conversion in detail, and investigated the role of Notch signaling and its interaction with the Jak/Stat pathway in the Drosophila optic lobe. In the early larval optic lobe, prior to neuroblast conversion, both Notch and Stat activity is found ubiquitously in the optic anlagen. Subsequently, the expression domains of Jak/Stat and Notch signaling separate spatially, such that Notch activity remains high in the medial OOA, and Jak/Stat, in the lateral OOA. During the third instar, a wave of enhanced Notch activity progressing over the OOA from medial to lateral controls the gradual NE-NB conversion. Epithelial cells at the medial edge of the OOA, shortly prior to becoming neuroblasts, express high levels of Delta, which activates the Notch pathway and thereby maintains the OOA in an epithelial state. Loss of Notch, as well as Jak/Stat, signaling results in a premature NE-NB conversion of the OOA, which in turn has severe effects on optic lobe patterning.
MATERIALS AND METHODS
Fly Stocks
Flies were grown at 25°C using standard fly media unless otherwise noted. The following mutant and transgenic strains were used in this study: Nts1 (Xu et al., 1992), Stat92EF (Baksa et al., 2002); Stat92E85C9, UAS-Su(H)DN/CyO, Actin-GFP (gift from Utpal Banerjee; Nagaraj and Banerjee, 2007), UAS-Stat92ERNAi (Kim et al., 2007), upd-GAL4 (Halder et al., 1995), dome-GAL4 (PG14, from Stephane Noselli), esg-GAL4 (Goto and Hayashi, 1999), E(spl)m8-lacZ (Lecourtois and Schweisguth, 1995), 10XStat92EGFP (Bach et al., 2007), nrv2-gal4, UAS-GFPS65T (Paul Salvaterra; Bloomington #6795), UAS-myr-RFP (Henry Chang; Bloomington #7118) and tub-PGAL80ts (Ron Davis; Bloomington #7018). E(spl)m8-lacZ is a well-documented reporter for Notch transcriptional activity, containing Suppressor of Hairless binding sites. Stat92E85C9 is a strong hypomorphic allele of Stat92E.
Temperature Shifts and Lineage Tracing Experiments
For temperature-shift experiments, embryos were collected and raised at 18°C. Hatching first-instar larvae of the Stat92E85C9/Stat92EF; esg-gal4, UAS-myr-RFP/UAS Su(H)DN; tub-PGAL80ts/+; and Nts1/Nts1 genotype were shifted to the restrictive temperature (30°C) for various time intervals (e.g. 0–48 hrs, 0–96 hrs and 48–96 hrs). After shifting to restrictive temperatures for certain time intervals, larvae were grown at 18°C. Wandering third instar were dissected and fixed according to standard procedures (Ashburner 1989).
Lineage expression analyses were done with upd-gal4 using the Gal4 Technique for Real-time and Clonal Expression (G-TRACE), containing the following genotype: UAS-Flp, UAS-dsRedStinger, ubi-p63E-FRT-stop-FRT-eGFP, allowing for marking of both cells in real-time and those derived from a particular lineage using fluorescent proteins (Evans et al., 2009).
Immunohistochemistry and BrdU Labeling
Samples were fixed in 4% formaldehyde in phosphate buffer saline (PBS, Fisher-Scientific, pH = 7.4; Cat No. #BP399-4). Tissues were permeabilized in PBT (phosphate buffer saline with 0.1% Triton X-100, pH = 7.4) and immunohistochemistry was performed using standard procedures (Ashburner 1989). The following antibodies were provided by the Developmental Studies Hybridoma Bank (Iowa City, IA): mouse anti-Dachshund (mAbdac2-3, 1:600), mouse anti-Delta (1:10), mouse anti-Neurotactin (BP106, 1:10), rat anti-DN-Cadherin (DN-EX #8, 1:20), rat anti-DE-cadherin (DCAD2, 1:500), mouse anti-FasciclinII (1D4, 1:500), mouse anti-Repo (8D12; 1:20). We also used mouse anti- β-Galactosidase (β-Gal; Promega, Cat No. #25580601; 1:100), rabbit anti- β-Gal (MP Biomedicals, LLC, Cat No. #0856032; 1:100), guinea pig anti-Delta (gift from Marc Muskavitch, 1:1000), rabbit anti-Deadpan (Dpn, gift from Yuh Nung Jan; 1:500), rat anti-Crumbs (Crb, gift from Elisabeth Knust; 1:2000) and mouse anti-BrdU (BD Biosciences, Cat No. #347580). For Crb staining, polyclonal antisera was pre-adsorbed overnight prior to immunostaining. Secondary antibodies, IgG (Jackson ImmunoResearch; Molecular Probes) were used at the following dilutions: Cy3-conjugated anti-guinea pig (1:250) and anti-rabbit (1:200); Fluorescein (FITC)-conjugated anti-mouse (1:200), anti-guinea pig (1:250) and anti-rabbit (1:200); Cy5-conjugated anti-rabbit (1:100) and anti-mouse (1:100); AlexaFluor 488-conjugated anti-mouse (1:500), Alexa 568-conjugated anti-rabbit (1:300), and 546-conjugated anti-mouse (1:500). Phalloidin 546 and Phalloidin-Rhodamine (Molecular Probes; Cat No. #A22283, #R415), used to visualize actin filaments were diluted in PBTA (pH = 7.4, phosphate buffer saline, 0.1% Triton X-100, 2% BSA, 5% Normal Goat Serum, NGS), 1:100. TO-PRO-3 (Invitrogen, 1 μM in PBTA) was used as a nuclear stain. Tissues were mounted in Vectashield mounting medium (Vector, Burlingame, CA; #H1000).
For BrdU labeling, larvae were fed for the duration of the pulse with medium to which 1mg/ml of BrdU was added. Subsequently larvae were transferred to normal food for the duration of the chase. For short pulses directly prior to fixation, dissected wandering third-instar larvae were incubated in BrdU at room temperature (Sigma, 70ug/ml) in PBS for 30 min. Samples were fixed in 4% formaldehyde in PBS and washed in 0.3% PBT. Denaturation in 2N HCl for 30 min was followed by a second fixation with 4% formaldehyde in 0.1% PBT. Standard immunohistochemistry was performed as described (Ashburner 1989).
Confocal Microscopy
Staged Drosophila larval and adult brains labeled with suitable markers were viewed as whole-mounts by confocal microscopy [Biorad MRC 1024ES microscope using Biorad Lasersharp version 3.2 software; lenses: 40× oil (numerical aperture 1.0; WD 0.17); and LSM 700 Imager M2 using Zen 2009 (Carl Zeiss Inc.); lenses: 40× oil (numerical aperture 1.3)]. Complete series of optical sections were taken at 2-μm intervals. Captured images were processed by ImageJ (National Institutes of Health, http://rsbweb.nih.gov/ij/) and Adobe Photoshop.
Generation of three-dimensional models
Digitized images of confocal sections were imported into the Amira (http://www.amiravis.com). Since sections were taken from focal planes of one and the same preparation, there was no need for alignment of different sections. All models were generated using the Amira software package. Objects, including the different domains of the optic anlagen and the emerging neuropile compartments of the optic lobe, were manually segmented on the series of confocal images imported into Amira. These domains are visible based on cell size, cell shape, and texture in brain preparation labeled with anti-Neurotactin antibody, or with Phalloidin. Following segmentation, the program then generated surfaces which could be rendered in different colors and degrees of transparency.
RESULTS
Structure and development of the larval optic lobe
The structure of the optic lobe primordium of the larva is highly dynamic and, towards the later stages, very complex. As a result, we currently have only a rudimentary understanding of how the different neuropiles and cell types of the adult optic ganglia map onto the larval optic lobe primordium. Moreover, the dynamic changes in shape that characterize the optic lobe at the different larval stages make it very difficult to interpret mutant phenotypes of genes controlling optic lobe development. We will in the following depict the normal development of the larval optic lobe, focusing on the outer optic anlage and its derivatives, the distal (outer) medulla and the lamina (Fig. 1).
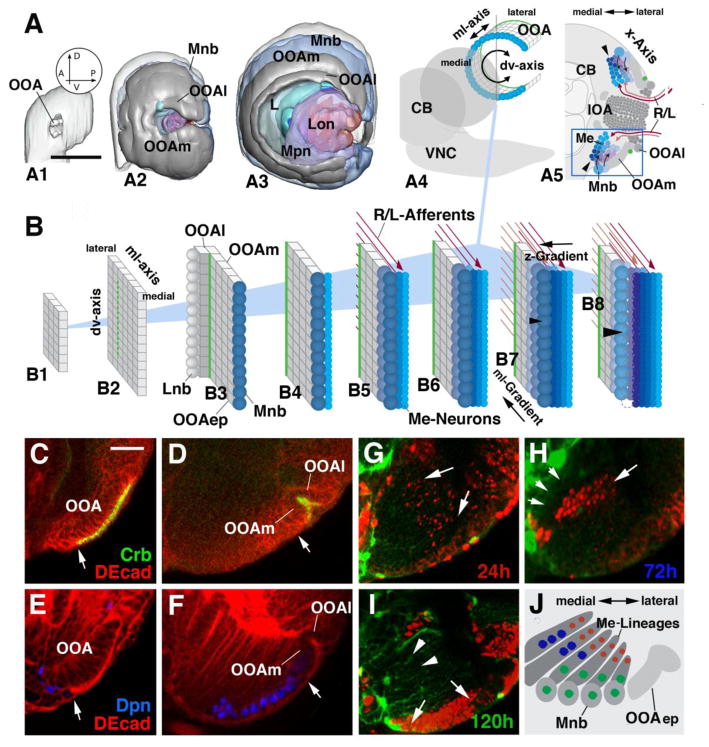
Figure 1.
Topology of the developing optic lobe in the larva. (A1–A3) Digital 3D models of larval optic lobe in lateral view. Orientation is indicated by arrows in circular inset in A1 (A, anterior; P, posterior; D, dorsal; V, ventral). (A1) First instar (12h); (A2) Early third instar (72h); (A3) Late third instar (120h). Parts of the optic lobe, in these models and models of subsequent figures, are shown in different colors: light gray, outer optic anlage (OOA); dark gray, inner optic anlage (IOA); cyan, lamina neuroblasts and lamina neurons; dull blue, neuroblasts forming from outer optic anlage (distal medulla neuroblasts); bright blue, distal medulla neurons; magenta, neuroblasts forming from inner optic anlage; purple, neurons of proximal medulla (Mpn); red, neurons of lobula complex (Lon). (A3–A5): Schematic depiction of OOA relative to larval brain (CB: central brain; VNC: ventral nerve cord). The OOA is curved along the dorso-ventral axis (A4), so that a frontal section (A5; plane of sectioning indicated by gray line in A4) cuts through the OOA twice. (B1–B8): Schematic depiction of the outer optic anlage (OOA) at different larval stages (B1: early first instar; B8: late third instar). The OOA forms a rectangular epithelial layer appended to the lateral surface of the brain. For clarity sake, the OOA epithelium (OOAep) is depicted as a flat layer. The boundary between lateral OOA (OOAl) and medial OOA (OOAm) is shown as a green line. Medulla neuroblasts (Mnb) arising at the medial edge of the OOAm (B3–B8) are colored dull blue. Neuroblasts formed early (B3) are in darker shade, later neuroblasts (B5, B7) in lighter shade. Neuroblasts divide perpendicular to the plane of the epithelium (B4–B8) and give rise to the neurons of the distal medulla (Me-neurons), depicted in shades of bright blue. Neurons born first (B4) are in light blue; those born later are in increasingly dark shades of blue (B6, B8). Afferent axons from the retina and lamina (R/L-Afferents) are symbolized by red arrows (early arriving afferents, dark red; later afferents, light red).
(C–F) Expression of markers for epithelial optic anlage (anti-Crb) and neuroblasts (anti-Dpn) in early third instar larva (C, E) and late third instar larva (D, F). Only ventral arm of the OOA is shown (see boxed area in panel A5). White arrows point at area of transition from epithelium (to the right of arrow) to neuroblast (to the left of arrow). OOA, outer optic anlage; OOAl, lateral domain of outer optic anlage; OOAm, medial domain of outer optic anlage. (G–J): BrdU pulse-chase experiments addressing pattern of proliferation in the OOAm. Panels show part of cross section of late third instar brain (120h after hatching) including OOAm, neuroblasts derived from it (Mnb), and columnar lineages of medulla neurons (Me-neurons) proliferated from the neuroblasts. The arrangement of these elements is schematically depicted in panel J. BrdU-positive cells are labelled by antibody (red); green signal corresponds to glia expressing GFP driven by Nrv2-Gal4. In panel G, a BrdU pulse was given between 0 and 24h after hatching; H: 48–72h; I: 96–120h. Scale bars: 40μm (A1–A3); 10μm (C–I)
The OOA of the early larva starts out as an expanding rectangular sheet of epithelial cells, formed dorso-ventrally oriented columns of cells (Fig. 1A1, B1). Starting at the late first instar and continuing throughout larval life, the OOA epithelium bends along the dorso-ventral-axis (Fig. 1A2–4). As a result, cells are aligned in C-shaped curves (Fig. 1A4). What this spatial transformation means when looking at optic lobes sectioned along the “standard” frontal plane, as shown in Fig. 1A5 is that the OOA is sectioned twice, once dorsally, and once ventrally.
During the second larval instar, the OOA becomes subdivided into two domains, visibly separated by a furrow called lamina furrow (green line in Fig. 1A4, 1B1–8). Cells lateral of this furrow (OOAl) give rise to the lamina; the much larger medial domain (OOAm) form the distal medulla. At around the time when the lamina furrow divides the OOA into a lateral and medial domain, epithelial cells along the edges of these domains convert into asymmetrically dividing medulla neuroblasts (Mnb; Meinertzhagen and Hanson, 1993; Egger et al., 2007; Fig. 1B3). As shown in Fig. 1C–F, this transition can be followed effectively by labelling optic lobes with anti-Crumbs (Crumbs being expressed at the apical membrane of all ectodermally derived tissues; Tepass et al., 1990; Fig. 1C, D) and anti-Deadpan (a marker for neuroblasts; Bier et al., 1992; Fig. 1E, F). Once cells have converted to neuroblasts, they “bud off” progeny in the direction perpendicular to the plane defining the OOA. Because of this directed proliferation, neurons born first come to lie at ever increasing distances from the neuroblast/OOA (Fig. 1B4–8, shown only for OOAm). At the same time as the medulla neuroblasts divide, new rows of neuroblasts appear as, one by one, rows of epithelial cells along the medio-lateral-axis convert into neuroblasts (Fig. 1B5–8). In the late larva, medulla neuroblasts start to disappear. Thus, the lineages at the medial edge of the optic lobe, which had been the first to appear, are no longer capped by a neuroblast (arrowheads in Fig. 1A5 and 1B8). The fate of the medulla neuroblasts after they cease to divide has not yet been followed in detail. Similar to neuroblasts of the central brain, they are likely to undergo programmed cell death (Cenci and Gould, 2005).
The correlation between neuron position and birth date can be visualized by BrdU pulse-chase experiments, shown in Fig. 1G–J. Early pulses (24h) result in faint labelling of medulla neurons located deep (arrows in Fig. 1G; red circles in Fig. 1J). In this experiment, BrdU is taken up by all cells of the epithelial OOA which, around 24h, divide symmetrically. As the epithelium converts into neuroblasts, all neuroblasts “inherit” the (by then already diluted) label. When neuroblasts start their rapid asymmetric division, only the first born neurons receive enough BrdU to maintain detectable label; these are the neurons located deeply. Pulses administered at mid-larval stages (72h; Fig. 1H) result in strong labelling of neurons located in the medial medulla at deep and intermediate levels (arrow in Fig. 1H; blue circles in Fig. 1J). In this experiment, the BrdU pulse reaches the OOA at a stage when the epithelial cells have all but ceased to divide, and the medial cells have converted into rapidly dividing neuroblasts. These are the cells that incorporate BrdU. Due to the rapid division/dilution of the label, only early born neurons, located deeply, receive enough label. Late pulses, followed by immediate fixation, result in labelling of most neuroblasts and superficially located neurons (arrows in Fig. 1I; green circles in Fig. 1J). At this stage, the most medial lineages no longer proliferate (arrowheads in Fig. 1I).
The description of OOA development above indicates that two spatio-temporal gradients are in the OOAm. One gradient, directed along the medio-lateral axis of the OOAm (“ml-gradient”), describes the sequence in which rows of neuroblasts are formed; the second gradient, directed from the surface inward, perpendicular to the plane of the OOA (“z-axis”), underlies the order in which each neuroblast produces neurons (“z-gradient”; Fig. 1B7). The ml-gradient correlates with the anterior-posterior axis of the medulla. Thus, axons that grow towards the first-born OOAm neurons, derived from the most medial row of neuroblasts, are the R7/8 axons originating from posterior retina, as well as L- neurons from the posterior lamina (Meinertzhagen and Hanson, 1993; Fig. 1B5). The next set of axons, arriving later, captures neurons of the next (more lateral) row of OOAm neuroblasts, etc etc. What this implies is that the ordered progression of NE-NB conversion may match the progression of ingrowing axons, and that this matching may be important for the formation of an ordered medulla neuropile. The significance of the z-gradient has not yet been investigated. It seems likely that, similar to what is known for lineages of the central brain and ventral nerve cord, it accounts for the sequential generation of different neuronal cell types (Grosskortenhaus et al., 2005).
Fig. 2 shows the different components of the late larval optic lobe primordium in more detail. The epithelial part of the OOA (blue solid lines in Fig. 2A) is flanked medially and laterally by neuroblasts (blue circles) that produce the distal medulla (Md) and lamina (L), respectively. The medial neuroblasts generate lineages of the distal medulla that are directed centro-laterally (hatched lines with arrows in Fig. 2A). As explained in the previous section, the oldest lineage (l1) is the one situated furthest medially; the youngest, most recently borne one (ln) is the one furthest laterally. Within each lineage, central neurons (nc) are older than peripheral ones (np). Neurons form bundles of axons that gather at the base of the lineages. Along with ingrowing axons from lamina neurons (L) and retinal axons (not shown), this mass of fibers give rise to the neuropile of the distal medulla (Mdnp; shaded blue).
Figure 2.
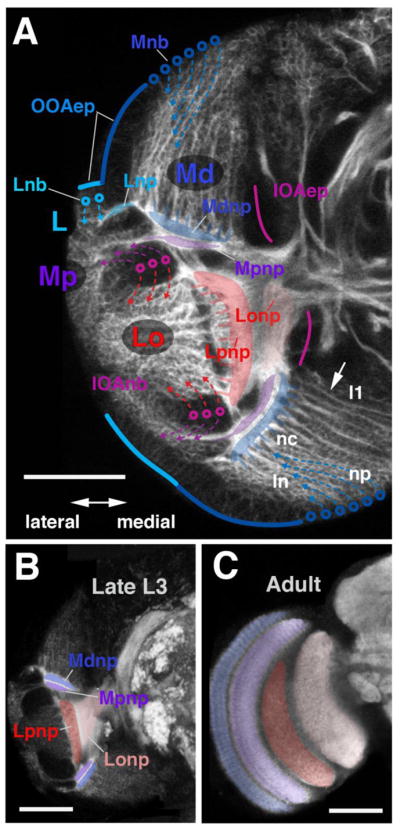
Fatemap of the late larval optic lobe. (A) Confocal cross-section of late larval optic lobe of left brain hemisphere, labelled with anti-Neurotactin (white). Epithelial inner and outer optic anlagen (IOAep; magenta line; OOAep, blue line) are only faintly positive for anti-Neurotactin and appear dark. Neuroblasts arising from the outer optic anlage (Lnb, cyan circles; Mnb, blue circles) and inner optic anlage (IOAnb, magenta circles) are moderately strongly labelled; neurons and outgrowing axons are strongly labelled. Arrows on hatched lines indicate direction in which neurons are given off by their respective neuroblasts. Neurons of the distal medulla (Md) form discrete lineages, each one emitting a bundle of axons towards centrally (arrow). Tips of these axons gather in a plexus that forms the forerunner of the distal medulla neuropile (Mdnp, shaded blue). Lineages located medially belong to neuroblasts formed from the OOAm first (l1); the further lateral a lineage is located (ln), the later the corresponding neuroblast was formed. In each given lineage, neurons born early are located centrally, far from the surface (nc); late born neurons are peripherally (np). Neuroblasts of the IOA give off neurons outwardly (purple arrows) and inwardly (red arrows). The former makes up the proximal medulla (Mp) and the latter makes up the lobula and lobula plate (Lo; see also digital models in panel E). Axons of both of these neuron populations grow medially and form the proximal medulla neuropile (Mpnp, shaded in purple), the lobula plate neuropile (Lpnp, red) and the lobula neuropile (Lonp, pink). (B, C) Confocal cross-sections of late larval brain (B) and adult brain (C), labelled with anti-DNcadherin to visualize optic neuropiles (white). Left hemisphere, lateral to the left, dorsal up. Optic lobe neuropiles of the larva and adult are rendered in corresponding colors; abbreviations and color code as in A. Bars: 40μm
The inner optic anlage (IOA) also undergoes a NE-NB conversion, bending along the dv-axis, similar to what has been described above for the OOA. The details of this transformation will be described elsewhere (Ngo et al., in prep.). Briefly, in the late larva, the IOA consists of a C-shaped epithelial component (IOAep; red lines in Fig. 2A; note that, as for the OOA, the IOA sectioned along the frontal plane appears twice, once ventrally, and once dorsally). Further laterally, neuroblasts derived from the IOA (IOAnb; red circles) form a mass of cells that is also bent, and therefore seen twice in a frontal section. The IOA neuroblasts produce two populations of neurons. Neurons pushed anteriorly (or outward, taking into account the curvature of the IOA) become the proximal medulla (Mp); those pushed interiorly, or centrally, become the lobula and lobula plate (Lo; Fig. 2A). Fig. 2B–C illustrate the arrangement of the optic lobe neuropiles in the late larva, and their corresponding adult counterparts.
Activity of Notch and Jak/Stat in the larval optic lobe primordium
A pivotal step in the development of the optic lobe is the formation of neuroblasts from the OOA epithelium. As established for neuroblasts formation in the embryo, the number of neuroblasts emerging at any given time point appears to be tightly regulated. It has been recently documented that the Jak/Stat pathway plays a pivotal role in the transition from epithelial cells to neuroblasts (Yasugi et al., 2008; Reddy et al., 2010), where it negatively regulates the proneural gene, l’sc. As Notch is a common player during selection of neural progenitors, we sought to investigate the function of Notch signalling and its interaction with the Jak/Stat pathway.
In the early larva, expression of the Notch ligand Delta and Notch activity (monitored by E(spl)m8-lacZ; Lecourtois and Schweisguth, 1995), as well as Stat activity (using 10XStat92GFP; Bach et al., 2007), are found ubiquitously at moderate levels in the OOA and IOA (Fig. 3A–D). Delta and E(spl)m8 are also expressed in all neuroblasts of the central brain and their early progeny; Stat activity is highest in glial cells. At the time when the OOA has expanded and is beginning to differentiate into a lateral and medial domain, Stat becomes increasingly restricted to the lateral domain (OOAl; Fig. 3E). In the late larva, Stat is exclusively in the OOAl and the lamina progenitors/lamina neurons derived from it (Fig. 3I). The Jak/Stat ligand upd, after an initial phase of widespread expression in the OOA and IOA, also become restricted to the OOAl and lamina (Fig. 3H). The restriction of expression can be confirmed experimentally by using the G-TRACE construct (Gal4 Technique for Real-time and Clonal Expression; Evans et al., 2009), which combines the Gal4/UAS system in concert with the FLP/FRT recombination system (Theodosiou and Xu, 1998) to visualize both real-time and lineage-traced gene expression pattern. While real-time upd expression is restricted to the OOAl and lamina during late larval development (marked by dsRedStinger, in red; in Figure 3H), a large proportion of the optic lobe is labelled by EGFP, demonstrating that these cells are derived from cells expressing upd at an earlier developmental stage. Consistent with the real-time expression of upd-gal4, dome-gal4 real-time expression is also restricted to the OOAl (Fig. 3L).
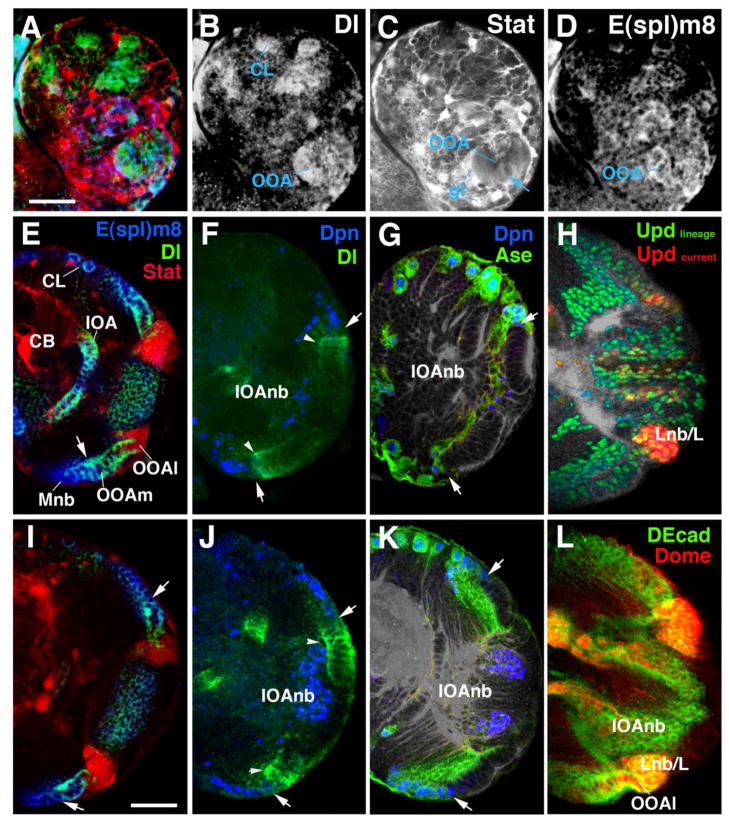
Figure 3.
Spatio-temporal pattern of Notch and Jak/Stat signalling activity in the larval optic lobe. (A–D) Confocal section of first instar brain hemisphere showing outer optic anlage (OOA) and central brain (CB). In this and all other panels, lateral is to the right, dorsal is up. Expression levels of Delta (Notch ligand, in green in panel A) and E(spl)m8 (read-out of Notch signalling activity; blue in A) are strong throughout the OOA and central brain lineages (CL). Stat92EGFP (reporter of Jak/Stat signalling; red in A) is expressed most strongly in glia (gl) surrounding neurons and OOA, but appears at moderate level throughout the OOA as well (arrow in C). (E–G) Confocal section of brain hemisphere of early third instar (72h after hatching). (H–L) Sections of late third instar (96–120h after hatching). High levels of Jak/Stat activity (E, I; red color) become restricted to the lateral rim of the OOA (OOAl), which will give rise to the lamina. Delta expression is found throughout the epithelial OOA (E, F, I, J; green color); it is highest at the medial margin of the OOA where the NE-NB conversion takes place (arrowheads in F, J). High levels of E(spl)m8 are seen in the OOAm and the medulla neuroblasts Mnb) derived from it (E, I; blue color). A lacZ reporter for the proneural gene asense (ase) is expressed right behind (medial of) the Delta peak, overlapping with the neuroblast marker Deadpan (G, K). In panels F–K, the line of NE-NB conversion is indicated by white arrow. (H) Confocal section of third instar brain hemisphere showing expression of the G-TRACE construct driven by upd-Gal4. At this stage, upd-Gal4 drives dsRed-Stinger (shown in red) in the OOAl. Widespread expression of GFP in the OOA and most of its derivatives (green) demonstrates that upd-Gal4, at an earlier stage, was expressed in the entire OOA. (L): Expression of the receptor Domeless (Dome), using dome-gal4>GFP (in red) in outer optic anlage, with peak levels in OOAl and lamina primordium (Lnb/L).
Other abbreviations: CB, central brain; CL, lineage of central brain; IOA, inner optic anlage; IOAnb, neuroblasts derived from inner optic anlage.
Bars: 20μm (A–D); 30μm (E–L)
At the stage when the NE-NB conversion begins, the Delta/Notch pathway becomes dynamically centered around this transition zone, following the ml-gradient of the OOA. Delta remains always restricted to the epithelial part of the OOAm (Fig. 3E, F, I, J). Laterally, it partially overlaps with the domain of Stat activity, i.e., it reaches into part of the OOAl, but does not extend all the way to its lateral rim. Medially, Delta expression shows a peak level in epithelial cells about to convert to neuroblasts (arrowheads in Fig. 3F, J). These strongly Delta-positive cells flank the already formed neuroblasts, which express the markers Deadpan (Dpn) and Asense (Ase; Egger et al., 2007; Wallace et al., 2000; Fig. 3G, K). The region of transitioning epithelial cells which shows peak expression of Dl have been previously shown to also express L’sc (Reddy et al., 2010). Additionally, Notch activity, monitored by expression of E(spl)m8, is high in the OOAm, overlapping with the expression of Delta. In contrast to Delta, E(spl)m8 stays on in neuroblasts and their progenies (Fig. 3E, I).
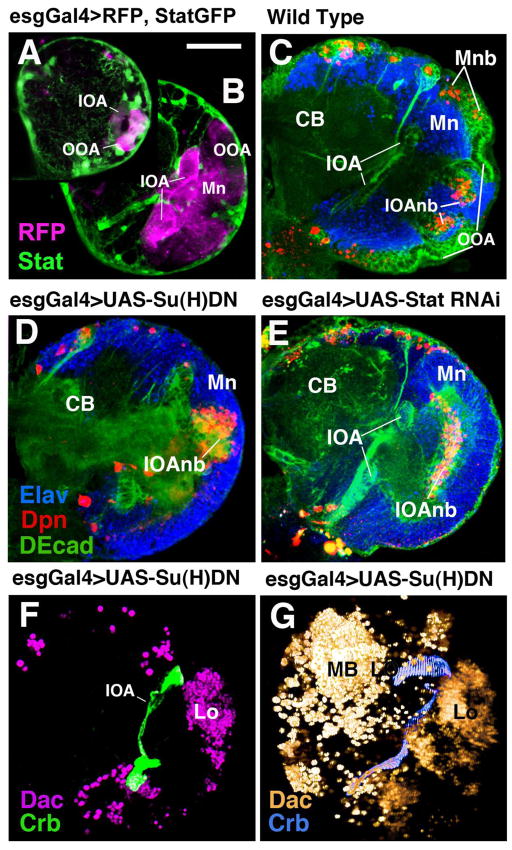
Loss of Notch leads to a precocious release of neuroblasts from the OOA
The dynamic expression of Delta and E(spl)m8 in the optic anlagen suggests that Notch signalling is involved in the ordered release of neuroblasts from the anlage. In order to investigate the function of Notch during larval neurogenesis, we used the temperature-sensitive Nts1 allele. Raising embryos at the permissive temperature resulted in wild-type early larvae; these were shifted to the restrictive temperature (30°C, dissected at later larval stages, and stained with markers for neurons, neuropile, and optic lobe epithelia. For the latter, we used an antibody against Crumbs which highly specifically labels the apical surfaces of the optic anlagen (Tepass et al., 1990; see Fig. 1C, D above). In wild-type, Crumbs expression labels the C-shaped belt that demarcates the outer optic anlage (Fig. 4A, C, R). In Nts1 mutant brains, the Crumbs-positive domain was strongly decreased in size and did not show its typical C-shape (Fig. 4B, D, S). This reduced size of the optic anlagen in late larval brains is caused by the premature conversion of epithelial cells into neuroblasts. Optic lobe neuroblasts and their progeny (GMCs and neurons) are positive for BP106 (Neurotactin, Nrt) and Deadpan (Dpn), whereas the optic anlagen are distinctively BP106-negative and Dpn-negative (Fig. 4E, J). The misshapen optic lobes of Nts1 mutant larvae are covered by Dpn-positive neuroblasts (Fig. 4F, I) and their progeny. As in wild-type controls, DN-Cadherin (DNcad in green, Fig. 4J, K) comes on in the deep layers of the medulla primordium, suggesting that differentiation of these cells has set in normally. At the same time, the evenly distributed columns of neurons and axons that represent the nascent medulla columns in the wild-type brain (Mdc in Fig. 4J) are disrupted in the mutant. Here, medulla neurons form more irregularly sized clusters with thicker axon bundles emanating from them (Fig. 4K).
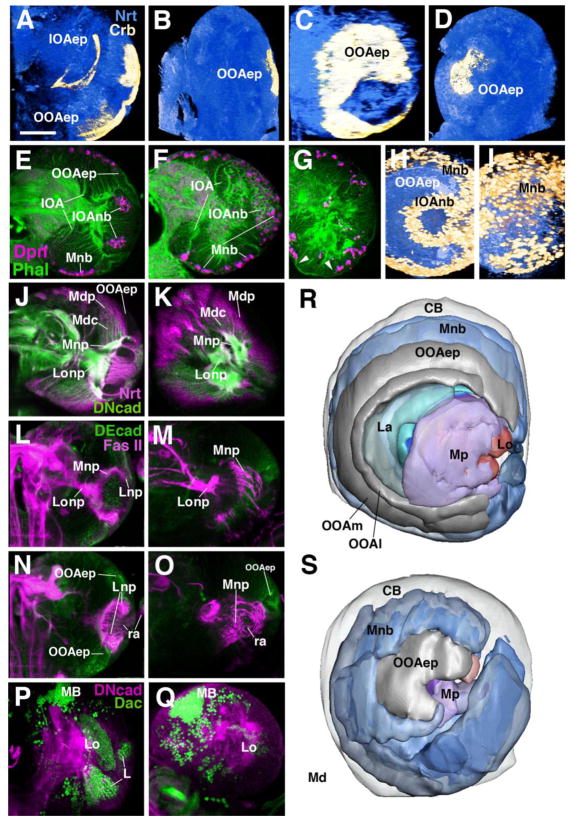
Figure 4.
Loss of Notch signalling causes a premature NE-NB conversion and leads to structural defects in the optic lobe. Photographic panels (A–Q) are volume renderings (A–D; H–I) or frontal confocal sections (all others) of late third instar brain hemispheres labelled with different markers. Panels of left column (A, E, J, L, N, P) and panels C and H show wild-type control; panels of middle column (B, F, K, M, O, Q) and panels D and I are from Nts1 larvae raised at the restrictive temperature from first to third instar. (A–D) Abrogation of Notch signalling results in loss of the OOA epithelium, labelled with anti-Crumbs (Crb) antibody (yellow). Brain neurons are labelled with anti-Neurotactin (Nrt; blue) for reference. (E–K) Neuroblasts and neurons of the medulla, marked by anti-Deadpan (Dpn, E–I) or anti-Nrt (J, K) cover the entire lateral surface of the optic lobe. This phenotype is most clearly visible in volume renderings shown in lateral view (H, I). In wild-type (H), Dpn-positive medulla neuroblasts (Mnb), shown in yellow color, occupy a C-shaped band medial (in this view: “peripheral”) of the epithelial outer optic anlage (OOAep); Dpn-positive neuroblasts derived from the inner optic anlage (IOAnb) form a central cylinder. In Nts1 (I), the OOAep has converted prematurely to medulla neuroblasts (Mnb). Mdp, proximal medulla neurons; Mdc, central medulla neurons. (L–O) Double labelling with anti-Fasciclin II (FasII, magenta) to mark fiber tracts and anti-DE Cadherin (DEcad, green) to mark the epithelium of the OOA. Panels L and M show a plane of section through the center of the optic lobe neuropile; N and O show the optic lobe neuropile at an anterior plane. Most retinal afferents (ra) terminate in the lamina neuropile in wild-type (Lnp in L and N). In mutant, retinal afferents project in irregular tangles of fibers to the deeper medulla neuropile (Mnp in M and O). Note strongly decreased optic lobe epithelium (OOAep) in mutant. (P, Q) Loss of lamina neurons in Nts1 mutants. Double-labelling with anti-DN cadherin (green, neuropile marker) and anti-Dachshund [Dac, magenta; labels neurons of lamina (L), as well as lobula (Lo) and mushroom body (MB)]. (R, S) Digital 3D models of late third-instar larval brain hemisphere of wild-type (R) and Nts1 mutants (S). Models are shown in lateral view, anterior is to the left, dorsal is up. Models represent a view of the optic lobe surface, which is formed by the epithelial outer optic anlage (OOAep) and, more medially, the medulla neuroblasts (Mnb) derived from it. Laterally, and surrounded by the OOA, is the primordium of the Lamina (La), proximal medulla (Mp) and lobula complex (Lo). Color code as in model shown in Fig. 2. Other abbreviations: CB, central brain; OOAl, lateral domain of outer optic anlage; OOAm, medial domain of outer optic anlage.
Bar: 50μm
In a significant fraction of Nts mutant brain, medulla neuroblasts derived from the OOA had all but disappeared, and a mass of tightly packed neurons covered the entire lateral surface of the brain (Fig. 4G; see also below for mutations in Jak/Stat signalling). This suggests that the time interval during which medulla neuroblasts of Nts-mutant optic lobes are active (i.e., from their birth from the OOAm to their disappearance) maybe the same as in wild-type. Because they are born earlier in the Notch mutant, they also disappear earlier.
Notch related defects in optic lobe connectivity
During the middle of the third instar larval stage, photoreceptor axons R1–R6 start growing into the brain and make connections with cells of the lamina (Selleck and Steller, 1991; Meinertzhagen and Hanson, 1993). R7/R8 axons continue past the lamina and terminate in the distal medulla; lamina neurons (L-neurons) also extend axons towards the distal medulla. The medulla neurons, in turn, connect to the lobula complex and the central brain. To explore the retinal axons connectivity defects within the optic lobe that is associated with Nts1 mutants, we labelled the fiber tracts with Fasciclin II (FasII). These include the retinal axons, lamina axons, the posterior optic tract (medulla to central brain), and the axons from the lobula to the central brain (Nassif et al., 2003).
Not surprisingly, connectivity defects were profound in Nts1 mutants, given that Notch is known to be required for patterning of retinal cell types (Cagan and Ready, 1989). Previous studies had shown that perturbation of Notch or Delta results in a neurogenic phenotype: most cells within the atonal (ato) intermediate group differentiates and express the fate of R8 cells (reviewed by Frankfort and Mardon, 2002). As a result, later born R1–6 and R7 are either reduced or absent in neurogenic mutants (Cagan and Ready, 1989). In Nts mutants, we observed that R8 neurons form axons which do not properly bundle into thin fascicles. Instead, these R8 neurons form thick and irregular bundles that completely bypass the lamina and terminate at a deep level within the medulla primordium (Fig. 4L–O). Aside from the fact that it receives more massive input from the retina, the medulla primordium is also misshapen due to the fact that medulla neuroblasts/neurons are born in an abnormal temporal pattern. Thus, in wild-type, the epithelial OOA grows to a large size and forms the “dome” covering the entire lateral surface of the brain (see previous section). Subsequently, neuroblasts are released in a well-ordered succession from the margin of the OOA, resulting in the medulla primordium with a given (large) surface area and (small) depth. In the Nts1 mutant the OOA does not grow to a large size because the OOA epithelium prematurely converts into neuroblasts. As a direct result of such premature conversion, the medulla primordium as a whole and the medulla neuropile in particular, is larger in depth and smaller in surface area (Fig. 4R, S).
The effect of loss of Notch function on the lamina primordium is complex. FasII labeling of nascent lamina neurons is visible in the wild-type OOAl. Axons of these neurons fasciculate with the afferent retinal axons (Fig. 4L, N; note that it is not possible to distinguish between retinal and lamina-derived axons in anti-FasII-labeled brains). In Nts mutants, FasII-positive neurons appear to be absent, where it cannot be detected in the immediate vicinity of the rudimentary OOA (Fig. 4M, O). This interpretation is further confirmed by the absence of the lamina neuronal marker, Dachshund (Dac). In wild-type, Dac expression appears at a low level in the OOAl and is strongly upregulated in postmitotic lamina neurons (Fig. 4P). This lamina-specific expression of Dac is reduced or absent in Nts1 mutant brains (Fig. 4Q).
Loss of Jak/Stat signaling mimics many aspects of reduced Notch signaling in the larval optic lobe
The use of molecular markers that switch expression as epithelial cells convert into neuroblasts had shown that the Jak/Stat pathway, similar to Notch signaling, may act as an inhibitor of neuroblast formation (Yasugi et al., 2008). To verify that the structural phenotype ensuing from loss of Jak/Stat signaling follows this prediction, we used markers for optic lobe epithelium, neuroblasts, and neurons in the background of a temperature-sensitive mutation of Stat92E (Stat92EF) with a strong Stat92E hypomorph (Stat92EF/Stat92E85C9). Late larvae that had developed at the restrictive temperature from hatching onward showed absence or significant reduction of the OOA epithelium as visualized by anti-Crb labeling (Fig. 5A–D). As observed in Nts, the Dac-positive band of lateral cells representing the primordium of the lamina was greatly reduced (Fig. 5C, D).
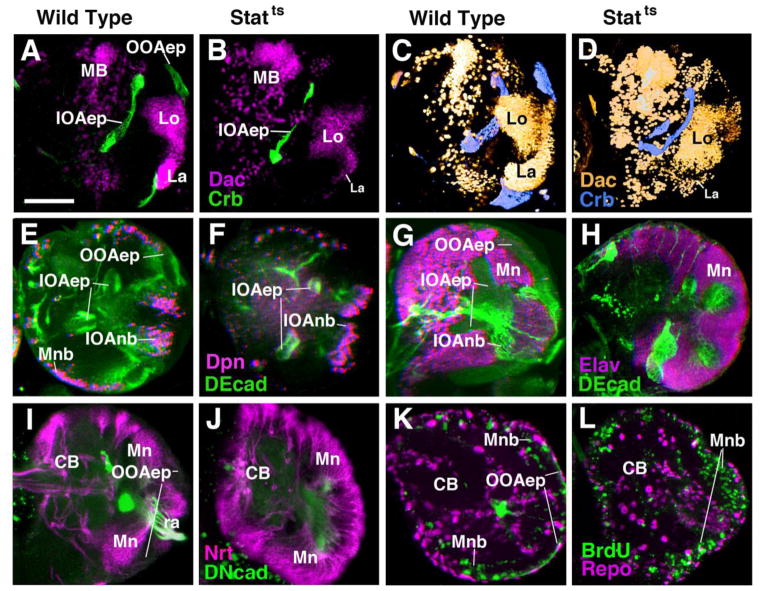
Figure 5.
Requirement of Stat signalling during optic lobe development. All panels except C and D show frontal confocal sections of late third instar brain hemispheres; medial is to the left, dorsal is up. C and D are volume renderings of brain hemispheres, antero-lateral view, lateral to the right. Panels of first column (A, E, I) and third column (C, G, K) show wild-type controls; panels of second column (B, F, J) and fourth column (D, H, L) are from Stat92EF/Stat92E85C9 larva raised at the restrictive temperature from first to third instar. (A–D) Labelling with anti-Crumbs (Crb, green in sections A and B; blue in volume renderings C and D) visualizes loss of epithelial outer optic anlage (OOAep) in mutant. By contrast, Crb-positive inner optic anlage (IOAep) is present, although deformed. Second label represents Dachshund (Dac) expressed in lamina (La) and lobula complex (Lo) in wild type; labelling of lamina is greatly reduced in mutant. (E, F) Labeling with anti-DEcad (green) and anti-Deadpan (Dpn, magenta). (G, H) Labeling with anti-DEcad (green) and anti-Elav (magenta; neuronal marker). Note that epithelial outer optic anlage (OOAep in E) and characteristic zone of medulla neuroblasts (Mnb in E) is absent in mutant (F). Entire lateral optic lobe is covered by Elav-positive medulla neurons (Mn in H). (I, J) Labeling with anti-Neurotactin (Nrt; magenta) illustrates loss of OOAep and overabundance of irregularly shaped clusters of medulla neurons (Mn).
(K, L) Pulse of BrdU applied to late third instar brains results in increase in BrdU-labeled cells (medulla neuroblasts, Mnb) covering the entire periphery of the optic lobe in Stat92EF/Stat92E85C9.
Bar: 50μm
Lineages of medulla neurons produced by prematurely converted neuroblasts occupied the entire surface area of the optic lobe primordium (Fig. 5E–F; I, J). Dpn-positive neuroblasts were typically strongly reduced or absent (Fig. 5F): due to their earlier time of birth, neuroblasts completed their proliferatory activity earlier than in wild-type and consequently also disappeared earlier. Loss of stat signal resulted in premature differentiation, as shown by the increase in ELAV-positive cells as compared to wild-type (Fig. 5G–H). Moreover, Stat-mutant brains dissected at an earlier stage (mid-third instar) had optic lobes with an increased number of neuroblasts (data not shown); similar to what is shown in the previous section for Nts mutants. The premature epithelial to neuroblast conversion of the OOA was confirmed by applying short 30-min BrdU pulses to Stat92EF/Stat92E85c9 larvae. In wild-type controls, BrdU incorporation is mostly confined to the medial OOA where cells have converted into neuroblasts, which cycle much more rapidly than the epithelial cells (Reddy et al., 2010; Fig. 5K). In Stat92EF/Stat92E85C9 mutants, BrdU incorporation is seen over the entire surface of the OOA, supporting the conclusion that these cells have prematurely converted into rapidly cycling neuroblasts (Fig. 5L).
Knock-down of Notch and Stat activity specifically in the optic lobe causes premature epithelium-neuroblast conversion
Both Notch and Jak/Stat activity are required not only in the optic lobe, but also in many cells of the central brain and other organs of the larva. To confirm that the optic lobe defects resulting from global inhibition of Notch or Jak/Stat signaling are indeed the result of a local requirement for these signaling activities in the optic lobe itself, we used a Gal4 driver line, esg-Gal4 (Goto and Hayashi, 1999), to direct the expression of mutant constructs to the optic lobe. esg-Gal4 is expressed at high levels in both optic anlagen (IOA, OOA) and their derivatives from early stages onward (Fig. 6A, B). The line is not expressed significantly in the central brain; outside the CNS, esg-Gal4 is seen in numerous adult-specific progenitor cells (e.g., those of the midgut; Ohlstein and Spradling, 2006) that are not involved in the normal functioning of the larva. In the head, esg is expressed in a narrow belt of cells surrounding the eye field, but not in retinal photoreceptors (Lim and Tomlinson, 2006). To inhibit Notch signaling, we expressed a dominant-negative form of Su(H) under the control of esg-Gal4 (Nagaraj and Banerjee, 2007); for the Jak/Stat pathway, we used a Stat92E RNAi construct placed under UAS control (Kim et al., 2007). The resulting phenotypes resembled the ones described above for the Nts and Statts mutants, respectively. The epithelial outer optic anlage converted prematurely into neuroblasts that budded of neurons (Fig. 6D, E) compared to wild-type (Fig. 6C). Also the loss of Dac-positive lamina neurons resembled the phenotype described for Nts and Statts (Fig. 6E, F).
Figure 6.
Optic lobe-directed expression of mutant constructs using the esg-Gal4 driver line. (A, B): Frontal confocal sections of first instar (A) and early third instar (B) brain showing RFP expression, driven by esg-Gal4, in optic lobe (magenta). Stat92EGFP is labeled in green. (C) Frontal confocal section of third instar wild-type brain labeled with anti-DEcad (green), anti-Elav (blue), and anti-Deadpan (red). CB, central brain; IOA, inner optic anlage; IOAnb, neuroblasts of inner optic anlage; Mn, medulla neurons; Mnb, medulla neuroblasts; OOA, outer optic anlage. (D, E): Frontal confocal sections of late third instar brain in which a dominant-negative Su(H) construct [Su(H)DN] (D) or Stat92E-RNAi construct (E) under UAS control was driven by esg-Gal4. Note loss of epithelial outer optic anlage and premature growth of medullary neurons. (F, G) Labeling with anti-Dachshund (Dac) illustrates strong reduction of lamina primordium (La) in confocal section (F) and volume rendering (G). MB, mushroom body (central brain).
Bar: 40μm
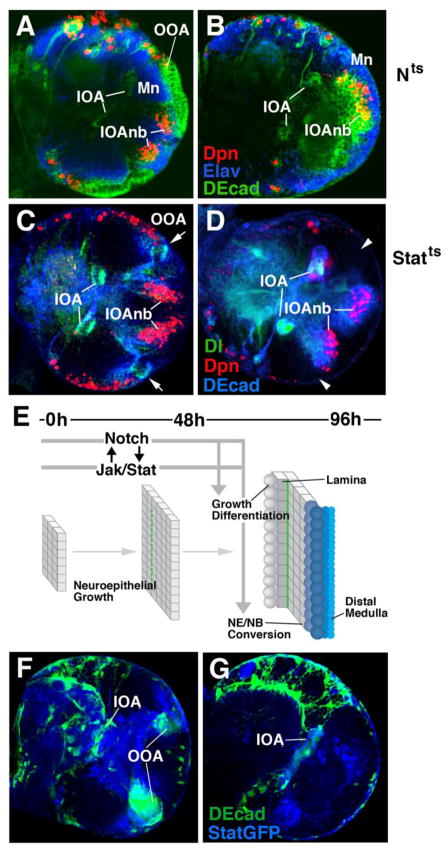
Notch and Stat activity are required during the late larval stage and are mutually interdependent
Notch and Jak/Stat are both active throughout the development of the optic lobe. Specifically, both activities are seen in a fully overlapping pattern in the IOA and OOA of the early larva (see Fig. 3A–D). To further understand the temporal requirement for the two signaling pathways, we carried out temperature-shift experiments. Extended shifts (0h–48h) carried out in first and second instar larvae (before 48h after hatching) had no overt effects on the structure of the optic lobe seen either in larvae dissected right after the temperature shift, or in larvae left to develop at the permissive temperature until 96h (Fig. 7A, C). Only shifts of 24h or more applied during the third larval instar (after 48h) caused premature epithelium-neuroblast conversion as described in the previous sections (Fig. 7B, D). These results indicate that the early growth phase (by symmetric division) of the optic anlagen does not depend on Notch or Stat activity, even though both are expressed. Specifically, loss of Notch or Stat is unable to convert the early optic anlagen into neuroblasts prior to the mid-larval stage.
Figure 7.
Temporal requirement of Notch and Stat signaling in optic lobe development. (A–D) Frontal confocal sections of late third instar brains (equivalent of 96h at 25°C) mutant for Nts (A, B) and Statts (C, D). In panels of the left, larvae were raised at the restrictive temperature from hatching until 48h later; in right panels, larvae grew at the restrictive temperature between 48h and 96h. (E) Timeline and schematic depiction of Notch/Stat function in optic lobe development. Despite their expression from early larval stages onward, Notch and Stat activity are only indispensable during the second half of the larval period, coinciding with the NE-NB conversion of the OOA. Thus, only late heat treatment caused noticeable abnormal phenotype, consisting of loss of epithelial OOA (arrowheads in D) and superficial position of medulla neurons (Mn in A, B). Peak expression of Delta, seen in narrow band along fringe of IOA and OOA in wild type or early heat-treated Stat-ts mutant (C; arrows) is only found in the IOA in late heat-treated Stat-ts (D), suggesting a requirement of Stat activity for maintaining high Dl levels. Conversely, as shown in panels F and G, persistent Notch activity is required to maintain Stat in the OOA (F: wild-type expression of Stat92EGFP reporter; G: Stat92EGFP reporter in optic lobe where Notch activity was inhibited by an esgGal4> Su(H)DN).
Bar: 40mm
To test whether Jak/Stat has the potential to inhibit Notch activation in the OOAm, we visualized the expression of the Notch ligand, Delta in these mutants. Loss of Stat during the second half of the larval period resulted in downregulation of Delta in the OOA (Fig. 7D). The altered pattern of Delta expression was particularly conspicuous, since expression in the IOA was unchanged in the Stat92EF/Stat92E85C9 mutants, and contrasted sharply with Delta reduction in the OOA. By contrast, we found Delta expression to be unchanged in early larvae mutant for Stat (data not shown). Consistent with this observation, loss of Stat prior to NE-NB conversion did not affect the expression of Delta (Fig. 7C).
As Delta expression is compromised in Stat-ts mutants, we also asked whether the converse is true; that is, if Stat signaling can be attenuated when Notch signaling is reduced. To address this, we drove Su(H)DN using esg-gal4 and placed Stat92EGFP reporter in the background to assess how Jak/Stat signaling is modulated. As expected, the Su(H)DN in the OOAm is reminiscent of what we have observed before, with the loss of DE-cadherin (marking the OOAep) in the mutant (Fig. 7G). Compared to wild-type, there is a severe reduction of Stat expression in the outer optic anlagen (Fig. 7F, G). Stat signaling is completely abrogated in the OOAl as well.
Together, these data suggest that there is a temporal requirement for both Jak/Stat and Notch signaling to ensure proper epithelial-to-neuroblast conversion in the OOA (Fig. 7E). Both signals are dispensable during early development, during the time of expansion in the epithelium; although they are continuously expressed during that time. Stat activity is needed to maintain the Dl-induced wave of Notch signaling activity along the NE-NB conversion front. Conversely, Notch is also necessary to maintain Stat in the OOA. Both signals, thus work in cooperation with one another to allow for the formation of the highly ordered medulla structure characteristic for the optic lobe. Given the changing expression dynamics of Notch signaling and Jak/Stat signaling, nevertheless, the interaction between the two is most likely indirect.
DISCUSSION
Neural progenitors give rise to the diversity of cell types seen in the central nervous system (CNS). Intrinsic factors expressed in progenitors, as well as extrinsic cues from neighboring cells specify cell fate. In both vertebrates and invertebrates, the specification of cell types follows a highly invariant spatio-temporal pattern. Typically, one can distinguish an early phase where the pool of progenitors (e.g., the neuroepithelium of the neural tube in vertebrates) expands by symmetric cell division. Subsequently, progenitors start leaving the pool of expanding cells and either directly differentiate into specific cell types, or undergo asymmetric divisions where one daughter cell keeps the properties of a progenitor, whereas the other differentiates (reviewed by Zhong and Chia, 2008).
Neurogenesis in the Drosophila optic lobe follows a similar pattern. Segregating from the embryonic neuroectoderm as a small epithelial placode, the optic lobe anlagen undergo a phase of growth by symmetric cell division in the early larva, followed by a highly ordered transition into asymmetrically dividing neuroblasts. The medio-lateral gradient that characterizes this transition in the OOA is correlated with the posterior-anterior gradient of eye development: photoreceptor axons of the earliest developing (posterior) row of ommatidia arrive first and capture the first born neurons, formed (in case of the medulla) from the medial edge of the OOA. Later born axons occupy medulla neurons forming later, at increasingly lateral levels (Meinertzhagen and Hanson, 1993). It is to be assumed that this temporal match between target neuronal development and afferent axonal development plays an important role for correctly wiring the optic lobe; this hypothesis, though, requires rigorous testing.
Notch activity controls the epithelium-neuroblast transition in the optic lobe
We show in this paper that the Notch pathway is critically involved in the ordered NE-NB conversion. The most significant effect resulting from decreasing Notch function in the larval brain was the reduction in size of the epithelial optic anlagen, as shown by the loss of the epithelial marker, Crb. It is therefore likely that the function of Notch in the optic anlagen is to maintain its undifferentiated, neuro-epithelial state. The clonal analysis of Reddy et al. (2010) led to the same conclusion. This would match a similar function of Notch in the embryonic neuroectoderm, where Notch activity is also required for cells to stay epithelial (reviewed in Campos-Ortega and Knust, 1990). The only difference is the topology of the neuroblast (i.e., cell that moves out of the epithelium): in the embryonic neuroectoderm, neuroblasts are mostly scattered cells, surrounded on all sides by epithelial cells. In the optic anlagen, there is a continuous front where all epithelial cells convert to neuroblasts. However, this difference aside, the way in which Notch signaling acts and is controlled during the NE/NB conversion could be quite similar in the embryonic neurectoderm and the late larval optic anlagen.
Surprisingly, Notch activity, despite of its continued expression throughout development, appears to be dispensable during the earlier phase of optic lobe development during which the epithelial optic anlagen grow by symmetric mitosis. Neither early temperature shift experiments with Nts, nor temporally restricted optic lobe expression of Su(H)DN resulted in premature neuroblast formation. Also the active lifespan of the optic lobe neuroblasts appear to be independent of Notch activity. Optic lobes of late Nts larvae raised at the restrictive temperature were mostly devoid of (Dpn-positive) neuroblasts, and lineages or overall volume of the medulla primordium were not noticeably enlarged.
Taken together, our findings in conjunction with other studies (Yasugi et al., 2008; Reddy et al., 2010) suggest the following model of Notch signaling in the larval optic lobe. Moderate levels of the Notch ligand Dl, as well as Notch activity, are present in the entire optic lobe anlage of the early larva. Starting during the mid larval stage, the proneural gene l’sc is expressed at the medial margin of the OOA (Yasugi et al., 2008). This expression sets in motion a cascade of events that result in the ordered NE/NB conversion. L’sc locally upregulates Delta and other proneural genes (ase; Wallace et al., 2000; this study) that promote first the conversion of OOA epithelium to neuroblasts, followed by rapid asymmetric division and neuronal differentiation. At the same time, once cells have converted to neuroblasts, L’sc and Delta are downregulated, even though N stays on in a dynamic manner in neuroblasts and neurons. L’sc remains high in a laterally moving band of cells at the medial OOA margin. A second mechanism that may act on the localized Dl upregulation has been proposed in the recent study by Reddy et al. (2010), who propose that the cell cycle arrest in the OOAm, caused by activation of the Fat/Hippo pathway, is prerequisite for the accumulation of Dl. Throughout the third larval instar, a continued, interdependent expression of L’sc and Delta in the OOA could be the mechanism that accounts for the slow, gradual release of neuroblasts from the OOA margin. Thus, L’sc is known to upregulate Delta in other neural precursors (e.g., Hinz et al., 1994), and this could be the case also in the OOA. The L’sc induced peak in Delta levels at the medial OOA margin would then signal to its neighbors laterally, increasing Notch activity, and thereby preventing a premature advance of L’sc towards lateral.
How initiation and maintenance of L’sc is controlled is still unclear. Wingless (Wg), a known activator of proneural genes in other tissues (Gonzalez-Gaitan and Jackle, 1995; Rulifson et al., 1996) is expressed in a fairly restricted pattern in the apices of the OOA (Kaphingst and Kunes, 1994). It is possible that a long range effect of Wg could be responsible for L’sc activation along the OOA margin.
Notch and the maintenance of the undifferentiated state in the developing nervous system
A large number of studies in vertebrate and invertebrate systems alike suggest that the fundamental role of N in the developing nervous system is to maintain cells in an undifferentiated (neuroepithelial) state at any given moment. Cells released from N activity enter a differentiative pathway (typically accompanied by structurally visible changes, such as a switch from epithelial cell to neuroblast, and/or a switch in mitotic behavior (symmetric vs asymmetric). The temporally controlled release/birth of neurons from the neuroepithelium is often tied to different cell fates. This has been shown very convincingly in the retina of vertebrates and Drosophila. For example, in the vertebrate retina, the first wave of differentiation results in ganglion cells, the second wave of differentiation at a later point includes photoreceptors, followed by bipolar cells, and others (Young, 1985). If N activity is reduced at an early time point, the number of ganglion cells produced increases massively, at the expense of later born cell types (Austin et al., 1995; Dorsky et al., 1997; Jadhav et al., 2005; Silva et al., 2002; Cau and Blader, 2009). In Drosophila, the first retinal cell type to be born behind the morphogenetic furrow is the R8 photoreceptor. If at the time of R8 specification, N function is decreased, the number of R8 cells is increased, and other cell types born later are decreased (Cagan and Ready, 1989). With later pulses of N depletion, one gets different phenotypes; what they all have in common is that the cell types born at the time of the pulse are increased in number, the ones born later decreased.
In the Drosophila OOA investigated in this paper, it is the temporal progression of neuroblast formation that is controlled by N activity is linked to the coordinated growth between eye (and lamina) and medulla, derived from the OOA. However, it is well possible that the temporal progression is also tied into the control of different cell fates. The way in which the multitude of different medulla cell types map onto the larval optic lobe is not clear. It is most likely that (as in the lineages of the ventral nerve cord) most cell types are specified along the z-axis, which would imply that each part of the OOA (in the medio-lateral and dorso-ventral dimension) would produce the same cell types. However, it is well possible that some cell types which are actually not found in all medulla columns, such as wide field tangential neurons (Hausen 1984; Taghert et al., 2000), are produced by different parts of the OOA. Such cell types then might be affected by premature or delayed conversion of the OOA into neuroblasts; identifying specific markers that label cell types at early stages, and using such markers in the background of Notch loss or overactivity, will help clarifying this question.
A role of the Notch pathway has been described for later stages in neural development, that is, the specification of neurons from ganglion mother cells (GMCs). Asymmetric neuroblast proliferation in the ventral nerve cord and brain produces a series of GMCs which each divides one more time into two, often different, neurons/glial cells (reviewed by Karcavich 2005). It has been shown that this fate choice between sibling pairs depends on Notch activity, both during embryonic and post-embryonic stages (Spana and Doe, 1996; Skeath and Doe, 1998; Truman et al., 2010). Such may also be the case for the neuroblasts emerging from the OOA. The relatively high level of the Notch reporter, E(spl)m8-lacZ maintained in the OOA-derived lineages would speak for a continued role of Notch in these cells; however, detailed investigations of the neurogenesis of these lineages need to be carried out in order to address the potential later Notch function.
Interdependency of Notch and Jak/Stat Activity during the development of the nervous system and other organs
As previously reported (Yasugi et al., 2008; Reddy et al., 2010), we find that reduction in Stat activity causes a premature loss of the epithelial state of the OOA. This is accompanied by accelerated proliferation and gross abnormalities in the architecture of the optic lobe neuropile, as also seen in Notch mutant brains. Furthermore, continued activity of Stat in the epithelial OOA is dependent on Notch, and vice versa. The mutual interaction between both signaling pathways is most likely indirect, mediated via a number of intermediate steps. Thus, Delta levels are normal in optic lobes of Statts mutant brains up to 48h after hatching. It is only during later stages, when the structurally visible premature change of OOA epithelium to neuroblasts occurs in the Statts mutant, that Delta expression is reduced. It remains to be seen what are the intermediate genetic events that interconnect the signaling activities of the Notch and Stat pathway.
The larval optic lobe represents but one of many scenarios in which interdependency between Notch and Stat have been reported. The types of genetic interactions between these pathways appear to be as diverse as the developmental events or cell fates which they control. For example, in the Drosophila ovary, mutual inhibition Notch and Stat set up the boundary between the stalk (Jak/Stat dependent) and the main-body follicle cells (Notch-dependent; Assa-Kunik et al., 2007). In the adult midgut, Notch is necessary for the differentiation of cells derived from the intestinal stem cells (ISCs) into enteroblasts and enterocytes (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007). Thus, high Notch activity in one of the daughter cells derived from an ISC division prompts these cells to become an enteroblast (EB), which then either differentiates into enterocyte (EC) or enteroendocrine cell (EE). High Notch activity in the EB promotes the EC fate; low Notch activity allows for the formation of EEs. Jak/Stat signaling intersects with the Notch pathway at multiple steps: on the one hand side, it acts upstream in an activating manner. Thus, under stressful conditions (e.g. bacterial infection or JNK-induced stress), Stat functions to induce Notch to allow for self-renewal and proliferation (Buchon et al., 2009; Jiang et al., 2009). In addition, Jak/Stat is required during the differentiation of different ISC-derived cell types (Beebe et al., 2010; Lin et al., 2010; Liu et al., 2010). The same kind of dynamic and complex relationship between the two signaling pathways can be seen in the eye, where Stat can function both upstream and downstream of Notch (Chao et al., 2004; Gutierrez-Avino et al., 2009). A more recent report even suggests that Jak/Stat can function to inhibit Notch as well (Flaherty et al., 2009). From all these studies, it is clear that many of the intermediates which link Notch and Jak/Stat signaling are still unknown. One can envision scenarios where subtle differences in the spatial distribution and timing of signals may contribute to how Stat and Notch interact during development. The Drosophila optic lobe is likely to present a highly favorable system to address these complexities which impact the role of the two signaling pathways.
Acknowledgments
We thank J. Lovick for the critical comments on the manuscript and members of the Hartenstein laboratory for helpful discussions. We also thank M. Kim for the technical assistance. We are grateful to Y.H. Sun, N. Perrimon, Y.N. Jan, M. Muskavitch, S. Noselli, E. Knust, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for fly strains and antibodies. This work was supported by NIH grant (RO1 NS29357-15). K.T. Ngo, J. Wang, G. Vo, B. Asem participated as undergraduate research students in the UCLA Undergraduate Research Consortium in Functional Genomics, supported by a Howard Hughes Medical Institute Professor’s Award to U. Banerjee. J.M. Olson was supported by the Howard Hughes Medical Institute Professor’s award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner M. Drosophila, a laboratory manual. New York: Cold Springs Harbor Laboratory Press; 1989. [Google Scholar]
- Artavanis-Tsakonas S, Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991;7:403–408. doi: 10.1016/0168-9525(91)90264-q. [DOI] [PubMed] [Google Scholar]
- Assa-Kunik E, Torres IL, Schejter ED, Johnston DS, Shilo BZ. Drosophila follicle cells are patternedby multiple levels of Notch signaling and antagonism between Notch and JAK/STAT pathways. Development. 2007;134:1161–1169. doi: 10.1242/dev.02800. [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Bach EA, Ekas L, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Curr Biol. 1997;7:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- Bier E, Vaessin H, Younger-Shepherd S, Jan LY, Jan YN. deadpan, an essential pan-neural gene in Drosophila, encodes a helix-loop-helix protein similar to the hairy gene product. Genes Dev. 1992;6:2137–2151. doi: 10.1101/gad.6.11.2137. [DOI] [PubMed] [Google Scholar]
- Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, Stat92E, regulates follicle differentiation during oogenesis. Dev Biol. 2002;243:166–175. doi: 10.1006/dbio.2001.0539. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Cabrera CV. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interaction between scute, Notch, and Delta. Development. 1990;109:733–742. [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Knust E. Molecular analysis of a cellular decision during embryonic development of Drosophila melanogaster: epidermogenesis or neurogenesis. Eur J Biochem. 1990;190:1–10. doi: 10.1111/j.1432-1033.1990.tb15538.x. [DOI] [PubMed] [Google Scholar]
- Campuzano S, Modelell J. Patterning of the Drosophila nervous system: the achaete-scute complex. Trends Genet. 1992;8:202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Cau E, Blader P. Notch activity in the nervous system: to switch or not switch? Neural Dev. 2009;4:36. doi: 10.1186/1749-8104-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci C, Gould AP. Drosophila Grainyhead specifies late programmes of neural proliferation by regulating the mitotic activity and Hox-dependent apoptosis of neuroblasts. Development. 2005;132:3835–3845. doi: 10.1242/dev.01932. [DOI] [PubMed] [Google Scholar]
- Chan YM, Jan YN. Conservation of neurogenic genes and mechanisms. Curr Opin Neurobiol. 1999:582–588. doi: 10.1016/S0959-4388(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signaling through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131:3839–3847. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- Delidakis C, Artavonis-Tsakonas S. The Drosophila Enhancer of split [E(spl)] locus encodes seven independent helix-loop-helix proteins. Proc Natl Acad Sci. 1992;89:8731–8735. doi: 10.1073/pnas.89.18.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky R, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signaling. Nature. 1997;385:67–69. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Egger B, Boone JQ, Stevens NR, Brand AH, Doe CQ. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2007;5:1. doi: 10.1186/1749-8104-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Chell JM, Brand AH. Insights into neural stem cell biology from flies. Philos Trans R Soc Lond B Biol Sci. 2008;363:39–56. doi: 10.1098/rstb.2006.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Olson JM, Ngo KT, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran PT, Do MT, Yackle K, Cespedes A, Hartenstein V, Call GB, Banerjee U. G-TRACE: a rapid, in vivo screening technique for Gal4-expression based cell lineage analysis in Drosophila. Nature Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach KF, Hiesinger PR. Optic lobe development. Adv Exp Med Biol. 2008;628:115–136. doi: 10.1007/978-0-387-78261-4_8. [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Technau G. Cell degeneration in the developing optic lobe of the sine oculis and small-optic lobes mutants of Drosophila melanogaster. Dev Biol. 1984;104:219–239. doi: 10.1016/0012-1606(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Zavadil J, Ekas LA, Bach EA. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of Notch signaling by the JAK/STAT pathway. Dev Dyn. 2009;238:2235–2253. doi: 10.1002/dvdy.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. 2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gaitan M, Jackle H. Invagination centers within the Drosophila stomatogastric nervous system anlage are positioned by Notch-mediated signaling which is spatially controlled through wingless. Development. 1995;121:2313–2325. doi: 10.1242/dev.121.8.2313. [DOI] [PubMed] [Google Scholar]
- Goto S, Hayashi S. Proximal to distal cell communication in the Drosophila leg provides a basis for an intercalary mechanism of limb patterning. Development. 1999;126:3407–3413. doi: 10.1242/dev.126.15.3407. [DOI] [PubMed] [Google Scholar]
- Green P, Hartenstein AY, Hartenstein V. The embryonic development of the Drosophila visual system. Cell Tissue Res. 1993;273:583–598. doi: 10.1007/BF00333712. [DOI] [PubMed] [Google Scholar]
- Grosskortenhaus R, Pearson BJ, Marusich A, Doe CQ. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev Cell. 2005;8:193–202. doi: 10.1016/j.devcel.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, Hanakawa Y, Hashimoto K, Nakajima K, Sakanaka M. Suppression of Stat3 promotes neurogenesis in culture neural stem cells. J Neurosci Res. 2005;81:163–171. doi: 10.1002/jnr.20561. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Analysis of the role of basic helix-loop-helix transcription factors in the development of neural lineages in the mouse. Biol Cell. 1995;84:3–6. doi: 10.1016/0248-4900(96)81312-8. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and four-jointed. EMBO. 2009;10:1051–1058. doi: 10.1038/embor.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hausen K. In: Photoreception and vision in invertebrates. Ali MA, editor. New York: Plenum Press; 1984. pp. 523–559. [Google Scholar]
- Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Hofbauer A, Campos-Ortega JA. Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Roux’s Arch Dev Biol. 1990;198:264–274. doi: 10.1007/BF00377393. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Signals transmitted along retinal axons in Drosophila: Hedgehog signal reception and the cell circuitry of lamina cartridge assembly. Development. 1998;125:3753–3764. doi: 10.1242/dev.125.19.3753. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL. Notch1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokin/JAK/STAT mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Sasai Y, Akazawa C, Ishibashi M, Takebayashi K, Shimizu C, Tomita K, Nakanishi S. Regulation of the mammalian neural development by helix-loop-helix transcription factors. Crit Rev Neurobiol. 1995;9:177–188. [PubMed] [Google Scholar]
- Kaphingst K, Kunes S. Pattern formation in the visual centers of the Drosophila brain: wingless acts via decapentaplegic to specify the dorsalventral axis. Cell. 1994;78:437–448. doi: 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Karcavich RE. Generating neuronal diversity in the Drosophila central nervous system: a view from the ganglion mother cells. Dev Dyn. 2005;232:609–616. doi: 10.1002/dvdy.20273. [DOI] [PubMed] [Google Scholar]
- Kim LK, Choi UY, Cho HS, Lee JS, Lee WB, Kim J, Jeong K, Shim J, Kim-Ha J, Kim YJ. Down-regulation of NF-kappaB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 2007;5:e238. doi: 10.1371/journal.pbio.0050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Schrons H, Grawe H, Campos-Ortega JA. Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics. 1992;132:505–518. doi: 10.1093/genetics/132.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtois M, Schweisguth F. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of Enhancer of split Complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Jimenez F, Dietrich U, Campos-Ortega JA. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Lim HY, Tomlinson A. Organization of the peripheral fly eye: the roles of Snail family transcription factors in peripheral retinal apoptosis. Development. 2006;133:3529–3537. doi: 10.1242/dev.02524. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- Liu W, Singh SR, Hou SX. JAK/STAT is restrains by Notch to control cell proliferation in the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Carmena A, Jimenez F. Neurogenic genes control gene expression at the transcriptional level in early neurogenesis and in mesectoderm specification. Development. 1995;121:219–224. doi: 10.1242/dev.121.1.219. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. In: The development of Drosophila. Bate CM, Martinez Arias A, editors. II. New York: Cold Spring Laboratory Press; 1993. pp. 1363–1491. [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. Combinatorial signaling in the specification of primary pigment cells in the Drosophila eye. Development. 2007;134:825–831. doi: 10.1242/dev.02788. [DOI] [PubMed] [Google Scholar]
- Nassif C, Noveen A, Hartenstein V. Early development of the Drosophila brain: III. The pattern of neuropile founder tracts during larval period. J Comp Neurol. 2003;455:417–434. doi: 10.1002/cne.10482. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Posakony JW. Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell. 1994;76:415–418. doi: 10.1016/0092-8674(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Reddy BV, Rauskolb C, Irvine KD. Influence of Fat-Hippo and Notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137:2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gomez M, Ghysen A. The expression and role of a proneural gene, achaete, in the development of the larval Drosophila nervous system. EMBO J. 1993;12:1121–1130. doi: 10.1002/j.1460-2075.1993.tb05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS. Wingless refines its own expression domain on the Drosophila wing margin. Nature. 1996;384:72–74. doi: 10.1038/384072a0. [DOI] [PubMed] [Google Scholar]
- Selleck SB, Steller H. The influence of retinal innervation on neurogenesis in the first optic ganglion of Drosophila. Neuron. 1991;6:83–99. doi: 10.1016/0896-6273(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Selleck SB, Gonzales C, Glover DM, White K. Regulation of the G1-S transition in postembryonic neuronal precursors by axon ingrowth. Nature. 1992;355:253–255. doi: 10.1038/355253a0. [DOI] [PubMed] [Google Scholar]
- Schrons H, Knust E, Campos-Ortega JA. The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for neural and epidermal progenitor cells. Genetics. 1992;132:481–503. doi: 10.1093/genetics/132.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AO, Ercole CE, McLoon SC. Regulation of ganglion cell production by Notch signaling during retinal development. J Neurobiol. 2002;15:511–524. doi: 10.1002/neu.10156. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB. Regulation of proneural gene expression and cell fate during neuroblast segregration in Drosophila embryo. Development. 1992;114:939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Doe CQ. Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development. 1998;125:1857–1865. doi: 10.1242/dev.125.10.1857. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17:21–26. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Roberts ME, Renn SCP, Jacobs PS. Metamorphosis of tangential visual system neurons in Drosophila. Dev Biol. 2000;222:471–485. doi: 10.1006/dbio.2000.9724. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Theodosiou NA, Xu T. Use of FLP/FRT system to study Drosophila development. Methods. 1998;14:355–365. doi: 10.1006/meth.1998.0591. [DOI] [PubMed] [Google Scholar]
- Truman JW, Moats W, Altman J, Marin EC, Williams DW. Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development. 2010;137:53–61. doi: 10.1242/dev.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K, Liu T-H, Vaessin H. The Pan-Neural bHLH proteins Deadpan and Asense regulate mitotic activity and Cdk inhibitor dacapo expression in the Drosophila optic lobes. Genesis. 2000;26:77–85. doi: 10.1002/(sici)1526-968x(200001)26:1<77::aid-gene10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Xu T, Caron LA, Fehon RG, Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992;115:913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Umetsum D, Murakami S, Sato M, Tabata T. Drosophila optic lobe neuroblast is triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development. 2008;135:1471–1480. doi: 10.1242/dev.019117. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Zhong W, Chia W. Neurogenesis and asymmetric cell divison. Curr Opin Neurobiol. 2008;18:4–11. doi: 10.1016/j.conb.2008.05.002. [DOI] [PubMed] [Google Scholar]