Abstract
The area of dynamic treatment regimes (DTR) aims to make inference about adaptive, multistage decision-making in clinical practice. A DTR is a set of decision rules, one per interval of treatment, where each decision is a function of treatment and covariate history that returns a recommended treatment. Q-learning is a popular method from the reinforcement learning literature that has recently been applied to estimate DTRs. While, in principle, Q-learning can be used for both randomized and observational data, the focus in the literature thus far has been exclusively on the randomized treatment setting. We extend the method to incorporate measured confounding covariates, using direct adjustment and a variety of propensity score approaches. The methods are examined under various settings including non-regular scenarios. We illustrate the methods in examining the effect of breastfeeding on vocabulary testing, based on data from the Promotion of Breastfeeding Intervention Trial.
Key words and phrases: Bias, confounding, dynamic treatment regime, inverse probability of treatment weighting, non-regularity, propensity scores
1. INTRODUCTION
Dynamic treatment regimes present a framework for developing individually tailored treatment policies that are particularly useful for chronic disorders like mental illnesses, substance abuse, HIV infection, cancer, and so on. More precisely, a dynamic treatment regime (DTR), or adaptive treatment strategy, is a sequence of decision rules where each decision rule takes a patient’s treatment and covariate history at each interval of clinical intervention as inputs, and outputs a recommended treatment. The ultimate goal of research in this arena is to come up with a DTR that leads to maximum benefit to the participants, that is, to formulate an “optimal” DTR in some well-defined sense. Formally, a DTR is often said to be optimal if it optimizes the mean outcome at the end of the final interval of treatment.
There are a number of methods of analysis and inference that have been developed to estimate the optimal dynamic treatment regime. Parametric and Bayesian approaches have been proposed (Bellman, 1957; Bertsekas & Tsitsiklis, 1996; Thall, Millikan, & Sung, 2000; Arjas & Saarela, 2010), however it is the semi-parametric methods that have gained the widest use in the statistical community, with techniques such as iterative minimization of regrets (Murphy, 2003), G-estimation (Robins, 2004), machine-learning methods (Pineau et al., 2007), regret-regression (Henderson, Ansell, & Alshibani, 2010), and even marginal structural models (Robins, Hernán, & Brumback, 2000; Hernán et al., 2006; van der Laan & Petersen, 2007; Robins, Orellana, & Rotnitzky, 2008). In particular, Q-learning (Murphy, 2005; Chakraborty, 2011) is a popular choice because of the ease of implementation and its connections with other methods such as G-estimation (Chakraborty, Murphy, & Strecher, 2010).
One important limitation of Q-learning is that it has been studied and implemented only in the context of randomized trials (Zhao, Kosorok, & Zeng, 2009; Chakraborty, Murphy, & Strecher, 2010; Zhao et al., 2011; Shortreed et al., 2011; Song et al., 2012; Chakraborty & Moodie, 2012; Laber et al., 2012). However, the development of DTRs is often exploratory, and it is useful to explore potentially optimal DTRs using large samples with observational data, which may later be assessed in a confirmatory study in which individuals are randomized to one of a small number of regimes of interest. It has been assumed that, in principle, Q-learning can be used for observational data. We examine several approaches to Q-learning in an observational (non-randomized) treatment setting, where all confounding covariates are assumed to be measured. In particular, we use direct adjustment and a variety of propensity score approaches, including regression and inverse probability weighting. We illustrate the methods by applying Q-learning to determine whether there is an optimal strategy for breastfeeding which will maximize a vocabulary score using data from the Promotion of Breastfeeding Intervention Trial. In the trial, subjects were randomized to a breastfeeding encouragement program or standard care, but all women in the study initiated breastfeeding and weaning time was not randomized. While the “treatment” of interest (breastfeeding) was influenced by the randomization status, it was by no means determined by it.
2. ESTIMATING TREATMENT RULES BY Q-LEARNING
2.1. Notation and Data Structure
We frame this work in a two-interval setting, however the methods considered extend naturally to any finite number of treatment intervals. Longitudinal data on a single patient are given by the trajectory (C1, O1, A1, C2, O2, A2, Y), where Cj and Oj (j = 1, 2) denote the covariates measured prior to treatment at the beginning of the jth interval, Y is the observation at the end of interval 2, and Aj (j = 1, 2) is the treatment assigned at the jth interval subsequent to observing Oj. We distinguish two types of covariates: those variables Oj that interact with treatment, called tailoring or prescriptive variables, and those variables Cj that do not interact with treatment but that potentially confound the relationship between the treatment and outcome. Note that Oj may also be a confounding variable, that is, a common cause of both treatment and the outcome. We do not consider that case in this work since tailoring variables are typically included in the models used for Q-learning, and so any confounding effects of tailoring variables are naturally accounted for in this way.
The data set consists of a random sample of n patients. Define the history at each interval as: H1 = (C1, O1), H2 = (C1, O1, A1, C2, O2). We consider studies in which there are two possible treatments at each interval, Aj ∈ {−1, 1}, and the receipt of treatment Aj depends on the observed value of covariates Cj. The outcome Y observed at the end of the second treatment interval may be a single measurement or a known function, f (·), of some or all covariates measured throughout the study. A two-interval DTR consists of two decision rules, (d1, d2), with dj ≡ dj(Hj) ∈ 𝒜j, where 𝒜j is the set of possible treatments at the jth interval.
2.2. Basic Q-learning With Linear Regression Models
Q-learning is closely related to dynamic programming, in that estimation begins at the last interval, and the optimal treatment at each interval is then found by estimating the impact of treatment in that interval on a “pseudo-outcome" which is constructed by assuming all subsequent treatments are optimal; the pseudo-outcome is simply a predicted counterfactual outcome under optimal treatment in the future intervals. We begin by describing the basic implementation of Q-learning, that assumes randomized treatment. That is, we suppose that there are no confounding variables Cj, so that H1 = O1, H2 = (O1, A1, O2). Define the Q-functions (Sutton & Barto, 1998; Murphy, 2005) for the two intervals as follows:
If perfect knowledge were available to the analyst, the true multivariate distribution of the data would be known, which would in turn imply known Q-functions. Were that the case, the optimal DTR (d1, d2) could be deduced using backwards induction, so that
In practice, however, the true Q-functions are not known, and hence must be estimated from the data. A simple and often reasonable approach is to assume a linear model for the Q-functions, so that the interval-j (j = 1, 2) Q-function is modelled as
| (1) |
where Hj0 and Hj1 are two (possibly different) summaries of the historyHj. We use Hj0 to contain the collection of variables that have a predictive effect on the outcome that is not modified by the treatment, and Hj1 to denote the components of the history that do interact with treatment. Both Hj0 and Hj1 include a constant, or intercept, term. Then the Q-learning algorithm consists of the following steps:
- Interval 2 parameter estimation: Using regression, find
Interval 2 optimal rule: By substitution, d̂2(h2) = argmaxa2 Q2(h2, a2; β̂2, ψ̂2).
- Interval 1 pseudo-outcome: By substitution,
- Interval 1 parameter estimation: Using regression, find
Interval 1 optimal rule: By substitution, d̂1(h1) = argmaxa1 Q1(h1, a1; β̂1, ψ̂1).
The estimated optimal DTR using Q-learning is given by (d̂1, d̂2). Note that the pseudo-outcome, Ŷ1i, is the (expected) value of the second-interval Q-function under the optimal treatment.
2.3. Q-Learning in Non-Regular Settings
It is now well-understood (Robins, 2004; Moodie & Richardson, 2010; Chakraborty, Murphy, & Strecher, 2010; Chakraborty & Moodie, 2012; Song et al., 2012) that most estimators of DTR parameters, including those found by Q-learning and G-estimation, are non-regular. This phenomenon is a consequence of the non-differentiability of the pseudo-outcome with respect to the estimated second-interval parameters, ψ2. The basic Q-learning algorithm is sometimes referred to as a “hard-max” estimator because of the sharp point of non-differentiability caused by employing a maximum operation, which in this case is the absolute value function.
The estimator ψ̂1 is a function of the estimated pseudo-outcome, Ŷ1i, and so it is also a non-smooth function of ψ̂2. It is therefore the case that the asymptotic distribution of ψ̂1 does not converge uniformly (Robins, 2004) over the space of the parameters ψ = (ψ1, ψ2). In fact, we can be more precise: the asymptotic distribution of is normal (and the estimator is therefore regular) when ψ2 is such that , but is non-normal if . Furthermore, the transition between the two asymptotic distributions is abrupt. This non-regularity results in bias in the estimator ψ̂1, as well as poor coverage of Wald type confidence intervals (Robins, 2004; Moodie & Richardson, 2010). Even the usual bootstrap confidence intervals can perform badly (Chakraborty, Murphy, & Strecher, 2010).
A handful of approaches have been proposed to reduce the problems of bias and/or incorrect coverage (Moodie & Richardson, 2010; Chakraborty, Murphy, & Strecher, 2010; Song et al., 2012; Laber et al., 2012; Chakraborty et al., 2012). We focus on the soft-thresholding approach of Chakraborty, Murphy, & Strecher (2010), which has shown good performance in terms of both bias and coverage in non-regular settings. The basic idea of the soft-thresholding approach is to shrink the problematic term in the pseudo-outcome towards zero. Thus, the soft-thresholding implementation of Q-learning replaces the pseudo-outcome of the basic (hard-max) Q-learning algorithm with
where x+ = x𝕀[x > 0] stands for the positive part of a function, and λi (> 0) is a tuning parameter associated with the ith subject in the sample. A data-driven choice of the tuning parameter λi is obtained from a Bayesian approach to the problem: taking , where Σ̂2/n is the estimated covariance matrix of ψ̂2 yields an approximate empirical Bayes estimator (Chakraborty, Murphy, & Strecher, 2010). The basic Q-learning algorithm is otherwise unchanged.
2.4. Q-Learning for Observational Data
In more general estimation problems where interest lies in assessing, for example, a treatment effect from a non-randomized study, many confounding-adjustment methods rely on the propensity score (PS). The basic approach requires the construction of a propensity score or treatment model—often a predicted probability resulting from a logistic regression of treatment on covariates—followed by some form of adjustment, such as regression or an unadjusted regression in a sample which uses inverse probability of treatment weights (IPTW).
Formally, the propensity score is defined to be
where A is a binary treatment and X is a collection of measured covariates (Rosenbaum & Rubin, 1983). The PS is said to be a balancing score, in that treatment received is independent of known covariates given the propensity score. This property is used to obtain unbiased estimates of the treatment effect based on conditional expectation modelling of the outcome given the propensity score. Common approaches include regression-based estimators, where some function of the propensity score is included as a covariate in the regression, or an estimate is obtained using a matched subsample of the data. Unbiasedness can be achieved if the PS is correctly specified; in particular, it is key that all confounding variables are available (measured) and included in the PS model.
In an inverse probability of treatment weighted analysis, weighting is used to achieve a pseudo-sample in which treatment is not confounded by the variables included in the PS, which is used to construct the weights (Robins, Hernán, & Brumback, 2000). That is, treatment receipt does not depend on confounders in the pseudo-sample. As with the propensity score adjustment methods and indeed all models for observational data, some assumptions are required for IPTW estimators of treatment effects to be unbiased. In particular, we require no unmeasured confounding, correct specification of the PS with respect to confounding variables and that the PS is neither 0 nor 1 to ensure the resulting weights are well-defined.
In what follows, we will compare the basic implementation of Q-learning with four approaches to confounder-adjustment. Recall that the basic Q-learning algorithm does not make any covariate adjustment, so that H1 = O1, H2 = (O1, A1, O2). Three of the adjustment methods adapt Q-learning by redefining the history vectors, H1 and H2. The fourth approach relies on weighting. Specifically, letting PS1 = P(A1 = 1|C1), PS2 = P(A2 = 1|C1, C2) denote the interval-specific propensity scores, we consider accounting for confounding by:
including covariates as linear terms in the Q-function (we refer to this as linear adjustment): H1 = (C1, O1), H2 = (C1, C2, O1, A1, O2),
including the propensity score as a linear term in the Q-function: H1 = (PS1, O1), H2 = (PS2, O1, A1, O2),
including quintiles of PSj as covariates in the jth interval Q-function, and
inverse probability of treatment weighting with H1, H2 defined as in the basic Q-learning.
For simplicity, we focus on the IPTW estimator which uses unstabilized weights, defined as w1 = 𝕀[A1 = 1]/PS1 + (1 −𝕀[A1 = 1])/(1 − PS1) at the first interval and w2 = w1 * {𝕀[A2 = 1]/PS2 + (1 −𝕀[A2 = 1])/(1 − PS2)} at the second interval.
3. SIMULATION STUDY
In this section, we consider a simulation study to compare the performances of several competing methods. In particular, we contrast no covariate adjustment (the basic Q-learning algorithm), with adjustment by (1) including covariates as linear terms in the Q-function; (2) including the PS directly in the Q-function; (3) including quintiles of the PS as covariates in the Q-function; and (4) IPTW.
The methods of adjustment are implemented using both the basic (hard-max) and the soft-thresholding versions of Q-learning. In addition to finding the performance of the point estimates, we will compute percentile bootstrap confidence intervals to assess coverage rates of 95% confidence intervals. The generative model and the analysis model are straightforward adaptations of the corresponding models described in Chakraborty et al. (2010), with the addition of time-varying confounding variables, Cj.
3.1. Generative Models: Primary Simulations for Confounding by Covariates
We consider a single continuous confounder, Cj, at each interval, where C1 ~ 𝒩(0, 1) and C2 ~ 𝒩(η0 + η1C1, 1) for η0 = −0.5, η1 = 0.5. Treatment assignment is dependent on the value of the confounding variable: P[Aj = 1|Cj] = 1 − P[Aj = −1|Cj] = expit(ζ0 + ζ1Cj), j = 1, 2 where expit(x) = exp(x)/(1 + exp(x)). In simulations where treatment was randomly allocated, ζ0 = ζ1 = 0, while for confounded treatment, ζ0 = −0.8, ζ1 = 1.25. The binary covariates which interact with treatment to produce a personalized rule are generated via
Let μ = E[Y|C1, O1, A1, C2, O2, A2], and ε ~ 𝒩(0, 1) be the error term. Then Y = μ + ε, with
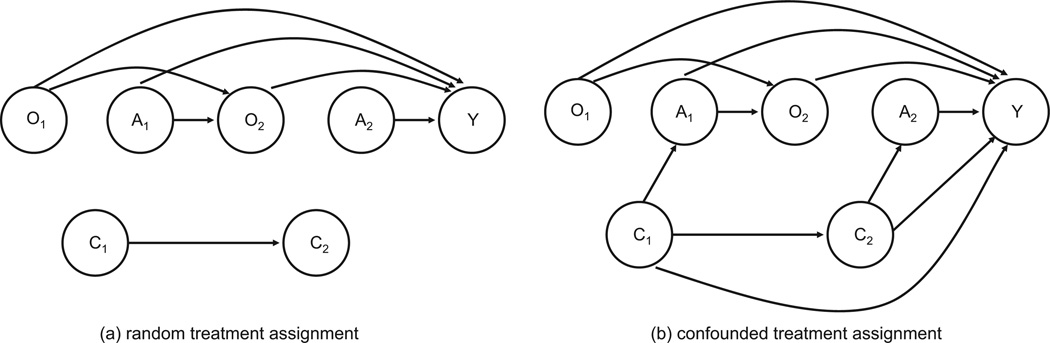
The parameters will be varied in the examples to follow. Note that confounding can be removed from the data generation by setting η1 = 0 or γ1 = γ5 = 0. See Figure 1 for a depiction of the data generating mechanism using causal diagrams.
Figure 1.
Causal diagram for generative model for simulations: (a) random treatment assignment and (b) confounded treatment assignment.
Parameters were chosen to consider two regular settings and two non-regular settings. Non-regularity in interval 1 parameter estimators is a consequence of non-uniqueness in the optimal treatment at the second interval for some non-zero fraction of the population. In terms of the above data-generating model, non-regularity occurs when p = P[γ6 + γ7O2 + γ8A1 = 0] > 0; we use p to describe the degree of non-regularity. Estimators also exhibit non-regular behaviour when γ6 + γ7O2 + γ8A1 is near zero; we therefore also consider the standardized effect size, ϕ = E[γ6 + γ7O2 + γ8A1]/var[γ6 + γ7O2 + γ8A1] (Chakraborty, Murphy, & Strecher, 2010; Song et al., 2012).
The first regular setting was designed to have a large treatment effect, with γ = (0, γ1, 0, −0.5, 0, γ5, 0.25, 0.5, 0.5) and δ = (0.1, 0.1) so that p = 0 and ϕ = 0.3451. In the second regular setting, the treatment effect at the second interval is small (ϕ = 0.1/0) and first-interval estimators exhibit bias due to the near-non-regularity of the scenario: γ = (0, γ1, 0, 0, 0, γ5, 0.1, 0, 0) and δ = (0.5, 0.5). The two non-regular scenarios differed in both the degree of non-regularity and the standardized effect size. We considered non-regularity which was moderate (γ = (0, γ1, 0, −0.5, 0, γ5, 0.5, 0, 0.5) and δ = (0.5, 0.5) so that p = 0.5 and ϕ = 1) and severe (γ = (0, γ1, 0, 0, 0, γ5, 0, 0, 0) and δ = (0.5, 0.5) so that p = 1 and ϕ = 0/0). In the random treatment settings, we took γ1 = γ5 = 0, and in the confounded treatment settings, γ1 = γ5 = 1.
3.2. Generative Models: Additional Simulations for Confounding by Covariates
We considered three additional simulations, focusing on adjustment by inclusion of covariates in the Q-function model, inclusion of the PS as a linear term in the Q-function model, and IPTW. In the first simulation, treatment was randomly allocated, but the variables C1 and C2 both predicted the outcome, Y. As in the confounded scenarios, we set γ1 = γ5 = 1, but fixed ζ0 = ζ1 = 0. That is, in this setting, treatment was not confounded but the Q-function models were incorrectly specified for all methods of PS-adjustment and IPTW due to the omission of C1 and C2 from the models. This setting was considered to verify whether the results from the random treatment setting could be attributed to the lack of confounding or the lack of dependence of the outcome on the variables C1 and C2.
We also considered two confounded treatment settings where the confounders C1 and C2 were binary rather than continuous. For these scenarios, the data generation of Figure 1b was followed, but here P[C1 = 1] = 1 − P[C1 = −1] = 1/3 and P[C2 = 1] = 1 − P[C2 = −1] = expit(0.6C1). In the first, γ1 = γ5 = 1 whereas in the second γ5 = 1 but γ1 = 0 so that C2 was a predictor of Y, but C1 was not.
3.3. Generative Models: Confounding by Counterfactuals
In the data-generating scenarios described above, only a model that includes the confounding variables as linear terms in the Q-function will be correctly specified. We therefore pursue an alternative approach to generating data that will allow us to examine the performance of the adjustment methods on a more even playing field by creating data for which all adjustment methods contain as a sub-model a correctly-specified model for the Q-function. We accomplish this by generating data in which the choice of treatment depends on the counterfactual outcomes.
In particular, the data are created by generating the outcome under each of the four potential treatment paths ((−1,−1), (−1,1), (1,−1), and (1,1)):
Generate the first-interval tailoring variable, O1, using .
Generate the potential value of the second-interval tailoring variable, O2(A1), using P[O2 = 1|O1, A1] = 1 − P[O2 = −1|O1, A1] = expit(δ1O1 + δ2A1) for each possible value of A1. Thus, we generate the potential second-interval value that would occur under A1 = −1 and that which would occur under A1 = 1.
- Generate the vector of potential outcomes, Y = μ + ε, where ε is a multivariate normal error term with mean (0, 0, 0, 0)T and a covariance matrix that takes the value 1 on its diagonal and 0.5 on all off-diagonals, and
where O1 is the 4 × 1 vector consisting of O1 from step (1) repeated four times, A1 = (−1, −1, 1, 1), O2 = (O2(−1), O2(−1), O2(1), O2(1)) using the potential values generated in step (2), and A2 = (−1, 1, −1, 1).(2) Set the confounders to be C1 = Y̅ and C2 = max(Y).
From among the four possible treatment paths and corresponding potential outcomes, select the “observed” data using P[Aj = 1|Cj] = 1 − P[Aj = −1|Cj] = expit(ζ0 + ζ1Cj), j = 1, 2.
The vector of δs was set to (0.1, 0.1), while the vector of γs was taken to be (0, 0, −0.5, 0, 0.25, 0.5, 0.5), indicating a regular (large effect) scenario. In simulations where treatment was randomly allocated, ζ0 = ζ1 = 0, while for confounded treatment, ζ0 = 0.2, ζ1 = 1. As can be observed from Equation (2), the Q-functions will not depend on the values of C1 and C2 so that any model for the Q-function that includes O1, A1, O2, A2 and the appropriate interactions will be correctly specified. However the observed or selected treatment depends on C1 and C2, which are functions of the potential outcomes, hence the treatment-outcome relationship is confounded by these variables.
3.4. Results for Confounding by Covariates
We focus our attention on the parameter ψ10, the parameter in the analytic model for the first-interval Q-function defined in Equation (1) which corresponds to the main effect of the treatment A1. The true value of the parameter is fully determined by the γ and δ vectors (see Chakraborty, Murphy, & Strecher (2010) for the values, or Chakraborty & Moodie (2012) for the complete derivation).
Performance of the five different Q-learning approaches under random treatment allocation for both the hard-max and soft-thresholding implementations are given in Table 1, for a sample size of 250. Table 2 provides the results for the confounded treatment settings, and Table 3 provides the results from the additional scenarios considered. In Table 2, where we vary sample size, results are presented for the most appropriate analytic approach (hard-max for the regular, large effect setting, and soft-thresholding for the other three settings) to isolate the effects of confounding on the performance of the different methods of adjustment in Q-learning.
Table 1.
Performance of Q-learning adjustment methods for n = 250 when treatment is randomly assigned, as per Figure 1a: bias, Monte Carlo variance (MC var), mean squared error (MSE), and coverage of 95% bootstrap confidence intervals.
| Hard-max | Soft thresholding | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjustment method | Bias | MC var | MSE | Cover | Bias | MC var | MSE | Cover |
| Regular setting (large effect) | ||||||||
| None | 0.0001 | 0.0076 | 0.0076 | 92.7 | 0.0028 | 0.0077 | 0.0077 | 95.2 |
| Linear | 0.0001 | 0.0076 | 0.0076 | 94.5 | 0.0028 | 0.0077 | 0.0077 | 95.4 |
| PS (linear) | 0.0000 | 0.0076 | 0.0076 | 94.2 | 0.0027 | 0.0076 | 0.0076 | 95.4 |
| PS (quintiles) | 0.0004 | 0.0077 | 0.0077 | 94.5 | 0.0029 | 0.0077 | 0.0077 | 95.4 |
| IPW | −0.0001 | 0.0076 | 0.0076 | 93.9 | −0.0011 | 0.0079 | 0.0078 | 95.5 |
| Regular setting (small effect) | ||||||||
| None | 0.0071 | 0.0055 | 0.0056 | 96.6 | 0.0058 | 0.0045 | 0.0046 | 95.2 |
| Linear | 0.0070 | 0.0056 | 0.0056 | 96.5 | 0.0057 | 0.0046 | 0.0046 | 95.3 |
| PS (linear) | 0.0069 | 0.0055 | 0.0056 | 96.5 | 0.0057 | 0.0046 | 0.0046 | 95.7 |
| PS (quintiles) | 0.0072 | 0.0055 | 0.0055 | 96.9 | 0.0057 | 0.0046 | 0.0046 | 95.6 |
| IPW | 0.0070 | 0.0055 | 0.0056 | 96.4 | 0.0058 | 0.0046 | 0.0046 | 95.3 |
| Non-regular (moderate) setting | ||||||||
| None | −0.0420 | 0.0071 | 0.0088 | 89.2 | −0.0202 | 0.0067 | 0.0071 | 92.8 |
| Linear | −0.0423 | 0.0071 | 0.0086 | 89.3 | −0.0204 | 0.0067 | 0.0071 | 92.7 |
| PS (linear) | −0.0421 | 0.0071 | 0.0088 | 89.2 | −0.0203 | 0.0067 | 0.0071 | 92.8 |
| PS (quintiles) | −0.0425 | 0.0071 | 0.0089 | 89.5 | −0.0206 | 0.0068 | 0.0071 | 93.1 |
| IPW | −0.0420 | 0.0071 | 0.0088 | 89.5 | −0.0202 | 0.0067 | 0.0071 | 93.1 |
| Non-regular (severe) setting | ||||||||
| None | −0.0015 | 0.0053 | 0.0053 | 95.7 | −0.0005 | 0.0045 | 0.0045 | 94.5 |
| Linear | −0.0011 | 0.0054 | 0.0054 | 95.7 | −0.0003 | 0.0045 | 0.0045 | 94.7 |
| PS (linear) | −0.0011 | 0.0054 | 0.0054 | 95.7 | −0.0002 | 0.0045 | 0.0045 | 94.6 |
| PS (quintiles) | −0.0007 | 0.0054 | 0.0054 | 95.8 | −0.0003 | 0.0045 | 0.0046 | 94.8 |
| IPW | −0.0013 | 0.0054 | 0.0054 | 95.4 | −0.0002 | 0.0045 | 0.0045 | 94.1 |
Table 2.
Performance of Q-learning adjustment methods when treatment is confounded, as per Figure 1b: bias, Monte Carlo variance (MC var), mean squared error (MSE), and coverage of 95% bootstrap confidence intervals.
| n = 250 | n = 500 | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjustment method | Bias | MC var | MSE | Cover | Bias | MC var | MSE | Cover |
| Regular setting (large effect) | ||||||||
| None | −0.7201 | 0.0256 | 0.5441 | 0.2 | −0.7194 | 0.0151 | 0.5325 | 0.0 |
| Linear | −0.0027 | 0.0116 | 0.0116 | 95.6 | 0.0010 | 0.0065 | 0.0065 | 95.0 |
| PS (linear) | −0.2534 | 0.0233 | 0.0875 | 64.0 | −0.2594 | 0.0134 | 0.0809 | 35.5 |
| PS (quintiles) | −0.3151 | 0.0213 | 0.1206 | 42.6 | −0.3226 | 0.0121 | 0.1162 | 14.0 |
| IPW | −0.4304 | 0.0189 | 0.2042 | 7.6 | −0.4213 | 0.0109 | 0.1884 | 0.3 |
| Regular setting (small effect) | ||||||||
| None | −0.4365 | 0.0436 | 0.2341 | 38.0 | −0.4427 | 0.0226 | 0.2186 | 12.2 |
| Linear | 0.0009 | 0.0095 | 0.0095 | 96.1 | 0.0036 | 0.0044 | 0.0044 | 95.9 |
| PS (linear) | −0.2537 | 0.0207 | 0.0851 | 62.3 | −0.2493 | 0.0105 | 0.0727 | 29.4 |
| PS (quintiles) | −0.2892 | 0.0191 | 0.1028 | 48.2 | −0.2872 | 0.0097 | 0.0923 | 17.2 |
| IPW | −0.2544 | 0.0265 | 0.0912 | 59.0 | −0.2563 | 0.0174 | 0.0831 | 44.9 |
| Non-regular (moderate) setting | ||||||||
| None | −0.9667 | 0.0337 | 0.9681 | 0.0 | −0.9712 | 0.0184 | 0.9616 | 0.0 |
| Linear | −0.0233 | 0.0121 | 0.0127 | 93.9 | −0.0093 | 0.0058 | 0.0058 | 93.9 |
| PS (linear) | −0.2988 | 0.0259 | 0.1152 | 47.8 | −0.2704 | 0.0125 | 0.0856 | 25.2 |
| PS (quintiles) | −0.3653 | 0.0237 | 0.1571 | 28.5 | −0.3378 | 0.0111 | 0.1252 | 8.4 |
| IPW | −0.5768 | 0.0251 | 0.3578 | 1.2 | −0.5362 | 0.0128 | 0.3003 | 0.0 |
| Non-regular (severe) setting | ||||||||
| None | −0.4490 | 0.0380 | 0.2395 | 33.9 | −0.4513 | 0.0226 | 0.2264 | 12.0 |
| Linear | −0.0025 | 0.0090 | 0.0090 | 96.4 | −0.0026 | 0.0045 | 0.0045 | 94.6 |
| PS (linear) | −0.2517 | 0.0191 | 0.0824 | 60.7 | −0.2555 | 0.0104 | 0.0757 | 28.3 |
| PS (quintiles) | −0.2900 | 0.0169 | 0.1011 | 46.5 | −0.2942 | 0.0097 | 0.0963 | 14.8 |
| IPW | −0.2606 | 0.0301 | 0.0980 | 66.5 | −0.2640 | 0.0171 | 0.0867 | 42.8 |
Soft-thresholding results are presented for all settings except the first; the hard-max results are shown for the regular setting with a large effect.
Table 3.
Performance of Q-learning adjustment methods under additional scenarios: bias, Monte Carlo variance (MC var), mean squared error (MSE), and coverage of 95% bootstrap confidence intervals.
| Hard-max | Soft thresholding | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjustment method | Bias | MC var | MSE | Cover | Bias | MC var | MSE | Cover |
| Non-regular (severe) setting, random treatment | ||||||||
| None | 0.0015 | 0.0220 | 0.0220 | 96.9 | 0.0032 | 0.0185 | 0.0185 | 96.1 |
| Linear | −0.0014 | 0.0094 | 0.0094 | 95.8 | −0.0020 | 0.0086 | 0.0086 | 94.8 |
| PS (linear) | −0.0018 | 0.0138 | 0.0138 | 97.4 | −0.0012 | 0.0129 | 0.0129 | 97.2 |
| IPW | −0.0001 | 0.0136 | 0.0136 | 96.9 | 0.0012 | 0.0115 | 0.0115 | 96.6 |
| Non-regular (severe) setting, Cj binary | ||||||||
| None | −0.3947 | 0.0265 | 0.1823 | 29.2 | −0.3782 | 0.0278 | 0.1708 | 33.3 |
| Linear | 0.0010 | 0.0099 | 0.0099 | 96.2 | 0.0048 | 0.0092 | 0.0093 | 95.1 |
| PS (linear) | −0.2739 | 0.0197 | 0.0947 | 51.0 | −0.2692 | 0.0186 | 0.0911 | 50.2 |
| IPW | −0.2145 | 0.0213 | 0.0673 | 63.5 | −0.2026 | 0.0163 | 0.0573 | 62.6 |
| Non-regular (severe) setting, Cj binary | ||||||||
| None | −0.0839 | 0.0157 | 0.0227 | 90.0 | −0.0774 | 0.0171 | 0.0231 | 90.8 |
| Linear | −0.0029 | 0.0101 | 0.0101 | 95.6 | 0.0006 | 0.0092 | 0.0092 | 94.8 |
| PS (linear) | −0.0043 | 0.0129 | 0.0129 | 95.8 | −0.0006 | 0.0118 | 0.0118 | 94.7 |
| IPW | −0.0505 | 0.0150 | 0.0176 | 92.5 | −0.0465 | 0.0122 | 0.0144 | 92.7 |
In the first two scenarios, C1 and C2 predict Y, while in the third, C2 predicts Y but C1 does not.
As we might hope, all methods perform well when treatment is randomly allocated, whether or not the covariates Cj predict the outcome (Tables 1 and 3). In settings where treatment is confounded by the continuous confounders C1 and C2, only adjustment by the covariates themselves provides unbiased estimates with appropriate coverage by the percentile-based bootstrap confidence intervals (Table 2).
When the confounders are binary, all methods of adjustment except the correctly specified model which adjusts for confounders linearly in the Q-function model again exhibit bias. There is one situation in which this is not the case: if there exists a single confounder at each interval, and only C2 but not C1 affects Y (i.e., γ1 = 0); in this case, including the PS in the Q-function model performs as well as including C2 in the model, since the PS acts as a re-scaled version of C2. Bias and undercoverage are exhibited by the unadjusted and IPTW estimators, however this is not as great as in other scenarios where there is a greater degree of confounding.
The question then arises as to whether these results are indeed as unexpected as they first appear: why is it that these classic methods of causal adjustment yield bias in the estimates of DTR parameters? We note that using these methods of adjustment, we do in fact obtain unbiased estimates of the data-generating parameters γ associated with the treatment A2 (i.e., the variables contained in H21), as is predicted by theory. However, we do not obtain unbiased estimators of the true pseudo-outcome (Figure 2) under the mis-specified (PS-based) models or, crucially, an accurate representation of the dependence of the pseudo-outcome on A1. For example, in the sample corresponding to Figure 2a, the difference in the mean true pseudo-outcome for A1 = 1 versus A1 = −1 is 1.50; the difference in mean pseudo-outcome as estimated by linear adjustment, linear PS adjustment, and IPTW are, respectively, 1.49, 1.40, and 0.29. When treatment is randomly assigned—even when Y depends on C1 and C2—the observed dependence of the pseudo-outcome on A1 does not vary widely, even though the pseudo-outcome is poorly estimated by some methods (Figure 2b): the difference in mean pseudo-outcome as estimated by linear adjustment, linear PS adjustment, and IPTW are, respectively, 0.03, 0.02, and −0.02 as compared to −0.01 for the true pseudo-outcome.
Figure 2.
True versus estimated pseudo-outcome in simulated data using linear adjustment, propensity-score (linear) adjustment, and inverse probability of treatment weighting, n = 10,000 in a regular (large effect) setting. (a) Confounding treatment (see Table 2, first scenario). (b) Randomly assigned treatment and C1, C2 predictive of Y.
3.5. Results for Confounding by Counterfactuals
Here again, we consider the estimates of the parameter ψ10 under the counterfactual-based data generation approach described in Section 3.3. Performance of the five different Q-learning approaches under random and confounded treatment allocation for sample sizes 250 and 1,000 are given in Table 4. From these results, it is clear that ignoring the confounding variables results in significant bias. All methods of adjusting for confounding provide considerably improved estimates in terms of both bias and coverage for small samples, but in large samples, evidence points again to including the confounding covariate as a linear term as the optimal choice. This form of adjustment provides the best performance, perhaps because this approach does not require any additional model fitting (i.e., no additional variability is introduced to the estimation procedure due to the estimation of the propensity score), although IPTW performs well too in both small and large samples. These results suggest that many standard adjustment methods can be used to reduce or eliminate bias provided the Q-function can be correctly specified.
Table 4.
Performance of Q-learning adjustment methods under the confounding by counterfactuals simulations: bias, Monte Carlo variance (MC var), mean squared error (MSE), and coverage of 95% bootstrap confidence intervals.
| Randomized treatment | Confounded treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjustment method | Bias | MC var | MSE | Cover | Bias | MC var | MSE | Cover |
| n = 250 | ||||||||
| None | 0.0020 | 0.0082 | 0.0082 | 94.0 | 0.2293 | 0.0080 | 0.0605 | 26.4 |
| Linear | 0.0011 | 0.0032 | 0.0032 | 95.1 | 0.0051 | 0.0039 | 0.0039 | 93.8 |
| PS (linear) | 0.0010 | 0.0052 | 0.0052 | 96.2 | 0.0548 | 0.0060 | 0.0090 | 89.4 |
| PS (quintiles) | 0.0008 | 0.0056 | 0.0056 | 96.1 | 0.0779 | 0.0061 | 0.0121 | 83.2 |
| IPW | 0.0004 | 0.0046 | 0.0046 | 93.9 | 0.0108 | 0.0075 | 0.0076 | 92.8 |
| n = 1000 | ||||||||
| None | −0.0012 | 0.0022 | 0.0022 | 93.4 | 0.2246 | 0.0021 | 0.0525 | 0.5 |
| Linear | 0.0001 | 0.0009 | 0.0009 | 93.5 | 0.0037 | 0.0010 | 0.0010 | 93.5 |
| PS (linear) | −0.0002 | 0.0014 | 0.0014 | 95.5 | 0.0446 | 0.0015 | 0.0035 | 77.0 |
| PS (quintiles) | −0.0004 | 0.0015 | 0.0015 | 95.7 | 0.0699 | 0.0015 | 0.0064 | 55.0 |
| IPW | −0.0008 | 0.0012 | 0.0012 | 93.6 | 0.0018 | 0.0018 | 0.0018 | 93.6 |
These simulations provide a useful demonstration of the methods of adjustment in principle, however it is not clear whether these results generalize to real-data scenarios as it is difficult to conceive of a situation in which counterfactual outcomes could be measured and used as covariates.
3.6. Additional Remarks
In addition to the regression and weighting approaches considered above, we considered propensity score matching. Caliper matching with replacement into pairs was accomplished using the Matching package in R (Sekhon, 2011); the matching was performed separately at each interval. We found that the estimators exhibited variability that was considerably larger than that of all other estimators. Furthermore, in the simulations where data generation was counterfactual-based, bias appeared to be of the same magnitude as the unadjusted Q-learning, however the origin of this bias is different: matching on the propensity score in this fashion targets estimation of the average treatment effect on treated (ATT) rather than the average treatment effect, which is the parameter of interest.
Finally, we note that it is typical to stabilize weights (Robins, 1999) in IPTW analyses to improve efficiency. We did implement this, but no substantial difference was noted in the estimators’ performance (results not shown).
4. BREASTFEEDING AND VOCABULARY TEST RESULTS: THE PROBIT STUDY
4.1. Background and Study Details
There have been a considerable number of studies from developed countries which have suggested higher cognitive scores on IQ and other tests among both children and adults who were breastfed compared with those who were formula-fed, although these findings have been nearly exclusively drawn from observational studies (Anderson, Johnstone, & Remley, 1999). Kramer and colleagues conducted the largest ever randomized trial assessing the impact of a breastfeeding promotion intervention on a variety of growth, infection, cognitive, and metabolic outcomes: the PROmotion of Breastfeeding Intervention Trial (PROBIT) (Kramer et al., 2001, 2002, 2003, 2004, 2007). Based on an intention-to-treat analysis, this trial produced strong evidence that prolonged and exclusive breastfeeding improves children’s cognitive development (Kramer et al., 2008). In our present analysis, we examine evidence that breastfeeding actually received—rather than the breastfeeding promotion intervention—increases verbal cognitive ability, and whether there is any advantage to tailoring breastfeeding habits to infant growth to improve this outcome.
In PROBIT, hospitals and affiliated polyclinics in the Republic of Belarus were randomized to a breastfeeding promotion intervention modelled on the WHO/UNICEF Baby-Friendly Hospital Initiative or to standard care. All study infants were born in one of 31 Belarusian maternity hospitals from June 17, 1996, to December 31, 1997, at term, weighing at least 2,500 g, initiated breastfeeding, and were enrolled during their postpartum stay. This resulted in the recruitment of 17,046 mother-infant pairs who were followed regularly for the first year of life. In a later wave of PROBIT, follow-up interviews and examinations were performed on 13,889 (81.5%) children at 6.5 years of age. One of the components of these visits was the administration of the Wechsler Abbreviated Scales of Intelligence (WASI), which consists of four subtests: vocabulary, similarities, block designs, and matrices. We focus our analysis on the vocabulary subtest.
4.2. Analysis
We focussed our attention on two key treatment intervals: birth to three months of age, and three to six months. The exposure of interest for our analysis is “any breastfeeding” measured in each of the intervals, which we will refer to as a “treatment” for consistency with the development of the previous sections. That is, A1 takes the value 1 if the child was breastfed up to three months of age (and is set to −1 otherwise), and A2 is the corresponding quantity for any breastfeeding up to six months of age. Note that any breastfeeding allows for exclusive breastfeeding, or breastfeeding with supplementation with formula or solid foods. The outcome, Y, is the vocabulary subtest score on the WASI measured at age 6.5 years.
We considered a single tailoring variable at each interval which measures the sex-specific size of the child. Specifically, we took O1 to be the birthweight of the infant, and O2 to be the infant’s three-month weight.
Four approaches were compared: no adjustment, adjustment via inclusion of potential confounders as linear terms in the Qj model, adjusting for the propensity score as a linear term in the Qj model, and inverse-probability weighting. Specifically, we fit the following Q-learning models:
where the matrix of confounders Cj (with columns Cj1, …, CjK for j = 1, 2) was empty for both the no-adjustment and IPTW approach. In the direct adjustment approach, Cj consisted of intervention group, geographical region, maternal characteristics (education, smoking status, age and age-squared, whether previous children had been breastfed for three months), whether there was a family history of allergy, whether the birth was by caesarean section, and weight at the start of the interval. Finally, for the propensity-score adjustment approach, Cj was the propensity score, a one-dimensional summary of the confounders used in the direct adjustment approach.
To appropriately account for the variability of the complete estimation procedure, a non-parametric bootstrap procedure was employed.
4.3. Results
The decision rule parameter estimates are given in Table 5. With the exception of ψ10, all four analytic approaches led to parameter estimates that agreed in terms of their sign, but in many cases differed with respect to statistical significance. Note that for a linear Q-function with a single variable used to tailor the treatment decision, the treatment rules at each interval can be characterized by the threshold −ψj0/ψj1. The optimal decision is determined by the rule: treat with Aj = 1 when (ψj0 + ψj1Oj) > 0, in other words, treat when Oj > −ψj0/ψj1 (for ψj1 > 0). For example, the PS-adjusted estimates yield the regime:
breastfeed to 3 months if birthweight exceeds 2.60 kg,
breastfeed to 6 months if 3-month weight exceeds 6.35 kg.
This corresponds to a rule which suggests that 98% of the infants in the study should be breastfed until 3 months to maximize vocabulary score at age 6.5, and 33% should be breastfed until 6 months (Figure 3). Note that ψ20 and ψ21 are not significantly different from zero, which suggests that breastfeeding decisions from 3 to 6 months will neither significantly increase nor decrease the vocabulary score.
Table 5.
Estimates (percentile bootstrap 95% CI) for decision rule parameters in the PROBIT example.
| None | Linear | PS | IPW | |
|---|---|---|---|---|
| Hard-max | ||||
| ψ10 | 0.612 (0.163, 0.996) | −0.556 (−0.971, −0.165) | −0.510 (−1.151, 0.201) | 0.674 (0.160, 1.140) |
| ψ11 | 0.031 (−0.044, 0.142) | 0.057 (−0.003, 0.139) | 0.184 (0.020, 0.347) | 0.046 (−0.037, 0.142) |
| ψ20 | −2.493 (−7.829, 2.783) | −4.964 (−9.491, −0.533) | −3.366 (−8.622, 1.741) | −2.614 (−9.768, 4.615) |
| ψ21 | 0.648 (−0.188, 1.495) | 0.790 (0.089, 1.515) | 0.532 (−0.304, 1.357) | 0.435 (−0.672, 1.554) |
| Soft thresholding | ||||
| ψ10 | 0.649 (0.203, 1.052) | −0.428 (−0.875, −0.112) | −0.424 (−1.082, 0.248) | 0.742 (0.187, 1.178) |
| ψ11 | 0.024 (−0.057, 0.144) | 0.026 (−0.015, 0.121) | 0.163 (0.003, 0.326) | 0.029 (−0.040, 0.140) |
| ψ20 | −2.493 (−7.829, 2.783) | −4.964 (−9.491, −0.533) | −3.366 (−8.622, 1.741) | −2.614 (−9.768, 4.615) |
| ψ21 | 0.648 (−0.188, 1.495) | 0.790 (0.089, 1.515) | 0.532 (−0.304, 1.357) | 0.435 (−0.672, 1.554) |
Figure 3.
Visualizing the optimal rule as a function of infant weight in the PROBIT study:—unadjusted; – – linear adjustment; … PS adjusted; · – · IPW. For values of infant weight where the line lies above 0, breastfeeding is considered optimal (zero is indicated by the horizontal grey line). Rugplot along x-axis indicates the observed distribution of the tailoring variable in the sample. (a) Interval 1: breastfeeding between 0 and 3 months as a function of birthweight. (b) Interval 2: breastfeeding between 3 and 6 months as a function of 3-month weight.
The unadjusted analysis suggests decision rules that would indicate breastfeeding all infants up to 3 months, and over 99% of the sample to 6 months. The linear adjustment analysis does not recommend breastfeeding for any infants in the sample up to 3 months, but suggests breastfeeding 38% of the sample from 3 to 6 months to maximize verbal IQ at 6.5 years. Note, however, that breastfeeding is such that infants do not recommence breastfeeding once weaned. Thus, for an infant to breastfeed from 3 to 6 months, it is necessary to have done so up until 6 months. The dependency of breastfeeding on not having already weaned an infant can be incorporated into the second-interval Q-function by including an interaction between A1 and A2; we did not pursue this in the current analysis as the simpler (fewer parameters) Q-function models did not suggest any significant or substantial findings. Finally, the IPTW analysis recommends breastfeeding all infants in the sample up to 3 months, and 49% from 3 to 6 months to maximize verbal IQ at 6.5 years. Estimates and decision rules from the hard-max estimation procedure were not substantially different. We also examined exclusive breastfeeding as the treatment of interest (i.e., no formula, other milks or liquids, or solid food supplementation); the results were similar.
5. CONCLUSION
In the care of virtually all conditions which are not acute, estimating the best sequence of actions or treatments should not be done in a series of single interval studies. Due to the complexity and cost of running a sequentially randomized trial, most complex regimes must first be explored through observational data, and hence it is key to develop inferential techniques that are easily interpreted and readily implemented. Q-learning is a particularly appealing approach due to the ease with which it can be implemented. However, as we have demonstrated, standard approaches to causal adjustment can lead to serious bias and unacceptably poor coverage if the model for the Q-function is not correctly specified. Where the dimension of the confounding variables permits, we therefore recommend directly including covariates into the models for the Q-functions. Where the relationships between the potential confounders and the outcome are poorly understood, it may be necessary to employ splines of polynomial functions to ensure an adequate model specification.
ACKNOWLEDGEMENTS
This work is supported by Operating Grants from the Canadian Institutes of Health Research. Dr. Moodie is supported by a Natural Sciences and Engineering Research Council (NSERC) University Faculty Award. Dr. Chakraborty would like to acknowledge support from the National Institutes of Health grant R01 NS072127-01A1 and the Calderone Junior Faculty Prize from the Mailman School of Public Health of Columbia University.
We would like to thank Dr. Susan Murphy for insightful comments and ideas. We also thank the two anonymous reviewers for their questions and suggestions.
BIBLIOGRAPHY
- Anderson J, Johnstone B, Remley D. Breast-feeding and cognitive development: A meta-analysis. American Journal of Clinical Nutrition. 1999;70(4):525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- Arjas E, Saarela O. Optimal dynamic regimes: Presenting a case for predictive inference. The International Journal of Biostatistics. 2010;6 doi: 10.2202/1557-4679.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellman R. Dynamic Programming. Princeton: Princeton University Press; 1957. [Google Scholar]
- Bertsekas D, Tsitsiklis J. Neuro-Dynamic Programming. Belmont, MA: Athena Scientific; 1996. [Google Scholar]
- Chakraborty B. Dynamic treatment regimes for managing chronic health conditions: A statistical perspective. American Journal of Public Health. 2011;101(1):40–45. doi: 10.2105/AJPH.2010.198937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty B, Laber EB, Zhao Y. Inference for optimal dynamic treatment regimes using an adaptive m-out-of-n bootstrap scheme. 2012 doi: 10.1111/biom.12052. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty B, Moodie EM. Estimating optimal dynamic treatment regimes with shared decision rules across stages: An extension of Q-learning. 2012 Submitted. [Google Scholar]
- Chakraborty B, Murphy S, Strecher V. Inference for non-regular parameters in optimal dynamic treatment regimes. Statistical Methods in Medical Research. 2010;19(3):317–343. doi: 10.1177/0962280209105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Ansell P, Alshibani D. Regret-regression for optimal dynamic treatment regimes. Biometrics. 2010;6:1192–1201. doi: 10.1111/j.1541-0420.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic & Clinical Pharmacology & Toxicology. 2006;98:237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- Kramer M, Aboud F, Miranova E, Vanilovich I, Platt R, Matush L, Igumnov S, Fombonne E, Bogdanovich N, Ducruet T, Collet J, Chalmers B, Hodnett E, Davidovsky S, Skugarevsky O, Trofimovich O, Kozlova L, Shapiro S. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Archives of General Psychiatry. 2008;65:578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet J, Vanilovich I, Mezen I, Ducruet T, Shishko G, Zubovich V, Mknuik D, Gluchanina E, Dombrovsky V, Ustinovitch A, Ko T, Bogdanovich N, Ovchinikova L, Helsing E. Promotion of breastfeeding intervention trial (PROBIT): A randomized trial in the Republic of Belarus. Journal of the American Medical Association. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Guo T, Platt RW, Sevkovskaya Z, Dzikovich I, Collet J, Shapiro S, Chalmers B, Hodnett E, Vanilovich I, Mezen I, Ducruet T, Shishko G, Bogdanovich N. Infant growth and health outcomes associated with 3 compared with 6 months of exclusive breastfeeding. American Journal of Clinical Nutrition. 2003;78:291–295. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Guo T, Platt RW, Shapiro S, Collet J, Chalmers B, Hodnett E, Sevkovskaya Z, Dzikovich I, Vanilovich I. Breastfeeding and infant growth: Biology or bias? Pediatrics. 2002;110:343–357. doi: 10.1542/peds.110.2.343. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Guo T, Platt RW, Vanilovich I, Sevkovskaya Z, Dzikovich I, Michaelsen KF, Dewey K. Feeding effects on growth during infancy. Journal of Pediatrics. 2004;145:600–605. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet J, Martin R, Davey Smith G, Gillman M, Chalmers B, Hodnett E, Shapiro S. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. American Journal of Clinical Nutrition. 2007;86:1717–1721. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- Laber EB, Qian M, Lizotte D, Murphy S. Statistical inference in dynamic treatment regimes. 2012 Submitted. [Google Scholar]
- Moodie E, Richardson T. Estimating optimal dynamic regimes: Correcting bias under the null. Scandinavian Journal of Statistics. 2010;37:126–146. doi: 10.1111/j.1467-9469.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. Optimal dynamic treatment regimes (with discussions) Journal of the Royal Statistical Society, Series B. 2003;65:331–366. [Google Scholar]
- Murphy S. A generalization error for Q-learning. Journal of Machine Learning Research. 2005;6:1073–1097. [PMC free article] [PubMed] [Google Scholar]
- Pineau J, Bellernare M, Rush A, Ghizaru A, Murphy S. Constructing evidence-based treatment strategies using methods from computer science. Drug and Alcohol Dependence. 2007;88:S52–S60. doi: 10.1016/j.drugalcdep.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM. Marginal structural models versus structural nested models as tools for causal inference. In: Halloran ME, Berry D, editors. Statistical Models in Epidemiology: The Environment and Clinical Trials. New York: Springer-Verlag; 1999. pp. 95–134. [Google Scholar]
- Robins JM. Optimal structural nested models for optimal sequential decisions. In: Lin D, Heagerty P, editors. Proceedings of the Second Seattle Symposium on Biostatistics; Springer; New York. 2004. pp. 189–326. [Google Scholar]
- Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Robins JM, Orellana L, Rotnitzky A. Estimation and extrapolation of optimal treatment and testing strategies. Statistics in Medicine. 2008;27:4678–4721. doi: 10.1002/sim.3301. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Sekhon J. Multivariate and propensity score matching software with automated balance optimization: The matching package for R. Journal of Statistical Software. 2011;42(7):1–52. [Google Scholar]
- Shortreed S, Laber E, Lizotte D, Stroup T, Pineau J, Murphy S. Informing sequential clinical decision-making through reinforcement learning: An empirical study. Machine Learning. 2011;84(1):109–136. doi: 10.1007/s10994-010-5229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Wang W, Zeng D, Kosorok M. Penalized Q-learning for dynamic treatment regimes. 2012 doi: 10.5705/ss.2012.364. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Barto A. Reinforcement Learning: An Introduction. Cambridge: MIT Press; 1998. [Google Scholar]
- Thall P, Millikan R, Sung H. Evaluating multiple treatment courses in clinical trials. Statistics in Medicine. 2000;30:1011–1128. doi: 10.1002/(sici)1097-0258(20000430)19:8<1011::aid-sim414>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- van der Laan MJ, Petersen ML. Causal effect models for realistic individualized treatment and intention to treat rules. The International Journal of Biostatistics. 2007;3 doi: 10.2202/1557-4679.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kosorok M, Zeng D. Reinforcement learning design for cancer clinical trials. Statistics in Medicine. 2009;28:3294–3315. doi: 10.1002/sim.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zeng D, Socinski M, Kosorok M. Reinforcement learning strategies for clinical trials in nonsmall cell lung cancer. Biometrics. 2011;67:1422–1433. doi: 10.1111/j.1541-0420.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]