Abstract
Active and passive immunizations with Aβ and Aβ antibodies successfully reduced AD pathology and improved cognitive functions in an AD mouse model. However, human clinical trials of vaccination with synthetic Aβ(AN1792), were halted due to brain inflammation, presumably induced by T cell-mediated immune response. In this study, we used Picha pastoris to produce a recombinant peptide vaccine, r4 × Aβ15(recombinant 4 × Aβ15), four tandem repeats of Aβ(1-15) interlinked by spacers . Wild-type mice were injected subcutaneously with CFA/IFA as adjuvant. r4 × Aβ15 vaccine elicited high titer anti-Aβ antibodies which bound to Aβ plaque in brain tissue from Tg2576 mouse. The antibody isotype was mainly IgG(1), indicating anti-inflammatory Th2 type. There was no splenocyte proliferation against Aβ peptide, which indicates that the r4 × Aβ15 vaccine does not induce Aβ-specific T cellular immune response. Thus, r4 × Aβ15 vaccine may be a safe and efficient vaccine for AD.
Keywords: Alzheimer disease (AD), C57BL/6 mice, P.pastoris, T cellular immune response, r4×Aβ15 vaccine
Introduction
The accumulation of β-amyloid peptides (Aβ) is strongly assoiated with the development of Alzheimer disease.1 Active and passive immunization studies performed in transgenic mouse models of amyloid deposition have demonstrated that antibodies against β-amyloid are able to reduce amyloid load and improve cognition.2-5 These results have raised the hope that Alzheimer disease could be treated by immunotherapy and prevented by vaccination. As T-cell mediated adverse reactions were observed in humans immunized with the whole β-amyloid peptide,6 immunogens that are devoid of β amyloid T epitopes are considered. The B cell epitope(s) in humans,7 monkeys,8 and mice9 is located within the Aβ1-15 region, while the T cell epitope has been mapped within Aβ15-42.10,11 Thus, Aβ fragments spanning the B cell epitope but not the T cell epitope may be safer, as a potentially deleterious anti-Aβ cellular immune response may be avoided. Recent studies suggest that immunization with B cell epitope is effectively in generating anti-Aβ antibodies against full-length Aβ. These strategies include Aβ epitope peptide presentation in tandem and /or branched structures either alone or in conjunction with known strong Th cell epitopes.12-19 However, most of these immunogens, especially Aβ15 based immunogen, are chemically synthetic, requiring complicated purification and modification of protein, which is expensive and time-consuming. Second, multicopies of Aβ15 in these immunogens were either directly synthesized to a new T cell helper epitope or directly linked by a tandem repeat, which might create new B cell epitopes, and result in the production of newly unknown antibodies.20
Producing immunogen using bacteria or yeast is economical, convenient and easy to be scaled. Compared with E.coli, Picha has a high chance of reaching high production levels and secretion of the product, which facilitates purification.21,22 To overcome the low immunogenicity of Aβ15, we designed a four tandem of repeats of Aβ15 interlinked by spacers which would avoid the irrelevant epitopes and make the 4 × Aβ15 fold freely, hence expose the B cell epitope fully.23 Therefore, in this study, we used P.pastoris to produce a recombinant peptide vaccine designed to elicit a specific Aβ immune response in the absence of a T cell response. The r4 × Aβ15 obtained from P.pastoris was used to immunize wild type C57BL/6 mice with CFA/IFA as adjuvant. The r4 × Aβ15 vaccine immunized mice were found to have generated antibodies which bound to Aβ plaque in brain tissue from a Tg2576 mouse.
Results
Construction of expression plasmid pGAPZαA4 × Aβ15
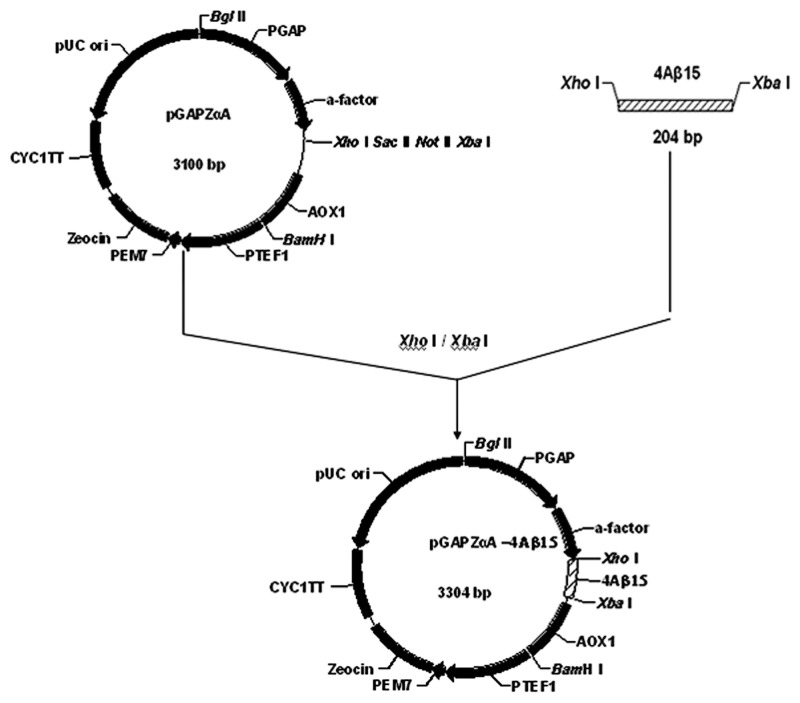
As described in Section 2.2, the DNA fragment encoding foldable 4 × Aβ15 with spacers were cloned by PCR and then fused to downstream of Kex2 cleavage site in the pGAPZaA to yield plasmid pGAPZαA4 × Aβ15(Fig. 1) The nucleotide sequence inserted in plasmid pGAPZαA4 × Aβ15 had been verified by sequencing (Fig. 2).
Figure 1. Construction of the pGAPZαA4 × Aβ15 plasmid.
Figure 2. Neucleotide and deduced amino acid sequence of 4 × Aβ15 and spacer genes in the expression vector of pGAPZαA4 × Aβ15 (bold letters are spacer region)
Expression of 4 × Aβ15 in P. pastoris
SDS-PAGE analysis showed the genetic strain with 2,000 Zeocin resistance was the highest-yield expressing clone (data not shown). Therefore, strain with 2,000 Zeocin resistance was chosen for further study. As shown in Figure 3A, compared with the negative control, an 18-kDa secreted protein band was detectable at 24 h of culture following Coomassie blue R250 staining, and protein expression gradually reached the maximal level at 96 h. Western blot analysis revealed that the expressed peptide was recognized by the mouse anti-human Aβ42 monoclonal antibody, and the sample at 96 h culture showed the strongest signal (Fig. 3B). In other words, the r4 × Aβ15 peptide was successfully expressed and secreted into the culture medium, and the highest production of r4 × Aβ15 was assumed to occur at the 96 h culture. The production of r4 × Aβ15 reached about 600 mg/l after 96 h induction.
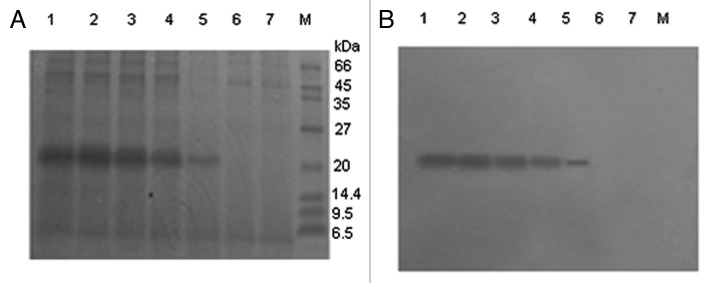
Figure 3. SDS-PAGE and western blot of expression products of pGAPZαA transformed in GS115. Samples were subjected to 12% SDS-PAGE and stained with Coomassie brilliant blue (A), and the duplicate gel was electroblotted onto a nitrocellulose membrane for Western analysis (B). M, Protein molecular weight marker. Lanes 1–5, 20 μl culture supernatant from a transformant at different culture time points: 120, 96, 72, 48, 24 h. Lane 6, culture supernatant from strain transformed with pGAPZαA empty plasmids as control. Lane 7, culture supernatant from P. pastoris GS115 as control. No band cross-reacting with the antibody was detected in the supernatant from the negative strain transformed with pGAPZαA empty plasmid.
Purification of r4 × Aβ15
The supernatant was first purified by 30–70% saturated ammonium sulfate precipitation. The precipitant was analyzed by SDS-PAGE and Bandscan software. The results indicate that the purity of r4 × Aβ15 is the highest, reaching 80% after 70% saturated ammonium sulfate precipitation of culture supernatant (Fig. 4A). Precipitation with 70% saturated ammonium sulfate was selected as the first step in purification of the secreted r4 × Aβ15. After precipitation, the product from the first purification step was dialyzed to remove the ammonium sulfate so as to avoid affecting the UNOsphere Q column used in the next step. The dialyzed product was then further purified on a UNOsphere Q column. The purity of the purified protein was evaluated to be 95% by densitometric scanning (Fig. 4B). About 114 mg purifed protein was obtained from 500 ml of culture.
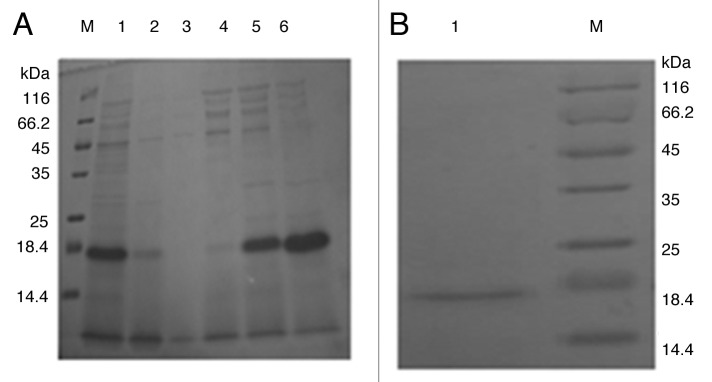
Figure 4. Purification of r4 × Aβ15. (A) Purification of r4 × Aβ15 by ammonium sulfate precipitation. M, protein molecular weight marker. Lane 1, r4 × Aβ15 was expressed and secreted into the culture supernatant. Lanes 2–6, 30–70% saturated ammonium sulfate precipitation of culture supernatant. Band scan analysis indicates that the purity of r4 × Aβ15 is the highest, reaching 80% after 70% saturated ammonium sulfate precipitation of the culture supernatant. (B) r4 × Aβ15 was further purified on a UNOsphere Q column. M, protein molecular weight markers. Lane 1, sample of purified r4 × Aβ15.
Protein identification by mass spectrometry and amino acid sequencing
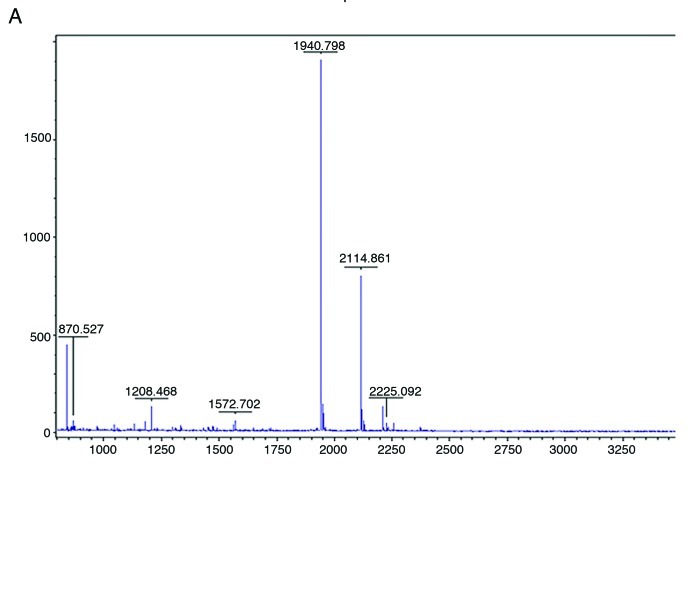
Peptide mass mapping is a particularly successful technique for the identification of proteins, especially for those recombinant protein whose apparent MW is bigger than its theoretical MW, so we chose this method to identify the products.30-33 The gel pieces containing 18 kDa protein(r4 × Aβ15) were excised to be analyzed by MALDI-TOF-TOF-MS and their peptide mass fingerprints (PMF) were obtained (Fig. 5A). The matched mass values overlapped each other (Fig. 5B). Sequence coverage reached 60%, and the molecular weight of r4 × Aβ15 was 8.588 kDa, consistent with the molecular mass of 4 × Aβ15 (8.585 kDa) calculated by Antheprot 5.0 software (results not shown). The results of amino acid sequencing showed that the N-terminal sequence of r4 × Aβ15 is DAEFR, which is identical to the N-terminal end of 4 × Aβ15. The reason for the apparent deviation of recombinant protein could be glycosylation, but there is no single N-glycosylation site in r4 × Aβ15. So abnormal migration rate of r4 × Aβ15 in SDS-PAGE may results from a high incidence of small amino acids or low SDS binding by extremely hydrophilic proteins.34
Figure 5. Identification of expressed 4 × Aβ15 protein with MALDI-TOF-TOF-MS (A) Peptide mass fingerprinting (PMF) obtained of the GS115/ pGAPZαA4 × Aβ15 product by MALDI-TOF-TOF-MS. The peaks are the individual peptide fractions of the protein and are indicated by the corresponding m/z values (molecular weight). The peak height is the peptide signal intensity. (B) Mascot search results: Sequence coverage reaches 60%. The matched peptides are shown shaded.
r4 × Aβ15 vaccine effectively induces high titers of anti-Aβ antibodies
We immunized C57BL/6 mice biweekly with r4 × Aβ15 vaccine. After the third immunization, serum titers were determined, and anti-Aβ antibodies were detected. With the increasing number of vaccinations, the antibody levels increased. After the last immunization, plasma anti-Aβ antibodies reached 77.37 ± 13.15 μg/mL. All of the C57BL/6 mice treated with Aβ42 developed antibodies (95.85 ± 15.27 μg/ml) against Aβ42, but the antibody titers in the PBS group were at a background level (Fig. 6A). Immunoreactivity of anti-sera against GST-Aβ42 was determined by western blotting analysis. It showed that the sera from C57BL/6 mice immunized with r4 × Aβ15 reacted strongly with GST-Aβ42 (31 kDa), which was similar to the immunostaining with monoclonal anti-Aβ antibody (Fig. 6B).
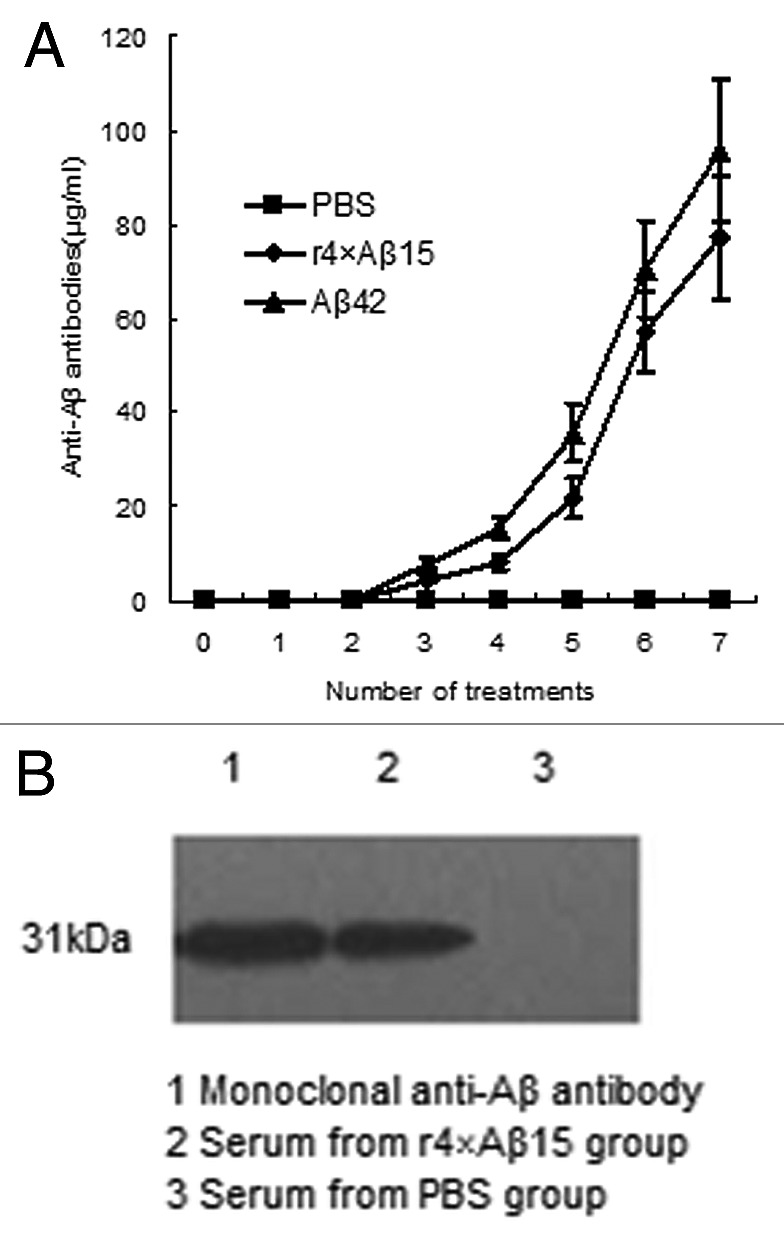
Figure 6. Immune response against human Aβ42 in C57BL/6 mice immunized withr4 × Aβ15. (A) Anti-Aβ42 antibody titers in C57BL/6 mice were assayed by ELISA at the indicated immunization points. (B) The sera from C57BL/6 immunized with r4 × Aβ15 reacted strongly with GST-Aβ42 (31kDa) by western blotting analysis.
IgG1 predominates in induced anti- Aβ antibodies
Immunoglobulin isotype-specific ELISAs identified the principle anti-Aβ isotype-specific as IgG1, with lower amounts of IgG2a, and IgG2b (Fig. 7A). In mice, the production of IgG1 is primarily induced by Th2 cytokines, while IgG2a is produced through Th1 cytokines. The IgG1/IgG2a ratio for the r4 × Aβ15 group and Aβ42 group are 11.95 ± 1.96 and 4.44 ± 0. 86 respectively (Fig. 7B). Thus, these results indicate that r4 × Aβ15 induced a highly Th2-biased immune response in C57BL/6 mice.
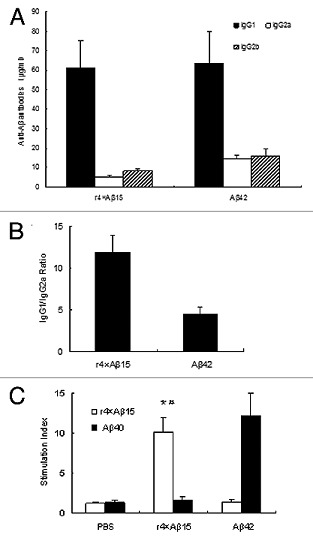
Figure 7. Detection of IgG1, IgG2a and IgG2b subclasses of anti-Aβ antibodies and T cell proliferation in mice immunized with the r4 × Aβ15 vaccines and Aβ42. (A) Isotyping in sera from immunized mice after seven immunizations with r4 × Aβ15 vaccines and Aβ42. (B) IgG1/IgG2a ratios were calculated based on the data present in A. (C) T cell proliferation in mice immunized with the r4 × Aβ15 vaccines and Aβ42 and PBS. Splenocytes isolated from immunized mice showed a high SI in response to stimulation with their corresponding immunogen peptide. *p < 0.01, compared with the PBS group. #p > 0.05, compared with the Aβ42 group.
Anti- Aβ42 antibody combine with Aβ plaque in brain of Tg7526 mouse
To analyze whether these antibodies can bind to Aβ plaques in brain of Tg2576 mouse, we used pooled sera from immunized mice to react with sections of brain of Tg2576 mouse. Results showed antiserum from r4 × Aβ15 group bound to Aβ plaques in the brain sections of brain tissue from Tg2576 mouse (Fig. 8A), which is similar to the binding of monocloning antibody to Aβ plaque(Fig. 8B). The PBS group sera from these mice did not bind to the Aβ plaques (Fig. 8C).
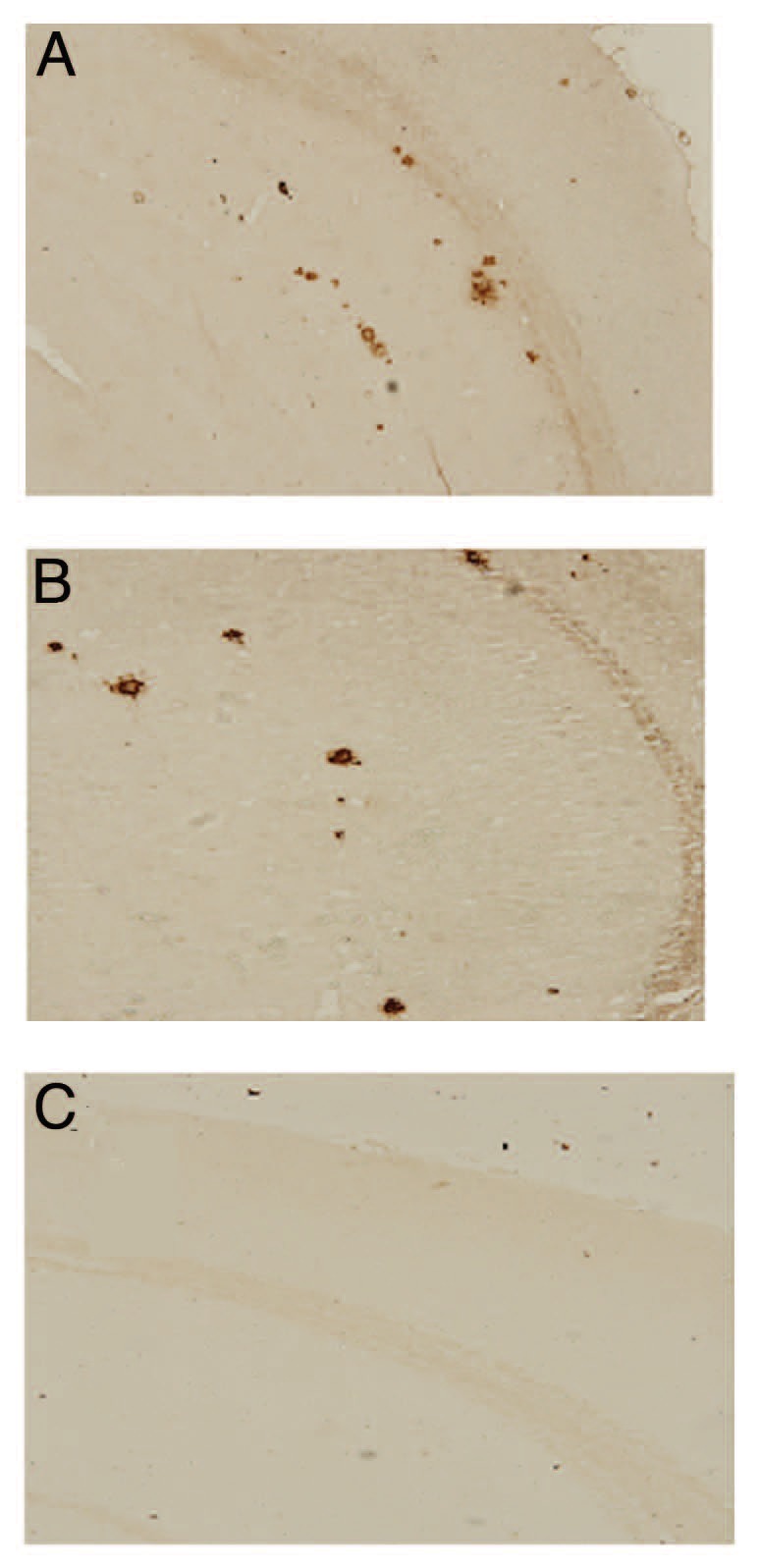
Figure 8. Sera from C57BL/6 mice immunized with r4 × Aβ15 react with amyloid plaques in the brain slice of Tg2576 transgenic mice (ABC immunohisto-chemical method). Serial brain sections from Tg2576 transgenic mice were stained with (A) serum from C57BL/6 mice treated with r4 × Aβ15 (B) Aβ monoclonal antibody (positive control) (C) serum from C57BL/6 mice treated with PBS.
r4 × Aβ15 and Aβ-specific T cell responses in splenocytes from immunized mice
To determine whether the cellular immune response was directed to Aβ, splenocytes from immunized C57BL/6 mice were restimulated with r4 × Aβ15 and Aβ40. the highest stimulation indexes (SIs) were observed in splenocytes restimulated with their corresponding immunogen(Fig. 7C), but no significant SI was induced in the PBS group (p < 0.01). Immunization with Aβ42 induced robust in vitro T cell proliferation after restimulation of the cultures with Aβ40. meanwhile, splenocytes isolated from mice immunized with r4 × Aβ15 also induced strong T cell proliferation after stimulation with r4 × Aβ15, but not with Aβ40 (p > 0.05).
Discussion
Since Schenk invented Aβ immunotherapy for Alzheimer disease,35 numerous AD vaccines have been developed. Good AD vaccine requires not only to be safe and effective but also to be produced less costly in less time. In this study, we aim to produce a r4 × Aβ15 vaccine by P.pastoris constitutive expression system, which is cost-effective and easy to be scaled. What’s more, we investigated the humoral and cellular immune response of r4 × Aβ15 vaccine in C57BL/6 mice.
P. pastoris is an efficient host for the expression and secretion of heterologous proteins. The commonly used expression system for P. pastoris includes inducible expression system and constitive expression system. pGAP, a constitutive promoter, has been used for the expression of more and more heterologous proteins in P. pastoris.36-38 Compared with inducible expression system, such as, pAOX1, pGAP-based expression system is more suitable for large-scale production because the hazard and cost associated with the storage and delivery of large volume of methanol are eliminated. So in this study, we choose pGAP to express 4 × Aβ15. To avoid irrelevant epitopes caused by extra amino acids at N-terminal and His-tags at C-terminal of r4 × Aβ15, we introduced Kex2 signal cleavaged site right before the cDNA encoding 4 × Aβ15 and a stop codon TCA right before the C-terminal His-tag. The sequencing results confirmed that r4 × Aβ15 without additional residues at the N-terminal end. Multiple copy integration of recombinant genes into P.pastoris has been demonstrated to increase the expression of the desired gene in some cases.39,40 The level of Zeocin resistance roughly depends on the number of recombinant genes integrated according to the instructions provided in the pGAPZαA manual. In this study, we also found obvious difference with regard to protein expression among the colonies exhibiting Zeocin resistance in different degrees (data not shown). At the optium condition (96 h fermentation, 2,000 Zeocin resistance), the production of r4 × Aβ15 is about 600 mg/l, which was higher than other recombinant protein production by P.pastoris.41,42 After two-step purification, about 228 mg/l of r4 × Aβ15 with a purity up to 95% was obtained, resulting in a 56% recovery of the initial r4 × Aβ15. The final yield of purified r4 × Aβ15 produced by P. pastoris is about 50 times higher than in E. coli.24
To develop a peptide vaccine, it is very important to improve the immunogenicity of peptides. Since Aβ15 alone is not an effective immunogen.43 Thus, using Aβ15 as the unit peptide, we attempted to overcome the low immunogenecity of the peptide by constructing a cDNA encoding four tandem repeats of Aβ15 interlinked by amino acid spacers and make it express in P.pastoris. The r4 × Aβ15 was used to immunize C57BL/6 mouse, eliciting high titers antibodies against Aβ, which indicate r4 × Aβ15 has the same immunogenecity as those synthesized Aβ15-based immunogens reported44,45. In addition, antibodies generated by immunization with the r4 × Aβ15 vaccine bound to Aβ plaques in brain tissues from a Tg2576 mouse. As binding of antibodies to the region of Aβ42 coincides with the ability of antibodies to bind native plaques in brain tissue,46,47 this result implied that the antibodies elicited by r4 × Aβ15 would be effective in binding native plaques in brain tissue.
Since T help 1(Th1) immune responses activate encephalitogenic T cells and induce continuous inflammation in the central nervous system, vaccine inducing Th2-immune would be more promising. Antibody isotyping has been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines to the humoral response.48 Thus we measured anti- Aβ42 IgG2a and IgG1 antibodies in the sera of immunized mice. Data indicate Mice in r4 × Aβ15 group produced anti-Aβ antibodies composed predominantly of isotype IgG1, which suggests r4 × Aβ15 induced a Th2–biased response, indicating a lower probability of inducing inflammation.
A safe and effective vaccine for AD requires not only therapeutic levels of anti-Aβ antibodies but also the prevention of an adverse T cell-mediated, proinflammatory autoimmune response. Splenocyte proliferation assay in immunized C57BL/6 mice was performed to determine whether r4 × Aβ15 induced an Aβ-specific T cell response. The results showed that the highest SIs were observed in splenocytes restimulated only with their corresponding immunogen. As we know, Aβ42 itself possesse both B and T cell epitopes, immunizing with Aβ42 induced Aβ-specific T cell proliferative response after stimulation with Aβ40.Importantly, strong T cell proliferation was observed in splenocytes isolated from the r4 × Aβ15 group after stimulation with r4 × Aβ15, but not with Aβ40. These results demonstrated that r4 × Aβ15 did not induce Aβ-specific T cell response, which suggested that 4 × Aβ15 contains new T cell eptiopes, but not Aβ T cell epitopes. Despite r4 × Aβ15 did not induce Aβ-specific T cell response, it induces a proliferative response in the splenocyte antigen coculture, which reflects T cell activation specific for the four tandem repeat. This probably represents T cell activation. To further exclude r4 × Aβ15 immunization of humans would result in autoimmunity, the following were considered. First, In case it’s a TCR-mediated (classical nomical) response, it is necessary to exclude that 4 × Aβ15 shares any relevant sequence homology with any protein expressed in humans, for 4 × Aβ15 might trigger an autoimmune response by virtue of homology sequences between 4 × Aβ15 and human proteins. Therefore, we performed a genomic research by NCBI blast and results show no significant similarity between 4 × Aβ15 and human proteins was found. Second, in case the T-cell response is of a superantigen-type, it is necessary to exclude that this would occur with human MHC and TCR moieties. Because HLADRB1*1501, as reported by Vicotor,49 is a highly prevalent allele that promotes strong proliferation of Aβ-reactive T cells in both humans and mice, we used NetMHCIIpan soft (version 2.1, www.cbs.dtu.dk PservicesPNetMHCIIpan) to predict peptide of 4 × Aβ15 binding to HLADRB1*1501. Results show that no high binding affinity peptide of 4 × Aβ15 was detected. These two results indicate this immunogen may avoid an autoreactive T cell response.
It should be noted that the novelty of this study is limited to the four tandem repeats of Aβ1–15 and the use of Picha pastoris, which ensures a high peptide yield. In addition, the limitation of this study is that T cell epitopes and B cell epitopes were not mapped. Notwithstanding its limitation, this study does suggest that the r4 × Aβ15 was an effective immunogen in C57BL/6 mice. Meanwhile, a strong humoral immune response, resulting in predominantly Th2-biased immunoglobulins without Aβ-specific T cell reactivity was seen, suggesting that this immunogen may avoid an autoreactive T cell response. Taken together, these data indicate that r4 × Aβ15 may have potential as a safe and effective AD vaccine and can be produced at a low cost.
Materials and Methods
Cell, vectors, host strains and reagents
The pGAPZαA vector was kindly provided by Pro. Peng Zhou (Institute of Tropical Bioscience and Biotechnology, CATAS). Plasmid pQE304 × Aβ15 was constructed by our lab.24 Restriction enzyme and T4DNA were purchased from Takara. Primers were synthesized by Sangon Biotechnology Corp. Mouse anti-human Aβ1–42 monoclonal antibody was purchased from Sigma.
Construction of recombinant plasmid pGAPZαA4 × Aβ15
In the previous study, our lab has used templated PCR to synthesize a cDNA encoding a four tandem repeats of Aβ1–15 which was interlinked by spacers. Gly-Gly links the first Aβ15 and the second Aβ15 as well as the third and the fourth Aβ15. Gly-Ser-Ser-Gly (SacI recognition site) links the second and the third. The cDNA was cloned into pQE304 × Aβ15 and expressed in M15 (E.coli),24 but the expression level is very low, so we try to make the cDNA express in P.pastoris. To express mature 4 × Aβ15 with its native N-terminus, the signal sequence with kex2 cleavage site (Lys-Arg) was introduced upstream of the N-terminal 4 × Aβ15 protein sequence by PCR. The forward primer (5′- CCG CTC GAG AAA AGA GAT GCA GAA TTC CGA CAT- 3′) contained a Xho I site (underlined). The reverse primer (5′-GC TCT AGA TCA TTG ATG ATG AAC TTC ATA-3′) contained a XbaI site (underlined) and a stop codon (bold sequence). Using pQE304 × Aβ15 as template, cDNA encoding 4 × Aβ15 was amplified .The PCR product was digested with XhoI and XbaI and then subcloned into Xho I-XbaI site of pGAPZαA to yield expression plasmid named pGAPZαA4 × Aβ15 using T4 ligase. Escherichia coli DH5α was transformed with this recombinant plasmid and selected on Low salt LB plates containing 25 μg/ml Zeocin. Single colonies were selected and the sequences of the isolated plasmids were analyzed to verify the presence of the correct insert.
Expression of 4 × Aβ15 in P.pastoris
The recombinant expression plasmids were linearized with AvrII and transformed into competent P. pastoris strain GS115 by electroporation using a Micropulser (Bio-Rad). Transformants were selected on YPD plates containing 100 μg/ml Zeocin. The resistant clone was further screened by PCR and sequencing analysis. To highly express 4 × Aβ15, a confirmed positive clone was further cultured on YPD plates containing 500, 1000, 2000, 3000 μg/ml Zeocin. High-resistance clones were obtained from YPD plates containing 500, 1000, 2000 μg/ml Zeocin, and their expression level was analyzed by 12% SDS-PAGE. The highest-yield clone was cultured for 5 d. The supernatant was collected daily and analyzed by performing SDS-PAGE followed by western blotting analysis.
Protein analysis by SDS-PAGE and western blotting
The r4 × Aβ15 protein was electrophoresed on a 12% polyacrylamide gel with 5% stacking gel. The gel was then stained with Coomassie brilliant blue and the protein lane scanned using a gel image analysis system to calculate the percentage of r4Aβ15. The proteins separated by electrophoresis were transferred from the gel onto a NC (nitrocellulose) membrane (Millipore) using a semi-dry electroblotting apparatus (Bio-Rad) at 30 mA for 2 h. The membrane was blocked by incubating with a solution containing 1% BSA for 2 h, and then incubated with mouse anti-human Aβ1–42 monoclonal antibody (1:2000 dilution, Sigma). After washing twice for 5 min each, the membrane was incubated with goat anti-mouse IgG conjugated to HRP(horseradish peroxidase), diluted 1:500 in washing buffer. The bound antibody was detected using DAB.
Liter-scale production and purification
Using the highest-yield expressing colony, 10 mL of YPD was inoculated and the culture was incubated at 28°C overnight. Next day, 2 mL of this overnight culture was used to inoculate 500 mL of YPD in a 2 L flask, and the culture was grown at 28°C for 4 d. The culture supernatant was harvested by centrifugation at 5,000 rpm and precipitated with 70% saturated ammonium sulfate. Then the precipitant was dissolved into sterilized ddH2O and dialyzed to remove ammonium sulfate. After dialysis, adjust the precipitants to pH 8.0 using 1N NaOH. Then it was loaded onto a UNOsphere Q column (5 mL, Bio-Rad) at a rate of 5 mL/min. The UNOsphere Q column was equilibrated with 25 mL 20 mmol Tris-Hcl (pH 8.0), then washed with the same buffer at a rate of 7.5 mL/min. The bound protein was eluted with a linear salt gradient (0–0.5 M NaCl). The eluted fractions containing r4 × Aβ15 were separated by 12% SDS-PAGE. Gel densitometry was used to quantify the proportion of purifed proteins among the eluates. The quantity of the purified protein was determined by the Bradford method.25
Protein identification by mass spectrometry and N-terminal amino acid sequence
The purified protein was run by 12% SDS–PAGE. After staining with 0.1% Coomassie brilliant blue R-250/50% methanol, the corresponding band was excised and digested with trypsin which cleaves on the carbonyl side of lysine or arginine and the fragments analyzed by MALDI-TOF-TOF-MS. The peptide mass fingerprinting (PMF) can then be used to search databases to identify the protein.26,27 The N-terminal sequence of purified r4 × Aβ15 was determined by automated Edman degradation performed on a protein sequencer model 491 (Applied Biosystems Company) at the National Center of Biomedical Analysis.
Immunological Properties of r4 × Aβ15 Vaccine
Animals and immunization
Six- to eight-week old wild type C57BL/6 mice were from the Animal Centre of SunYat-sen University, with six mice in each treatment group. Aβ42 was synthesized in Shanghai Sangon. Each group were immunized seven times (biweekly) subcutaneously (s.c.) with 200 μl of a 1:1 mixture of antigen (100 μg Aβ42, 100 μg purified r4 × Aβ15, PBS)and adjuvant . Complete Freund’s Adjuvant(CFA) was used in the first injection, and Incomplete Freund’s Adjuvant(IFA)in subsequent shots. Blood was collected from the tail seven days after antigen injection.
Detection of anti-human Aβ antibodies in the serum from immunized mice
ELISA (ELISA) was performed as described previously,28 96-microwell plates were coated with 5 μg/ml glutathione S-transferase- Aβ42 (GST- Aβ42) proteins extracted from the bacteria. Anti-Aβ antibody titers in the mouse sera were determined using 9F1 antibody (quantitive monoclonal anti-Aβ antibody, Calbio-chem, Germany) as the standard. OD450 values that were twice greater than background (usually > 0.08) were considered positive. To determine the specific isotypes produced, the mouse MonoAB ID kit (Zymed) was used according to manufacturer's instructions. IgG1/IgG2a ratios were assessed, and a Th2 response was defined as an IgG1/IgG2a ratio exceeding 1.29
Detection of Aβ plaques in a Tg2576 mouse
Sera from immunized mice were screened for the ability to bind to Aβ plaques in an Tg2576 mouse brain. Briefly, pooled sera were added to the deparaffinized and hydrated sections of Tg2576 mouse brain. The titer of mice antisera were 1:100 dilution. As a negative control, we used the same dilution of PBS group sera as a positive control, monoclonal anti-human Aβ antibody 9F1 was used to immunostain plaques in brain sections of the Tg2576 mouse. Binding of antibodies to the brain sections was determined via using SABC-HRP/DAB(Mouse IgG)(EIAab Science Co.,Ltd,Wuhan, China), according to manufacturer recommendations. A digital camera was used to view the plaques at 100 × image magnification
Splenocyte proliferation assay
Splenocyte were isolated and harvested using standard methods as previously reported.30 Aβ40 and r4 × Aβ15 were added to cultures in triplicate at a final concentration of 10 µg/ml. To measure proliferation, 1 μCi of 3H-thymidine was added to cells at 72 h. Eighteen hours later, cells were harvested and thymidine incorporation determined using a liquid scintillation counter, A stimulation index was calculated using the following formula: CPM of well with antigen/CPM with no antigen; SI > 2.0 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Quanrong Shang for critical reading of the manuscript. This research was supported by funds from the National Natural Science Foundation of PR China (No.30400512).
Glossary
Abbreviations:
- Aβ
amyloid β
- AD
Alzheimer disease
- ELISA
Enzyme-linked immunoabsorbent assay
- GST
glutathione S-transferase
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- DAB
diaminobenzidine SI, stimulation index
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/20472
References
- 1.Näslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci U S A. 1994;91:8378–82. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan D, Gitter BD. Evidence supporting a role for anti-Abeta antibodies in the treatment of Alzheimer’s disease. Neurobiol Aging. 2004;25:605–8. doi: 10.1016/j.neurobiolaging.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Weksler ME, Gouras G, Relkin NR, Szabo P. The immune system, amyloid-beta peptide, and Alzheimer’s disease. Immunol Rev. 2005;205:244–56. doi: 10.1111/j.0105-2896.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 4.Frazer ME, Hughes JE, Mastrangelo MA, Tibbens JL, Federoff HJ, Bowers WJ. Reduced pathology and improved behavioral performance in Alzheimer’s disease mice vaccinated with HSV amplicons expressing amyloid-beta and interleukin-4. Mol Ther. 2008;16:845–53. doi: 10.1038/mt.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–9. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 6.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat Rev Immunol. 2006;6:404–16. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 7.Geylis V, Kourilov V, Meiner Z, Nennesmo I, Bogdanovic N, Steinitz M. Human monoclonal antibodies against amyloid-β from healthy adults. Neurobiol Aging. 2005;26:597–606. doi: 10.1016/j.neurobiolaging.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–97. doi: 10.1016/S0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–6. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 10.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with β-amyloid. Int Immunol. 2003;15:505–14. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, et al. Increased T cell reactivity to amyloid β protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–22. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, et al. Epitope and isotype specificities of antibodies to β -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, et al. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci. 2007;27:12721–31. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, et al. Short amyloid-β (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–28. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamikonyan G, Necula M, Mkrtichyan M, Ghochikyan A, Petrushina I, Movsesyan N, et al. Anti-A β 1-11 antibody binds to different β-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J Biol Chem. 2007;282:22376–86. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–6. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 17.Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, et al. Dendrimeric Abeta1-15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007;28:813–23. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, et al. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–28. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Novel Abeta immunogens: is shorter better? Curr Alzheimer Res. 2007;4:427–36. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- 20.Perkins DL, Berriz G, Kamradt T, Smith JA, Gefter ML. Immunodominance: intramolecular competition between T cell epitopes. J Immunol. 1991;146:2137–44. [PubMed] [Google Scholar]
- 21.Wan L, Cai HW, Yang H, Lu YR, Li YY, Li XW, et al. High-level expression of a functional humanized single-chain variable fragment antibody against CD25 in Pichia pastoris Appl Micro boil Biotechnol. 81(2008):33–41. [DOI] [PubMed]
- 22.Cregg JM, Vedvick TS, Raschke WC. Recent advances in the expression of foreign genes in Pichia pastoris. Biotechnology (N Y) 1993;11:905–10. doi: 10.1038/nbt0893-905. [DOI] [PubMed] [Google Scholar]
- 23.Livingston B, Crimi C, Newman M, Higashimoto Y, Appella E, Sidney J, et al. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 24.Lu L. Optimized Construction and over expression of a prokaryotic expressionvector of a tandem repeat of four Aβ1-15 sequences. Dissertation, Sun Yat-sen University. 2006:22-28. [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Blackstock WP, Weir MP. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999;17:121–7. doi: 10.1016/S0167-7799(98)01245-1. [DOI] [PubMed] [Google Scholar]
- 27.Yates JR., 3rd Mass spectrometry. From genomics to proteomics. Trends Genet. 2000;16:5–8. doi: 10.1016/S0168-9525(99)01879-X. [DOI] [PubMed] [Google Scholar]
- 28.Qu BX, Xiang Q, Li L, Johnston S A, Hynan LS, Rosenberg RN. Abeta(42) gene vaccine prevents Abeta (42) deposition in brain of double transgenic mice. J Neurol Sci. 2602007):204–13. [DOI] [PMC free article] [PubMed]
- 29.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–58. [PubMed] [Google Scholar]
- 30.Seabrook TJ, Iglesias M, Bloom JK, Spooner ET, Lemere CA, Lemere CA. Differences in the immune response to long term Abeta vaccination in C57BL/6 and B6D2F1 mice. Vaccine. 2004;22:4075–83. doi: 10.1016/j.vaccine.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 31.Gygi SP, Aebersold R. Mass spectrometry and proteomics. Curr Opin Chem Biol. 2000;4:489–94. doi: 10.1016/S1367-5931(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 32.Patterson SD, Aebersold R. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis. 1995;16:1791–814. doi: 10.1002/elps.11501601299. [DOI] [PubMed] [Google Scholar]
- 33.Patterson SD. Matrix-assisted laser-desorption/ionization mass spectrometric approaches for the identification of gel-separated proteins in the 5-50 pmol range. Electrophoresis. 1995;16:1104–14. doi: 10.1002/elps.11501601187. [DOI] [PubMed] [Google Scholar]
- 34.Werten WT. Marc, WisselinkH, Wouter, Jansen-van den Bosch, Tanja J et al. Protein engineerning. Oxford University Press, New York, (2001):447-454. [Google Scholar]
- 35.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 36.Oledzka G, Dabrowski S, Kur J. High-level expression, secretion, and purification of the thermostable aqualysin I from Thermus aquaticus YT-1 in Pichia pastoris. Protein Expr Purif. 2003;29:223–9. doi: 10.1016/S1046-5928(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 37.Goodrick JC, Xu M, Finnegan R, Schilling BM, Schiavi S, Hoppe H, et al. High-level expression and stabilization of recombinant human chitinase produced in a continuous constitutive Pichia pastoris expression system. Biotechnol Bioeng. 2001;74:492–7. doi: 10.1002/bit.1140. [DOI] [PubMed] [Google Scholar]
- 38.Chen GH, Yin LJ, Chiang IH, Jiang ST. Expression and purification of goat lactoferrin from Pichia pastoris expression system. J Food Sci. 2007;72:M67–71. doi: 10.1111/j.1750-3841.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 39.Scorer CA, Clare JJ, McCombie WR, Romanos MA, Sreekrishna K. Rapid selection using G418 of high copy number transformants of Pichia pastoris for high-level foreign gene expression. Biotechnology (N Y) 1994;12:181–4. doi: 10.1038/nbt0294-181. [DOI] [PubMed] [Google Scholar]
- 40.Vassileva A, Chugh DA, Swaminathan S, Khanna N. Effect of copy number on the expression levels of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Protein Expr Purif. 2001;21:71–80. doi: 10.1006/prep.2000.1335. [DOI] [PubMed] [Google Scholar]
- 41.Wang AP, Wang S, Shen MQ, Chen F, Zou Z, Ran X, et al. High level expression and purification of bioactive human α-defensin 5 mature peptide in Pichia pastoris. Appl Microbiol Biotechnol. 2009;84:877–84. doi: 10.1007/s00253-009-2020-x. [DOI] [PubMed] [Google Scholar]
- 42.Shen M, Wang Q, Mu X, Xu H, Yan W.Expression, purification and characterization of recombinant human beta-amyloid 1-42 in Pichia pastoris. Protein expr purify. 2009; 2:84-8. [DOI] [PubMed]
- 43.Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CAA. Abeta1-15 is less immunogenic than Abeta1-40/42 for intranasal immunization of wild-type mice but may be effective for “boosting”. Vaccine. 2003;21:2197–206. doi: 10.1016/S0264-410X(02)00754-5. [DOI] [PubMed] [Google Scholar]
- 44.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, et al. Epitope and isotype specificities of antibodies to β -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:10273–8. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–9. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 47.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 48.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr., Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 49.Zota V, Nemirovsky A, Baron R, Fisher Y, Selkoe DJ, Altmann DM, et al. HLA-DR alleles in amyloid β-peptide autoimmunity: a highly immunogenic role for the DRB1*1501 allele. J Immunol. 2009;183:3522–30. doi: 10.4049/jimmunol.0900620. [DOI] [PubMed] [Google Scholar]