Abstract
Introduction
Epimedium species (aka horny goat weed) have been utilized for the treatment of erectile dysfunction in Traditional Chinese Medicine for many years. Icariin (ICA) is the active moiety of Epimedium species.
Aim
To evaluate the penile hemodynamic and tissue effects of ICA in cavernous nerve injured rats. We also studied the in vitro effects of ICA on cultured pelvic ganglia.
Methods
Rats were subjected to cavernous nerve injury and subsequently treated for 4 weeks with daily gavage feedings of a placebo solution of normal saline and Dimethyl sulfoxide (DMSO) vs. ICA dissolved in DMSO at doses of 1, 5, and 10 mg/kg. A separate group underwent a single dose of ICA 10 mg/kg 2 hours prior to functional testing. Functional testing with cavernous nerve stimulation and real-time assessment of intracavernous pressure (ICP) was performed at 4 weeks. After functional testing, penile tissue was procured for immunohistochemistry and molecular studies. In separate experiments, pelvic ganglia were excised from healthy rats and cultured in the presence of ICA, sildenafil, or placebo culture media.
Main Outcome Measure
Ratio of ICP and area under the curve (AUC) to mean arterial pressure (MAP) during cavernous nerve stimulation of subject rodents. We also assayed tissue expression of neuronal nitric oxide synthase (nNOS), eNOS: endothelial nitric oxide synthase (eNOS), calponin, and apoptosis via immunohistochemistry and Western blot. Serum testosterone and luteinizing hormone (LH) were assayed using enzyme-linked immunosorbant assay (ELISA). Differential length of neurite outgrowth was assessed in cultured pelvic ganglia.
Results
Rats treated with low-dose ICA demonstrated significantly higher ICP/MAP and AUC/MAP ratios compared with control and single-dose ICA animals. Immunohistochemistry and Western blot were revealing of significantly greater positivity for nNOS and calponin in penile tissues of all rats treated with ICA. ICA led to significantly greater neurite length in cultured specimens of pelvic ganglia.
Conclusion
ICA may have neurotrophic effects in addition to known phosphodiesterase type 5 inhibiting effects.
Keywords: Complementary Medicine, Erectile Dysfunction, Cavernous Nerve Injury, Icariin, Neurotrophins, Testosterone
Introduction
The various species of the plant genus Epimedium have been utilized in Traditional Chinese Medicine (TCM) for centuries to treat a variety of human illnesses. Colloquially known as “horny goat weed” or “yin yang huo,” these plants have, as the name implies, been of particular interest for their perceived efficacy in the management of sexual concerns.
Recent investigations into the properties of these plants have suggested that the most metabolically active extract of Epimedium is icariin (ICA), a flavonol glycoside obtained from the aerial part of the plant [1]. ICA has been demonstrated to exert inhibitory activity against phosphodiesterase type 5 (PDE5) in vitro [2,3]. Modification of native ICA by addition of two hydroxyethyl ethers moieties enhances the PDE5 inhibitory activity 80-fold, near the level that is observed with sildenafil [3]. In addition to an erectogenic role, it has been suggested that ICA has testosterone-mimetic properties [4]. These effects lend credence to the use of ICA for the management of sexual problems.
ICA has been demonstrated to enhance eNOS expression and NO production in human endothelial cells as well as decrease caspase-3 expression and cellular apoptosis in response to hydrogen peroxide [5,6]. ICA has also been demonstrated to increase the intracavernous pressure (ICP) response triggered by cavernous nerve stimulation in rats; these effects were abolished by inhibitors of nitric oxide synthase and guanylate cyclase [7]. Furthermore, ICA has been used successfully to ameliorate both castration-related and arteriogenic impairment of erectile function and decline in penile neuron nNOS content in a rodent model [8,9]. To our knowledge, the utility of icariin for potentiation of erection in an animal model of radical pelvic surgery has not been explored. This is of particular interest given recent evidence suggesting that the commonly utilized regimen of routine dose therapy with commercially available PDE5 inhibitors may not lead to better erectile function outcomes after prostatectomy [10].
In this study we administered ICA as a daily supplement or a single dose in healthy 12-week-old rats that had undergone cavernous nerve crush injury. At study conclusion, subjects underwent functional testing of erectile hemodynamics during cavernous nerve stimulation as well as histological and molecular assessment of penile tissues. We also explored the neurotrophic effects of ICA in an in vitro rat major pelvic ganglion culture system.
Methods
ICA was procured by three-fold ethanol extraction of the aerial part of Epimedii herba (specifically, E. koreanum, E. sagittatum, and/or E. brevicornum). The product was dried with a vacuum. The dried extract was suspended in water and partitioned successively with n-hexane, CHCl3, and n-butyl alcohol. The n-butyl alcohol fraction was subjected to silica gel column chromatography to isolate ICA, which was further purified by repeated recrystallization with methanol. The final product was 98.8% ICA as determined by high-performance liquid chromatography analysis [11].
Fifty-eight healthy 12-week-old male Sprague Dawley rats (Harlan Laboratories, Indianapolis, Indiana, USA) were obtained and housed two per cage in standard rodent cages. All animals were given standard rat chow and tap water ad libitum and maintained in a temperature- and humidity-controlled room on 12-hour light/dark cycles. Live animals were handled in accordance with our institution's policy on animal husbandry; approval for all procedures on live animals was obtained from our Institutional Animal Care and Use Committee.
Cavernous nerve exposure with or without injury was performed as previously described [12]. Briefly, animals were anesthetized with isoflurane by inhalation. A laparotomy was performed and the cavernous nerves identified in the periprostatic space in all rats. In positive control animals (n = 12) no further intervention was performed. In all other animals the cavernous nerve was crushed distal to the major pelvic ganglion with a specially designated needle driver for a period of 2 minutes; this procedure was repeated on the contralateral side. After cavernous nerve crush the laparotomy was closed in two layers with absorbable suture. All animals received an opioid and an nonsteroidal anti-inflammatory drug (NSAID) by intraperito-neal (IP) injection in the perioperative period.
Starting on the day of nerve injury and continuing for 4 weeks, rats were given daily gavage feedings of a 50:50 mix of normal saline and DMSO in which was dissolved ICA at concentrations of 0 (positive and negative control groups, n = 12 each), 1 mg/mL (n = 10), 5 mg/mL (n = 12), and 10 mg/mL (n = 12). Animals were treated with the appropriate solutions at ICA doses of 0 (sham and negative control) 1 mg/kg, 5 mg/kg, or 10 mg/kg. Animals were weighed weekly and dosing was adjusted as appropriate. To facilitate gavage feedings animals were briefly exposed to isoflurane 2% in an induction chamber; after the animal was mildly sedated an olive tip 16-gauge feeding needle was advanced atraumatically into the animal's esophagus and the prescribed treatment was administered. To assess the effect of a single dose of ICA 10 mg/kg on penile hemodynamics, a separate group of six rats underwent cavernous nerve crush and received no treatment other than a single dose of ICA 10 mg/kg 2 hours prior to sacrifice and cavernous nerve stimulation.
At the 4-week time point all rats were anesthetized with ketamine and midazolam (100 and 5 mg/kg, respectively) by IP injection. All rats received a dose of saline/DMSO ± ICA at the appropriate concentration 2 hours prior to anesthesia. A repeat laparotomy was performed and the cavernous nerves were identified. The penis was denuded of overlying skin and cannulated with a heparinized 23-gauge needle connected to a real-time continuous pressure transducer. The cavernous nerves were then stimulated with a bipolar steel electrode; stimulation parameters were 50-second continuous trains at 20 Hz, 1.5 mAmp. Real-time response of the erectile tissue was determined by change in ICP. The maximum change in ICP was utilized for further analysis; additionally, the area under the curve (AUC) for all ICP assessments was quantitated by determining the number of pixels below the ICP curve during cavernous nerve stimulation using Image-Pro Plus v 5.1 (Media Cybernetics, Bethesda, MD, USA).
After functional testing, systemic blood pressure was measured via aortic cannulation. Mean arterial pressure (MAP) was calculated by the formula MAP = (diastolic blood pressure + [(systolic blood pressure—diastolic blood pressure)/3]). Serum samples were obtained at the time of aortic cannulation. Whole blood was aspirated into a collection tube and allowed to clot for 30 minutes. Clotted blood was then centrifuged at 1,000 rpm for 15 minutes. Serum was decanted from the collection tube and stored at -80 centigrade until use. Serum testosterone levels in eight randomly selected rats from each group were assayed using the Parameter Testosterone Assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Serum LH levels from the same rats were assayed using a rat LH ELISA kit (Cusabio, Newark, DE, USA) according to the manufacturer's instructions.
After aortic cannulation, animals were euthanized with IP pentobarbital (200 mg/kg) and bilateral thoracotomy. Penile tissues were harvested for immunohistochemistry (stored in 3% formaldehyde with 0.05% picric acid for 4 hours followed by 30% sucrose in phosphate buffered saline) at 4°C until use.
A segment of the penis from all subject rats was fixed in optimal cutting temperature fixative and subsequently sectioned at 5 microns in a cryostat. Tissue sections were stained with mouse anti-nNOS (1:800; BD Transduction Laboratories, Franklin Lakes, NJ, USA), mouse anti-eNOS (1:11,000; Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-calponin antibody (1:500; Abcam, Cambridge, MA, USA) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; Roche Diagnostics Corporation, Indianapolis, IN, USA) using standard technique. Slides were examined by an observer blinded to treatment group. nNOS positivity was ascertained by counting positive fibers in four random high-power fields (hpf; 400×) of the midline dorsal neurovascular bundle of the penis. eNOS positivity was quantified by selection of four random 200× fields of the corporal bodies. Calponin positivity was ascertained by selection of four random 200× fields of the corporal bodies. TUNEL positivity was calculated by counting positive nuclei in four random hpf fields of the corporal bodies. All staining intensity was measured using Image-Pro Plus v 5.1 (Media Cybernetics, Bethesda, MD, USA).
For Western blot analysis, cellular protein samples from the penis were prepared by homogenization of cells in a lysis buffer containing 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium docecyl sulfate, aprotinin (10 μg/mL), leupeptin (10 μg/mL), and phosphate buffered saline. Cell lysates containing 20 mcg of protein were electrophoresed in sodium docecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Millipore Corp, Bedford, MA, USA). The membrane was stained with Ponceau S to verify the integrity of the transferred proteins and to monitor the unbiased transfer of all protein samples. Detection of target proteins on the membranes was performed with an electrochemiluminescence kit (Amersham Life Sciences Inc, Arlington Heights, IL, USA) with the use of primary antibodies for nNOS, eNOS, Calponin, and Caspase3 (all antibodies at 1:500 concentration, Abcam Inc, Cambridge, MA, USA) After hybridization of secondary antibodies, the resulting images were analyzed with ChemiImager 4,000 (Alpha Innotech Corporation, San Leandro, CA, USA) to determine the integrated density value of each protein band.
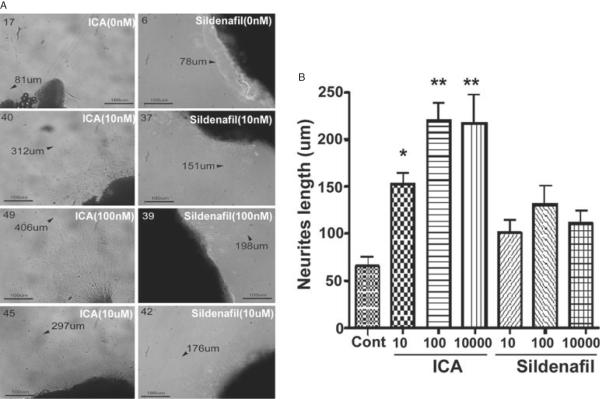
A separate group of twelve 12-week-old Sprague-Dawley rats underwent isoflurane anesthesia for harvest of the major pelvic ganglia (MPG) for the purpose of cell culture as previously described [13]. After harvest rats were euthanized with IP pentobarbital (200 mg/kg) and bilateral thoracotomy. Each dorsocaudal region of the MPG (from which the cavernous nerve originates) of subject rats was sectioned into three pieces and plated on a sterile cell culture well in a matrigel medium for a total of 72 specimens. All MPG fragments were incubated in Dulbecco's Modified Eagle medium (Sigma-Aldrich) in a humidified chamber with 5% CO2. To each culture well was added ICA or sildenafil at 10 nM, 100 nM, and 10 uM. Control specimens were incubated with phosphate buffered saline. At 48 hours, MPG fragments were examined under the dissecting microscope for neurite outgrowth. The longest neurite from each segment was measured; mean neurite length for each treatment group was calculated.
All data are reported as mean ± standard deviation. For statistical analysis, one-way anova was performed followed by Bonferroni multiple comparison's test for between-group differences. All calculations were performed using Prism version 4.2 (Graphpad Software, La Jolla, CA, USA). Statistical significance was set at P < 0.05.
Results
A gavage feeding mishap led to a single fatality in the sham surgery group during the study period. Additionally, a lethal reaction to ketamine anesthesia at the time of functional testing occurred in four rats (one each from all the ICA groups and the positive control group). In these subjects it was impossible to perform functional testing. After accounting for fatalities prior to functional testing the total number of rats per study group were 10, 11, 9, 11, and 11 for the sham, negative control, ICA 1, 5, and 10 mg/kg groups. Furthermore, tissues from four rats in the 5 mg/kg group became desiccated during storage and were not usable for immunohistochemistry; for this reason immunohistochemistry was not performed in tissues procured from rats in the 5 mg group.
ICP/MAP Assessment
Mean ICP/MAP and AUC/MAP ratio for all groups is presented in Figure 1A, B. Both measures were significantly lower in injured, untreated animals and single-dose ICA animals compared with uninjured, sham-positive controls (P < 0.01). Animals in the ICA 1 and 10 mg/kg treatment groups had significantly higher ICP/MAP ratios relative to control animals; animals that received ICA 5 mg/kg tended to have a higher mean ICP/MAP ratio, but the difference was not statistically significant (95% confidence interval [CI]—0.8187–0.03132). The difference in mean ICP/MAP ratio between the routine ICA treatment groups and the single-dose treatment group did not attain statistical significance, although this value trended toward significance for all three treatment groups. Animals that received ICA 1 mg/kg had significantly higher AUC/MAP ratios relative to both control animals and single-dose ICA animals (P < 0.05); there were no other significant differences in AUC/MAP ratios between groups.
Figure 1.
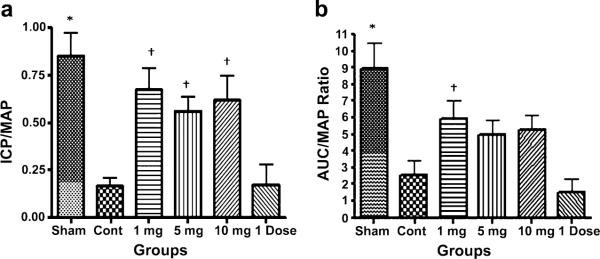
ICP/MAP assessment in cavernous nerve injured rats. (A) mean ICP/MAP ratio in all groups; (B) mean AUC/MAP ratio in all groups (*P < 0.01 vs. control group and single dose group, †P < 0.05 vs. control group and single dose group).
AUC = area under the curve; ICP = intracavernous pressure; MAP = mean arterial pressure.
nNOS Western Blot and Immunohistochemistry
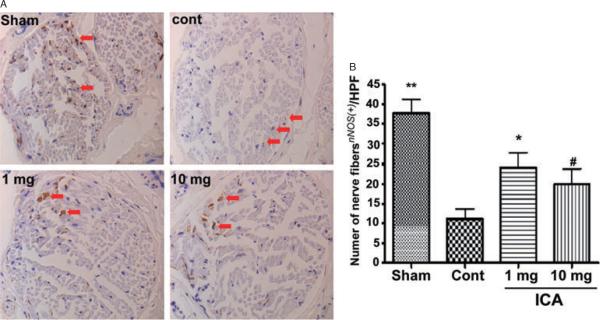
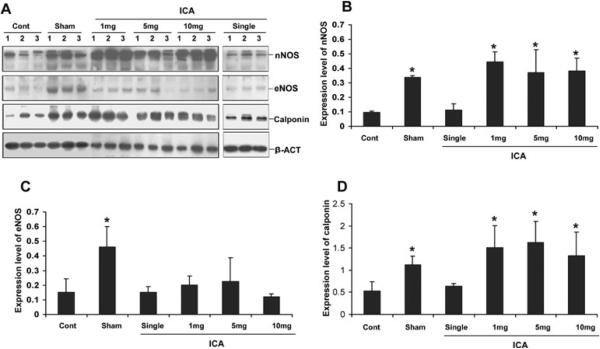
Representative images of nNOS staining are presented in Figure 2A. The mean number of nNOS positive fibers per hpf for each group is presented in Figure 2B. There was significantly greater positivity for nNOS fibers in the sham group compared with all others. The number of nNOS positive fibers was significantly lower in the control group relative to the 1 mg/kg group (P < 0.05, 95% confidence interval −22–−3) and nearly significantly lower compared with the 10 mg/kg group (P = 0.08, 95% CI —19—1). Western blot confirmed that the penile content of nNOS was significantly higher in all ICA treatment groups relative to control and single-dose ICA animals (Figure 3B).
Figure 2.
nNOS Immunohistochemistry. (A) representative images from rats in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups (arrows indicate small positive staining fibers); (B) mean nNOS positivity in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups (**P < 0.05 vs. all other groups, *P < 0.05 and #P < 0.08 vs. control group).
hpf = high-power fields; ICA = icariin.
Figure 3.
Western blot for nNOS, eNOS, and calponin. (A) Western blot normalized to alpha-actin; (B) mean nNOS positivity; (C) mean eNOS positivity; (D) mean calponin positivity (*P < 0.05 vs. control and single dose animals).
eNOS Western Blot and Immunohistochemistry
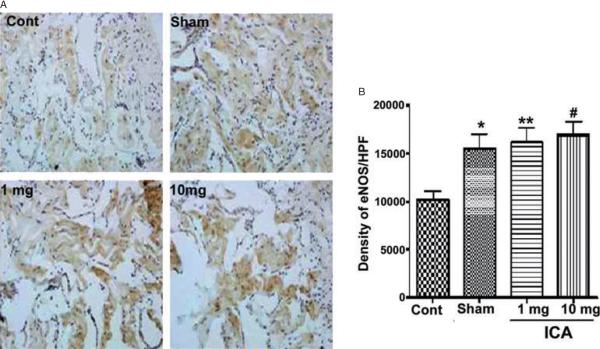
Representative images of eNOS staining are presented in Figure 4A. The mean intensities of alpha-actin positivity per 200× field for each group are presented in Figure 4A. The level of eNOS positivity in the control group was significantly lower relative to all other groups. There was no significant difference in positivity between ICA-treated and sham rats. Although histochemistry suggested greater eNOS content in ICA-treated rats, this finding was not confirmed on Western blot, which showed no significant differences between groups (Figure 3C).
Figure 4.
eNOS Immunohistochemistry. (A) representative images from rats in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups; (B) mean eNOS positivity in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups (*P < 0.05 relative to control animals).
ICA = icariin.
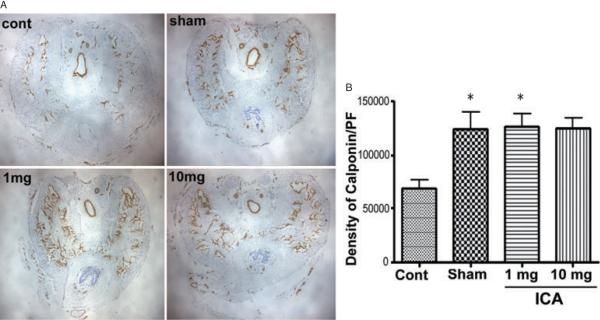
Calponin Western Blot and Immunohistochemistry
Protein expression of the smooth muscle marker calponin was significantly higher in sham animals and all ICA treatment groups relative to control animals (P < 0.05) (Figure 5A). Calponin staining was more intense in sham and ICA 1 mg/kg animals relative to untreated controls (Figure 5B). There was a trend toward lesser calponin positivity in control animals vs. 10 mg/kg animals, but the difference did not attain statistical significance (95% CI —113,900–1,816). Western blot confirmed that the penile content of calponin was significantly higher in all ICA treatment groups relative to control and single-dose ICA animals (Figure 3D).
Figure 5.
Calponin Immunohistochemistry. (A) Representative images from rats in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups. (B) mean calponin positivity in the sham, control, ICA 1 mg/kg, and ICA 10 mg/kg groups (*P < 0.05 relative to control animals).
ICA = icariin.
Caspase-3 Western Blot and TUNEL Immunohistochemistry
There were no significant differences in caspase-3 expression nor in TUNEL positivity between groups (data not shown).
Serum Testosterone and LH levels
Mean serum testosterone levels are presented in Figure 6A. Compared with testosterone levels reported in “sham animals” from other studies, our sham animals demonstrated serum testosterone levels that were similar [4] or slightly lower [14]. Testosterone levels in control and 1 mg/kg ICA-treated animals were higher than what was observed in sham animals, whereas higher doses of ICA were associated with lower serum testosterone levels. Serum testosterone in the 10 mg group was significantly lower than serum test-osterone in the control (P < 0.01, 95% CI for difference 1.2–5.8) and 1 mg/kg groups (P < 0.01, 95% CI for difference 1.8–4.8). No other differences between groups reached statistical significance. Serum LH levels are presented in Figure 6B. The standard deviation of the mean was large between groups; there was no significant difference in LH expression.
Figure 6.
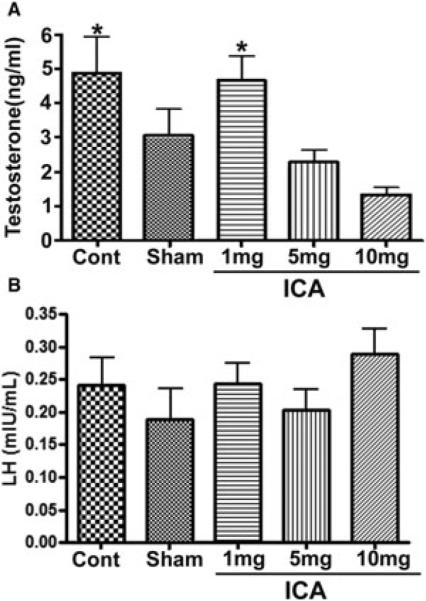
Gonadotropin and testosterone levels. (A) Serum testosterone level (*P < 0.05 relative to ICA 10 mg/kg animals); (B) serum LH level.
ICA = icariin.
In Vitro Assessment of Neurite Outgrowth
Representative images of neurite outgrowth from cultured MPG fragments are presented in Figure 7A. The mean length of neurites in the various groups is presented in Figure 7B. Neurite length was significantly enhanced in MPG fragments treated with ICA vs. control MPG. There was no significant difference in neurite length between control and sildenafil-treated MPG.
Figure 7.
Neurite outgrowth in cell culture under various treatment conditions. (A) Representative images from cultured major pelvic ganglia in the control (top two panels), ICA (left column) and sildenafil (right column). Arrows and numbers indicate length and position of longest neurite; (B) mean neurite length in the various treatment groups (*P < 0.05 vs. control, **P < 0.05 vs. control and all sildenafil groups).
ICA = icariin.
Discussion
ICA has garnered attention as a potential therapeutic agent for a variety of human disease states, including osteoporosis, congestive heart failure, and ischemic brain injury [15–18]. While ICA may have numerous applications in different fields of medicine, it is best known for its' putative role in potentiating sexual function by inhibition of PDE5I [2,3].
In the current study we provide functional evidence that ICA may have beneficial effects on erectile function after cavernous nerve injury. These effects may extend beyond PDE5 inhibition; we detected significantly higher nNOS expression and smooth muscle content in ICA-treated rats relative to untreated controls. Furthermore, it is suggested that eNOS was also up-regulated in endothelial cells of the penis after ICA treatment, although the evidence of this is somewhat less robust given the discrepancy between histochemical and Western blotting results for eNOS. Interestingly, it was low-dose ICA that produced the most consistently beneficial results.
Prior work has supported a role for ICA in enhancing erectile function in various animal models of erectile dysfunction (ED). Makarova et al. reported that ICA increases intromission and ejaculatory frequency and decreases latency period between ejaculations in aged (20–22 months) male rats treated with ICA at 300 or 750 mg/kg doses for 10 days [19]. Liu et al. reported that castrated rats treated with daily ICA over a 4-week period with a 7-day washout period had superior penile hemodynamics at the time of functional erectogenic testing compared with untreated castrated rats. The expression of nNOS in ICA-treated animals was higher than what was observed in controls, similar to the results of our study. Interestingly, in this study the 5 mg/kg dose produced a stronger ICP response relative to the 1 mg/kg dose [8].
In addition to a role in erectile physiology, a prior study suggested that ICA has been shown to correct hypogonadism induced by cyclophosphamide exposure in rats [4]. Zhang et al. reported that a higher concentration (200 mg/kg/day by oral gavage) of lower purity (40%) ICA was utilized for 7 days after cyclophosphamide treatment. Rodents in the ICA group had higher serum testosterone levels compared with animals not treated with cyclophosphamide and animals treated with cyclophosphamide alone [4]. These results are in contrast to the results of Liu et al. (who did not detect a difference in serum testosterone levels between ICA treatment animals and controls) [8] and the results of the current study, in which ICA at doses above 1 mg/kg appeared to have a dose-dependent suppressive effect on serum testosterone concentration. Differences in the purity and dosing interval of ICA as well as differences in the assay method may have played a role in these discrepancies.
Interestingly, in the one prior study demonstrating changes in serum T with ICA, there were no significant perturbations in LH and follicle stimulating hormone (FSH) level in rats in any of the treatment arms [4]; this result is similar to our own. It is implied that regardless of its effects on serum T, ICA does not impact pituitary LH expression. It is possible that ICA may influence aromatase or 5-alpha reductase activity in vivo and thereby modulate serum T levels without directly impacting LH secretion. It is clear that further research will be required to elucidate the impact and mechanisms of cavernous nerve injury and/or ICA therapy on T metabolism.
While both functional and molecular differences were apparent between our study groups, statistically significant differences were not observed in several key areas; the lack of significant difference in functional results between the higher-dose ICA groups and control animals is likely secondary to the small number of animals and subsequent lack of statistical power. However, it is apparent that even with the small number of animals low-dose ICA was effective at producing improved erectile responses. In the absence of a washout period we cannot definitively ascribe the observed differences in ICP/MAP ratios to a “penile rehabilitative” effect. It may be that the improvement noted was secondary to an acute PDE5 inhibitory effect of the drug on subject rats. The T1/2 of ICA has been reported to be approximately 1 hour [20]. Given the relatively low concentrations of ICA used in our study it seems likely that the remnant portion of ICA in the animal's tissues at the time of testing was low; nevertheless, we cannot rule out the possibility that a direct PDE5 inhibitor effect of the drug played a role in the ICP/MAP ratios observed. To address this possibility, we conducted an adjunctive study of single-dose ICA in six rats; values for ICP/MAP, AUC/MAP, and nNOS expression (by Western blot) were similar in these animals to controls, suggesting that single-dose ICA has marginal efficacy in this model system and supporting a rehabilitative role for ICA in this model system.
Cavernous nerve injury in our rats was associated with an increase (albeit an insignificant one) in serum testosterone relative to uninjured rats. This differs from a recent study that suggested that cavernous nerve injury is associated with centrally mediated hypogonadism in rats [14]. Variations in measurement method, rat strain, and mechanism of injury (transection in the other study compared to crush in ours) may have contributed to the observed differences and further research will be required to resolve this discrepancy. Furthermore, we did not detect any significant differences in apoptosis between sham and cavernous nerve injured animals; this contrasts with prior reports in which cavernous nerve injury increases expression of apoptotic markers [21–23]. However, in a study of the time course of apoptosis after cavernous nerve injury, User et al. reported a sharp increase in apoptosis after cavernous nerve injury that diminished to baseline over time, with the difference in apoptosis between injured and uninjured animals statistically significant but modest at 28 days post-injury with normalization of apoptotic markers at 60 days post-injury [24]. We theorize that cavernous nerve injury-mediated apoptosis had returned to near-baseline levels in all groups at the time of functional assay in this study, although we cannot rule out the possibility that a less severe injury was inflicted in our study relative to other similar studies.
Despite several important limitations, our study adds to the mounting evidence that ICA may play an important salubrious role with respect to maintenance of penile erectile function. In contrast to prior studies, our results suggest that very low-dose ICA (1 mg/kg in this study) may be of similar or even greater efficacy than higher doses. It is particularly intriguing that ICA had neurotrophic properties when cultured with nerve fragments. PDE5 inhibitors are the agents most commonly utilized for penile rehabilitation in humans [25], and to our knowledge no synthetic PDE5 inhibitor has been demonstrated to have significant neurotrophic effects, a finding confirmed in the sildenafil arm of our MPG culture experiments. Whether ICA would be a superior alternative to PDE5I remains to be determined; further studies of this interesting compound from nature may enhance our understanding and ability to treat men with ED.
Conclusions
Daily treatment with low-dose, purified ICA improves penile hemodynamic parameters 4 weeks after cavernous nerve injury in a rat model of ED. Improved functional outcomes in ICA-treated animals are associated with increased penile nNOS and smooth muscle content. Our findings are of interest not just as a validation of this traditional treatment for erectile problems but also as a new and potentially important means to study and treat nerve injuries in human patients.
Statement of Authorship.
Category 1
-
(a)
Conception and Design
Alan W. Shindel; Guiting Lin; Tom F. Lue; Zhong-Chen Xin
-
(b)
Acquisition of Data
Alan W. Shindel; Thomas M. Fandel; Yun-Ching Huang; Lia Banie
-
(c)
Analysis and Interpretation of Data
Alan W. Shindel; Benjamin N. Breyer; Ching-Shwun Lin; Tom F. Lue; Maurice M. Garcia
Category 2
-
(a)
Drafting the Article
Alan W. Shindel
-
(b)
Revising It for Intellectual Content
Alan W. Shindel; Yun-Ching Huang; Thomas M. Fandel; Zhong-Chen Xin; Guiting Lin; Tom F. Lue; Ching-Shwun Lin; Lia Banie; Benjamin N. Breyer; Maurice M. Garcia
Category 3
-
(a)
Final Approval of the Completed Article
Alan W. Shindel; Tom F. Lue; Guiting Lin; Zhong-Chen Xin; Thomas M. Fandel; Yun-Ching Huang; Lia Banie; Ching-Shwun Lin; Benjamin N. Breyer; Maurice M. Garcia
Acknowledgments
Funding for this study was provided by the California Urological Foundation. Salary support for one of the authors (AWS) during the time of the study was provided by the American Urological Association Foundation. We thank Lora Nunes and Guifang Wang for their assistance with immunohistochemistry.
Footnotes
Conflict of Interest: None.
References
- 1.Xin ZC, Kim E, Tian ZJ, Ling GT, Guo YL. Icariin on relaxation effect of corpus cavernosum smooth muscle. Chin Sci Bull. 2001;46:1186–90. [Google Scholar]
- 2.Ning H, Xin ZC, Lin G, Banie L, Lue TF, Lin CS. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology. 2006;68:1350–4. doi: 10.1016/j.urology.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Dell'Agli M, Galli GV, Dal Cero E, Belluti F, Matera R, Zironi E, Pagliuca G, Bosisio E. Potent inhibition of human phosphodiesterase-5 by icariin derivatives. J Nat Prod. 2008;71:1513–7. doi: 10.1021/np800049y. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZB, Yang QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8:601–5. doi: 10.1111/j.1745-7262.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang YK, Huang ZQ. Protective effects of icariin on human umbilical vein endothelial cell injury induced by H2O2 in vitro. Pharmacol Res. 2005;52:174–82. doi: 10.1016/j.phrs.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Xu HB, Huang ZQ. Icariin enhances endothelial nitric-oxide synthase expression on human endothelial cells in vitro. Vascul Pharmacol. 2007;47:18–24. doi: 10.1016/j.vph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Tian L, Xin ZC, Yuan YM, Fu J, Liu WJ, Wang LL. Effects of icariin on intracavernosal pressure and systematic arterial blood pressure of rat. Zhonghua Yi Xue Za Zhi. 2004;84:142–5. [PubMed] [Google Scholar]
- 8.Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L, Guo YL. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J Androl. 2005;7:381–8. doi: 10.1111/j.1745-7262.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 9.Tian L, Xin ZC, Liu WJ, Yang YM, Liu G, Chen L, Fu J, Wang LL. Effects of icariin on the erectile function and expression of nitrogen oxide synthase isoforms in corpus cavernosum of arterigenic erectile dysfunction rat model. Zhonghua Yi Xue Za Zhi. 2004;84:954–7. [PubMed] [Google Scholar]
- 10.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, Stief C. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–31. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 11.Xin ZC, Kim EK, Lin CS, Liu WJ, Tian L, Yuan YM, Fu J. Effects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activities. Asian J Androl. 2003;5:15–8. [PubMed] [Google Scholar]
- 12.Bella AJ, Hayashi N, Carrion RE, Price R, Lue TF. FK1706 enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Sex Med. 2007;4:341–6. doi: 10.1111/j.1743-6109.2007.00438.x. discussion 46-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Chen KC, Hsieh PS, Yeh CH, Lue TF, Lin CS. Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU Int. 2003;92:631–5. doi: 10.1046/j.1464-410x.2003.04439.x. [DOI] [PubMed] [Google Scholar]
- 14.Vignozzi L, Filippi S, Morelli A, Marini M, Chavalmane A, Fibbi B, Silvestrini E, Mancina R, Carini M, Vannelli GB, Forti G, Maggi M. Cavernous neurotomy in the rat is associated with the onset of an overt condition of hypogonadism. J Sex Med. 2009;6:1270–83. doi: 10.1111/j.1743-6109.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 15.Yin XX, Chen ZQ, Liu ZJ, Ma QJ, Dang GT. Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chin Med J (Engl) 2007;120:204–10. [PubMed] [Google Scholar]
- 16.Song YH, Li BS, Chen XM, Cai H. Ethanol extract from Epimedium brevicornum attenuates left ventricular dysfunction and cardiac remodeling through down-regulating matrix metalloproteinase-2 and -9 activity and myocardial apoptosis in rats with congestive heart failure. Int J Mol Med. 2008;21:117–24. [PubMed] [Google Scholar]
- 17.Nian H, Ma MH, Nian SS, Xu LL. Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine. 2009;16:320–6. doi: 10.1016/j.phymed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Zhang L, Chen ZB, Wu JY, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur J Pharmacol. 2009;609:40–4. doi: 10.1016/j.ejphar.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J Ethnopharmacol. 2007;114:412–6. doi: 10.1016/j.jep.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Lou YJ. Determination of icariin and metabolites in rat serum by capillary zone electrophoresis: Rat pharmacokinetic studies after administration of icariin. J Pharm Biomed Anal. 2004;36:365–70. doi: 10.1016/j.jpba.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP, Muller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, Tal R, Li PS, Cohen-Gould L, Scardino PT. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–36. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 22.Mulhall JP, Muller A, Donohue JF, Golijanin D, Tal R, Akin-Olugbade Y, Kobylarz K, Cohen-Gould L, Bennett NE, Scardino P. FK506 and erectile function preservation in the cavernous nerve injury model: Optimal dosing and timing. J Sex Med. 2008;5:1334–44. doi: 10.1111/j.1743-6109.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 23.Fandel TM, Bella AJ, Lin G, Tantiwongse K, Lin CS, Pohl J, Lue TF. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2008;5:1866–75. doi: 10.1111/j.1743-6109.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 24.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–9. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 25.Teloken P, Mesquita G, Montorsi F, Mulhall J. Post-radical prostatectomy pharmacological penile rehabilitation: Practice patterns among the international society for sexual medicine practitioners. J Sex Med. 2009;6:2032–8. doi: 10.1111/j.1743-6109.2009.01269.x. [DOI] [PubMed] [Google Scholar]