Abstract
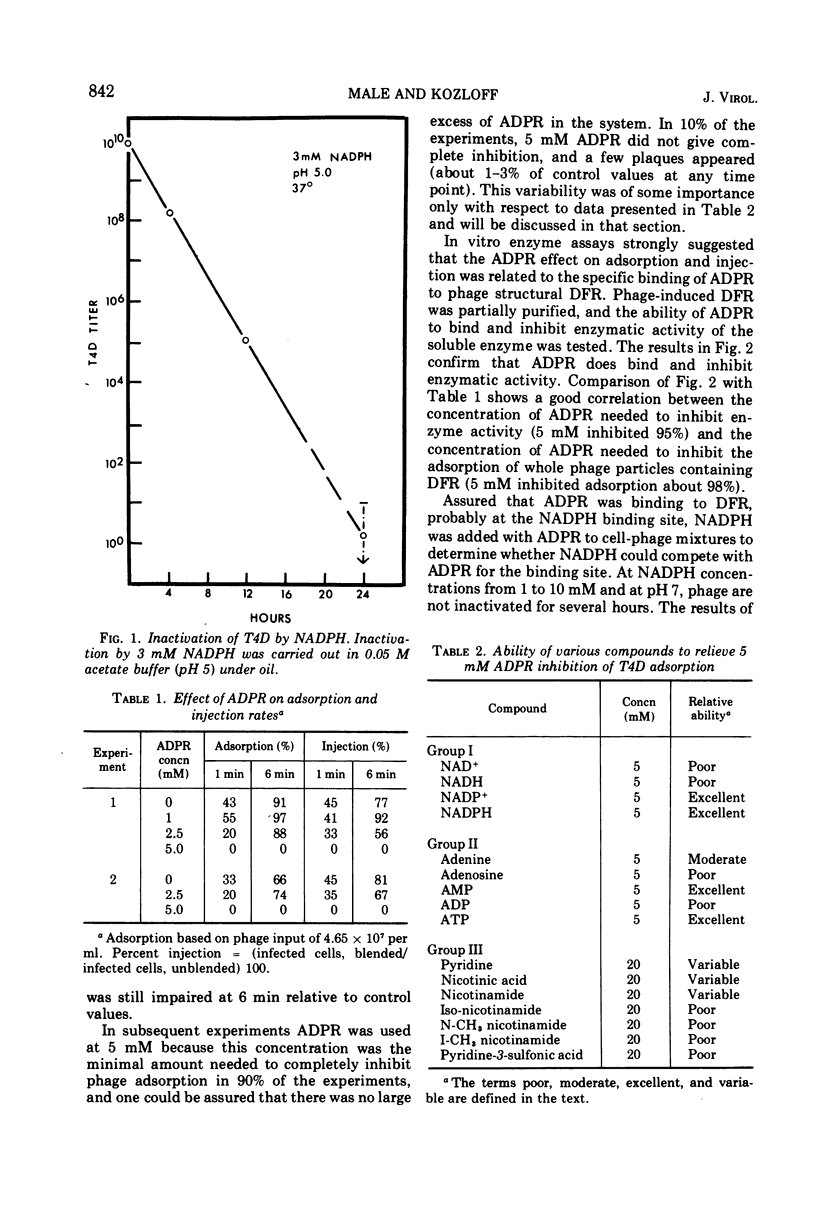
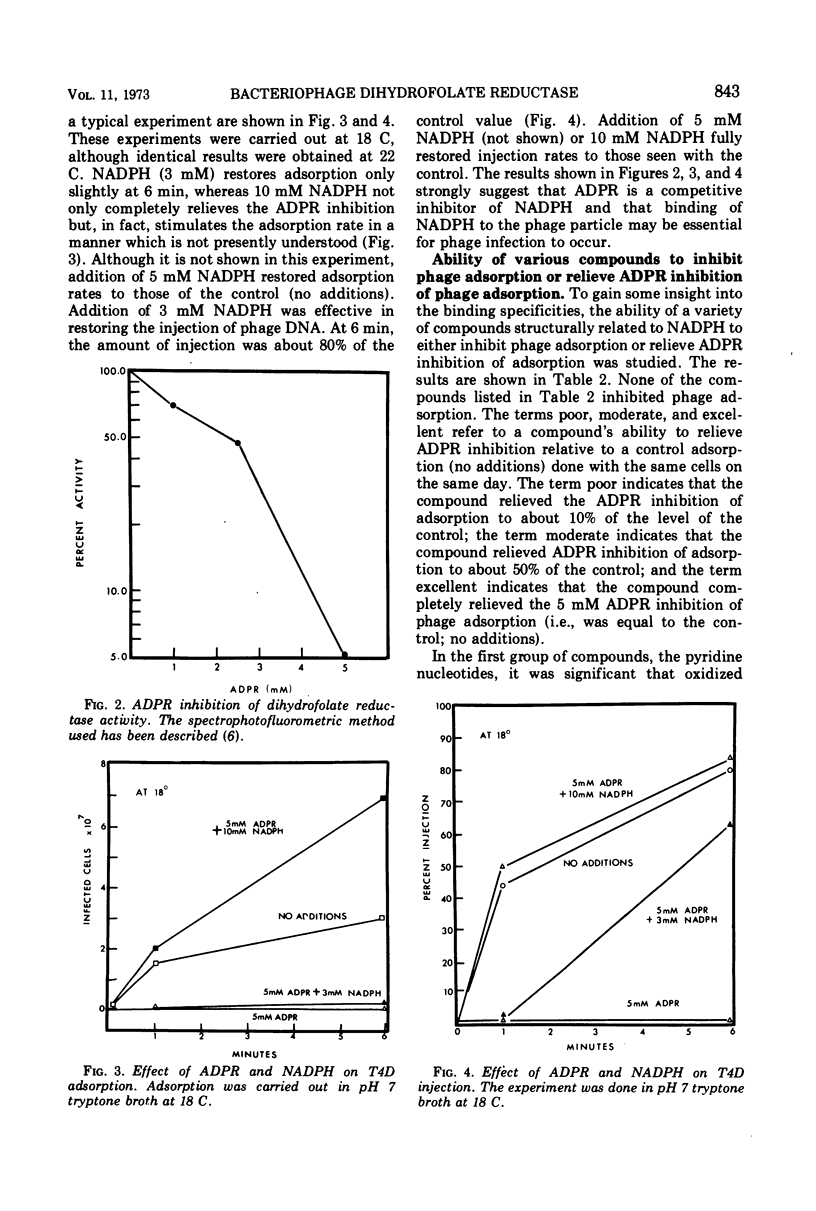
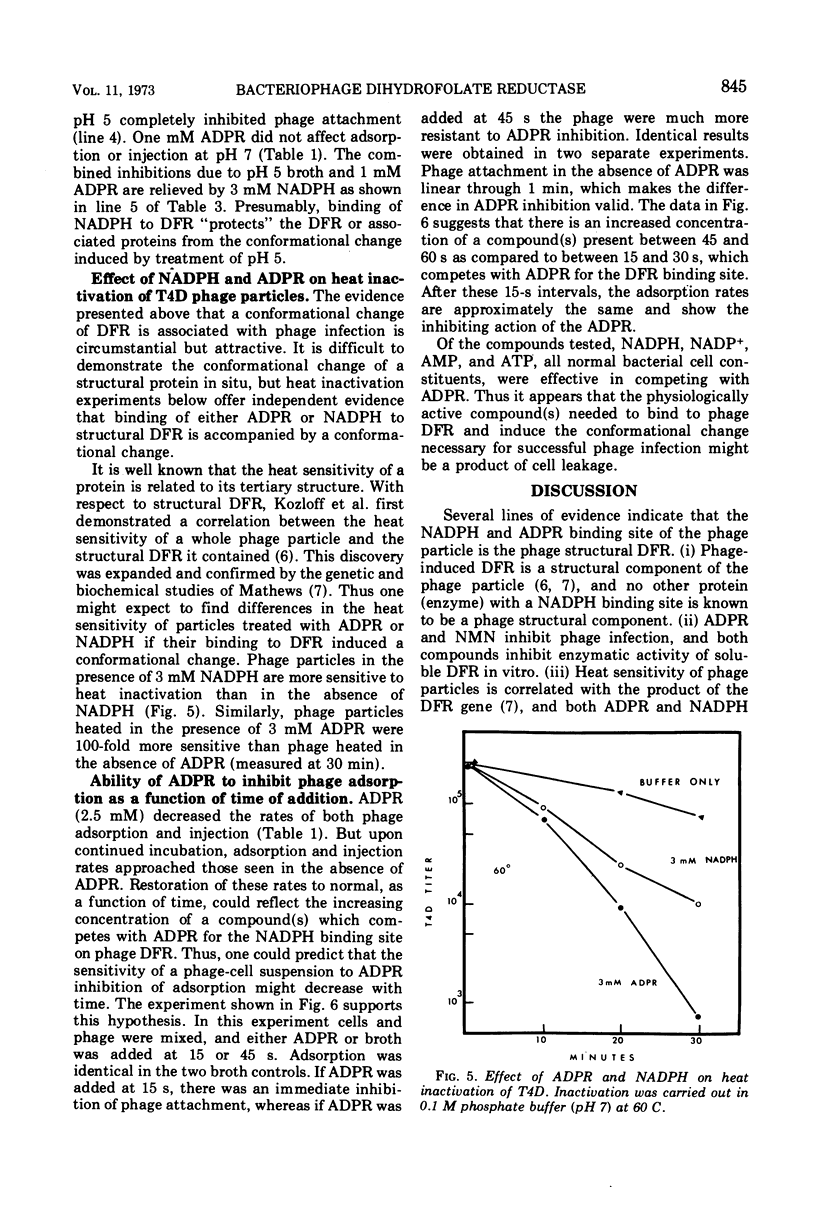
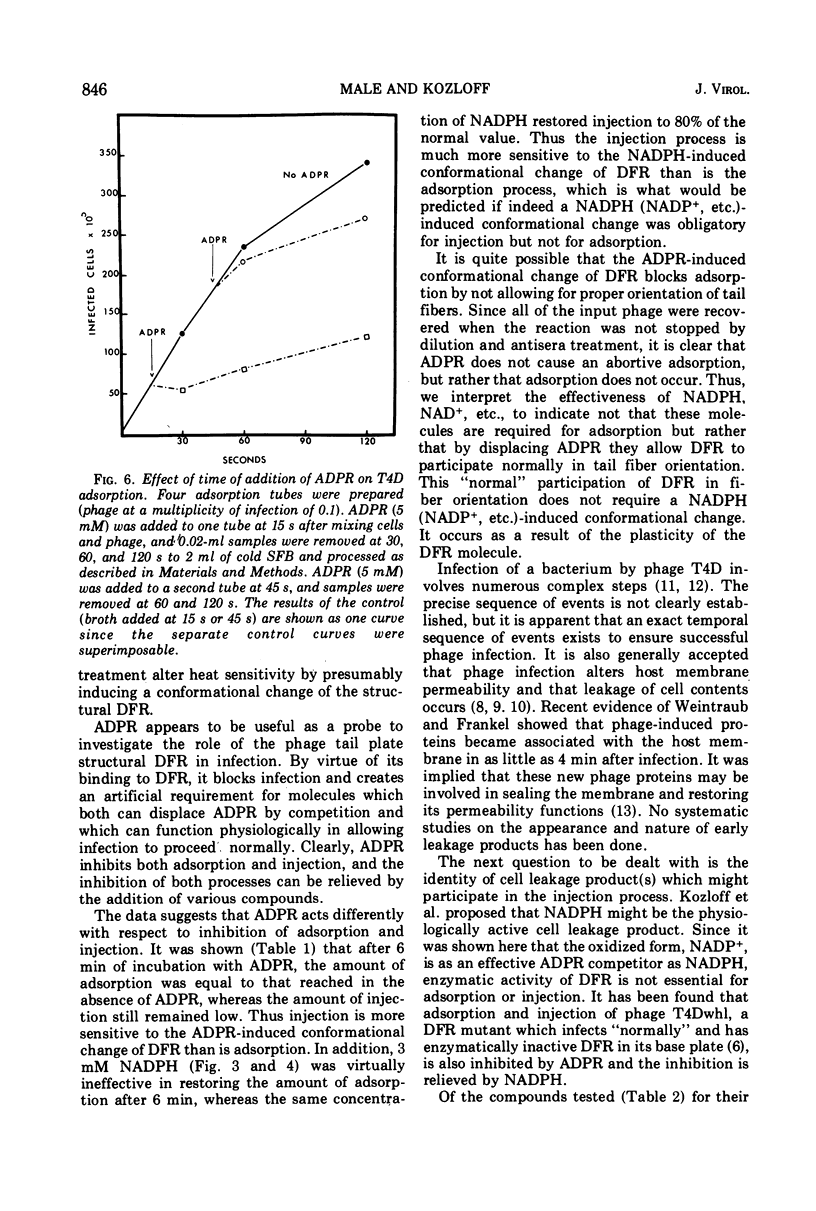
Various properties of the bacteriophage structural dihydrofolate reductase (DFR) have been examined to determine its function during phage infection. It has been found that a binding site for reduced nicotinamide adenine dinucleotide phosphate (NADPH), most likely on the DFR present in the phage tail plate, is required for phage viability. Attachment of adenosine diphosphoribose, an analogue of NADPH, to this site prevents phage adsorption and injection. This adenosine diphosphoribose inhibition can be competitively reversed by the addition of NADPH or oxidized nicotinamide adenine dinucleotide phosphate. It is suggested that, during phage infection, the host bacterial cell might leak compounds functionally similar to the pyridine nucleotides. These compounds have been shown to nonenzymatically change the conformation of the phage tail plate DFR which is apparently necessary for successful injection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchall J. J. Comparative studies of dihydrofolate reductase. Postgrad Med J. 1969 Nov;45(Suppl):29–32. [PubMed] [Google Scholar]
- Greenfield N. J., Williams M. N., Poe M., Hoogsteen K. Circular dichroism studies of dihydrofolate reductase from a methotrexate-resistant strain of Escherichia coli. Biochemistry. 1972 Dec 5;11(25):4706–4711. doi: 10.1021/bi00775a011. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I. T4 mutants unable to induce deoxycytidylate deaminase activity. Virology. 1966 Jun;29(2):339–345. doi: 10.1016/0042-6822(66)90041-9. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K., Rao N., Chapman V. A., DeLong S. S. Bacteriophage tail components. I. Pteroyl polyglutamates in T-even bacteriophages. J Virol. 1970 Jun;5(6):726–739. doi: 10.1128/jvi.5.6.726-739.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Verses C., Lute M., Crosby L. K. Bacteriophage tail components. II. Dihydrofolate reductase in T4D bacteriophage. J Virol. 1970 Jun;5(6):740–753. doi: 10.1128/jvi.5.6.740-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Identity of genes coding for soluble and structural dihydrofolate reductases in bacteriophage T4. J Virol. 1971 Apr;7(4):531–533. doi: 10.1128/jvi.7.4.531-533.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. I. An increase in host cell permeability induced by bacteriophage infection. J Exp Med. 1954 May 1;99(5):481–494. doi: 10.1084/jem.99.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. II. Demonstration of cyclic permeability change accompanying virus infection of Escherichia coli B cells. J Exp Med. 1955 Feb 1;101(2):151–175. doi: 10.1084/jem.101.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]


