Abstract
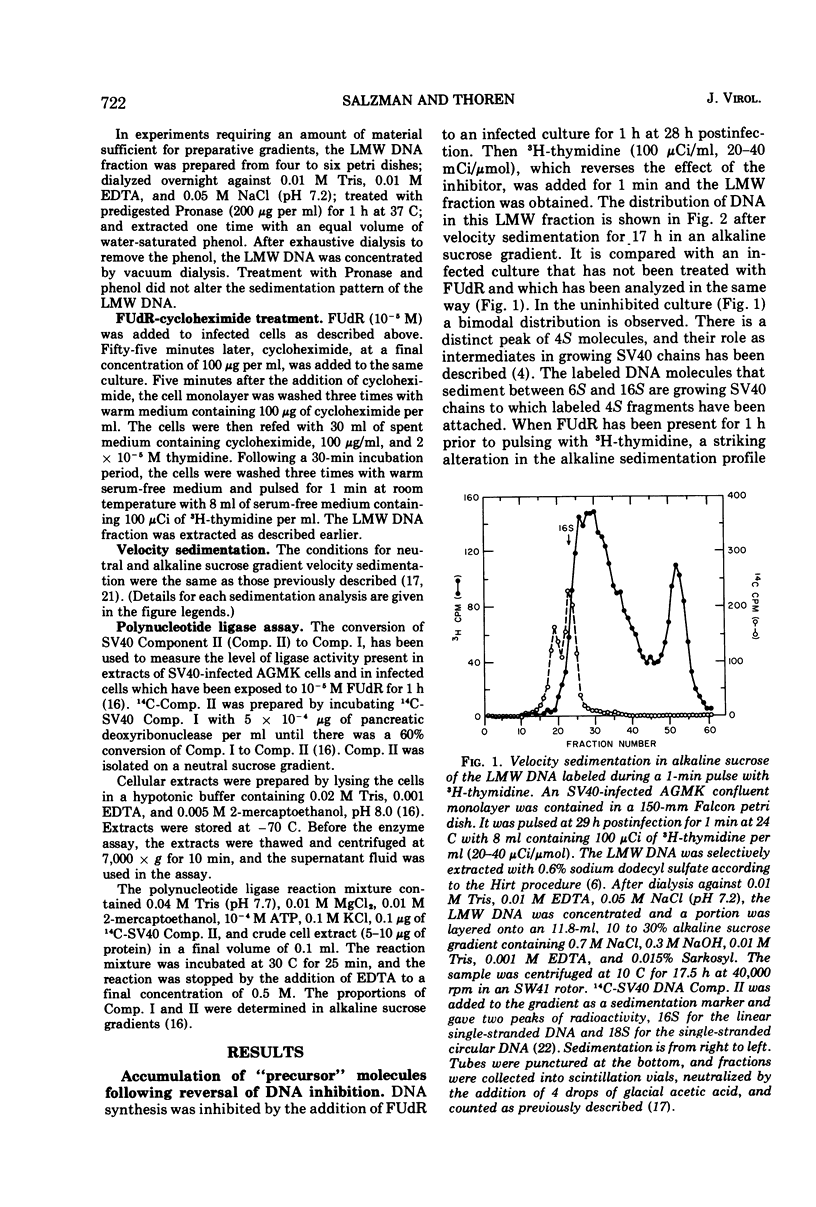
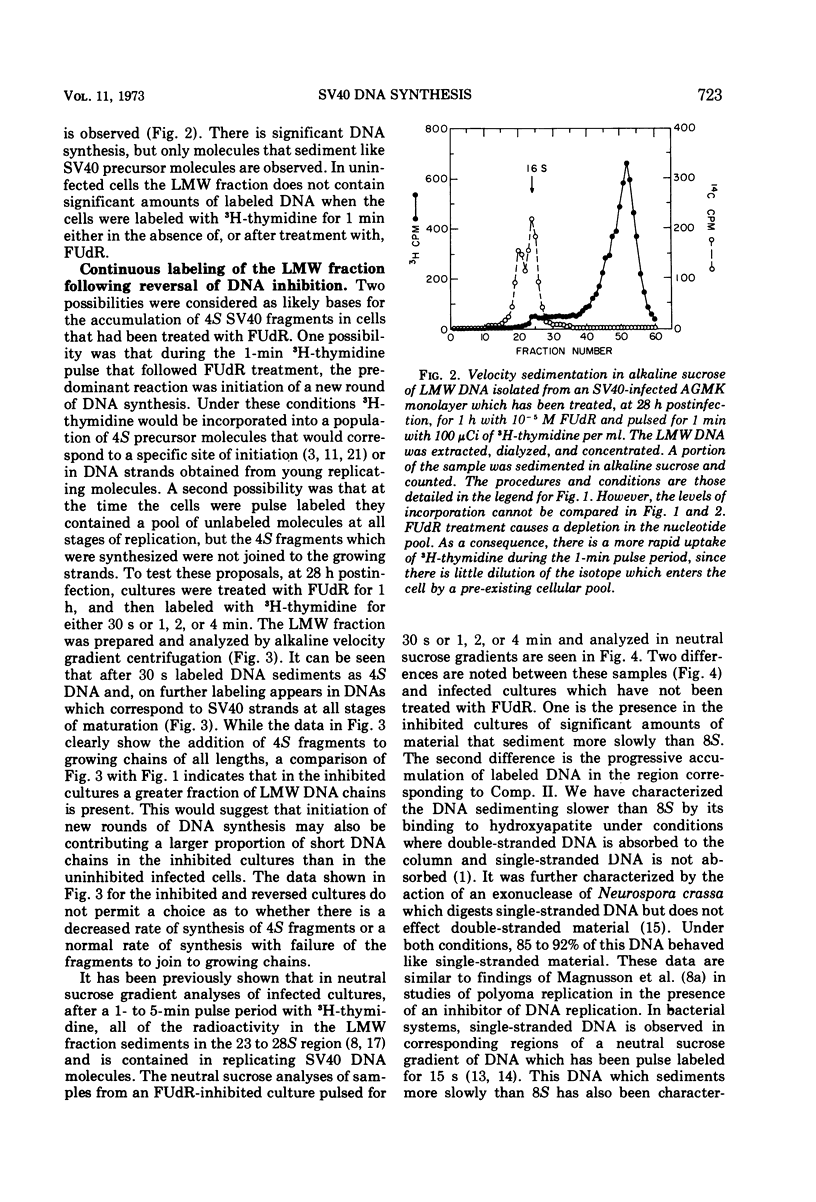
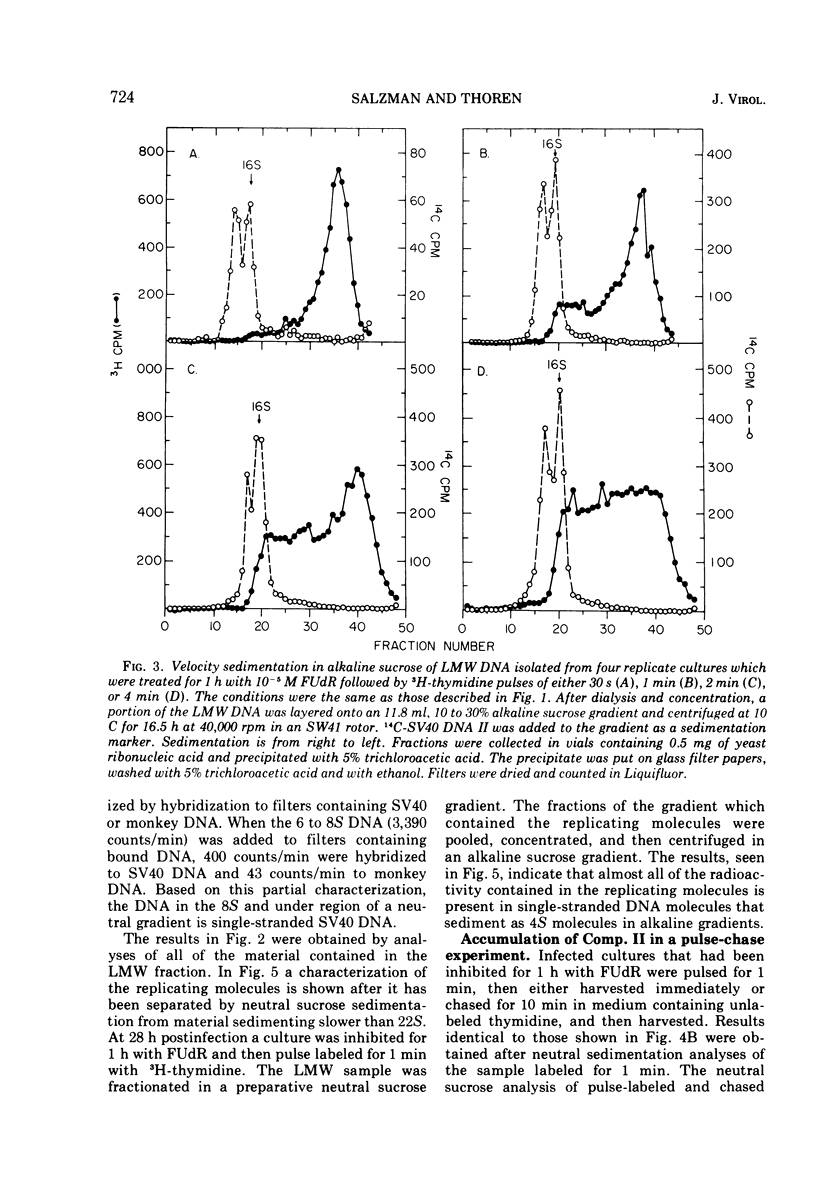
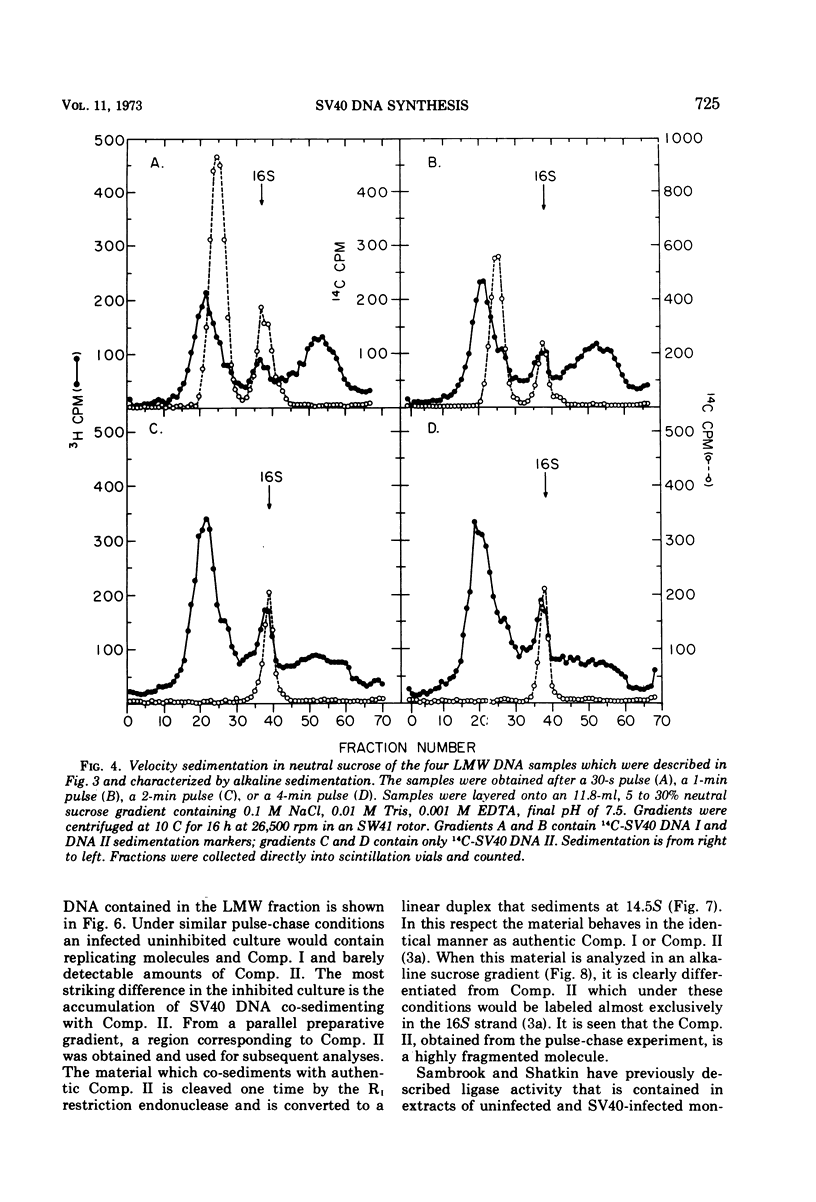
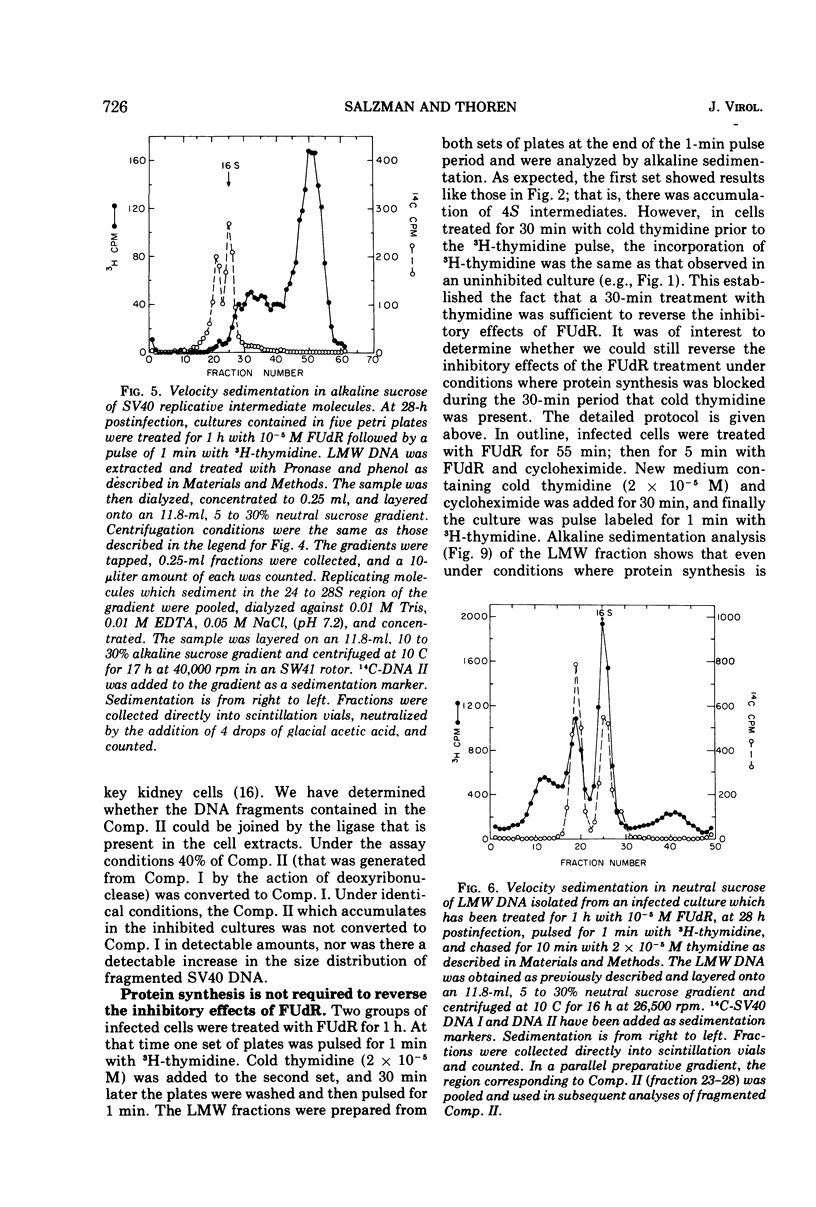
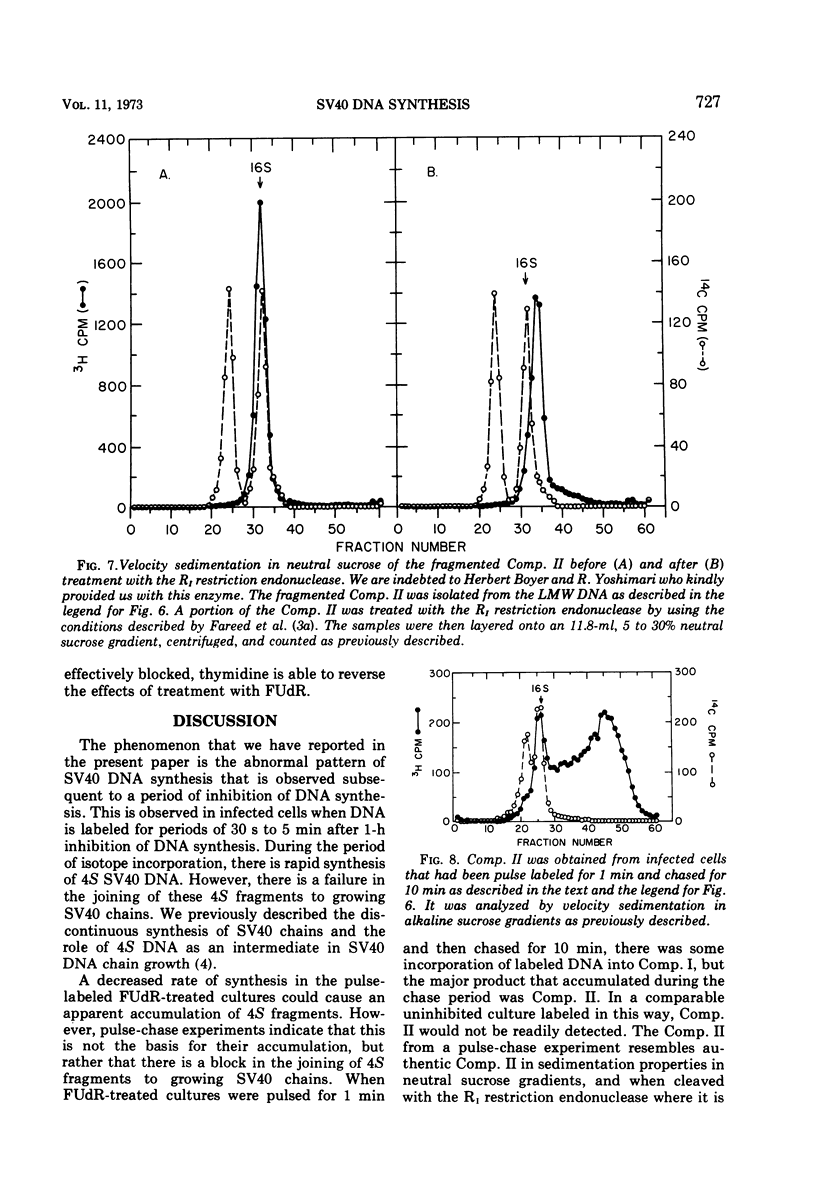
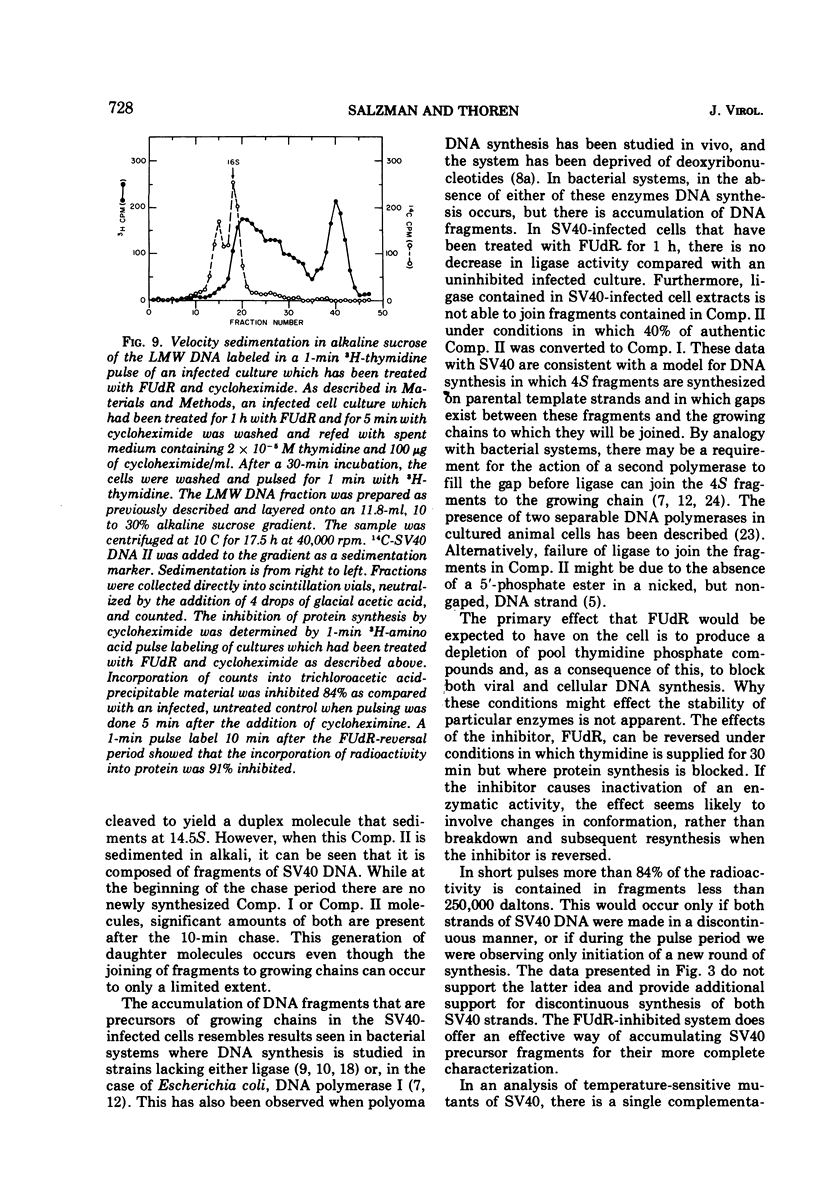
Viral DNA synthesis was inhibited for 1 h by the addition of 5-fluorodeoxyuridine (FUdR) to simian virus 40 (SV40)-infected cultures at 28 to 30 h postinfection. The subsequent addition of 3H-thymidine to the inhibited cultures reverses the effect of the inhibitor, and during a 1-min labeling period there is rapid synthesis of SV40 DNA. By alkaline sedimentation analysis, it is observed that in FUdR-treated cultures there is synthesis of 4S SV40 DNA intermediates but there is a block in the joining of these intermediates to growing SV40 chain cultures. In addition to 4S fragments that are associated with replicating SV40 molecules, there is accumulation of SV40 DNA in the 6 to 8S region which is observed in neutral sucrose gradients. In an inhibited culture that is pulsed for 1 min with 3H-thymidine and then chased for 10 min, accumulation of a Component II (Comp. II)-like material is observed. This Comp. II has the same neutral sedimentation characteristics and yields the same RI restriction endonuclease product as does authentic Comp. II. However, in alkali it is seen that it is composed of fragmented SV40 DNA. The basis for the failure of 4S fragments to join to growing SV40 chains is discussed. A model in which there is a requirement for two DNA polymerases and a ligase to permit SV40 DNA chain growth is proposed which is consistent with the data presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., Little J. W., Oshinsky C. K., Zimmerman S. B. Joining of DNA strands by DNA ligase of E. coli. Cold Spring Harb Symp Quant Biol. 1968;33:21–26. doi: 10.1101/sqb.1968.033.01.007. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Veomett G. E. A possible function of DNA polymerase in chromosome replication. Biochem Biophys Res Commun. 1970 Nov 25;41(4):973–980. doi: 10.1016/0006-291x(70)90180-4. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Frenkel G. D., Richardson C. C. A mutant of bacteriophage T7 deficient in polynucleotide ligase. J Biol Chem. 1971 Nov 25;246(22):6874–6879. [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IV. DNA synthesis in E. coli infected with ligase-negative mutants of phage T4. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1328–1335. doi: 10.1073/pnas.61.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. Z., Tenenhouse H., Fraser M. J. An exonuclease of Neurospora crassa specific for single-stranded nucleic acids. Biochim Biophys Acta. 1972 Jan 18;259(1):50–68. doi: 10.1016/0005-2787(72)90473-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Okazaki T., Okazaki R. Mechanism of DNA chain growth, II. Accumulation of newly synthesized short chains in E. coli infected with ligase-defective T4 phages. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1356–1362. doi: 10.1073/pnas.60.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren M. M., Sebring E. D., Salzman N. P. Specific initiation site for simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):462–468. doi: 10.1128/jvi.10.3.462-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Yudelevich A., Ginsberg B., Hurwitz J. Discontinuous synthesis of DNA during replication. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1129–1136. doi: 10.1073/pnas.61.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]



