Abstract
Costs of completing the recommended immunization schedule have increased over the last decade. Access to prophylactic vaccines may become limited due to financing obstacles within current delivery systems. Vaccine prices reflect research and development expenses incurred by vaccine manufacturers, including costs associated with evaluating candidate vaccines in human subjects. If the number of subjects in clinical trials is increasing over time and associated with vaccine price, this may help explain increases in prices of vaccine series. We examined whether: (A) the initial public- and private-sector prices for recommended prophylactic vaccine series licensed and recommended in the US increased from 2000–2011, (B) the number of human subjects per licensed vaccine increased during the time period, and (C) the number of human subjects was associated with the initial public–and private–sector prices of the vaccine series. In regression analyses of 13 vaccines, approval year was not significantly associated with the number of human subjects, initial public-sector prices, or initial private-sector prices. While the number of phase II subjects was not significantly associated with prices, the numbers of phase III and combined late phase (phases II + III) subjects were significantly associated with initial public- and private-sector series prices (p < 0.05). The association between number of subjects and initial prices demonstrated diminishing marginal increases in price with increasing numbers of subjects. These findings may help guide the number of subjects required by the FDA in clinical trials, in order to reduce expenses for manufacturers and thereby help mitigate increases in initial vaccine series prices.
Keywords: vaccines, clinical trials, human subjects, price, research and development
Introduction
Prophylactic vaccines have contributed to significant decreases in the morbidity and mortality of many common diseases over recent decades.1-3 However, as the number of recommended vaccines has increased, the cost of completing the recommended immunization schedule for a patient has become substantially more expensive.4-6 Some experts believe access and availability of routine vaccines for children and adolescents may become limited by financing obstacles under existing vaccine delivery systems.7 The increasing cost of vaccines also has a substantial impact on federal spending, given that approximately 50% of pediatric vaccine doses are paid for with public funds.7-9 The private insurance sector faces similar challenges: while most private insurers cover the cost of vaccines for children and adults, there are many private insurance plans that either exclude recommended immunizations or require significant cost-sharing.8,10
Included in vaccine prices are expenses for research and development incurred by vaccine manufacturers. A major component of these expenses is the cost of clinical trials involving human subjects conducted to evaluate safety, efficacy and effectiveness. According to pharmaceutical executives and industry experts, the number of subjects in clinical trials has been increasing over time.7,11 While larger clinical trials help manufacturers learn more about the safety of vaccines before they reach the market,12 additional human subjects make clinical trials more expensive for manufacturers.13 More specifically, phase II trials examining efficacy and phase III trials assessing effectiveness and safety tend to be the largest14 and most expensive for manufacturers.15
If the number of subjects enrolled in clinical trials for vaccines has been increasing over time and the number of subjects is associated with price, expenses related to clinical trials may help explain the observed rise in public- and private-sector vaccine prices. This paper presents an analysis of the number of human subjects in clinical trials and the pricing for prophylactic vaccines approved in the US from 2000–2011. In this paper, we examined whether: (A) initial public- and private-sector prices for recommended vaccines increased from 2000 through 2011, (B) the number of human subjects per licensed vaccine increased over the time period, and (C) the number of human subjects was associated with initial public- and private-sector prices of the vaccines.
Results
Characteristics of vaccines in the study sample
For the 13 vaccines in the study sample, inflation-adjusted Centers for Disease Control and Prevention (CDC) public-sector contract prices for the full immunization series ranged from $ 32.10 (Boostrix) to $ 367.00 (Prevnar 13). Inflation-adjusted private-sector prices for immunization series ranged from $39.36 (Boostrix) to $ 435.00 (Prevnar 13) (Table 1).
Table 1. Prophylactic vaccines licensed in US from 2000–2011, rank-ordered by year of licensure, with inflation-adjusted initial prices of immunization series and number of subjects enrolled in clinical trials per phase.
| Vaccine name | Approval year | Doses | 2010 Inflation adjusted CDC contract Price | CDC price of immunization series | 2010 Inflation adjusted private sector price | Private price of immunization series | phase II n | phase III n | Late phase (II+III) n |
|---|---|---|---|---|---|---|---|---|---|
| IPOL |
2000 |
4 |
$9.81 |
$39.24 |
$19.53 |
$78.12 |
361 |
2,358 |
2,719 |
| Prevnar |
2000 |
4 |
$56.03 |
$224.12 |
$73.45 |
$293.80 |
1,062 |
41,661 |
42,723 |
| Daptacel |
2002 |
5 |
$15.45 |
$77.25 |
$23.82 |
$119.10 |
7,471 |
10,575 |
18,046 |
| Boostrix |
2005 |
1 |
$32.10 |
$32.10 |
$39.36 |
$39.36 |
647 |
5,545 |
6,192 |
| Adacel |
2005 |
1 |
$34.33 |
$34.33 |
$39.92 |
$39.92 |
*2854 |
8,904 |
11,758 |
| Menactra |
2005 |
1 |
$75.92 |
$75.92 |
$91.55 |
$91.55 |
3,106 |
7,836 |
10,942 |
| RotaTeq |
2006 |
3 |
$56.24 |
$168.72 |
$68.41 |
$205.23 |
3,201 |
64,268 |
67,469 |
| Gardasil |
2006 |
3 |
$103.84 |
$311.52 |
$129.52 |
$388.56 |
4,047 |
22,938 |
26,985 |
| Zostavax |
2006 |
1 |
$113.51 |
$113.51 |
$152.86 |
$152.86 |
1,799 |
40,144 |
41,943 |
| Rotarix |
2008 |
2 |
$83.30 |
$166.60 |
$103.81 |
$207.62 |
6,374 |
80,427 |
86,801 |
| Cervarix |
2009 |
3 |
$96.08 |
$288.24 |
$128.75 |
$386.25 |
3,964 |
45,025 |
48,989 |
| Menveo |
2010 |
1 |
$79.75 |
$79.75 |
$103.41 |
$103.41 |
740 |
8,989 |
9,729 |
| Prevnar 13 |
2010 |
4 |
$91.75 |
$367.00 |
$108.75 |
$435.00 |
1,478 |
49,296 |
50,774 |
| |
|
|
|
|
|
mean |
2,854 |
29,844 |
32,698 |
| median | 2,453 | 22,938 | 26,985 |
The total number of subjects in Phase II trials for a vaccine ranged from 361 (IPOL) to 7,471 (Daptacel), with mean = 2,854 and median = 2,453. The number of subjects in Phase III trials for a vaccine ranged from 2,358 (IPOL) to 80,427 (Rotarix), with mean = 29,844 and median = 22,938. The total number of subjects in late phase (II + III) trials for a vaccine ranged from 2,719 (IPOL) to 86,801 (Rotarix), with mean = 32,698 and median = 26,985.
Year of licensure and vaccine prices
In regression analyses, year of licensure was not significantly associated with initial public- or private-sector prices for immunization series (Table 2).
Table 2. Association of initial public- and private-sector prices of immunization series with year of licensure in United States.
| β coefficient from regression analysis | R2 from regression model | p value | |
|---|---|---|---|
|
Year of licensure as a predictor of initial public-sector price for immunization series |
14.01 |
0.17 |
0.16 |
| Year of licensure as a predictor of initial private-sector price for immunization series | 15.17 | 0.13 | 0.13 |
Similarly, vaccines’ year of licensure was not associated with numbers of human subjects in the different clinical phases of vaccine development.
Numbers of subjects in clinical trials and initial vaccine prices
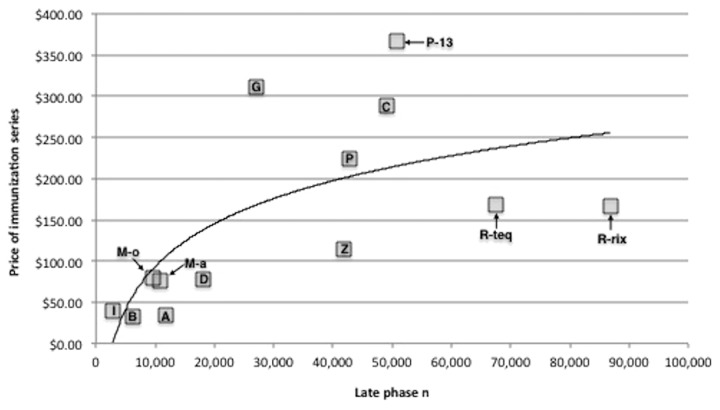
The number of Phase II subjects was not significantly associated with initial public-sector contract series price or initial private-sector series price. However, the number of phase III subjects and the number of combined late phase subjects were both significantly associated with initial public-sector and initial private-sector prices for immunization series (Table 3). The curvilinear nature of this relationship is illustrated in Figure 1.
Table 3. Association of initial public- and private-sector prices for immunization series with numbers of subjects in late phases of vaccine development.
| |
β coefficient |
R2 |
p value |
|---|---|---|---|
| Number of subjects and public immunization series price | |||
|
Phase 2 n |
34.07 |
0.08 |
0.36 |
|
Phase 3 n |
72.73 |
0.50 |
0.01 |
| Late Phase n | 75.08 | 0.47 | 0.01 |
| Number of subjects and private immunization series price | |||
|---|---|---|---|
|
Phase 2 n |
41.06 |
0.08 |
0.36 |
|
Phase 3 n |
86.84 |
0.48 |
0.01 |
| Late Phase n | 89.74 | 0.45 | 0.01 |
Figure 1. Association between the number of human subjects in late phase (II + III) clinical trials and inflation-adjusted initial public-sector price of the immunization series for newly licensed vaccines (2000–2011). All series prices are expressed in 2010 US dollars. Key for vaccines: I, IPOL; B, Boostrix; M-o, Menveo; M-a, Menactra; A, Adacel; D, Daptacel; G, Gardasil; Z, Zostavax; P, Prevnar; C, Cervarix; P-13, Prevnar 13; R-teq, RotaTeq; R-rix, Rotarix.
Estimated increase in initial dose price related to human subjects
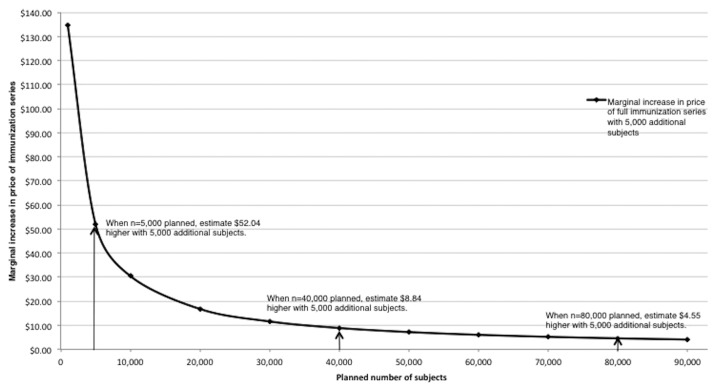
Based on the association between number of human subjects and initial prices observed (Fig. 1), we estimate a diminishing marginal increase in prices for immunization series with respect to additional subjects in clinical trials (Fig. 2). For example, if a vaccine developer plans late phase trials with 5,000 subjects, the projected initial public-sector series price would be $ 41.26; an increase of 5,000 late phase subjects is estimated to increase the initial public-sector price by $ 52.04 (126% in relative terms).
Figure 2. Estimated increase in initial public-sector price of the immunization series with increased number of planned late phase (II + III) subjects. Estimates from authors’ calculations, based on associations between numbers of subjects in phase II and III trials and introductory series prices.
By comparison, if a vaccine developer plans late phase trials with 40,000 subjects, the projected initial public-sector series price is $ 197.37; an additional 5,000 late phase subjects is estimated to increase the public-sector price by $ 8.84 (4.5% in relative terms). If a developer plans for 80,000 late phase subjects, the projected initial public-sector series price is $ 249.41 and an additional 5,000 late phase subjects is estimated to increase the initial public-sector series price by $ 4.55 (1.8%).
Discussion
This analysis, the first of which we are aware to critically examine the assumption that higher numbers of subjects are associated with increasing initial vaccine series prices, establishes a statistically significant association between the number of subjects in clinical trials and the price of immunization series in the United States from 2000–2011. In particular, we find that the number of phase III subjects is strongly associated with initial prices. Importantly, this association between number of subjects and series prices advances the field of vaccine economics beyond prior work4,5,7 that had observed that prices were increasing over time beyond inflation, but had not identified a specific mechanism or reason for the increase.
The apparent logarithmic association between the number of late phase subjects and vaccine prices implies a diminishing marginal increase in series price with a rise in the number of subjects. Although the Food and Drug Administration (FDA) mandate to judge safety and effectiveness does not include considerations of product development costs or market prices, our findings indicate implications of FDA requirements about numbers of subjects that can be considered by other stakeholders such as the CDC and private payers. Importantly, increases in numbers of subjects at the high end of trials would be expected to have much smaller ramifications for immunization series prices than increases in subjects for vaccines with otherwise smaller clinical trials (Fig. 2). With over 4 million children born in the US annually and approximately 50% of them eligible for vaccination through the federal Vaccines for Children program, increases in vaccine price translate to additional millions of dollars per year in federal spending.
Unexpectedly, an increase in the number of clinical trial subjects was not observed over the years included in this study. This finding is contrary to prevailing wisdom,7,11 and points to the need for further work in this area. One possible reason for this lack of an association is the wide range in the number of human subjects for prophylactic vaccine trials. Analyses of therapeutic drugs have established that there is a wide range in the duration of clinical phases across distinctive classes of medications.16 Similarly, it could be that certain complex target diseases for vaccines require a greater number of human subjects in order to prove safety and efficacy. If so, investigators who view an increase in the number of human subjects principally as a time trend may have instead been observing a timeframe in which vaccines targeting more complex diseases with larger clinical trials were approved later.
Similarly, it is necessary to address why certain vaccines had particularly large numbers of phase III subjects. In the case of pneumococcal conjugate vaccines or varicella zoster vaccine, large numbers of phase III subjects were likely needed to establish efficacy given a low event rate in the placebo group. In contrast, rotavirus vaccines licensed during this study period required particularly large phase III trials in order to demonstrate rare but clinically meaningful adverse events that were of concern due to an earlier rotavirus vaccine being withdrawn from the market several years earlier.
This study has limitations that must be acknowledged. In addition to the number of human subjects in clinical trials, there are other research and development expenses for vaccine manufacturers. These other expenses—such as the duration of clinical trials, capital investments and interest—were beyond the scope of this study. In addition, generally accepted assumptions for multivariate statistical tests suggest that a multivariate model should have approximately 10 cases per parameter. Consequently, with our sample size of 13 vaccines we were unable to run a multivariate regression of price with the number of subjects and licensure year simultaneously.
An additional limitation is that our sources often did not include phase I data. However, phase I trials are not as expensive as phase II or III trials for manufacturers because they have comparatively fewer subjects. Therefore, we believe that the inclusion of phase I data from these analyses would not substantively change the results.
These limitations notwithstanding, this study is the first to indicate that the number of subjects in phase III clinical trials and number of subjects in late phase trials are strongly associated with initial public- and private-sector series prices of prophylactic vaccines in the United States. There is a critical balance to be struck between cost and establishing the safety and efficacy of vaccines. While clinical trials with fewer human subjects would likely make for more affordable vaccine prices, smaller clinical trials are less likely to expose rare adverse effects or establish efficacy. Conversely, clinical trials with more human subjects would presumably increase confidence in the safety and effectiveness of a vaccine, but the associated increase in series price might lead to cost-related access barriers for people who wish to receive vaccinations. Our findings suggest potential benefits if the FDA and manufacturers were to work together to plan clinical trials that are large enough to establish safety and efficacy, but not so costly that vaccines are prohibitively expensive when they reach the market.
Methods
Sample
Prophylactic vaccines licensed in the US from 2000–2011 for which the government was a major purchaser were examined, using records from the FDA for all licensures for novel prophylactic vaccines in this time period. This recent timeframe was selected because FDA regulation of vaccine manufacturers evolves over time.17
Vaccines against seasonal influenza, H1N1 influenza and H5N1 influenza, were excluded due to significant differences in the FDA approval process of influenza vaccines. Hiberix was excluded because it is used as a booster dose. Ixiaro was excluded because it is primarily for travelers. TENIVAC was excluded because initial market prices were unavailable. The 13 vaccines that met the criteria for inclusion in the study were: inactivated polio (IPOL, Sanofi Pasteur), pneumococcal conjugate (Prevnar, Wyeth; Prevnar13, Pfizer), diphtheria-tetanus-acellular pertussis (DTaP)(Daptacel, Sanofi Pasteur), tetanus-diphtheria-acellular pertussis (Tdap)(Adacel, Sanofi Pasteur; Boostrix, GlaxoSmithKline), meningococcal conjugate (Menactra, Sanofi Pasteur; Menveo, Novartis), herpes zoster (Zostavax, Merck), rotavirus (RotaTeq, Merck; Rotarix, GlaxoSmithKline), and human papillomavirus (Gardasil, Merck; Cervarix, GlaxoSmithKline).
For each of the 13 vaccines examined in the study, initial public-sector series prices reported by the CDC were obtained for the year of introduction in the market. Twelve of the vaccines were purchased through the Vaccines for Children program. The remaining vaccine examined, Zostavax, was initially licensed for adults (≥ 60 y) but was also included as the government is the dominant payer for Zostavax through the Medicare program.
Data sources and measures
Year of licensure and the number of subjects in each phase of clinical trials were determined from approval documents submitted by pharmaceutical companies to the FDA Center for Biologics Evaluation and Research when seeking initial approval. These documents are available online through the FDA.18 From these documents, the numbers of subjects in each clinical trial for a particular vaccine were summed across trials by phase to provide the total numbers of subjects per phase for each vaccine.
Initial public-sector and initial private-sector dose prices were available in the CDC Contract Price List Archives.19 Public- and private-sector dose prices were adjusted for inflation to 2010 dollars using the Bureau of Labor Statistics Consumer Price Index Inflation Calculator.20 The vaccine prices for immunization series were calculated for each vaccine by multiplying the initial dose price by the number of doses recommended in FDA licensure documents.18
Data Analyses
phase I data was only available for 8 of the 13 vaccines studied, so these data were excluded. Every vaccine had phase II and phase III data available except Adacel, which did not provide the number of subjects in phase II clinical trials. The mean number of phase II subjects for the cohort (n = 2,854) was used as the number of phase II subjects for Adacel. A composite measure of “late phase” number of subjects was constructed by aggregating the number of phase II and phase III subjects. “Late phase” was utilized as a summary measure for the number of subjects in clinical trials.
Scatter plots of approval year vs. price, approval year vs. number of subjects, and number of subjects vs. price were created using Microsoft Excel 2010. These scatterplots were used to determine the appropriate data transformations, based on data frequency distributions, for testing the relationships between price measures and numbers of subjects in bivariate regressions. Log transformations were applied for the numbers of subjects. Bivariate linear regressions were conducted using Stata 12 (Stata Corporation). With findings from these analyses, we estimate the incremental change in series price for vaccines based on the number of subjects in clinical trials.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- FDA
Food and Drug Administration
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/20506
References
- 1.Davis MM, Butchart AT, Coleman MS, Singer DC, Wheeler JRC, Pok A, et al. The expanding vaccine development pipeline, 1995-2008. Vaccine. 2010;28:1353–6. doi: 10.1016/j.vaccine.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Ten great public health achievements--United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60:619–23. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Ten great public health achievements--United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241–3. [PubMed] [Google Scholar]
- 4.Davis MM, Zimmerman JL, Wheeler JRC, Freed GL. Childhood vaccine purchase costs in the public sector: past trends, future expectations. Am J Public Health. 2002;92:1982–7. doi: 10.2105/AJPH.92.12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis MM. Price as a double-edged sword in the golden era of vaccines. Hum Vaccin. 2010;6:689–93. doi: 10.4161/hv.6.9.13496. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ. The role of cost-effectiveness in U.S. vaccination policy. N Engl J Med. 2011;365:1760–1. doi: 10.1056/NEJMp1110539. [DOI] [PubMed] [Google Scholar]
- 7.Lindley MC, Shen AK, Orenstein WA, Rodewald LE, Birkhead GS. Financing the delivery of vaccines to children and adolescents: challenges to the current system. Pediatrics. 2009;124(Suppl 5):S548–57. doi: 10.1542/peds.2009-1542O. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Financing vaccines in the 21st century: assuring access and availability. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 9.Smith PJ, Lindley MC, Rodewald LE. Vaccination coverage amongst US children aged 19-35 months entitled by the Vaccines for Children program, 2009. Public Health Rep. 2001;126(Supplement 2):109–23. doi: 10.1177/00333549111260S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloan FA, Berman S, Rosenbaum S, Chalk RA, Giffin RB. The fragility of the U.S. vaccine supply. N Engl J Med. 2004;351:2443–7. doi: 10.1056/NEJMsb033394. [DOI] [PubMed] [Google Scholar]
- 11.US Congress Office of Technology Assessment. Pharmaceutical R&D: costs risks and rewards. Washington, DC: US Government Printing Office; 1993. [Google Scholar]
- 12.Ellenberg SS. Safety considerations for new vaccine development. Pharmacoepidemiol Drug Saf. 2001;10:411–5. doi: 10.1002/pds.616. [DOI] [PubMed] [Google Scholar]
- 13.DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87:272–7. doi: 10.1038/clpt.2009.295. [DOI] [PubMed] [Google Scholar]
- 14.US National Institutes of Health ClinicalTrials. gov. Understanding clinical trials. Bethesda, MD: National Library of Medicine; 2007-2011. Available from http://clinicaltrials.gov/ct2/info/understand
- 15.Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 2006;25:420–8. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 16.Kaitin KI, DiMasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000-2009. Clin Pharmacol Ther. 2011;89:183–8. doi: 10.1038/clpt.2010.286. [DOI] [PubMed] [Google Scholar]
- 17.Klein JO, Myers MG. Vaccine shortages: why they occur and what needs to be done to strengthen vaccine supply. Pediatrics. 2006;117:2269–75. doi: 10.1542/peds.2005-2427. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services Food and Drug Administration. Complete list of vaccines licensed for immunization and distribution in the US. Silver Spring, MD: Available from http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM093833
- 19.US Department of Health and Human Services Center for Disease Control and Prevention. Vaccines for Children: CDC vaccine price list archives. Atlanta, GA. Available from http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list-archives.htm
- 20.US Department of Labor Bureau of Labor Statistics. Consumer price index inflation calculator. Washington, DC. Available from http://www.bls.gov/data/inflation_calculator.htm
- 21.Jacobson RM, Adegbenro A, Pankratz VS, Poland GA. Adverse events and vaccination-the lack of power and predictability of infrequent events in pre-licensure study. Vaccine. 2001;19:2428–33. doi: 10.1016/S0264-410X(00)00467-9. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. Biologics license application process. Rockville, MD: US Department of Health and Human Services.
- 23.Honig P, Lalonde R. The economics of drug development: a grim reality and a role for clinical pharmacology. Clin Pharmacol Ther. 2010;87:247–51. doi: 10.1038/clpt.2009.298. [DOI] [PubMed] [Google Scholar]