Abstract
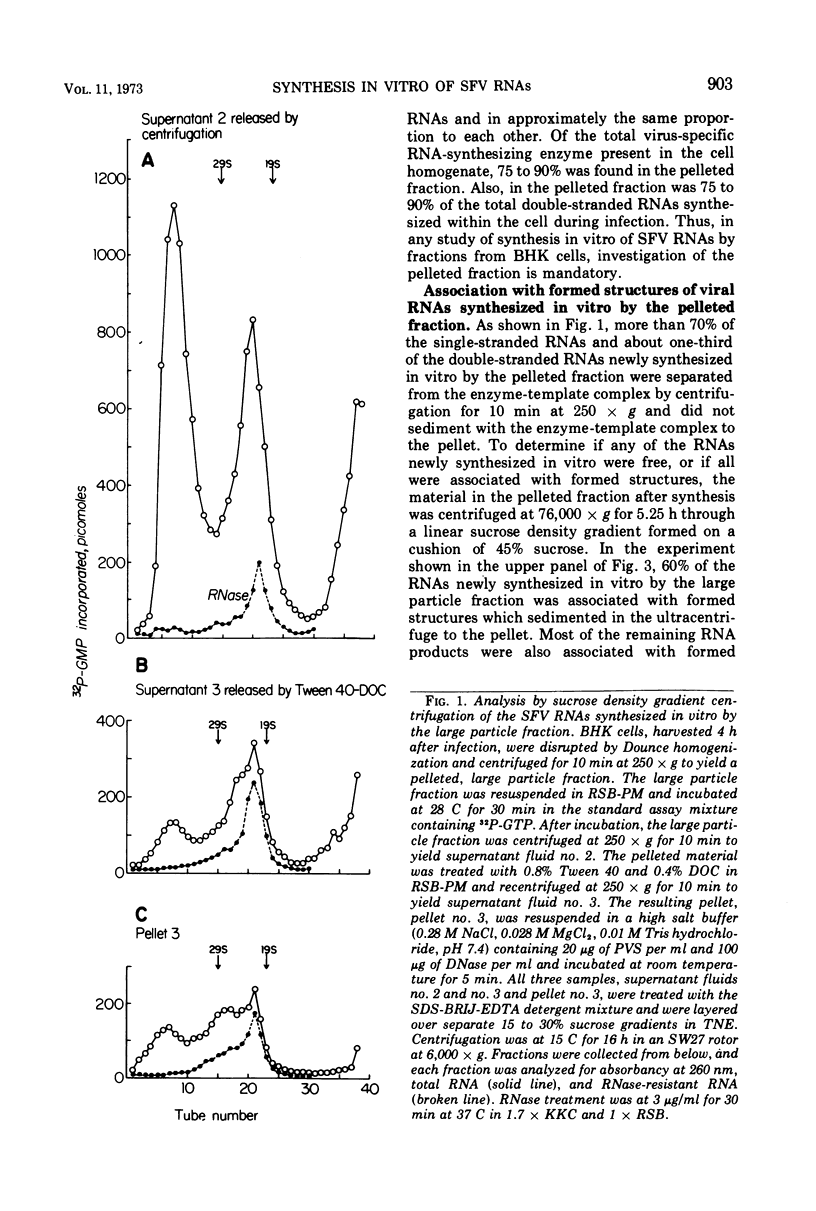
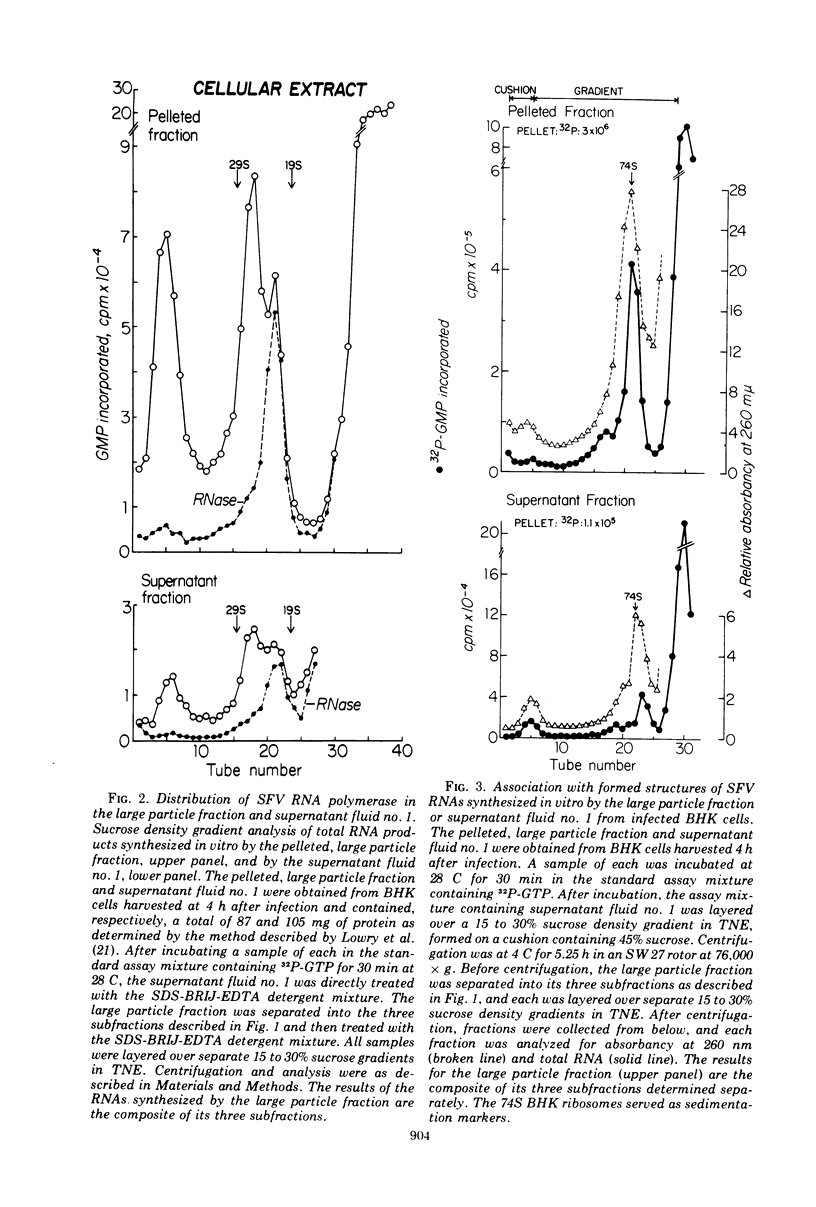
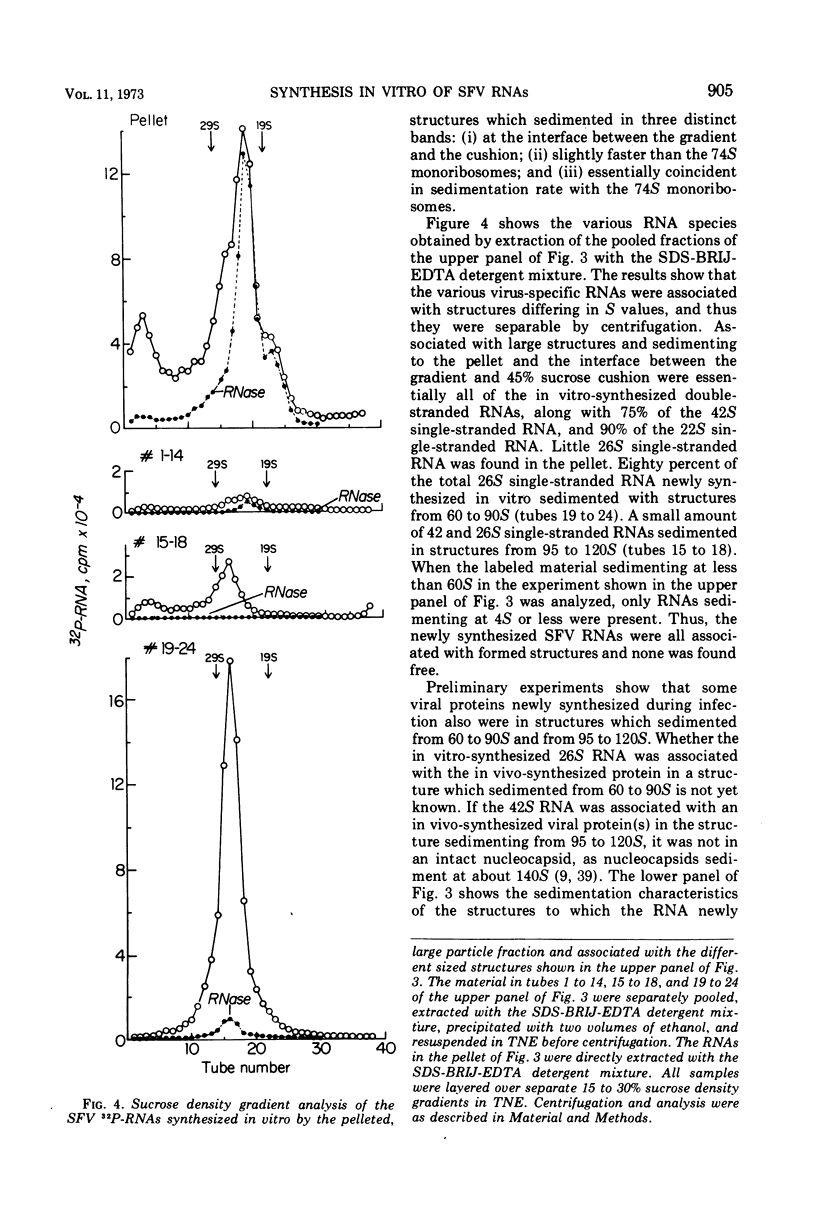
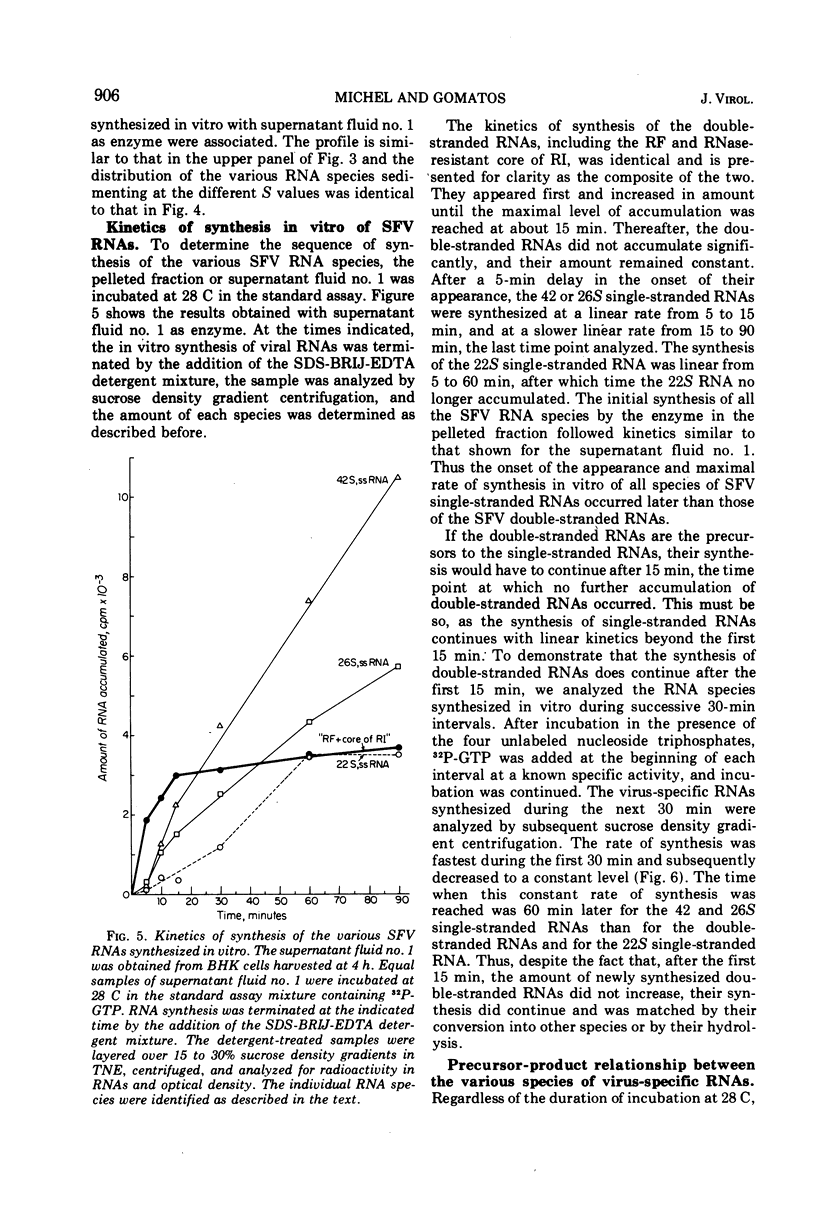
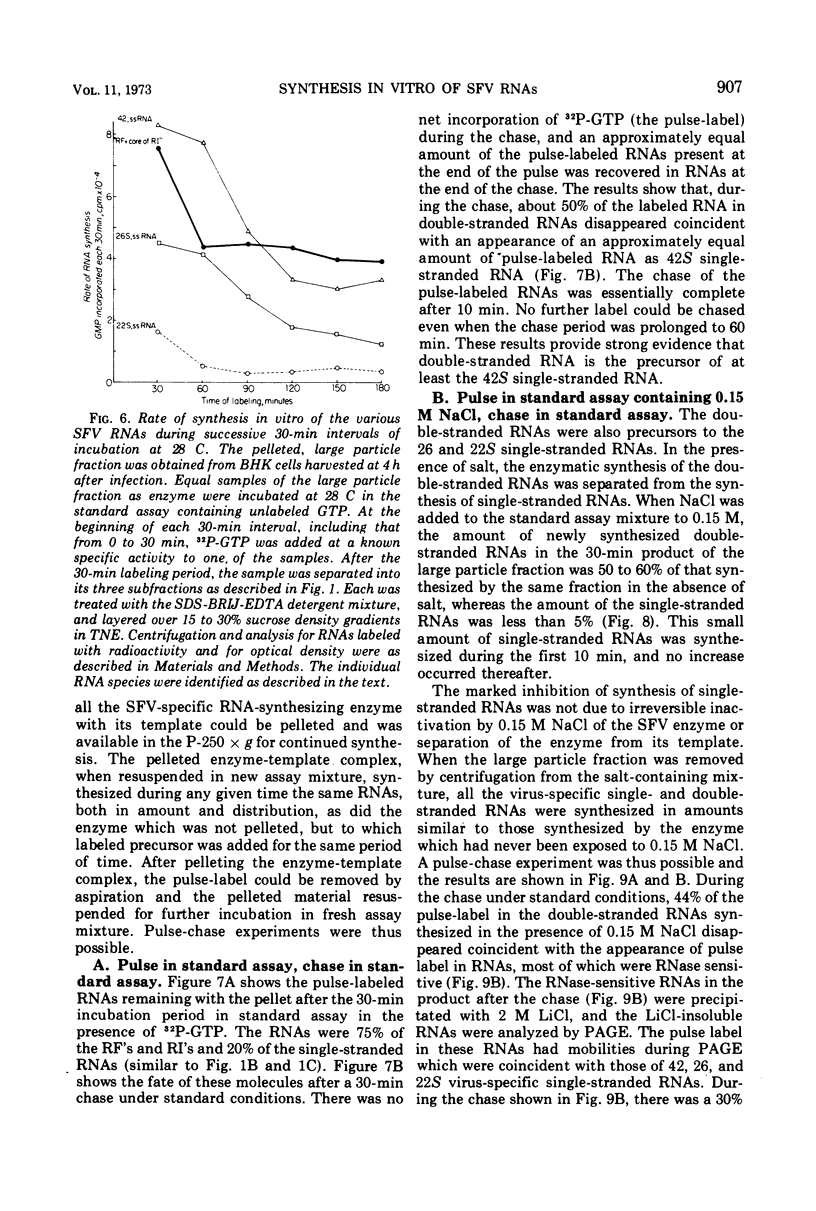
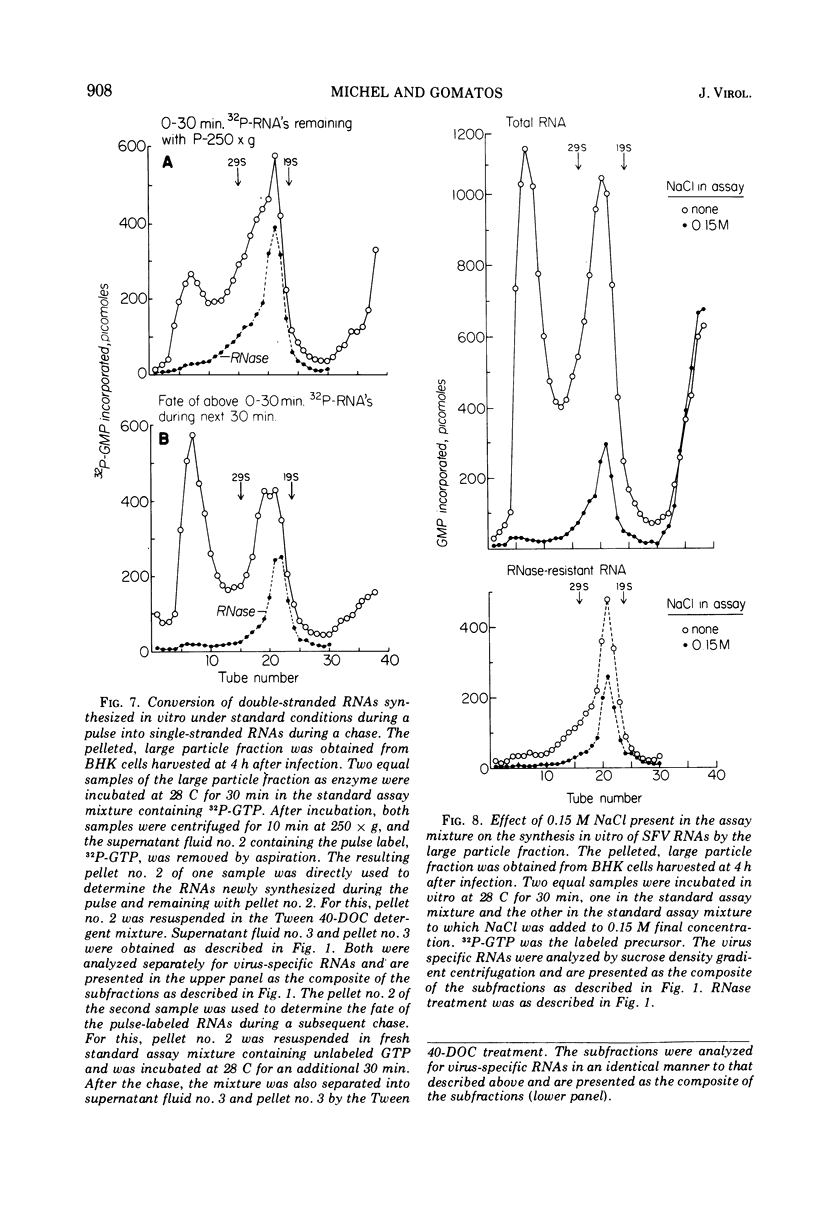
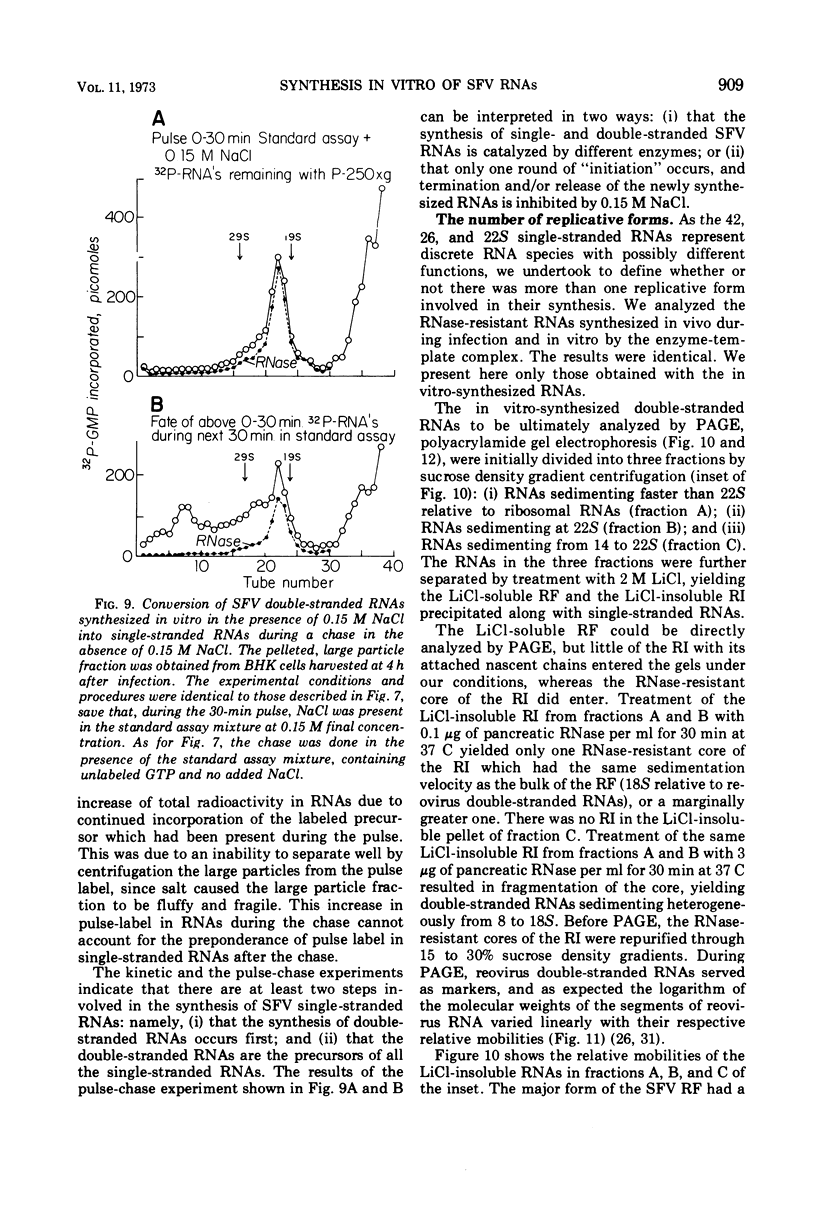
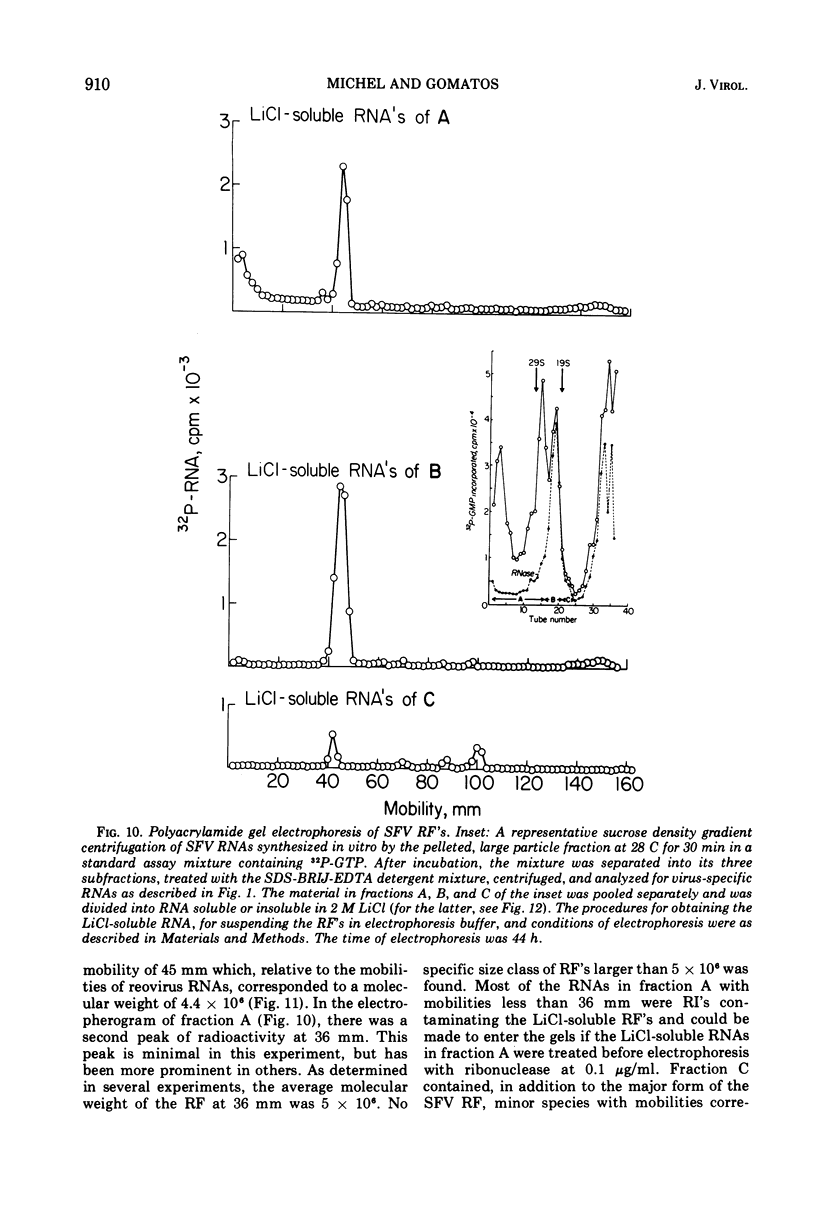
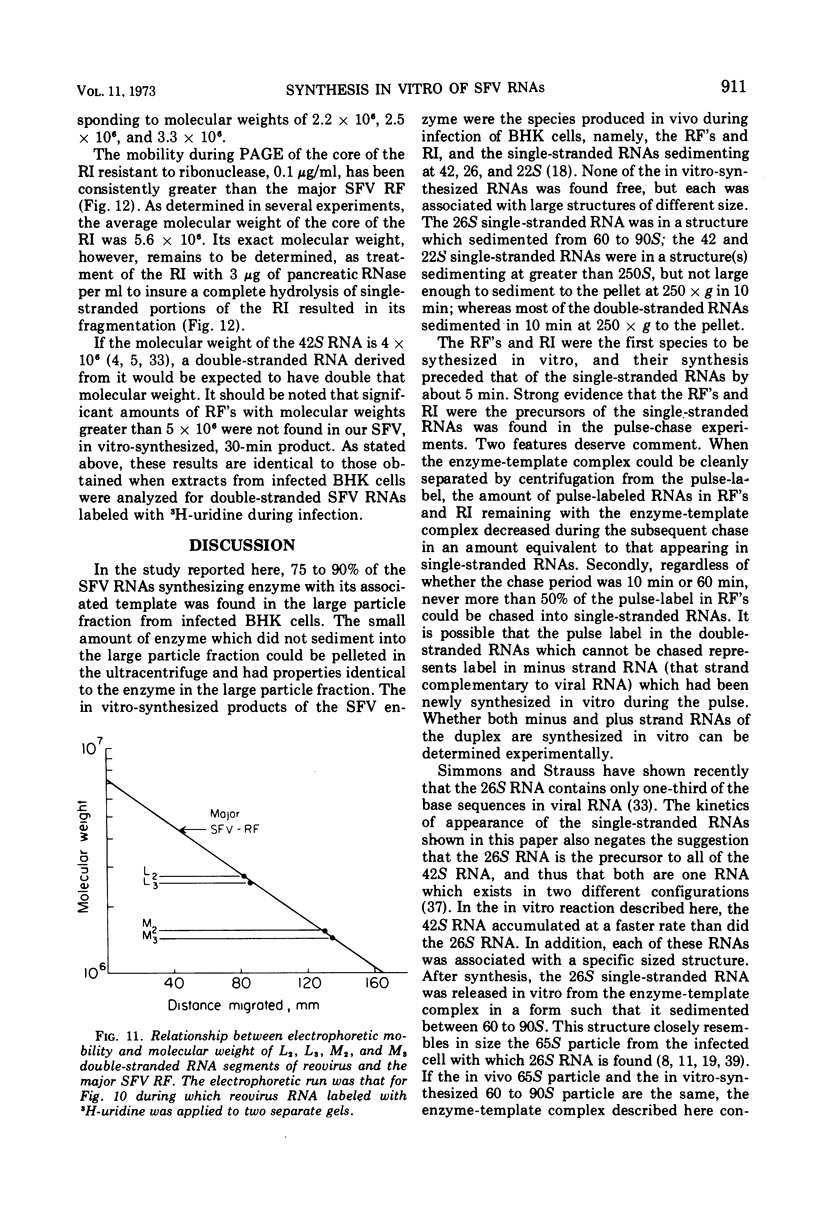
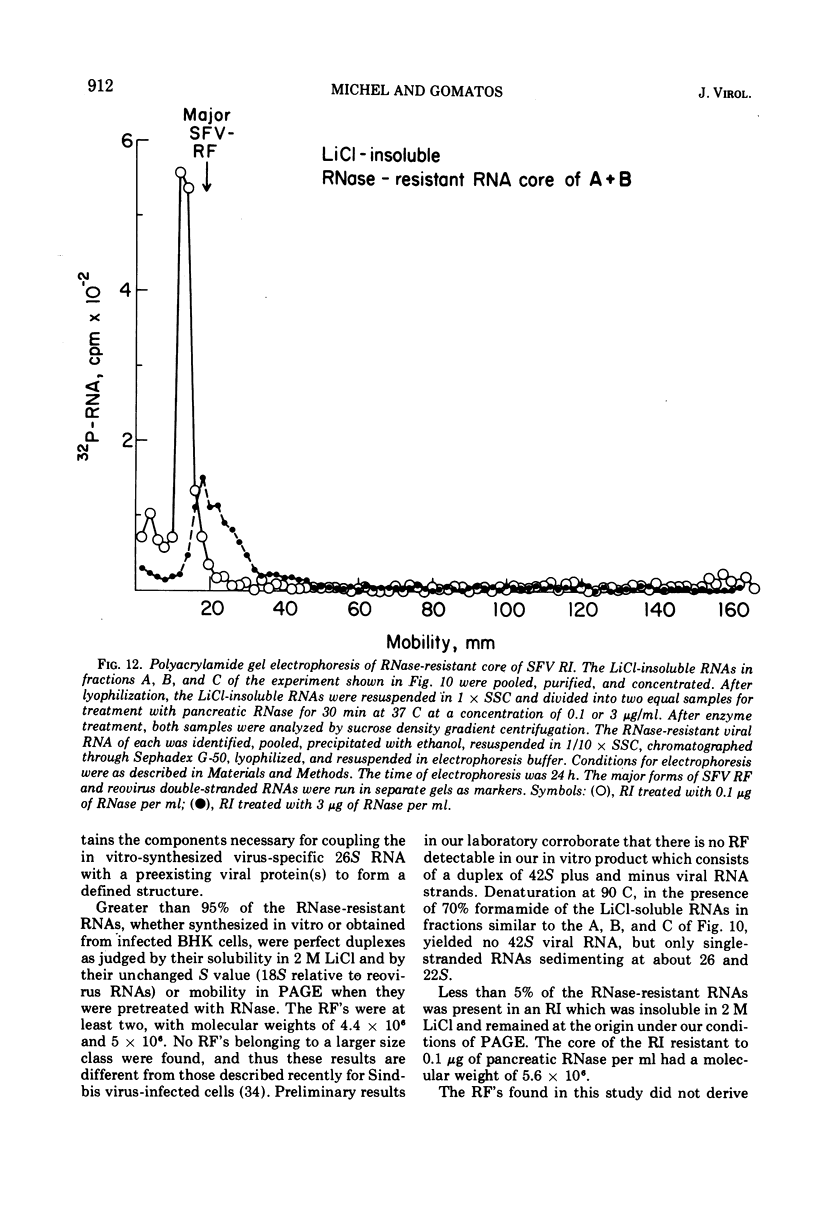
When Semliki Forest virus (SFV)-infected BHK cells were disrupted 4 h after infection, 75 to 90% of the total virus-specific RNA synthesizing enzyme was found in the large particle fraction, along with 75 to 90% of the in vivo-synthesized double-stranded RNAs. The RNA products of this enzyme-template complex in an in vitro system were double-stranded RNAs sedimenting predominantly at 18S, and single-stranded RNAs sedimenting at 42S, 26S, and 22S. The various virus-specific SFV RNAs synthesized in vitro were associated with different sized structures, and thus each was separable by differential centrifugation. Kinetic and pulse-chase experiments showed that the double-stranded RNAs were the precursors to the single-stranded RNAs. There were several double-stranded RNAs identified both in the in vitro product and also in extracts from infected cells. The major replicative form had a molecular weight of 4.4 × 106.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arif B. M., Faulkner P. Genome of Sindbis virus. J Virol. 1972 Jan;9(1):102–109. doi: 10.1128/jvi.9.1.102-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J Mol Biol. 1971 May 14;57(3):513–527. doi: 10.1016/0022-2836(71)90106-9. [DOI] [PubMed] [Google Scholar]
- Cartwright K. L., Burke D. Virus nucleic acids formed in chick embryo cells infected with Semliki Forest virus. J Gen Virol. 1970 Feb;6(2):231–248. doi: 10.1099/0022-1317-6-2-231. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusberg P. H., Robinson W. S. On the structure and replication of influenza virus. J Mol Biol. 1967 May 14;25(3):383–405. doi: 10.1016/0022-2836(67)90193-3. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Donaghue T. P., Faulkner P. Presence of poly (A) in the polyribosome-associated RNA of Sindbis-infected BHK cells. Nat New Biol. 1972 Jul 26;238(82):109–111. doi: 10.1038/newbio238109a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Berezesky I. K. Cytoplasmic fractions associated with Semliki Forest virus ribonucleic acid replication. J Virol. 1967 Apr;1(2):374–383. doi: 10.1128/jvi.1.2.374-383.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levin J. G., Grimley P. M., Berezesky I. K. Membrane-associated replication complex in arbovirus infection. J Virol. 1972 Sep;10(3):504–515. doi: 10.1128/jvi.10.3.504-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Replicative intermediate of an arbovirus. J Virol. 1968 Jun;2(6):547–552. doi: 10.1128/jvi.2.6.547-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Sreevalsan T. Membrane binding of input arbovirus ribonucleic acid: effect of interferon or cycloheximide. J Virol. 1970 Aug;6(2):169–175. doi: 10.1128/jvi.6.2.169-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Levin J. G., Berezesky I. K., Friedman R. M. Specific membranous structures associated with the replication of group A arboviruses. J Virol. 1972 Sep;10(3):492–503. doi: 10.1128/jvi.10.3.492-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen L., Gomatos P. J. A kinetic analysis of the synthesis in BHK 21 cells of RNAs specific for Semliki Forest virus. J Gen Virol. 1969 Sep;5(2):251–265. doi: 10.1099/0022-1317-5-2-251. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Isolation and identification of the virus-specified RNA species found on membrane-bound polyribosomes of chick embryo cells infected with Semliki Forest virus. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1254–1258. doi: 10.1016/0006-291x(72)90846-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL B. THE EXTRACTION OF RIBONUCLEIC ACID FROM POLIOVIRUS BY TREATMENT WITH SODIUM DODECYL SULFATE. Virology. 1964 Mar;22:360–367. doi: 10.1016/0042-6822(64)90026-1. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Martin E. M., Sonnabend J. A. Ribonucleic acid polymerase catalyzing synthesis of double-stranded arbovirus ribonucleic acid. J Virol. 1967 Feb;1(1):97–109. doi: 10.1128/jvi.1.1.97-109.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. M. Studies on the RNA polymrase of some temperature-sensitive mutants of Semliki Forest virus. Virology. 1969 Sep;39(1):107–117. doi: 10.1016/0042-6822(69)90352-3. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Zweerink H. J. Isolation and characterization of two types of bluetongue virus particles. Virology. 1972 Nov;50(2):495–506. doi: 10.1016/0042-6822(72)90400-x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Burge B. W., Coady H. M. Intracellular conversion of the RNA of sindbis virus to a double-stranded form. Virology. 1967 Oct;33(2):239–249. doi: 10.1016/0042-6822(67)90143-2. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972 Mar;47(3):711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- Qureshi A. A., Trent D. W. Saint Louis encephalitis viral ribonucleic acid replication complex. J Virol. 1972 Apr;9(4):565–573. doi: 10.1128/jvi.9.4.565-573.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. II. Separation and characterization of virus-specific RNA species. Virology. 1972 Sep;49(3):766–783. doi: 10.1016/0042-6822(72)90533-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E. Viral specific RNAs in infected cells. Nature. 1967 Jan 28;213(5074):365–367. doi: 10.1038/213365a0. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr, Dodson M. L., Jr, Hartman K. A. Replication of Western equine encephalomyelitis virus. I. Some chemical and physical characteristics of viral ribonucleic acid. J Virol. 1968 Jun;2(6):558–566. doi: 10.1128/jvi.2.6.558-566.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevalsan T., Yin F. H. Sindbis virus-induced viral ribonucleic acid polymerase. J Virol. 1969 Jun;3(6):599–604. doi: 10.1128/jvi.3.6.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lockart R. Z., Jr Maturation defects in temperature-sensitive mutants of Sindbis virus. J Virol. 1968 Jul;2(7):728–737. doi: 10.1128/jvi.2.7.728-737.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



