Abstract
BACKGROUND & AIMS
Dkk1 is a secreted antagonist of the Wnt/β-catenin signaling pathway. It is induced by inflammatory cytokines during colitis and exacerbates tissue damage by promoting apoptosis of epithelial cells. However, little is known about the physiologic role of Dkk1 in normal intestinal homeostasis and during wound repair following mucosal injury. We investigated whether inhibition of Dkk1 affects the morphology and function of the adult intestine.
METHODS
We used doubleridge mice (Dkk1d/d), which have reduced expression of Dkk1, and an inhibitory Dkk1 antibody to modulate Wnt/β-catenin signaling in the intestine. Intestinal inflammation was induced with dextran sulfate sodium (DSS), followed by a recovery period in which mice were given regular drinking water. Animals were killed before, during, or after DSS administration; epithelial homeostasis and the activity of major signaling pathways were investigated by morphometric analysis, bromo-2′-deoxyuridine incorporation, and immunostaining.
RESULTS
Reduced expression of Dkk1 increased proliferation of epithelial cells and lengthened crypts in the large intestine, which was associated with increased transcriptional activity of β-catenin. Crypt extension was particularly striking when Dkk1 was inhibited during acute colitis. Dkk1d/d mice recovered significantly faster from intestinal inflammation but exhibited crypt architectural irregularities and epithelial hyperproliferation compared with wild-type mice. Survival signaling pathways were concurrently up-regulated in Dkk1d/d mice, including the AKT/β-catenin, ERK/Elk-1, and c-Jun pathways.
CONCLUSIONS
Dkk1, an antagonist of Wnt/β-catenin signaling, regulates intestinal epithelial homeostasis under physiologic conditions and during inflammation. Depletion of Dkk1 induces a strong proliferative response that promotes wound repair after colitis.
Keywords: IBD, Crohn’s Disease, Mucosa, Intestinal Cell Signaling
The renewal of the intestinal epithelium is tightly regulated by integrated signaling pathways that control the proliferation, differentiation, and apoptosis of intestinal epithelial cells (IECs). Dysregulation of epithelial homeostasis has been observed in a variety of human diseases, including the inflammatory bowel diseases Crohn’s disease and ulcerative colitis1 as well as colorectal cancers.2 The Wnt/β-catenin pathway, which promotes IEC proliferation by stabilizing cytosolic β-catenin, has emerged as a key regulator of the intestinal stem cell niche.3 Dkk1 is a secreted protein that inhibits the canonical Wnt pathway by competitive binding to LRP family cell surface receptors, thereby decreasing β-catenin protein stability.4 We have recently shown that Dkk1 is induced by inflammatory cytokines during intestinal inflammation and that it exacerbates tissue damage by enhancing IEC apoptosis.5 In addition, it has previously been reported that ectopic overexpression of Dkk1 in the intestine causes dramatic mucosal injury by inhibition of epithelial cell proliferation and differentiation.6,7
These observations prompted us to investigate the physiologic role of Dkk1 during normal intestinal homeostasis and intestinal inflammation. We used 2 complementary experimental approaches to functionally inhibit Dkk1 activity in the intestine. Dkk1 hypomorphic doubleridge mice (designated Dkk1d/d) with systemically reduced Dkk1 expression8 were used to identify morphologic and functional changes in the intestine. Results from these studies were then validated by treating mice with an inhibitory Dkk1 antibody. We found that Dkk1 regulates colonic crypt length by inhibiting IEC proliferation, which was most striking in inflamed areas of the mucosa during experimental colitis. Conversely, epithelial hyperproliferation resulted in aberrant crypt morphology after recovery from intestinal inflammation, which was associated with pronounced activity of pro-survival signaling pathways. Based on these results, we conclude that Dkk1 contributes to intestinal epithelial homeostasis and maintains tissue morphology following colitis.
Materials and Methods
Animal Experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee at Emory University and performed according to National Institutes of Health guidelines. Dkk1d/d C57BL/6J mice were generously provided by M. H. Meisler (University of Michigan, Ann Arbor, MI) and J. Kearney (Vanderbilt University, Nashville, TN). Wild-type littermates served as controls. Additional wild-type mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and Harlan (Prattville, AL). Colitis was induced by treatment with 3% dextran sulfate sodium (DSS, lot 124156; USB Corp, Cleveland, OH) dissolved in tap water. In some experiments, mice received 10 mg/kg rat monoclonal anti-Dkk1 antibody (kindly provided by Amgen Inc, Thousand Oaks, CA), or isotype control (Sigma-Aldrich, St Louis, MO) by daily intraperitoneal injection. For cancer studies, mice received a single intraperitoneal injection of 7.5 mg/kg azoxymethane (Sigma), followed by 3 cycles of 2% DSS for 5 days with a recovery period of 1 week. Animals were killed after 10 weeks. Proliferation was determined by intraperitoneal injection of 1 mg 5-bromo-2′-deoxyuridine (BrdU; Sigma).
Antibodies and Reagents
The following primary antibodies were obtained from the following companies: Dkk1 (R&D Systems, Minneapolis, MN), BrdU (Roche Diagnostics, Indianapolis, IN), AKT pT308, AKT, ERK1/2 pT202/Y204, ERK1/2, Elk-1 pS383, c-Jun pS63 (Cell Signaling, Beverly, MA), Ki-67 (Dako, Carpinteria, CA), α–smooth muscle actin (Novus Biological, Littleton, CO), LRP6 (US Biological, Swampscott, MA), CD31 (Abbiotec, San Diego, CA), CD41 (Bio-Legend, San Diego, CA), B220, NK1.1, CD4, CD8, Ly6G, F4/80, CD11b, CD11c (Becton Dickinson, Franklin Lakes, NJ), PCNA, c-Myc, cyclin D1, Kremen1 (SCBT, Santa Cruz, CA), β-catenin, Villin (Invitrogen, Carlsbad, CA), cleaved Caspase-3 (R&D Systems, Minneapolis, MN), E-cadherin, β-actin, and α-tubulin (Sigma-Aldrich). The β-catenin pS552 antibody was provided by L. Li.9 Secondary antibodies were purchased from Invitrogen and Jackson ImmunoResearch (West Grove, PA). For Dkk1 colocalization studies, 5 μg of Dkk1 antibody was covalently labeled with Alexa Fluor 555 using the APEX kit (Invitrogen).
Mucosal Cell Isolation
Cells were isolated from pooled intestines of 10 mice per group, essentially as described previously.10 In brief, after removal of the epithelium, tissues were dissociated in EDTA-containing buffer followed by collagenase digestion. Cells were then labeled with antibodies against TCRβ, F4/80, CD45, CD11c, and CD19 (Becton Dickinson) and separated using a FACSAria II cell sorter (Becton Dickinson).
Measurement of Villus-Crypt Length
Villus and crypt length was determined by bottom-to-surface measurement of at least 50 correctly aligned villi or crypts of 3 or more mice per group. NIH ImageJ 1.38 software was used for analysis.
Statistics
GraphPad (La Jolla, CA) Prism 5 and Microsoft Excel software (Microsoft, Redmond, WA) were used for statistical analyses. Data were analyzed with Bonferroni’s post hoc test following one-way analysis of variance or 2-tailed Student t test. Statistical significance was assumed at P < .05. Differences in crypt and villus length are shown as percent changes ± 99% confidence interval. All other results are displayed as mean ± SEM.
Additional information on animal experiments as well as protocols for immunoblotting, fluorescence microscopy, immunohistochemistry, and cell culture can be found in the Supplementary Materials and Methods.
Results
Dkk1 Controls Colonic Epithelial Cell Proliferation and Crypt Length
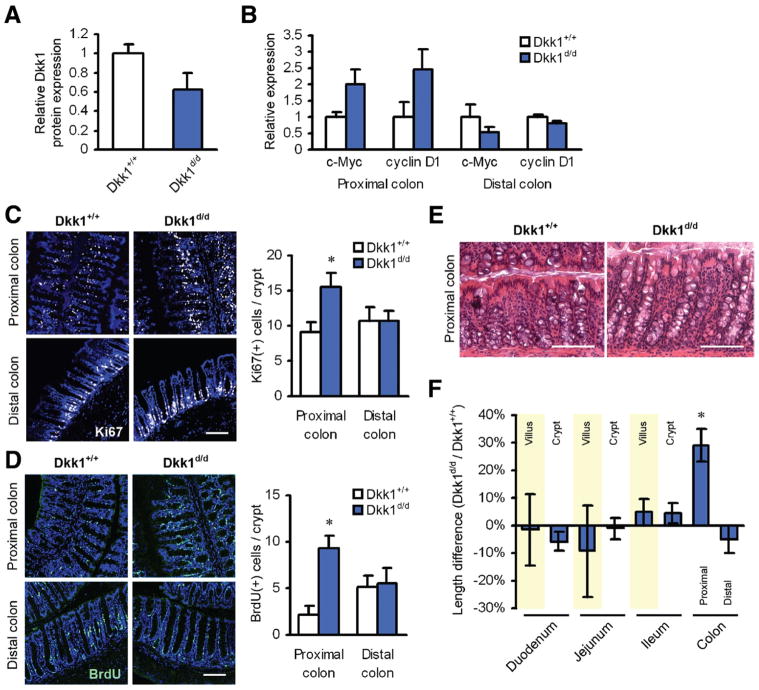
To determine the function of Dkk1 in the intestine, we first analyzed the expression of Dkk1 in colonic samples of Dkk1d/d and matched wild-type mice using an antibody with high affinity for the 29-kilodalton form of Dkk1. We observed a marked reduction of Dkk1 protein expression in total mucosal lysates of Dkk1d/d mice compared with controls (Figure 1A and Supplementary Figure 1A). Consistently, we found enhanced β-catenin signaling in the proximal colon compared with control animals, as evidenced by increased expression of the β-catenin/TCF transcriptional targets c-Myc and cyclin D1 (Figure 1B). Additionally, indirect evidence for decreased Dkk1 activity in Dkk1d/d mice came from the observation that the Dkk1 coreceptor Kremen1 was stabilized in the basal membrane of crypt epithelial cells, indicative of reduced ligand-receptor internalization11 (Supplementary Figure 1B). Reduction of Dkk1 expression did not lead to a significant change in the expression of other secreted Wnt inhibitors, as determined using a Wnt-specific RNA array (data not shown). Based on these observations, we hypothesized that reduced Dkk1 expression in Dkk1d/d mice may increase colonic epithelial cell proliferation. Indeed, we observed significantly higher IEC proliferation in the proximal colon of Dkk1d/d mice, as shown by Ki67 staining and BrdU labeling (Figure 1C and D). Morphometric analysis of the intestinal epithelium revealed a highly significant crypt elongation in the proximal colon of Dkk1d/d mice relative to controls (Figure 1E and F), whereas there was no difference in crypt depth and villus length in the small intestine or the distal colon (Figure 1F). These results suggest that basal mucosal Dkk1 expression contributes to normal epithelial homeostasis in the intestine by negatively regulating Wnt/β-catenin signaling in the ascending colon.
Figure 1.
Dkk1 regulates IEC proliferation, cell migration, and crypt length in the proximal colon. (A) Dkk1 protein expression in total mucosal lysates of naïve mice. (B) Relative protein expression of the β-catenin/TCF transcriptional targets c-Myc and cyclin D1. (C) IEC proliferation in the proximal colon of Dkk1d/d mice was significantly increased, as shown by Ki67 staining (white). Scale bar = 100 μm. (D) Increased proliferation was confirmed by BrdU incorporation (green) for 24 hours. Scale bar = 100 μm. (E) Representative H&E staining of tissue samples from the proximal colon. Scale bars = 50 μm. (F) Morphometric analysis of intestinal specimens revealed a significant crypt elongation in the proximal colon of Dkk1 hypomorphic mice. All quantitative data in this figure are derived from 3 mice per group. *P < .001.
Dkk1d/d Mice Exhibit Accelerated Mucosal Restitution Following Colitis
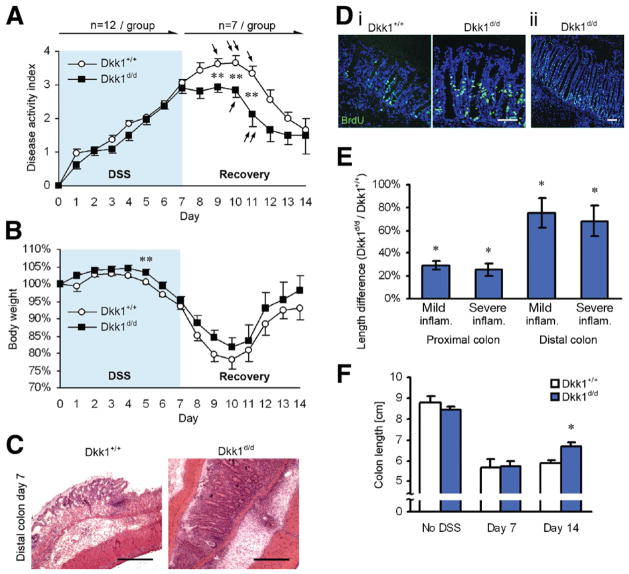
Because Dkk1 expression is strongly enhanced by inflammatory cytokines such as interferon gamma and tumor necrosis factor α,5,12 we next asked whether systemic reduction of Dkk1 during experimental colitis affects intestinal morphology and function. To this end, we challenged Dkk1d/d and control mice with DSS. We did not observe a difference in the overall clinical disease activity during acute colitis (Figure 2A); however, the relative body weight of Dkk1d/d mice was consistently higher during the entire observation period (Figure 2B), indicating a small clinical benefit for these animals. Histologic examination showed a comparable severity of inflammation at day 7 (Figure 2C) but increased crypt length in the colon of transgenic mice with signs of increased proliferation as shown by BrdU labeling (Figure 2D) and the occurrence of extremely long crypts (>500 μm; Figure 2Dii). Quantitative analysis of epithelial morphology revealed a highly significant increase in colonic crypt length in all segments of the large intestine in Dkk1d/d mice, in particular in the distal colon (Figure 2E). Strikingly, when animals were allowed to recover from DSS-induced colitis, disease activity rapidly decreased in Dkk1d/d mice (Figure 2A and B), most likely due to faster re-epithelization and increased IEC proliferation in the absence of mucosal Dkk1. Improved recovery was also reflected in an increased colon length on day 14 (Figure 2F). We have previously reported that Dkk1 induces IEC apoptosis in vitro and in vivo.5,13 Thus, to assess if reduced epithelial cell apoptosis may contribute to accelerated restitution following colitis, we investigated Caspase-3 activation in mucosal sections taken at different time points (Supplementary Figure 2). Although there was a trend toward reduced apoptosis in samples from Dkk1d/d mice, these changes did not reach statistical significance, suggesting that apoptosis is not the main mechanism regulating the observed effects.
Figure 2.
Dkk1d/d mice exhibit increased crypt length during colitis and faster recovery following mucosal injury. (A) Clinical disease activity index and (B) body weight graph of mice subjected to DSS-induced colitis for 7 days, followed by regular drinking water for 7 days. Arrows indicate animals that had to be killed due to severe morbidity. (C) H&E staining revealed comparable disease activity but increased crypt length in Dkk1 hypomorphic mice on day 7. Scale bars = 200 μm. (Di) BrdU incorporation (green) for 1 hour showed increased proliferation in elongated colonic crypts. (Dii) Dkk1d/d mice displayed excessively long crypts in inflamed areas of the distal colon. Scale bars = 100 μm. (E) Morphometric analysis showed a significant crypt lengthening in the entire colon on day 7. Measurements were grouped as mild or severe inflammation, based on histologic appearance. (F) Faster recovery of Dkk1d/d mice was supported by increased colon length at the end of the recovery period. Quantitative data in E and F are derived from 3 to 4 mice per group. *P < .001, **P < .05.
Induction of Dkk1 in Mucosal Cell Populations During Colitis Is Reduced in Dkk1d/d Mice
The previous observations suggested that reduced induction of Dkk1 expression in transgenic mice facilitates a more efficient epithelial wound repair by promoting epithelial cell proliferation. To confirm this hypothesis, we first determined Dkk1 protein expression during acute colitis, which we have previously shown to be increased approximately 3-fold during inflammation.5 As shown in Figure 3A, Dkk1 expression was reduced by approximately 80% in total mucosal lysates of Dkk1d/d mice (containing epithelial cells and stromal cells) compared with controls. LRP6 expression in IECs is strongly reduced during colitis, and loss of LRP6 inhibits epithelial cell proliferation.5 In agreement with these data, we observed that by Western blot, LRP6 expression was undetectable in mucosal samples of control mice treated with DSS, in contrast to Dkk1d/d mice (Figure 3A). Comparable expression of the Dkk1 coreceptor Kremen1 across all samples suggested that loss of LRP6 was indeed caused by Dkk1 induction, rather than enhanced Kremen expression (Figure 3A).14 We next sought to determine the cellular source of Dkk1 in the intestinal mucosa. Although we did see some Dkk1 expression in control tissue by immunoblot (Supplementary Figure 1A), Dkk1 signal intensity was below detection threshold by immunofluorescence microscopy. In tissues from mice treated with DSS for 7 days, however, coimmunostaining revealed Dkk1 protein expression in a wide variety of cell types, predominantly CD4+ and CD8+ T lymphocytes (Figure 3B and Supplementary Figure 3). Interestingly, we also observed strong Dkk1 staining in CD41+ platelets associated with other cells, most likely neutrophils, as described.15 Moderate Dkk1 expression was also seen in epithelial cells, myofibroblasts, macrophages, and dendritic cells (Supplementary Figure 3). Comparative analysis revealed that Dkk1 expression in Dkk1d/d mice was most strongly reduced in T cells (Table 1). No specific staining was observed when tissues were coincubated with recombinant mouse Dkk1 (data not shown). To confirm Dkk1 induction in mucosal leukocytes during colitis, we next amplified RNA from different cell populations in the intestinal mucosa. As can be seen in Figure 3C, no Dkk1 messenger RNA was detected in samples from healthy intestines. In striking contrast, intestinal inflammation resulted in an induction of Dkk1 in all investigated cell types (epithelial cells, macrophages, T cells, dendritic cells). Taken together, these data strongly suggest that a variety of different cell types, including IECs and lamina propria leukocytes, contribute to the total pool of secreted Dkk1 in the inflamed mucosa and may thus regulate tissue homeostasis during colitis.
Figure 3.
Reduced Dkk1 expression in Dkk1d/d mice during acute inflammation. (A) Immunoblot of mucosal lysates from 2 mice per group. Villin served as control for epithelial cell content. (B) Coimmunolocalization of Dkk1 (red) with the indicated markers (green). Strong Dkk1 staining was observed in CD8+ T lymphocytes and CD41+ platelets. Scale bar = 10 μm. Please see also Supplementary Figure 3 and Table 1 for additional information. (C) Polymerase chain reaction analysis of Dkk1 expression in isolated IECs and lamina propria leukocytes. MΦ, macrophages; T, T lymphocytes; DC, dendritic cells. Arrow indicates primer dimers.
Table 1.
Expression of Dkk1 in the Inflamed Intestinal Mucosa
| Marker (primary cell type) | Dkk1+/+ | Dkk1d/d |
|---|---|---|
| E-cadherin (epithelial cells) | + | +/− |
| B220 (B lymphocytes) | − | − |
| NK1.1 (natural killer cells) | − | − |
| CD8 (cytotoxic T lymphocytes) | +++ | + |
| α-SMA (myofibroblasts) | + | + |
| CD41 (platelets) | ++ | + |
| Ly6G (neutrophils) | − | − |
| CD4 (T-helper lymphocytes) | ++ | + |
| F4/80 (macrophages) | +/− | +/− |
| CD11b (monocytes/macrophages) | + | +/− |
| CD11c (dendritic cells) | + | − |
| CD31 (endothelial cells) | +/− | +/− |
NOTE. Tissue from mice treated with DSS for 7 days was analyzed by confocal microscopy (see also Supplementary Figure 3). Dkk1 fluorescence intensity was compared in 3 mice per group at identical instrument settings. Expression is displayed as strong (+++), intermediate (++), weak (+), present in some cells (+/−), and absent (−).
Differential Regulation of β-Catenin Signaling Pathways in Acute Colitis
To investigate signaling pathways other than Wnt/β-catenin that may regulate epithelial homeostasis during acute colitis, we examined the activity of the PI-3 kinase/AKT/β-catenin signaling pathway, which has been shown to promote IEC proliferation and crypt branching.9,16 Interestingly, AKT activity was decreased in Dkk1d/d mice during acute colitis, as indicated by reduced phosphorylation at Thr308 (Figure 4A) and reduced plasma membrane association of phospho-AKT (Figure 4B and Supplementary Figure 4A), whereas there was no discernible difference in naïve animals (data not shown). Consequently, immunoblot analysis revealed less AKT-mediated phosphorylation of β-catenin at Ser552 (Figure 4A). Subsequent microscopic analysis showed patchy phospho-β-catenin staining in Dkk1d/d mice, particularly at the crypt base (Figure 4B and Supplementary Figure 4A). Furthermore, mitotic cells with nuclear phospho-β-catenin were almost exclusively observed in control animals. Taken together, these results show that reduced expression of Dkk1 during acute colitis differentially regulates β-catenin signaling pathways. This observation suggests that the increases in IEC proliferation and crypt length observed in Dkk1d/d mice are caused by increased Wnt/β-catenin, but not AKT/β-catenin signaling.
Figure 4.

Reduced AKT/β-catenin signaling in Dkk1d/d mice during acute colitis. (A) AKT activity and AKT-mediated phosphorylation of β-catenin at residue 552 were determined by immunoblotting. (B) (Upper panels) Reduced AKT activity in Dkk1d/d mice during colitis was additionally shown by lack of plasma membrane association of AKT phospho-T308 (green). (Lower panels) Localization of β-catenin phospho-S552 (green) revealed patchy staining, particularly at the crypt base, in Dkk1d/d mice. Arrows indicate cells with nuclear phospho-β-catenin. Scale bars = 100 μm.
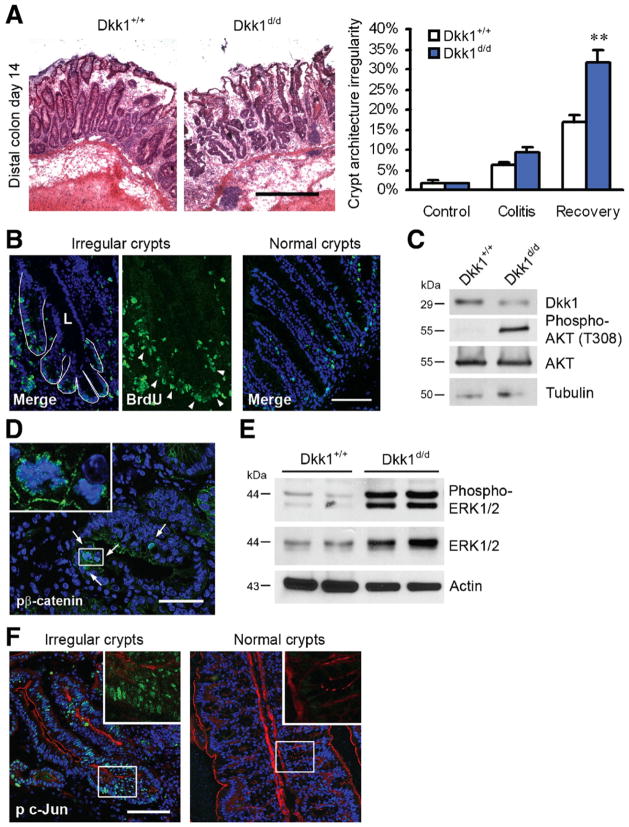
Irregular Crypts in Dkk1d/d Mice Show Evidence of Hyperproliferation and Pro-survival Signaling
Accelerated recovery from DSS-induced colitis in Dkk1d/d mice coincided with a significant increase in the number of crypts with architectural irregularities, including crypt branching and fission, as shown in Figure 5A. At the end of the observation period (day 14), there were almost twice as many crypts with structural abnormalities in Dkk1d/d mice compared with control animals (31.8% vs 17.0% of all crypts). These aberrant crypts were highly proliferative compared with morphologically normal tissue, as indicated by BrdU staining (Figure 5B). Multiple conserved signaling pathways cooperatively facilitate cell proliferation and migration following epithelial injury. To identify pathways that were differentially regulated in aberrant colonic crypts, we analyzed samples from Dkk1d/d and control mice by immunoblotting and confocal microscopy. Mucosal Dkk1 expression in Dkk1d/d mice was only slightly lower than in control animals on day 14 (Figure 5C), and confocal microscopy revealed comparable LRP6 expression in all mice (data not shown). In striking contrast to the results obtained during acute colitis, AKT phosphorylation in samples from Dkk1d/d mice was strongly increased (Figure 5C). Consistently, we observed a large number of enterocytes with active nuclear β-catenin pS552 in irregular crypts (control, 2.2 ± 0.2 cells/aberrant crypt; Dkk1d/d, 2.4 ± 0.2 cells/aberrant crypt; n = 73 per group) (Figure 5D), which may account for their excessive branching.9 In contrast, cells with nuclear β-catenin pS552 were sporadic in adjacent normal crypts. Previous reports have shown that Wnt and mitogen-activated protein kinase pathways function synergistically in the intestine and that signaling through ERK is required for IEC migration.17,18 Conversely, it has been reported that ERK promotes intestinal tumorigenesis by stabilizing the oncogene c-Myc.19 Although there was no significant difference in ERK protein expression and activity in naïve animals and on day 7 of DSS treatment (data not shown), we observed a remarkably higher ERK activity (>10-fold) in Dkk1d/d mice after recovery from colitis, which coincided with enhanced ERK protein expression (Figure 5E). Confocal microscopy revealed strong nuclear and cytoplasmic phospho-ERK staining in irregular crypts of Dkk1d/d mice compared with controls, which was reflected in slightly increased activity of downstream transcription factor Elk-1 in these animals (Supplementary Figure 5). Finally, we investigated the activity of the transcription factor c-Jun, which is phosphorylated by MKK and JNK family kinases in response to inflammatory stimuli and has been linked to epithelial homeostasis and intestinal tumorigenesis.20 We found that c-Jun activity was strongly enhanced throughout aberrant colonic crypts, whereas we detected no phospho-c-Jun in morphologically normal crypts in the same animal (Figure 5F) or in animals not challenged with DSS (data not shown). To demonstrate the role of AKT and ERK signaling in epithelial cell survival and migration, nontransformed IEC-6 cells were either scratch wounded or exposed to DSS and treated with AKT inhibitor triciribine or MEK inhibitor U0126 (Supplementary Figure 6A–C). Both inhibitors significantly reduced wound closure, but only the AKT inhibitor induced IEC apoptosis. In summary, these results suggest that following mucosal injury, Dkk1 regulates the activity of multiple pro-proliferation and pro-survival pathways to prevent crypt hyperproliferation and accumulation of structural abnormalities.
Figure 5.
Irregular crypts accumulate in Dkk1d/d mice during mucosal restitution. (A) H&E staining of colonic sections showed a large number of irregular crypts in Dkk1d/d mice after recovery from DSS-induced colitis. Scale bar = 200 μm. The ratio of irregular crypts to all crypts is displayed in the graph on the right. (B) Aberrant crypts were highly proliferative compared with morphologically normal crypts in the same sections, as indicated by BrdU incorporation for 1 hour (green, arrowheads). Multiple crypt branches opening into a central lumen (L) are outlined in white. Scale bar = 50 μm. (C) Immunoblotting revealed decreased mucosal Dkk1 expression but increased AKT activity in Dkk1d/d mice at day 14. (D) A large number of proliferating crypt epithelial cells positive for β-catenin phospho-S552 (green, arrows) were observed in irregular crypts. Scale bar = 50 μm. (E) ERK expression and activity was strongly increased in mucosal lysates from Dkk1d/d mice after recovery. (F) Activity of c-Jun, indicated by phosphorylation of S63 (green, with actin in red), was highly increased in irregular crypts. Scale bar = 50 μm. Quantitative data in A are derived from 3 mice per group. **P < .05.
Inhibition of Dkk1 Recapitulates Reduced Dkk1 Expression in Dkk1d/d Mice
To confirm that the observed results were caused by reduced Dkk1 signaling, we treated wild-type mice with an inhibitory Dkk1 antibody (Supplementary Figure 7A). In good agreement with the data from Dkk1d/d mice, daily treatment with the Dkk1 antibody for 1 week led to a significant increase in IEC proliferation in the proximal colon compared with control animals, as evidenced by Ki67 staining (Figure 6A). Additionally, we observed highly significant crypt lengthening, which was restricted to the proximal colon (Figure 6B). To investigate the effect of Dkk1 inhibition on acute colitis and recovery, we first challenged mice with DSS for 7 days. As with the transgenic mice, there was no clinical or histologic benefit for animals treated with Dkk1 antibody (Supplementary Figure 7B–E). However, morphometric analysis revealed a significant increase in crypt length in the proximal and distal colon of mice treated with Dkk1 antibody, predominantly in highly inflamed areas of the mucosa (Figure 6C). In agreement with the data from Dkk1d/d mice, sections of the inflamed mucosa showed little to no staining for phospho-β-catenin Ser552 when Dkk1 was inhibited (Figure 6D). Finally, we examined mice that were allowed to recover from DSS-induced colitis for an additional 7 days. We observed a substantial clinical benefit during the recovery period for animals that were treated with Dkk1 antibody and an overall body weight increase throughout the entire observation period (Figure 6E and F). Taken together, these results show that anti-Dkk1 treatment faithfully recapitulates systemically reduced Dkk1 expression in Dkk1d/d mice and confirm that the Wnt antagonist regulates epithelial homeostasis in the intestine.
Figure 6.
Inhibition of Dkk1 phenocopies genetic Dkk1 reduction in vivo. Wild-type mice treated for 1 week with a functional Dkk1 antibody showed an increased number of Ki67-positive crypt cells (A) and increased crypt length (B) in the proximal colon. (C) DSS-induced colitis led to a significant crypt lengthening in mice receiving Dkk1 antibody for 1 week. (D) Immunohistochemistry revealed decreased β-catenin phosphorylation at S552 in anti-Dkk1 treated mice during acute colitis. Scale bars = 100 μm. (E) Clinical disease activity index and (F) relative body weight of mice that received DSS for 7 days, followed by 7 days of regular drinking water. Animals were treated daily with the indicated antibody. n = 5 per group. Quantitative data in A and B are derived from 2 to 3 mice per group and data in C from 6 mice per group. *P < .001, **P < .05.
Dkk1d/d Mice Do Not Show Increased Susceptibility to Colitis-Associated Cancers
Because we observed increased epithelial cell proliferation and crypt distortion in Dkk1d/d mice, we speculated that systemic Dkk1 depletion might increase the susceptibility to intestinal cancers in chronic inflammation. We therefore challenged mice with azoxymethane and repeated cycles of DSS (Supplementary Figure 8A).21 Surprisingly, we observed rapid weight loss following azoxymethane treatment in the Dkk1d/d group, presumably due to increased hepatotoxicity of the drug (data not shown), which forced us to kill several of these animals. At the end of the observation period, all control animals had developed prominent colorectal adenocarcinomas and dysplastic foci (Supplementary Figure 8B–D). In striking contrast, no dysplasia or tumors were observed in the surviving Dkk1d/d mice. Histologic examination of mucosal sections showed residual inflammatory infiltrate in all animals; however, we observed increased epithelial regeneration in Dkk1d/d mice, as indicated by a reduced nuclei-cytoplasm ratio and a loss of goblet cells (Supplementary Figure 8E). Although the low number of animals in this experiment precludes us from reaching a definitive conclusion at this point, these results suggest that loss of Dkk1 does not increase the susceptibility to colitis-associated cancers.
Discussion
Previous reports from our laboratory and others have shown that the secreted Wnt antagonist Dkk1 is induced by inflammatory cytokines and that it exacerbates intestinal inflammation.5,12,13 These observations prompted us to investigate the physiologic role of Dkk1 during normal intestinal homeostasis and the recovery from mucosal injury. We found that in doubleridge mice with systemically reduced Dkk1 expression, β-catenin transcriptional activity and IEC proliferation were markedly increased, despite low basal expression of this Wnt antagonist in the intestine.22 This subtle imbalance in epithelial cell turnover was sufficient to cause a significant lengthening of colonic crypts. Remarkably, this phenotype was restricted to the proximal colon in naïve animals, whereas there were no differences in the small or distal large intestine. This discrepancy may be attributed to a differential responsiveness of segments of the digestive tract to Dkk17 or to the relative abundance of other Wnt agonists and antagonists in these tissues.22 Indeed, we and others have found that a number of Wnt signaling-related molecules are differentially expressed in the proximal and distal colon. These include Wnt agonists such as Wnt5a and Wnt1123,24 and, interestingly, Wnt/Dkk coreceptor LRP6, among others (S.K. and A.N., unpublished data November 2010). We therefore believe that the proximal colon may constitute a unique signaling niche that is more sensitive to Dkk1 signaling; however, this hypothesis remains to be addressed in future studies. Taken together, these observations identify Dkk1 as a novel regulator of normal tissue homeostasis in the adult organism. Furthermore, our data show that Dkk1 is required for the maintenance of epithelial morphology during intestinal inflammation. We observed that in the absence of Dkk1, there was a dramatic increase in crypt length compared with control mice, which was most striking in areas with histologically severe inflammation, which harbor particularly proliferative crypts.5,25
It is of considerable interest to determine the cellular source of mucosal Dkk1 during inflammation. Previous reports have localized Dkk1 to IECs,13 mammary epithelial cells,26 macrophages,5 osteoblasts,27 synovial cells,12 chon-drocytes,12 platelets,28 myofibroblasts,29 and endothelial cells.30 These data suggest that the inhibitor can be expressed by a wide variety of cell types, which is consistent with the observation that Dkk1 is a transcriptional target of the pleiotropic β-catenin/TCF signaling axis.31 Here we report that during acute inflammation, Dkk1 is strongly induced in a number of different cell types, in particular T lymphocytes and platelets. Interestingly, we also observed robust Dkk1 messenger RNA expression in dendritic cells, whose activity has recently been shown to be regulated by Wnt/β-catenin.32 These results strongly support that Dkk1 expression is induced in multiple cell types during intestinal inflammation and regulates epithelial homeostasis during acute colitis and recovery.
Investigating signaling pathways that regulate epithelial cell proliferation and apoptosis during intestinal inflammation and wound repair, we observed that suppression of Dkk1 function was associated with reduced AKT/β-catenin activity during acute inflammation. Phosphorylation of β-catenin by AKT leads to its transcriptional activation and has been suggested to induce crypt branching initiated by quiescent progenitor cells.9,33,34 Recent studies have indicated that activation of these inducible progenitors is involved in epithelial hyperproliferation and dysplasia in chronic colitis.35,36 In the current study, we found that proliferating IECs with AKT-mediated phosphorylation of β-catenin were rarely observed in acutely inflamed tissues when Dkk1 was systemically reduced or inhibited but were common in architecturally irregular crypts during the recovery from experimental colitis. Although we were not able to identify a mechanism by which Dkk1 may regulate AKT activity, the biphasic effect of Dkk1 depletion on the activity of AKT and β-catenin during acute inflammation and recovery suggests that this could be a secondary effect caused by changes in crypt epithelial homeostasis. This hypothesis remains to be addressed in future studies. In addition to the changes in AKT/β-catenin signaling, we saw a striking dys-regulation of mitogen-activated protein kinase signaling pathways in Dkk1d/d mice during epithelial restitution.
Taken together, these observations suggest that reduction of Dkk1 expression regulates epithelial homeostasis, primarily by enhancing β-catenin signaling, and that multiple pro-survival pathways may control epithelial cell survival and crypt remodeling during epithelial restitution. We conclude that Dkk1 is a critical regulator of epithelial homeostasis in the large intestine. Inflammation induces Dkk1 expression in a variety of cell types to regulate epithelial cell proliferation and crypt morphology during acute colitis.
Supplementary Material
Acknowledgments
The authors thank Drs M. Meisler and J. Kearney for providing the doubleridge mice, Amgen Inc for the Dkk1 antibody, and K. den Beste for technical assistance.
Funding
Supported by grants from the National Institutes of Health (DK 061379, DK 072564, and DK 079392 to C.A.P.; DK 055679 to A.N.), the Crohn’s & Colitis Foundation of America (to S.K.), and the American Gastroenterological Association (to P.N.).
Abbreviations used in this paper
- BrdU
5-bromo-2′-deoxyuridine
- DSS
dextran sulfate sodium
- IEC
intestinal epithelial cell
Footnotes
Conflicts of interest
The authors disclose no conflicts.
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.03.043.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 3.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 4.Glinka A, Wu W, Delius H, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 5.Nava P, Koch S, Laukoetter MG, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- 9.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning TL, Wang YC, Patel SR, et al. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 11.Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 12.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 13.Koch S, Capaldo CT, Samarin S, et al. Dkk-1 inhibits intestinal epithelial cell migration by attenuating directional polarization of leading edge cells. Mol Biol Cell. 2009;20:4816–4825. doi: 10.1091/mbc.E09-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Lu W, King TD, et al. Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One. 2010;5:e11014. doi: 10.1371/journal.pone.0011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissmuller T, Campbell EL, Rosenberger P, et al. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J Clin Invest. 2008;118:3682–3692. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng H, Shao J, Townsend CM, Jr, et al. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo F, Brooks DG, Ye H, et al. Mutated K-ras(Asp12) promotes tumourigenesis in Apc(Min) mice more in the large than the small intestines, with synergistic effects between K-ras and Wnt pathways. Int J Exp Pathol. 2009;90:558–574. doi: 10.1111/j.1365-2613.2009.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Owen CR, Sanders MA, et al. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology. 2006;131:1179–1189. doi: 10.1053/j.gastro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Hu LL, Gonzalez-Navajas J, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancho R, Nateri AS, de Vinuesa AG, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 22.Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Tai CC, Sala FG, Ford HR, et al. Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res. 2009;156:278–282. doi: 10.1016/j.jss.2009.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lickert H, Kispert A, Kutsch S, et al. Expression patterns of Wnt genes in mouse gut development. Mech Dev. 2001;105:181–184. doi: 10.1016/s0925-4773(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 25.Renes IB, Verburg M, Van Nispen DJ, et al. Epithelial proliferation, cell death, and gene expression in experimental colitis: alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int J Colorectal Dis. 2002;17:317–326. doi: 10.1007/s00384-002-0409-4. [DOI] [PubMed] [Google Scholar]
- 26.Forget MA, Turcotte S, Beauseigle D, et al. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Ueland T, Otterdal K, Lekva T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 29.Hughes KR, Sablitzky F, Mahida YR. Expression profiling of Wnt family of genes in normal and inflammatory bowel disease primary human intestinal myofibroblasts and normal human colonic crypt epithelial cells. Inflamm Bowel Dis. 2011;17:213–220. doi: 10.1002/ibd.21353. [DOI] [PubMed] [Google Scholar]
- 30.Smadja DM, d’Audigier C, Weiswald LB, et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol. 2010;30:2544–2552. doi: 10.1161/ATVBAHA.110.213751. [DOI] [PubMed] [Google Scholar]
- 31.Niida A, Hiroko T, Kasai M, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 32.Manicassamy S, Reizis B, Ravindran R, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 34.Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JB, Lee G, Managlia E, et al. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology. 2010;138:595–605. doi: 10.1053/j.gastro.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.