Abstract
Thymidylate synthetase, which appears after infection of Escherichia coli with bacteriophage T4, has been partially purified. The phage enzyme is immunologically distinct from the host enzyme and has a molecular weight of 50,000 in comparison to 68,000 for the host enzyme. A system has been developed to characterize T4 td mutants previously known to have impaired expression of phage thymidylate synthetase. For this system, an E. coli host lacking thymidylate synthetase was isolated. Known genetic suppressors were transduced into this host. The resulting isogenic hosts were infected with phage T4 td mutants. The specific activities and amounts of cross-reacting material induced by several different types of phage mutants under conditions of suppression or non-suppression have been examined. The results show that the phage carries the structural gene specifying the thymidylate synthetase which appears after phage infection, and that the combination of plaque morphology, enzyme activity assays, and an assay for immunologically cross-reacting material provides a means for identifying true amber mutants of the phage gene.
Full text
PDF


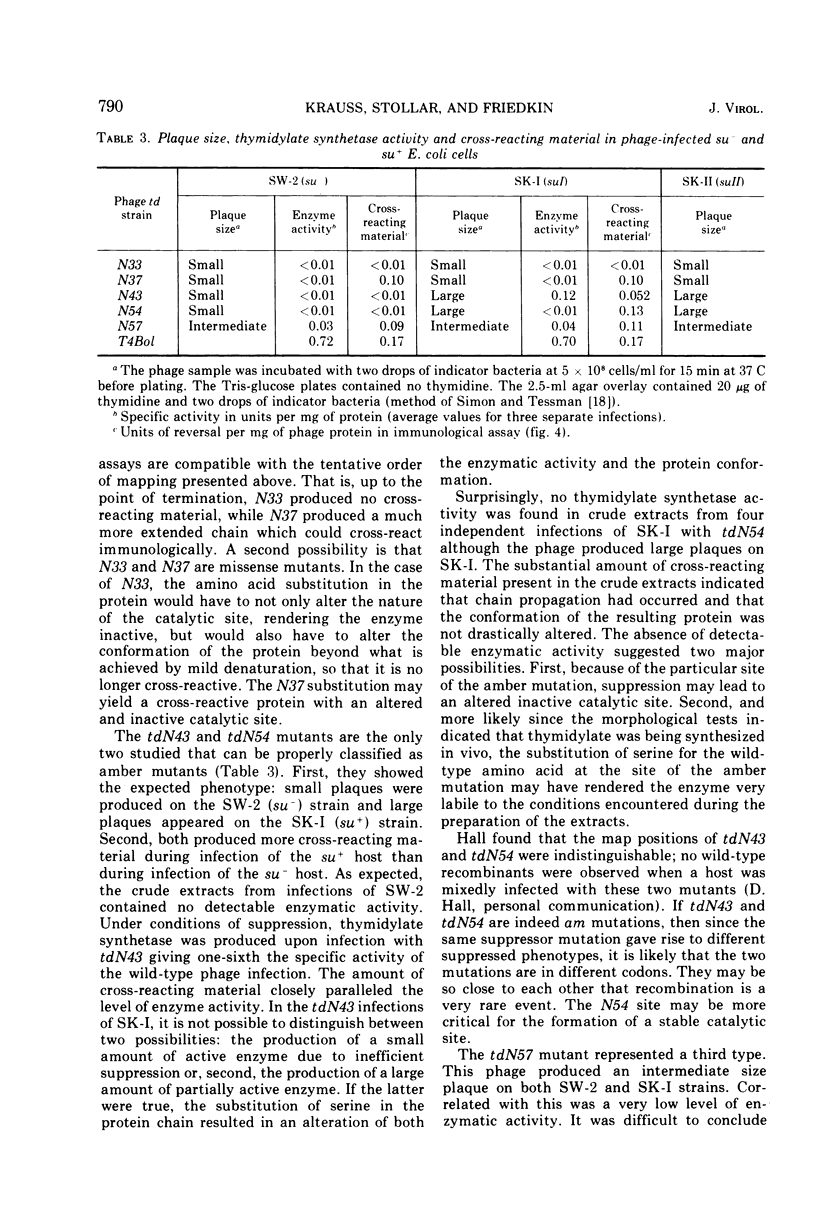

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNER H. D., COHEN S. S. Virus-induced acquisition of metabolic function. IV. Thymidylate synthetase in thymine-requiring Escherichia coli infected by T2 and T5 bacteriophages. J Biol Chem. 1959 Nov;234:2987–2991. [PubMed] [Google Scholar]
- Bertino J. B., Stacey K. A. A suggested mechanism for the selective procedure for isolating thymine-requiring mutants of Escherichia coli. Biochem J. 1966 Nov;101(2):32C–33C. doi: 10.1042/bj1010032c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. I. Enzymatic formation of 5-hydroxymethyldeoxycytidylate. J Biol Chem. 1959 Jun;234(6):1501–1506. [PubMed] [Google Scholar]
- Friedkin M., Donovan E. Reversible inactivation and reactivation of thymidylate synthetase. Adv Enzyme Regul. 1972;10:133–142. doi: 10.1016/0065-2571(72)90010-6. [DOI] [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L., DEWOLF S. Resolution of phage-initiated and normal host thymidylate synthetases of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:242–247. doi: 10.1073/pnas.48.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISLIUK R. L. Studies on the mechanism of formaldehyde incorporation into serine. J Biol Chem. 1957 Aug;227(2):805–814. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- Mathews C. K. Phage Growth and Deoxyribonucleic Acid Synthesis in Escherichia coli Infected by a Thymine-Requiring Bacteriophage. J Bacteriol. 1965 Sep;90(3):648–652. doi: 10.1128/jb.90.3.648-652.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- SHAPIRO D. M., EIGNER J., GREENBERG G. R. INABILITY OF THYMINE-DEPENDENT MUTANTS OF BACTERIOPHAGE T4 TO INDUCE THYMIDYLATE SYNTHETASE. Proc Natl Acad Sci U S A. 1965 Apr;53:874–881. doi: 10.1073/pnas.53.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHBA A. J., FRIEDKIN M. Direct spectrophotometric evidence for the oxidation of tetrahydrofolate during the enzymatic synthesis of thymidylate. J Biol Chem. 1961 Mar;236:PC11–PC12. [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]




