Abstract
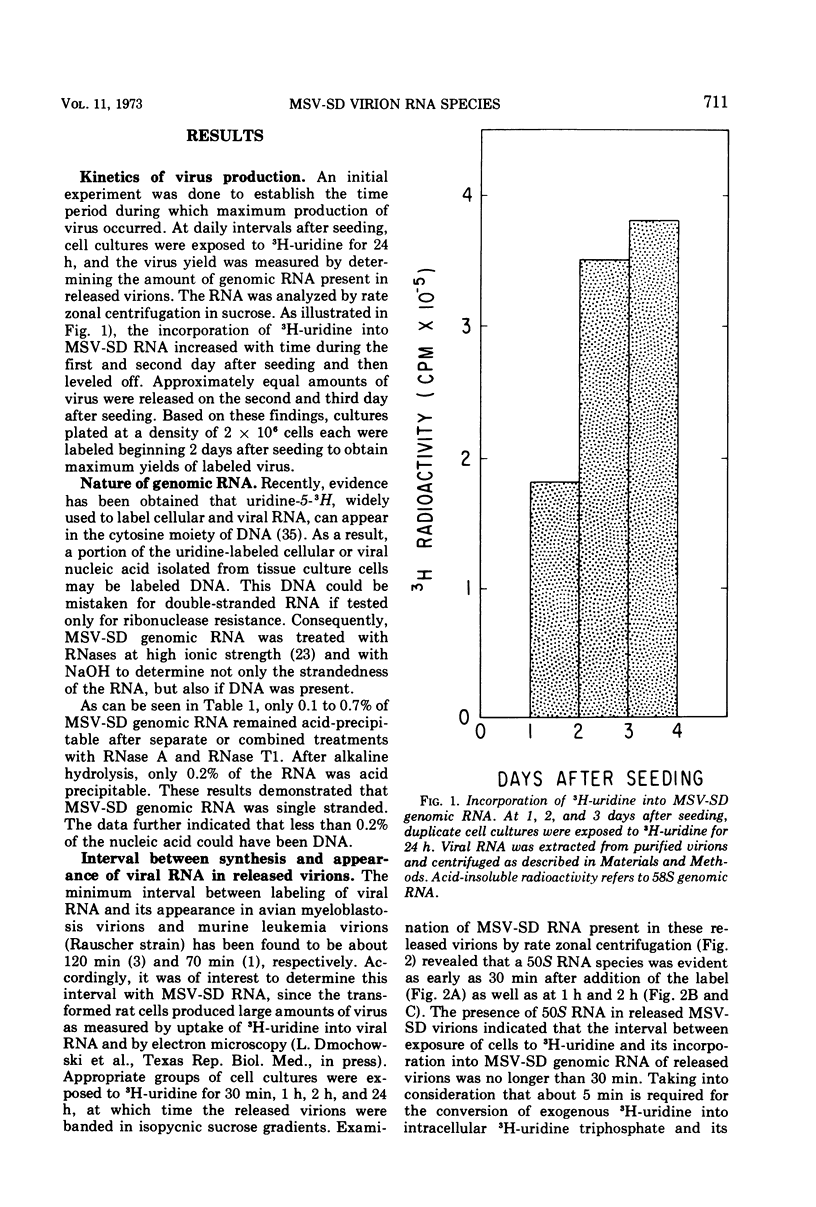
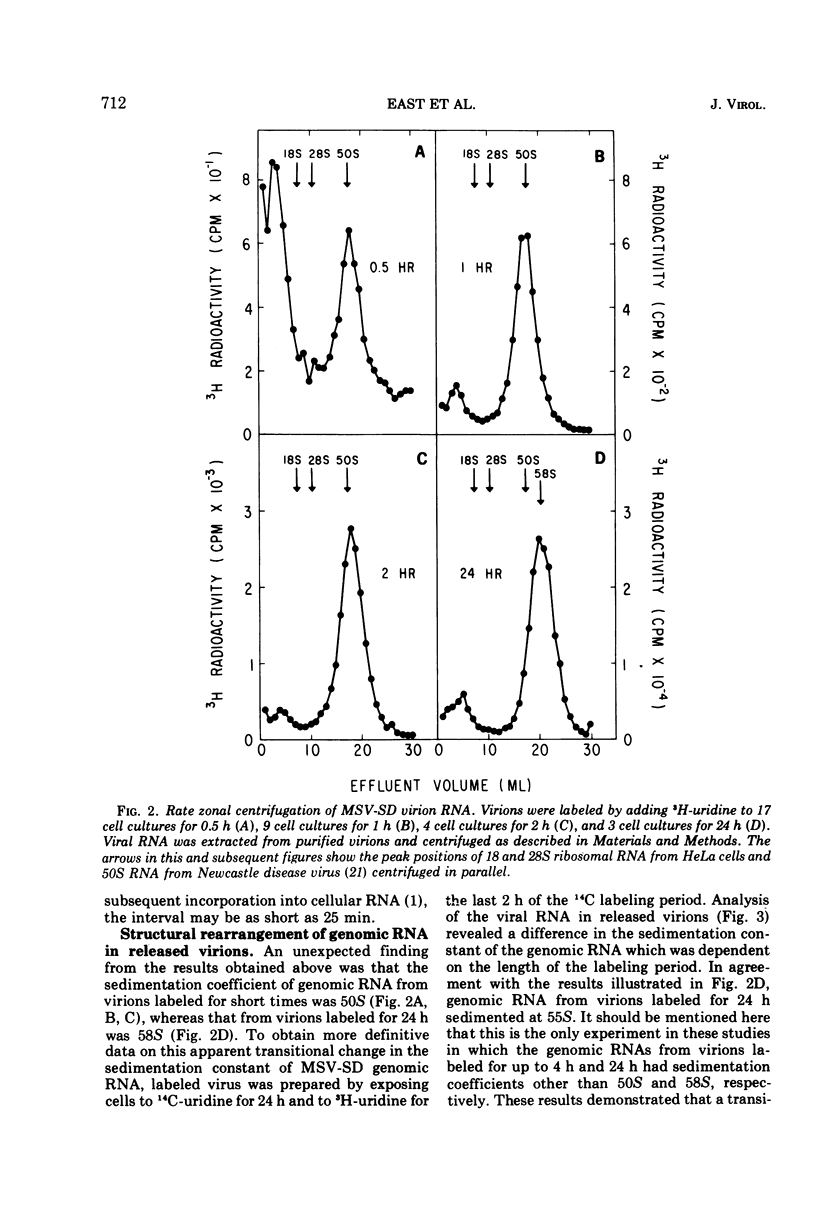
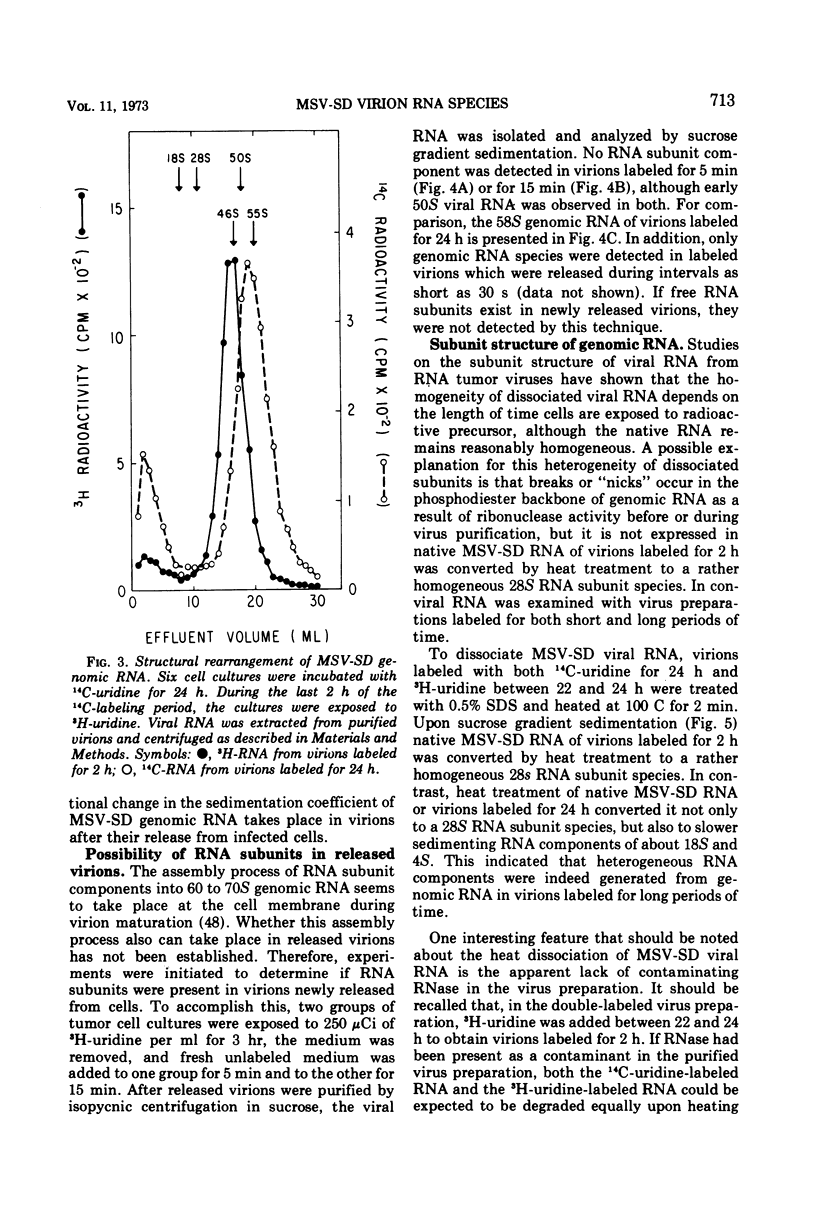
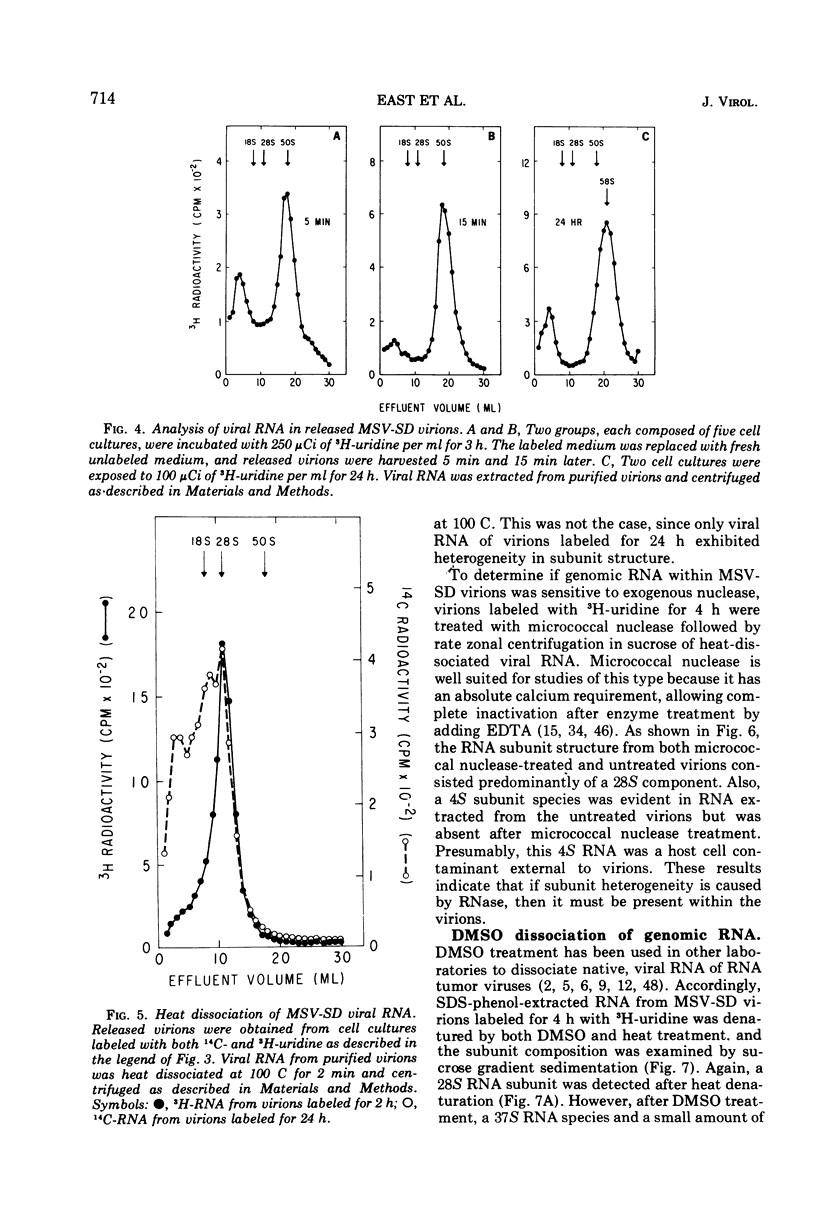
Two types of genomic, high-molecular-weight RNA species were found in Soehner-Dmochowski murine sarcoma virions released from virus-induced rat tumor cells grown in tissue culture. The type of RNA species observed depended on the length of exposure of the tumor cells to radioactive precursor. Early RNA of virions labeled up to 4 h with radioactive uridine had a sedimentation coefficient of 50S, and late RNA of virions labeled for 24 h had a sedimentation coefficient of 58S. Thermal transitions of early and late RNA indicated a difference in the configuration or structure of these two types of RNA. The late RNA may represent either a different configurational state of the early RNA or an aggregate molecule of two early RNA components joined together. Heat dissociation revealed that the major subunit of both RNA types was a 28S species, which was not susceptible to degradation by the addition of micrococcal nuclease to virions. A transitional, intermediate RNA species with a sedimentation coefficient of 37 to 40S was detected when early RNA was dissociated by dimethyl sulfoxide or heat at temperatures suboptimal for complete conversion. No free RNA subunit components were detected in virions harvested at intervals as short as 30 s or 5 min. A model for the assembly of genomic RNA from 28S RNA subunits is proposed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Weissmann C., Warner R. C. Replication of viral ribonucleic acid. IX. Properties of double-stranded RNA from Escherichia coli infected with bacteriophage MS2. J Mol Biol. 1966 May;17(1):145–173. doi: 10.1016/s0022-2836(66)80101-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. D., Duesberg P. H. Structure of Rauscher mouse leukaemia virus RNA. Nature. 1968 Oct 26;220(5165):396–399. doi: 10.1038/220396a0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowski L. Comparison of leukemogenic and sarcomagenic viruses at the ultrastructural level. Bibl Haematol. 1970;(36):62–82. doi: 10.1159/000391695. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Blair P. B. Isolation of the nucleic acid of mouse mammary tumor virus (MTV). Proc Natl Acad Sci U S A. 1966 Jun;55(6):1490–1497. doi: 10.1073/pnas.55.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Cardiff R. D. Structural relationships between the RNA of mammary tumor virus and those of other RNA tumor viruses. Virology. 1968 Dec;36(4):696–700. doi: 10.1016/0042-6822(68)90206-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. On the structure of RNA tumor viruses. Curr Top Microbiol Immunol. 1970;51:78–104. [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Pitts J. D., Whalley J. M., Clason A. E., Hay J. Isolation of the nucleic acid of feline leukemia virus. Virology. 1971 Jan;43(1):317–320. doi: 10.1016/0042-6822(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. I. Isolation and preliminary characterization of RNA from virus particles. J Mol Biol. 1966 Jun;18(1):195–203. doi: 10.1016/s0022-2836(66)80085-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. II. Preferential synthesis of RNA complementary to parental viral RNA by chick embryo cells. J Mol Biol. 1966 Jun;18(1):204–214. doi: 10.1016/s0022-2836(66)80086-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus complementary RNA: its relationship to the viral genome and its accumulation in the presence or absence of actinomycin D. Virology. 1967 Oct;33(2):227–233. doi: 10.1016/0042-6822(67)90141-9. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Manning J. S., Schaffer F. L., Soergel M. E. Correlation between murine sarcoma virus buoyant density, infectivity, and viral RNA electrophoretic mobility. Virology. 1972 Sep;49(3):804–807. doi: 10.1016/0042-6822(72)90537-5. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Mora P. T., McFarland V. W., Luborsky S. W. Isolation of the nucleic acid of Rauscher murine leukemia virus. Natl Cancer Inst Monogr. 1966 Sep;22:191–203. [PubMed] [Google Scholar]
- Naso R. B., Vidrine J. B., Wang C. S., Arlinglaus R. B. C-type virus particles produced by normal mouse spleen-thymus cultures. Virology. 1972 Feb;47(2):521–524. doi: 10.1016/0042-6822(72)90293-0. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi K. K. Nature of ribonuclease-resistant nucleic acid of chick embryo cells transformed by Schmidt-Ruppin strain of Rous Sarcoma Virus. Nat New Biol. 1971 Jan 6;229(1):25–27. doi: 10.1038/newbio229025a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Kingsbury D. W. Deoxycholate releases RNA from Rauscher murine leukemia virus. Proc Soc Exp Biol Med. 1970 Sep;134(4):1039–1042. doi: 10.3181/00379727-134-34939. [DOI] [PubMed] [Google Scholar]
- Soehner R. L., Dmochowski L. Induction of bone tumours in rats and hamsters with murine sarcoma virus and their cell-free transmission. Nature. 1969 Oct 11;224(5215):191–192. doi: 10.1038/224191a0. [DOI] [PubMed] [Google Scholar]
- Soehner R. L., Fujinaga S., Dmochowski L. Neoplastic bone lesions induced in rats and hamsters by Moloney and Harvey murine sarcoma viruses. Bibl Haematol. 1970;(36):593–599. doi: 10.1159/000391757. [DOI] [PubMed] [Google Scholar]
- Somers K., Kit S. Clonal isolation of murine sarcoma virus (MSV): characterization of virus produced from transformed cells. Virology. 1971 Dec;46(3):774–785. doi: 10.1016/0042-6822(71)90079-1. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S., Crosbie R. B., Hayes S. F., Keeler R. F. TRICINE-buffered tissue culture media for control of mycoplasma contaminants. Proc Soc Exp Biol Med. 1971 May;137(1):258–263. doi: 10.3181/00379727-137-35556. [DOI] [PubMed] [Google Scholar]
- VONHIPPEL P. H., FELSENFELD G. MICROCOCCAL NUCLEASE AS A PROBE OF DNA CONFORMATION. Biochemistry. 1964 Jan;3:27–39. doi: 10.1021/bi00889a006. [DOI] [PubMed] [Google Scholar]
- Watson J. D. The structure and assembly of murine leukemia virus: intracellular viral RNA. Virology. 1971 Sep;45(3):586–597. doi: 10.1016/0042-6822(71)90174-7. [DOI] [PubMed] [Google Scholar]
- Watson J., Ralph P., Sarkar S., Cohn M. Leukemia viruses associated with mouse myeloma cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):344–351. doi: 10.1073/pnas.66.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]



