Abstract
The effects of La Crosse virus (LACV) infection on blood feeding behavior in Aedes triseriatus (Say) and Aedes albopictus (Skuse) were investigated in the laboratory by measuring the size of the bloodmeal imbibed and the extent of refeeding by virus-infected and uninfected mosquitoes. LACV-infected Ae. triseriatus and Ae. albopictus took significantly less blood compared with uninfected mosquitoes. Twice as many virus-infected Ae. triseriatus mosquitoes refed compared with uninfected individuals (18 vs. 9%; P < 0.05); however, virus infection had no significant effect on the refeeding rate of Ae. albopictus. Reduction in bloodmeal size followed by an increased avidity for refeeding may lead to enhanced horizontal transmission of the LACV by its principal vector, Ae. triseriatus.
Keywords: Aedes triseriatus, Aedes albopictus, La Crosse virus, bloodmeal, refeeding
La Crosse encephalitis (Family Bunyaviridae, Genus Bunyavirus, CA serogroup, LACV) is the most common and important endemic mosquito-borne disease of children in the United States (Rust et al. 1999). The majority of human cases have occurred in the upper Midwestern states with more recent cases being reported in West Virginia and Tennessee (Jones et al. 1999). Estimates of the number of infections annually have been as high as 300,000, with the majority of the cases being undiagnosed because of the mild symptoms (Rust et al. 1999).
The principal vector of LACV is Aedes triseriatus (Say) (Pantuwatana et al. 1974). In the laboratory, Ae. triseriatus has demonstrated high-transovarial transmission and filial infection rates of LACV (Miller et al. 1977, Hughes et al. 2006). Recent findings of naturally infected Aedesalbopictus (Skuse) have implicated this species as a possible accessory vector of LACV (Gerhardt et al. 2001). In laboratory studies, Ae. albopictus was a more competent vector of LACV than Ae. triseriatus (Grimstad et al. 1989); however, it is unlikely for Ae. albopictus to be efficient in horizontal amplification of LACV because of its broad host range (Niebylski et al. 1994).
Alteration of feeding behavior of virus-infected mosquitoes can result in enhanced transmission (Kuno and Chang 2005). For example, Aedes aegypti (L.) mosquitoes infected with dengue-3 virus required 10 min longer or more to complete feeding compared with noninfected individuals (Platt et al. 1997). LACV infection also has been shown to modify the feeding behavior of Ae. triseriatus. Grimstad et al. (1980) described increased probing behavior and reduced rates of engorgement by several strains of orally infected Ae. triseriatus. The impairment seen in LACV-infected mosquitoes does not appear to result from decreased salivary gland function (Paulson et al. 1992), because mosquitoes that probed without feeding successfully transmitted virus (Grimstad et al. 1980). If alteration of the ability to blood feed results in increased host avidity or host-seeking, the frequency of host contact would be increased, thus enhancing horizontal transmission.
The purpose of the current study was to determine the effect of LACV infection on the blood feeding behavior of Ae. triseriatus and Ae. albopictus by measuring bloodmeal size and the propensity for refeeding.
Materials and Methods
Mosquito Species
Ae. triseriatus adult females were reared in the laboratory from eggs collected from Blacksburg, VA, in 2006 and 2007. Ae. albopictus adults were obtained from eggs collected in Wise County, VA, in 2006 and subsequently maintained as a laboratory colony. Mosquitoes were maintained at 24°C, 75% relative humidity (RH), and a photoperiod of 16:8 (L:D) h. To ensure uniformity in adult size, larvae were reared at a density of 250 larvae per container (33 × 17.5 × 11 cm) with 1,600 ml deionized water (DI), and fed a bovine liver powder solution (7.5 g/500 ml). The maximum likelihood estimation of infection rate of LACV for Ae. triseriatus in the county where our eggs were collected was 0.15/1,000 (95% CL = 0.01–0.72) based on adults reared from eggs collected in 2005 (B.T.J. and S.L.P., unpublished data).
Virus Isolates and Assays
The LACV isolate (VA0921075) used for the study came from adult Ae. triseriatus mosquitoes collected from Wise County, VA, in 1999 (Barker et al. 2003). The isolate was maintained in the laboratory by alternate passage through Ae. triseriatus mosquitoes and Vero cells before being used in this study. The titer of the stock virus was 1.0 × 108 plaque forming units (PFU) per milliliter.
Virus titers in mosquitoes were determined by plaque assay on Vero cells following the methods of Barker et al. (2003). To determine the average dose of virus delivered, five mosquitoes from each replicate were frozen immediately after inoculations and assayed. The mean inoculation dosages for Ae. triseriatus and Ae. albopictus were 3.7 × 103 and 3.4 × 103 PFU per mosquito, respectively. To ensure that virus infection in inoculated mosquitoes was consistent, five mosquitoes were frozen after the behavioral studies and later assayed for the virus. All inoculated mosquitoes were positive for LACV with mean titers of 2.2 × 106 PFU/mosquito for Ae. triseriatus and 3.3 × 106 PFU/mosquito for Ae. albopictus. All control mosquitoes, five from each replicate, were negative for LACV.
Virus Infection of Mosquitoes
For each replicate, ≈50 3-d old adult female mosquitoes were injected intrathoracically with 0.5 ml of the LACV and another 50 with a control medium using the methods of Rosen and Gubler (1974). The inoculation method ensured 100% infection and that individuals received similar infecting doses. Because control mosquitoes were inoculated, the possibility of trauma affecting feeding was controlled. Inocula consisted of LACV in M199 cell medium [(500 ml M199, 27 ml fetal bovine serum, 10 ml penicillin/streptomycin (5,000 IU/ml), 0.5 ml gentamycin (50 mg/ml), and 2 ml Amphotericin B (250 μl/ml)]; the control group was injected with only M199 cell medium. Mosquitoes were then placed in 5,000 ml plastic buckets with a screened top and fabric sleeve on the side, and held for 1 wk in the same chamber as our colonies. Cotton balls soaked in a 10% sugar solution were placed on top of the cage as a food source. Two days before the start of the experiment, the sugar source was removed and replaced with cotton balls soaked in DI water. Between 5–10% of the mosquitoes injected did not survive the 1 wk incubation period.
Measurement of Bloodmeal Size
A laboratory mouse was anesthetized with 15 ml of a ketamine/xylazine solution [9 parts Ketamine (100 mg/ml), 9 parts Xylazine (100 mg/ml), and 3 parts Acepromazine (10 mg/ml) to 70 parts saline], placed on top of the cage, and covered with a damp paper towel. All feedings were conducted in the colony chamber. Separate mice were used for the virus and control cages for each replication. After 20 min, the cage was checked to determine whether any mosquitoes were actively feeding. If there were any individuals still feeding, then the mouse was left on the cage until all feeding ceased. Ae. triseriatus females were held in the cage for 20 min after feeding, whereas Ae. albopictus females were held for at least 2 h to allow the blood to become more congealed and less likely to escape the gut during dissections (M. J. Klowden, personal communication). Adults were then removed with a hand-held aspirator and placed on ice until dissection.
The methods of Briegel et al. (1979) were used to determine bloodmeal size based on the conversion of hemoglobin into hemiglobincyanide (HiCN). Individual bloodmeals were removed and placed in a 1.5 ml eppendorf tube containing 1.0 ml of Drabkin’s solution. After the bloodmeals were thoroughly ground and incubated at room temperature for at least 1 h, a 200 μl sample was transferred to a FisherBrand 96-well flat bottom plate (Fisher, Pittsburgh, PA). Test samples were run in quadruplicate. A standard curve was developed for each mouse because the hemoglobin titer differed among animals. This curve allowed us to equate the optical density (OD) of a bloodmeal with its blood volume in microliters and was created by adding 20 μl of mouse blood to 2 ml of reagent and producing a dilution series (10, 8, 7, 5, 2.5, 1.25, 0.625, and 0.3125 μl) that was run in triplicate. Plates were read on a Dynex TRIAD Series Multimode Detector using Concert-TRIAD Series software (version 2.0.0.11) (Dynex Technologies, Inc., Chantilly, VA) at an absorbance scan of 540 nm to determine the OD for each sample. All samples were assayed on the day of feeding; none were frozen for later testing. Because wing length is directly correlated with body weight (Christophers 1960), mosquito size was determined by measuring the wing length of each mosquito using a dissecting scope and an ocular micrometer according to Packer and Corbet (1989). The entire experiment was replicated three times.
Refeeding Study
After inoculation, the virus-infected group and uninfected control group were separated into three cages, each containing ≈15–20 mosquitoes. At 7 d postinfection an anesthetized mouse was placed inside each cage. Feedings commenced in the late afternoon to coincide with the natural feeding periods for each species (Haddow et al. 2009). A moistened paper towel and piece of Plexiglas were placed on top of the cage with a sufficient opening so that the number feeding could be recorded while in the colony chamber. After a 15-min feeding period, the mouse was removed from the cage and all mosquitoes were collected, chilled on ice, and examined on a light box through a dissecting scope for the presence of fresh blood. The number of mosquitoes that fed was recorded and all individuals were immediately placed back into the colony chamber. A comparison was made between the number visually seen feeding and the number with fresh blood in their abdomen to ensure all feedings were recorded. Mosquitoes that did not take a bloodmeal were kept in the cage and were allowed to feed during subsequent feedings. Blood feedings were repeated at 2 and 24 h intervals. There was the possibility for a mosquito to take its initial bloodmeal during the 2 h feeding and then refeed during the 24 h feeding. Any female with fresh blood in the abdomen that had taken a previous blood-meal was recorded as having refed. A fresh bloodmeal was distinguished from a previous bloodmeal through the use of the light box. The number of refeeding mosquitoes recorded at the 2 and 24 h intervals were combined for analysis. New mice were used during each feeding. This experiment was replicated three times.
Ethics
The maintenance and care of experimental animals in these studies complied with the National Institutes of Health guidelines for the humane use of laboratory animals. The feeding of mosquitoes upon laboratory mice was done under protocol 07-074-ENT.
Statistical Analysis
Bloodmeal size for virus-infected and uninfected control mosquitoes were compared separately for Ae. albopictus and Ae. triseriatus using a one-factor analysis of covariance (ANCOVA) with mosquito wing length as the covariate. The ANCOVA was considered valid if the assumption of homogeneity of slopes for the linear relationships of bloodmeal size and wing length for the treatment levels was met, as would be the case for a nonsignificant interaction (P>0.05) between the covariate and the treatment (Ott and Longnecker 2001, Petraitis et al. 2001). Following the ANCOVA, we developed a frequency table for virus-infected and control mosquitoes of each species based on the categories of bloodmeal size in 0.5 μl intervals, with the frequency for each bloodmeal size category represented by the number of females taking a bloodmeal. We then plotted the cumulative percent of the total number of females against the categories of bloodmeal size and fitted the data to a logistic model (Brown and Rothery 1993),
| [1] |
where Y(x) is the cumulative percent of females, x is bloodmeal size, a is a rate constant, and b is the size of the bloodmeal taken by at least 50% of females, that is, the median bloodmeal size. The fit of the data to equation 1 was carried out using nonlinear least squares regression in TableCurve 5.01(Systat Software Inc. 2002). We also estimated the 95 and 84% CIs for the median bloodmeal size, b, and determined significant differences in b between the treatments for each species by the failure of the 84% CIs to overlap (Payton et al. 2003, Julious 2004).
The data on female refeeding were analyzed using a 2 × 2 contingency analysis (Zar 1984). The significance level for all tests was α = 0.05. Statistical analyses were carried out using JMP 8.0.1 (SAS Institute 2008).
Results
Bloodmeal Size
The results of the ANCOVA on the bloodmeal size data showed that the interaction of treatment and wing length was not significant for both Ae. albopictus (F = 2.3; df = 1,250; P = 0.13) and Ae. triseriatus (F = 0.01; df = 1, 235; P = 0.94). The assumption of homogeneity of slopes was therefore met in both cases. The effect of the covariate, wing length, on the bloodmeal size was also significant for both Ae. albopictus (F = 73.2; df = 1, 250; P < 0.001) and Ae. triseriatus (F = 46.5; df = 1, 235; P < 0.01). Therefore, the body size of individual mosquitoes within each species, as measured by wing length, had a significant effect on bloodmeal size. The analysis further showed that after accounting for the body size, females of both Ae. albopictus and Ae. triseriatus females inoculated with LACV took a significantly smaller mean bloodmeal (F = 14.5; df = 1, 250; P < 0.001 and F = 10.5; df = 1, 235; P < 0.01, respectively) compared with the control group (Table 1).
Table 1.
Mean wing length and mean (±SE) bloodmeal size for Aedes albopictus and Aedes triseriatus mosquitoes parenterally infected with La Crosse virus
| Species | Treatment | n | Mean wing length (mm) | Mean bloodmeal (μl) |
|---|---|---|---|---|
| Aedes albopictus | Control | 121 | 3.28a | 3.36 (±0.056)a |
| Virus-infected | 133 | 3.29a | 3.07 (±0.053)b | |
| Aedes triseriatus | Control | 106 | 4.14a | 5.71 (±0.123)a |
| Virus-infected | 133 | 4.17a | 5.17 (±0.110)b |
For each of the species, means in the same column followed by the same lowercase letter are not significantly different (P > 0.05).
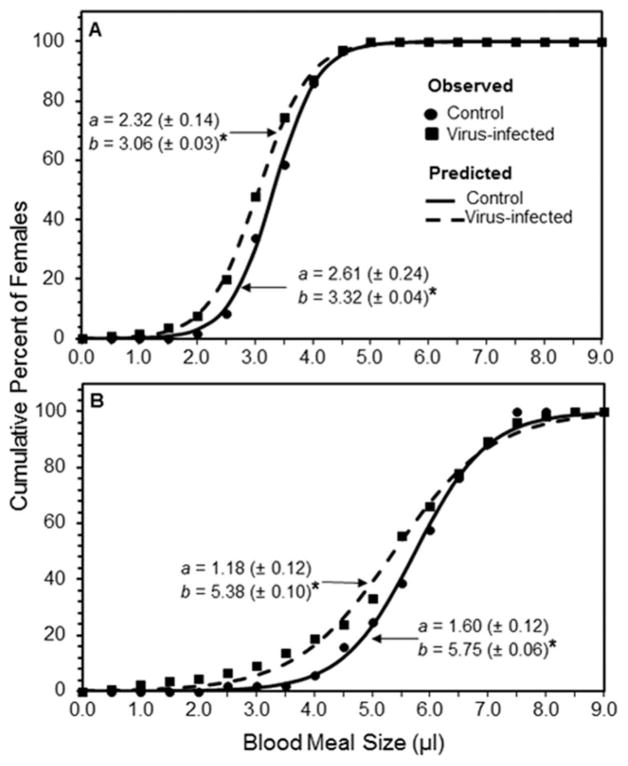
Figure 1A shows cumulative percentage of females of each species imbibing bloodmeals of varying sizes and the fit of the data to equation 1. For Ae. albopictus, 50% of females in the control and virus infected groups took bloodmeals of ≥3.32 μl and 3.06 μl, respectively. The nonoverlapping 84% CIs indicated a significant difference in median bloodmeal size between uninfected and virus-infected Ae. albopictus females.
Fig. 1.
Cumulative percent of La Crosse virus-infected and uninfected (control) female Aedes albopictus (A) and Aedes triseriatus (B) mosquitoes in relation to size of blood-meal (microliters). Data were fit to a logistic model, Y(x) = 100/(1 + e−a(x−b)) where a(±95% CL) is a rate constant and b (±95% CL) is the median bloodmeal size. R2 for all fitted curves is >0.99. Asterisk indicates a significant difference in the median bloodmeal size, b, between the treatment groups for each mosquito species based on nonoverlapping 84% CIs.
Figure 1B also shows that the median bloodmeal sizes for Ae. triseriatus females in the control and virus-infected groups were 5.75 and 5.38 μl, respectively. The nonoverlapping 84% CIs indicated that the median bloodmeal size for uninfected and virus-infected Ae. triseriatus females were significantly different.
Refeeding
No significant differences were detected in the proportion of uninfected (control) and virus-infected Ae. albopictus mosquitoes that refed (χ2 = 0.46; df = 1; P > 0.05). Of the 109 mosquitoes in the uninfected control group tested, 6% refed; only 8% of the 130 virus-infected mosquitoes returned for a bloodmeal.
A significantly greater proportion of virus-infected Ae. triseriatus females refed on a mouse (χ2 = 4.93; df = 1; P < 0.05) compared with the control group. Overall, 9% of 117 control females and 18% of 114 virus-infected Ae. triseriatus females refed.
Discussion
Our study showed that LACV infection affected blood feeding of both Ae. triseriatus and Ae. albopictus mosquitoes. Both species took smaller bloodmeals compared with uninfected siblings, and there was an increased probability of Ae. triseriatus mosquitoes taking multiple bloodmeals within a gonotrophic cycle. The latter increase in feeding frequency has the potential to enhance horizontal transmission.
Pathogen induced alterations of the feeding behavior of blood-feeding arthropods that result in an increase of transmission rate have been described for numerous parasite-vector systems (Schaub 2006). In mosquitoes, infection with Plasmodium affected the probing behavior of both laboratory infected (Rossignol et al. 1984, 1986) and naturally infected (Wekesa et al. 1992) individuals. Ae. aegypti infected with dengue virus resulted in extended periods of probing compared with uninfected ones (Platt et al. 1997).
Grimstad et al. (1980) reported increased probing frequency by LACV-infected Ae. triseriatus accompanied by reduced feeding success using visual assessment of the amount of blood imbibed. The HiCN method used in our study provided a quantitative measure of bloodmeal size that was unaffected by digestion or diuresis by the mosquito, and gives accurate readings up to 12 h postfeeding (Briegel et al. 1979). We also observed that LACV infection disrupted feeding behavior in both mosquito species with infected females desisting before they were fully engorged. The similar size of virus-infected and uninfected females, as determined by wing length, indicated that any differences in bloodmeal size were not the result of size differences in the mosquitoes. Although some viruses have been shown to be cytopathologic to salivary glands (Mims et al. 1966, Girard et al. 2005), LACV induced reduction in feeding efficacy is not the result of salivary gland impairment as Paulson et al. (1992) found no reduction in the ability of infected mosquitoes to locate blood and Grimstad et al. (1980) showed that mosquitoes that probed without feeding were able to transmit virus.
Many arboviruses have been shown to be neurotropic in the mosquito vector (Beaty and Thompson 1976, Girard et al. 2004) leading to speculation that altered feeding behavior in infected mosquitoes may be because of virus effects on the mosquito nervous system (Grimstad et al. 1980, Platt et al. 1997). In particular, neural disruption of the abdominal ganglia may be related to early termination of feeding because stretch receptors in the abdomen signal the brain that an adequate amount of blood has been imbibed to produce a batch of eggs (Gwadz 1969). These same stretch receptors also inhibit host-seeking behavior (Klowden and Lea 1979) and may explain the increased refeeding activity seen in infected Ae. triseriatus females. It is likely that virus infection impacts one or both of the endogenous regulatory mechanisms controlling host-seeking behavior after a bloodmeal (Klowden 1981, Klowden and Lea 1979). First, the distention caused by the bloodmeal triggers abdominal stretch receptors and then later a humoral factor is produced by vitellogenic females. Together, these mechanisms result in inhibition of host-seeking between a feeding event and subsequent oviposition.
A method of estimating the transmission rate by a vector is the vectorial capacity, the number of new infections delivered by a vector per case per day (Fine 1981). The equation for vectorial capacity, as modified for arboviruses, is C = ma2VPn/−log P (Reisen 1989) where m is the density of mosquitoes per host, a is number of bloodmeals taken on hosts per vector per day, V is vector competence, n is the extrinsic incubation period, and P is the probability of daily survival. This equation demonstrates that vectorial capacity of populations is dynamic and draws together aspects of what is known about the vector-host-pathogen relationship (Black and Moore 2005) including the biting habit of the infected vector. Our refeeding study with Ae. triseriatus showed that twice as many virus-infected females fed multiple times in a 24-h period compared with controls. This altered feeding behavior is likely to result in multiple contacts with competent hosts within one gonotrophic cycle thereby increasing vectorial capacity. Infected Ae. albopictus females imbibed significantly smaller bloodmeals than uninfected siblings but there was no concomitant increase in refeeding activity. It is possible that although the volume of blood was reduced, it still exceeded the threshold required to suppress host-seeking by this species (Klowden and Lea 1978).
Alteration of vector feeding behavior by a virus in a manner that leads to increased host contact will ultimately increase the probability of pathogen transmission. We have demonstrated this effect in the La Crosse virus–Ae. triseriatus system, but it is interesting to note that similar effects on vector behavior have been seen with other bunyaviruses. Turell et al. (1985) described reduced refeeding success in Rift Valley fever virus-infected Culex pipiens L. and Stafford et al. (2011) found that thrips (Frankliniella occidentalis) infected with Tomato spotted wilt virus demonstrated altered feeding behavior including nearly a three-fold increase in noningestion probes by infected males. These three viruses are members of different genera in the Family Bunyaviridae suggesting that alteration of vector feeding behavior is a conserved trait providing a selective advantage to the virus (Stafford et al. 2011). However, the manipulation of mosquito feeding behavior is not benign, as host defensive behavior is a significant cause of mosquito mortality (Edman and Scott 1987) and decreased blood ingestion would lead to a reduction in egg production (Turell et al. 1985). Thus, a trait that provides an advantage to the virus may concurrently be detrimental to the vector resulting in a conflict of interest between the two entities.
Future studies will examine the physiological mechanisms of this feeding alteration. Novak and Rowley (1994) demonstrated that serotonin modulates blood feeding by Ae. triseriatus. We have found that serotonin levels in the brains of Ae. triseriatus is altered in LACV-infected specimens as compared with uninfected controls when measured by high performance liquid chromatography (S.L.P. and F. Yang, unpublished data). More research is needed to elucidate this mechanism. Understanding how vector-borne pathogens induce behavioral changes in the vector to enhance transmission could lead to the development of new control strategies (Lefèvre and Thomas 2008).
Acknowledgments
The authors would like to express gratitude to J. R. Bloomquist for the use of scientific equipment. This research was supported by investigator-initiated small research Grant A1059466 from the National Institute of Allergy & Infectious Diseases (NIAID), National Institutes of Health (NIH). Its content is solely the responsibility of the authors and does not represent the official views of NIAID or NIH.
References Cited
- Barker CM, Paulson SL, Cantrell S, Davis BS. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in Southwestern Virginia. J Med Entomol. 2003;40:403–410. doi: 10.1603/0022-2585-40.4.403. [DOI] [PubMed] [Google Scholar]
- Beaty BJ, Thompson WH. Delineation of La Crosse virus in developmental stages of transovarially infected Aedes triseriatus. Am J Trop Med Hyg. 1976;25:505–512. doi: 10.4269/ajtmh.1976.25.505. [DOI] [PubMed] [Google Scholar]
- Black WC, IV, Moore CG. Population biology as a tool to study vector-borne diseases. In: Marquardt WC, editor. Biology of Disease Vectors. Elsevier Academic; London, United Kingdom: 2005. pp. 187–206. [Google Scholar]
- Briegel H, Lea AO, Klowden MJ. Hemoglobinometry as a method for measuring blood meal sizes of mosquitoes (Diptera: Culicidae) J Med Entomol. 1979;15:235–238. doi: 10.1093/jmedent/15.5-6.514. [DOI] [PubMed] [Google Scholar]
- Brown D, Rothery P. Models in biology: mathematics, statistics, and computation. Wiley; West Sussex, England, United Kingdom: 1993. [Google Scholar]
- Christophers SR. Aedes aegypti (L.). The yellow fever mosquito. Cambridge University Press; London, United Kingdom: 1960. [Google Scholar]
- Edman JD, Scott TW. Host defensive behaviour and the feeding success of mosquitoes. Insect Sci Appl. 1987;8:617–622. [Google Scholar]
- Fine PEF. Epidemiological principles of vector mediated transmission. In: McKelvey JJ Jr, Eldridge BF, Maramorosch K, editors. Vectors of Disease Agents. Praeger Scientific; New York, NY: 1981. pp. 77–91. [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;7:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard YA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoon Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- Girard YA, Popov V, Wen J, Han V, Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2005;42:429–444. doi: 10.1093/jmedent/42.3.429. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Ross QE, Craig GB., Jr Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. II Modification of mosquito feeding behavior by virus infection. J Med Entomol. 1980;17:1–7. doi: 10.1093/jmedent/17.1.1. [DOI] [PubMed] [Google Scholar]
- Grimstad PR, Kobayashi JF, Zhang MB, Craig GB., Jr Recently introduced Aedes albopictus in the United States: potential vector of La Crosse virus (Bunyaviridae: California serogroup) J Am Mosq Control Assoc. 1989;5:422–427. [PubMed] [Google Scholar]
- Gwadz RW. Regulation of blood meal size in the mosquito. J Insect Physiol. 1969;15:2039–2044. doi: 10.1016/0022-1910(69)90071-7. [DOI] [PubMed] [Google Scholar]
- Haddow AD, Gerhardt RR, Jones CJ, Odoi A. The mosquitoes of eastern Tennessee: studies on abundance, habitat preferences, and host-seeking behaviors. J Vector Ecol. 2009;34:70–80. doi: 10.1111/j.1948-7134.2009.00009.x. [DOI] [PubMed] [Google Scholar]
- Hughes MT, Gonzalez JA, Reagan KL, Blair CD, Beaty BJ. Comparative potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to transovarially transmit La Crosse virus. J Med Entomol. 2006;43:757–761. doi: 10.1603/0022-2585(2006)43[757:cpoata]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jones TF, Craig AS, Nasci RS, Patterson LER, Erwin PC, Gerhardt RR, Ussery XT, Schaffner W. Newly recognized focus of La Crosse encephalitis in Tennessee. Clin Infect Dis. 1999;28:93–97. doi: 10.1086/515087. [DOI] [PubMed] [Google Scholar]
- Julious SA. Using confidence intervals around individual means to assess statistical significance between two means. Pharm Stat. 2004;3:217–222. [Google Scholar]
- Klowden MJ. Initiation and termination of host-seeking inhibition in Aedes aegypti during oocyte maturation. J Insect Physiol. 1981;27:799–803. doi: 10.1016/0022-1910(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.) Am J Trop Med Hyg. 1978;27:827–831. doi: 10.4269/ajtmh.1978.27.827. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Abdominal distention terminates subsequent host-seeking behaviour of Aedes aegypti following a blood meal. J Insect Physiol. 1979;25:583–585. doi: 10.1016/0022-1910(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Kuno G, Chang GJJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre T, Thomas F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne disease. Infect Genet Evol. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Miller BR, DeFoliart GR, Yuill TM. Vertical transmission of La Crosse virus (California encephalitis group): transovarial and filial infection rates in Aedes triseriatus(Diptera: Culicidae) J Med Entomol. 1977;14:437–440. doi: 10.1093/jmedent/14.4.437. [DOI] [PubMed] [Google Scholar]
- Mims CA, Day MF, Marshall ID. Cytopathic effects of Semliki Forest viruses in the mosquito Aedes aegypti. Am J Trop Med Hyg. 1966;15:775–784. doi: 10.4269/ajtmh.1966.15.775. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Savage HM, Nasci RS, Craig GB., Jr Blood hosts of Aedes albopictus in the United States. J Am Mosq Control Assoc. 1994;10:447–450. [PubMed] [Google Scholar]
- Novak MG, Rowley WA. Serotonin depletion affects blood-feeding but not host-seeking ability in Aedes triseritatus (Diptera: Culicidae) J Med Entomol. 1994;31:600–606. doi: 10.1093/jmedent/31.4.600. [DOI] [PubMed] [Google Scholar]
- Ott RL, Longnecker M. An introduction to statistical methods and data analysis. 5. Duxbury, Thompson Learning Inc; Pacific Grove, CA: 2001. [Google Scholar]
- Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae) Ecol Entomol. 1989;14:297–309. [Google Scholar]
- Pantuwatana S, Thompson WH, Watts DM, Yuill TM, Hanson RP. Isolation of La Crosse virus from field collected Aedes triseriatus larvae. Am J Trop Med Hyg. 1974;23:246–250. doi: 10.4269/ajtmh.1974.23.246. [DOI] [PubMed] [Google Scholar]
- Paulson SL, Poirier SJ, Grimstad PR, Craig GB., Jr Vector competence of Aedes hendersoni (Diptera: Culicidae) for La Crosse virus: lack of impaired function in virus-infected salivary glands and enhanced virus transmission by sporozoite-infected mosquitoes. J Med Entomol. 1992;29:483–488. doi: 10.1093/jmedent/29.3.483. [DOI] [PubMed] [Google Scholar]
- Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1093/jis/3.1.34. (insectscience.org/3.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraitis PS, Beaupre SJ, Dunham AE. ANCOVA. Nonparametric and randomization approaches. In: Scheiner M, Gurevitch J, editors. Design and Analysis of Ecological Experiments. Oxford University Press; New York, NY: 2001. pp. 116–133. 2nd div. [Google Scholar]
- Platt KB, Linthicum KJ, Myint KSA, Innis BL, Lerdthusnee K, Vaughn DW. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Estimation of vectorial capacity: introduction. Bull Soc Vector Ecol. 1989;14:39–40. [Google Scholar]
- Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- Rossignol PA, Ribeiro JMC, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- Rossignol PA, Ribeiro JMC, Spielman A. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1986;35:277–279. doi: 10.4269/ajtmh.1986.35.277. [DOI] [PubMed] [Google Scholar]
- Rust RS, Thompson WH, Mathews CG, Beaty BJ, Chun RWM. La Crosse and other forms of California Encephalitis. J Child Neurol. 1999;14:1–14. doi: 10.1177/088307389901400101. [DOI] [PubMed] [Google Scholar]
- SAS Institute. JMP version 8.0. SAS Institute; Cary, NC: 2008. [Google Scholar]
- Schaub GA. Parasitogenic alterations of vector behavior. Int J Med Microbiol. 2006;296:37–40. doi: 10.1016/j.ijmm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Stafford CA, Walker GP, Ullman DE. Infection with a plant virus modifies vector feeding behavior. Proc Natl Acad Sci USA. 2011;108:9350–9355l. doi: 10.1073/pnas.1100773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Systat Software Inc. User’s Manual. Systat Software Inc; Richmond, CA: 2002. TableCurve 2D, version 5.01 for Windows. [Google Scholar]
- Turell MJ, Gargan P, II, Bailey CL. Culex pipiens (Diptera: Culicidae) morbidity and mortality associated with Rift Valley fever virus infection. J Med Entomol. 1985;22:332–337. doi: 10.1093/jmedent/22.3.332. [DOI] [PubMed] [Google Scholar]
- Wekesa JW, Copeland RS, Mwangi RW. Effect of Plasmodium falciparum on blood feeding behavior of naturally infected Anopheles mosquitoes in Western Kenya. Am J Trop Med Hyg. 1992;47:484–488. doi: 10.4269/ajtmh.1992.47.484. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 2. Prentice-Hall; Englewood Cliffs, NJ: 1984. [Google Scholar]