Abstract
Magnetic Resonance Imaging (MRI) brain scans were obtained from 19 infants at 7 months. Expressive and receptive language performance was assessed at 12 months. Voxel-based morphometry (VBM) identified brain regions where gray-matter and white-matter concentrations at 7 months correlated significantly with children’s language scores at 12 months. Early gray-matter concentration in the right cerebellum, early white-matter concentration in the right cerebellum, and early white-matter concentration in the left posterior limb of the internal capsule (PLIC)/cerebral peduncle were positively and strongly associated with infants’ receptive language ability at 12 months. Early gray-matter concentration in the right hippocampus was positively and strongly correlated with infants’ expressive language ability at 12 months. Our results suggest that the cerebellum, PLIC/cerebral peduncle, and the hippocampus may be associated with early language development. Potential links between these structural predictors and infants’ linguistic functions are discussed.
Keywords: Voxel-based morphometry, Infancy, Cerebellum, Hippocampus, Posterior Limb of the Internal Capsule (PLIC), Cerebral Peduncle, Expressive Language, Receptive Language
1. Introduction
Information regarding structural and functional brain development in the first two years of life is very limited (Knickmeyer et al., 2008). Adult studies have revealed associations between gray-matter and white-matter volumes and a variety of behavioral measures, including general intelligence, proficiency in a second language, and phonetic learning (Golestani, Molko, Dehaene, LeBihan, & Pallier, 2007; Haier, Jung, Yeo, Head, & Alkire, 2004; Mechelli et al., 2004). Only two such studies have been conducted with infants (Ortiz-Mantilla, Choe, Flax, Grant, & Benasich, 2010; Short, et al., in press) reflecting difficulties in data collection from infants and challenges faced in analyzing infant MR images (Knickmeyer et al., 2008). Infant MR images often exhibit motion artifact, and contrast between gray-matter and white-matter is variable across regions at birth and during early development, largely due to ongoing myelination processes in white-matter tissues during infant development (Barkovich, 2005; Prastawa, Gilmore, Lin, & Gerig, 2005; Shi et al., 2010). Despite these challenges, brain development between birth and age 2, and its relationship to behavior, remains of great interest. This early period may be the most important phase of postnatal brain development in humans and is likely critical in neurodevelopmental disorders such as autism (Knickmeyer et al., 2008).
The study of brain structure early in life, and its relationship to behavior, is particularly interesting in the field of language development. There is evidence that early language performance is a powerful predictor of later language and achievement (National Institute of Child Mental Health and Human Development, 2005). Interest in neural correlates of early language skills is strong in the area of child development (Kuhl & Rivera-Gaxiola, 2008). Studies on typically developing children indicate that brain and behavioral responses early in infancy predict later performance and learning. For example, behavioral and event related potential (ERP) measures of phonetic learning in the first year of life predict language skills between 14 and 30 months (Kuhl et al., 2008; Kuhl, Conboy, Padden, Nelson, & Pruitt, 2005; Rivera-Gaxiola, Klarman, Garcia-Sierra, & Kuhl, 2005; Tsao, Liu & Kuhl, 2004), language and pre-literacy skills at 5 years (Cardillo Lebedeva & Kuhl, 2009; Cardillo, 2010; Molfese & Molfese, 1997), and language and cognitive ability at 8 years (Molfese, 2000). In addition, variations in brain structure as early as at 6 months (i.e., size of the right amygdala) have been linked to longitudinal language outcomes up to 4 years (Ortiz-Mantilla et al., 2010), while white-matter microstructure has been associated with behavioral measures of working memory in 12 month old infants (Short et al., in press).
While such studies inform us about the relations between brain and behavior, determination of the source of language functions in the brain is a topic of continued research. Two brain areas have long been associated with language function in adults: Broca’s area, in the left inferior frontal lobe, and Wernicke’s area, in the left posterior temporal cortex (Papathanassiou, et al., 2000). Recent data suggest that multiple brain areas contribute to language processing in adults (e.g., Atallah, Frank, & O’Reilly, 2004; Doya, 1999, 2000; Hickok & Poeppel, 2007; Kuhl & Damasio, 2012; Opitz & Friederici, 2003), and research using modern brain imaging techniques has provided an expanded view of the brain areas associated with adult language processing (Murdoch, 2010). For example, in a recent study, an fMRI language localizer task was employed to identify 13 key regions implicated in high-level linguistic processing in adults (Fedorenko, Hsieh, Nieto-Castañón, Whitfield-Gabrieli, & Kanwisher, 2010). These key regions were distributed in the left frontal lobe, left temporal/parietal lobes, right temporal lobe, and cerebellum (Fedorenko et al., 2010). Within the last two decades, there has been a dramatic increase in the number of research studies that emphasize the importance of multiple brain areas (e.g., prefrontal cortex, cerebellum, hippocampus, basal ganglia) for cognitive and linguistic processing.
The foregoing studies showing that multiple brain areas are activated during language tasks have been conducted with adults. The brain areas involved developmentally, during the period in which language is acquired, are not yet clear, and this topic has been identified as an important area for research (Booth, Wood, Lu, Houk, & Bitan, 2007; Breitenstein et al., 2005; Doya, 1999, 2000; Gordon, 2007; Konczak & Timmann, 2007; Murdoch, 2010). Infant studies utilizing fMRI and MEG have shown that the left inferior frontal lobe (e.g., Broca’s area) is active during speech processing (Dehaene-Lambertz et al., 2010; Imada et al., 2006), however, no whole brain longitudinal study has yet examined which specific brain areas predict language development over time in infancy.
Our aim was to explore the brain structures associated with infants’ language development utilizing a longitudinal design. The 2nd year of life is characterized by an explosion of language, intensified connectivity of the two hemispheres, maturation of the prefrontal cortex and the cortical-subcortical network, and strengthened connections between the cortex and the limbic system (Herschkowitz, 2000). The present study examines brain structure early in the first year of life for evidence of structural predictors of early language acquisition. Specifically, we explore the relationship between early concentration of gray-matter and white-matter in the brain at 7 months and infants’ expressive and receptive language skills at 12 months. We utilize voxel-based morphometry (VBM) of MRI data to a) represent local concentration of gray-matter and white-matter (i.e., the probability of a specific tissue type within a region), and b) study correlations between local concentrations of gray-matter and white-matter in the whole brain and children’s later language outcomes (Ashburner & Friston, 2000; Whitwell, 2009).
2. Methods
2.1. Subjects
The 19 infants in the present study were drawn from a larger group enrolled in a comprehensive study of typically developing children which explored relationships among brain, behavior and environment across the first 2 years of life. An additional 10 subjects did not produce usable MRI data at 7 months due to failure to sleep at the imaging center (n = 2), waking during transition to the scanning bed or at the onset of the first scan (n = 6), and motion artifact (n = 2). One infant with usable MRI data did not return for behavioral measures at 12 months. The children were recruited from urban and suburban communities in the Seattle area (12 boys and 7 girls). Infants were born healthy and full-term (+ 2 weeks from due date), ranging in weight from 6 to 9 lbs. All came from English-speaking monolingual families, had uneventful pre- and perinatal circumstances, with no history of language impairment, hearing loss, or other neurological or psychiatric disorders.
Children visited the laboratory at about 7 months (Mean = 6.9, SD = .5, Range = 6.0 – 7.8, corrected for gestational age at birth) for brain imaging, and at about 12 months (Mean = 12.3, SD = .4, Range = 11.7 – 13.2, corrected for gestational age at birth) for standardized testing. A parental questionnaire was used to obtain socio-demographic data, as well as information about infant and maternal health and obstetrical history at the 7-month visit. A follow-up questionnaire provided additional infant health data at 12 months. The socioeconomic status (SES) was assessed by the Hollingshead Four Factor Index (Hollingshead, 1975) and was not related to language abilities at 12 months. Infants came from families classified as middle-to-upper-middle class (SES ranging from 39 to 66, mean = 54.41, SD = 7.05). The University of Washington Institutional Review Board approved this project and written informed consent was obtained from parents according to the principles explained in the Declaration of Helsinki. Parents were compensated for their time and infants received a toy after each visit.
2.2. Procedures
Successful structural MRI brain scans were collected by certified MR technicians during natural sleep. The MRI visits were scheduled for late mornings during naptime, so that a non-sedated naturally sleeping MRI could be acquired. Children were swaddled and moldable silicon ear protectors were placed in the outer ear when they arrived at the imaging center. Inside the imaging center, normal naptime routines were replicated (e.g., soft lullaby music, rocking chair, crib, other materials to encourage sleeping) to comfort the children. After the child was asleep, s/he was moved to the scanning bed where ear protection was placed over the ears and the head was stabilized in the standard coil with foam.
Magnetic Resonance Imaging (MRI) data were obtained as follows: Sagittal images (1.0 mm slice thickness) were acquired using a GE Signa 1.5 tesla scanner (version 5.8) (General Electric, Milwaukee, WI, USA), and a 3D fast spoiled gradient echo pulse sequence. These specific imaging parameters were utilized: TR (repetition time) 11.1 ms, TE (echo time) 2.2 ms, flip angle 25°, field of view 24 cm, pixel size dimension 0.94×0.94×1mm, reconstructed matrix size 256×256×124. The entire acquisition time was 4 min 36 s.
2.3. Standardized measures
The Mullen Scales of Early Learning (Mullen, 1995) is a standardized measure of early learning abilities that is widely used in research and clinical practice. The administration time typically varies with age and characteristics of the child. In the present case, a speech language pathologist with extensive experience administered the Mullen Scales. Test administration took approximately 15 minutes in our one-year-old children. This matches the time for test administration listed in the technical manual for children at this age (Mullen, 1995). Testing practices and guidelines for assessing and increasing children’s attention level are described in full detail in the manual, and were followed by the highly trained speech pathologist who administered our tests (see Mullen, 1995). The Mullen consists of five scales: Gross Motor, Visual Reception, Fine Motor, Receptive Language, and Expressive Language that have normative scores (T-scores) with a mean of 50 and a standard deviation of 10 (Mullen, 1995). In addition, the Mullen provides a single standardized composite score (i.e., early learning index score, mean = 100, SD = 15) that represents general intelligence. Test-retest reliability for receptive and expressive language scales within that interval (i.e., 1-to-2 weeks) was high, with correlations ranging from r = .82 to r = .85 (Mullen, 1995).
For the purposes of the current study, the Mullen Expressive and Receptive Language Scales served as language measures. The Mullen Expressive Language Scale measures voluntary babbling, production of at least three different consonant sounds, vocalization of two-syllable sounds, and participation in gesture/language games. The Mullen Receptive Language Scale measures infants’ attention to words and movement, recognition of their name, recognition of familiar names or words, and comprehension of inhibitory words such as no and stop (Mullen, 1995).
2.4. Data analysis: Voxel-based morphometry (VBM)
VBM was used to investigate voxel-wise differences in gray-matter and white-mater volume/topography related to language scores in infants. This approach is unbiased, in that it requires no a priori information about the location of possible differences in gray-matter or white-matter, and is not operator-dependant. It follows the optimized VBM protocol developed by Good et al. (2001). Our use of this methodology employs multiple comparisons across the whole brain, which in turn requires corrected p-values using a conservative statistical criterion. This conservative statistical threshold provides confidence in positive findings. However, negative results must be interpreted with caution, since significant associations may exist in regions of interest selected a priori but fail to survive the correction for whole brain multiple comparisons.
An MRI template was created for use in the VBM analysis using the structural MRIs of the 19 subjects enrolled in the present study. Several preprocessing procedures were performed on each subject’s T1 image as the first step in a multi-step process: N3 intensity non-uniformity correction (Sled, Zijdenbos, & Evans, 1998), intensity normalization (Nyul & Udupa, 1999), and midsagittal line (McAuliffe et al., 2001) and AC-PC plane alignment (Smith et al., 2004). Then, these images were linearly registered (6 parameter, rigid body) to the standard NIH MRI Study of Normal Brain Development average head template for ages 0 to 2 in MNI152-like coordinate system (Fonov, Evans, McKinstry, Almli, & Collins, 2009). Lastly, a symmetric diffeomorphic image normalization algorithm and iterative averaging method were applied to these registered T1 images to obtain an optimal custom average template (Avants, Epstein, Grossman, & Gee, 2008; Avants & Gee, 2004; Avants et al., 2011) (Figure 1).
Figure 1.
Average MRI template (A - coronal, B- sagittal, C- axial slices) for subjects aligned into anterior commissure-posterior commissure (AC-PC) plane and fitted into standard Talairach space. The crosshair is at the anterior commissure point.
The biology and developmental processes of the infant brain pose substantial segmentation challenges (Barkovich, 2005; Prastawa et al., 2005; Shi et al., 2010). Among these are low white-matter and gray-matter contrast to noise ratios (CNR) partly due to short scanning periods, the small size of the infant brain, motion artifacts during scanning, and incomplete myelination of white-matter (Prastawa et al., 2005). While 80% of total gray-matter volume is present around 1 year, white matter volume does not reach 80% until late childhood because the growth-rate of white matter slows over time, following a rapid rate of increase within the first 2 years (Groeschel, Vollmer, King, & Connelly, 2010). In brain regions with incomplete myelination, the T1 signal intensity is decreased, which may lead to underestimation of white-matter and overestimation of the gray-matter volume and thickness. Developmentally, myelin is present only in the cerebellum and brainstem at birth, and myelination of white-matter tissue proceeds rapidly after birth from back to front (Barkovich, 2005; Deoni, Dean III, O’Muircheartaigh, Dirks, & Jerskey, in press, 2012). These maturational processes result in segmentation errors during automatic tissue segmentation, which are not uniformly distributed in the brain due to differences in maturation and myelination. Segmentation errors are likely to be more common in brain regions in which myelination is incomplete until relatively late in development, such as the frontal and temporal lobes (Pujol et al., 2006). Consequently, VBM methodology is more likely to yield significant results in gray-matter and in brain regions where myelination occurs early in development.
To perform the behavioral correlations with relative local concentrations of gray-matter and white-matter, structural data was analyzed with FSL-VBM, a voxel-based morphometry style analysis (Ashburner & Friston, 2000; Good et al., 2001) carried out with FSL tools (Smith et al., 2004). First, structural images were brain-extracted using BET (Smith, 2002). Next, tissue-type segmentation was carried out using FAST4 (Zhang, Brady, & Smith, 2001). We performed automatic tissue segmentation without manual correction for each subject as described above, and this approach resulted in segmentation errors consistent with the known developmental process in the infant brain (Prastawa et al., 2005). The gray-matter concentrations yielded by automatic segmentation may not depend entirely on gray-matter volume/thickness. They may be influenced by cortical maturation which affects the T1 contrast, or be changed by the level of white-matter myelination. The interface between gray- and white-matter may thus not be perfectly segmented. However, we performed the segmentation algorithms in the exact same way across all infants in this study. Thus, all infant brains were segmented in the same reproducible manner and the effects of development on automatic tissue segmentation should be comparable across subjects yielding a consistent dataset to serve as input for further processing. See Figure 2 for T1-weighted raw MR images in original space (Figure 2, column 1) and gray-matter/white-matter probability images in original space (Figure 2, column 2) from a representative subject.
Figure 2.
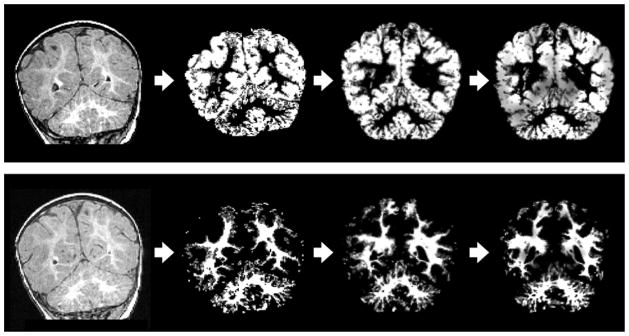
VBM analysis progression for subject 16356: T1-weighted raw MR image in original space (column 1), probability image in original space (column 2), probability image which has been coregistered to the study specific template (column 3), image corrected for the amount of local gray-matter or white-matter using the Jacobian transform (column 4). Row 1 shows VBM analysis progression for gray-matter at the level of the cerebellum (coronal), and row 2 shows VBM analysis progression for white-matter at the level of the cerebellum (coronal).
After tissue type segmentation, individual gray-matter and white-matter partial volume images were aligned to the respective partial volume images of our infant template using the registration tool FLIRT (Jenkinson & Smith, 2001; Jenkinson, Bannister, Brady, & Smith, 2002), followed by nonlinear registration using FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 2 mm. The nonlinear registration and smoothing compensate for imperfect alignment (e.g., in location or sulci shape) of cortical regions across individuals. See Figure 2 for VBM analysis modulated segmented images for gray-matter (Row 1) and white-matter (Row 2) from a representative subject.
Expressive and receptive vocabulary scores were correlated with relative local concentrations of gray-matter and white-matter across all subjects using FSL’s General Linear Model (GLM) tool (www.fmrib.ox.ac.uk/fsl/fsl/list) to define the design matrix, and p-values were corrected for multiple comparisons. MRI age and Mullen age (both corrected for gestational age at birth) were entered in the gray-matter and white-matter VBM analyses as covariates. The correlation inferences were tested using permutation methods with FSL’s Randomise (www.fmrib.ox.ac.uk/fsl/randomise). We ran 1000 two-tailed Monte Carlo permutation tests, (i.e., both positive and negative associations) for each of the correlations between relative local concentrations of gray-matter and white-matter and Mullen language (i.e., receptive and expressive) scores. Randomise uses permutation methods (also known as randomisation methods) for inference (thresholding) on statistic maps when the null distribution is not known. Randomise is a simple permutation program enabling modeling and inference using standard GLM design setup. A cluster-based test was used (Nichols & Holmes, 2002) with the Threshold-Free Cluster Enhancement (TFCE) option which is a method for finding “clusters” in the data without having to define clusters in a binary way.
3. Results
3.1. Standardized language measures
The Mullen total standard score for the sample (i.e., early learning composite) had a mean of 106.10 (SD = 10.13, N = 19) at 12 months, which fell within the average range (Composite Standard Score range = 85 through 115). In addition, at 12 months, the Mullen Expressive Language T-scores had a mean of 46.89 (SD = 9.43, N = 19), and the Mullen Receptive Language T-scores had a mean of 52.89 (SD = 6.74, N = 19), both of which fell within the average range (Scale T-score range = 40 through 60). Thirty six percent of the sample performed at the 50th percentile or higher in expressive language skills (range = 54 to 99), while 74% of the sample performed at the 50th percentile or higher in receptive language skills (range = 50 to 93).
3.2. Voxel-based morphometry (VBM) results
We used VBM to compare the relative local concentration of gray-matter or white-matter (i.e., the probability of a specific tissue type to other tissue types within a region) to standardized language measures. MRI age and Mullen age (corrected for gestational age at birth) were entered into VBM analyses as covariates to control for age effects. Two gray-matter correlations and two white-matter correlations with later language remained significant after application of the conservative statistical threshold that corrected for multiple comparisons.
3.2.1. Gray-Matter VBM results
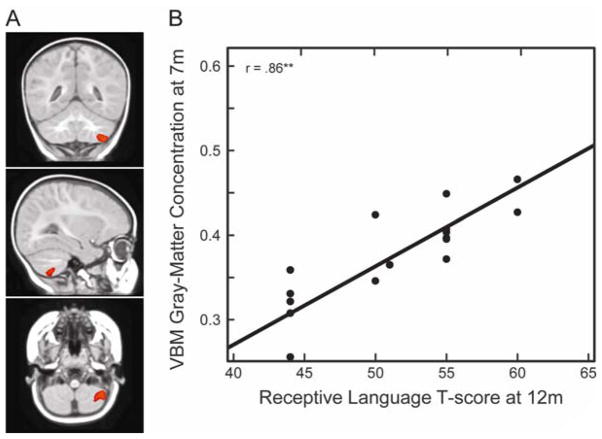
The whole brain VBM analysis showed that the distribution of gray-matter concentration in the right posterior cerebellum (regions VIIB and 8) at 7 months (Figure 3A) was strongly and positively associated with infants’ receptive language abilities at 12 months (x = 341, y = −32, z = −47; t = 7.85; Cluster size = 800 voxels; p < 0.00 corrected for multiple comparisons across the whole brain; N = 19). Figure 3B displays a plot of VBM gray-matter concentration at 7 months in this region and receptive language at 12 months.
Figure 3.
(A). Coronal (y = −32), sagittal (x = 34), and axial (z = −47) view of the right posterior cerebellum (regions VIIB and 8) at 7 months. (B) VBM gray-matter concentration at 7 months, measured as cubic millimeters of gray-matter per voxel in the right cerebellar region, as a function of receptive language skills at 12 months.
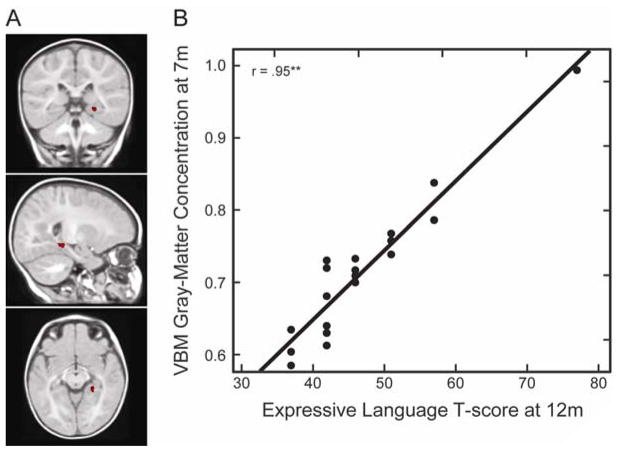
In addition, gray-matter concentration in the right hippocampus at 7 months (Figure 4A) was positively and strongly associated with expressive language skills at 12 months (x = 19, y = −25, z = −9; t = 10.67; Cluster size = 99 voxels; p < 0.00 corrected for multiple comparisons across the whole brain; N = 19). Figure 4B displays a scatter plot of VBM gray-matter concentration in this region at 7 months and expressive language at 12 months.
Figure 4.
(A). Coronal (y = −25), sagittal (x = 19), and axial (z = −9) view of the right hippocampus at 7 months. (B) VBM gray-matter concentration at 7 months, measured as cubic millimeters of gray-matter per voxel in the right hippocampus region, as a function of expressive language skills at 12 months.
3.2.2. White-matter VBM results
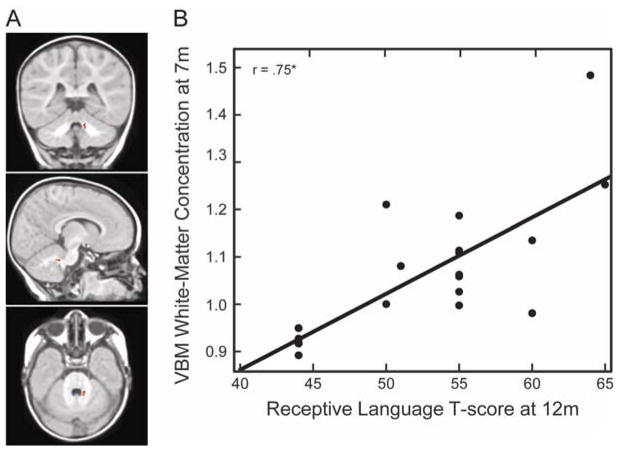
Interestingly, the distribution of white-matter concentration in the right inferior cerebellar peduncle at 7 months (Figure 5A) was also positively associated with infants’ receptive language abilities at 12 months—this is similar to the pattern observed for gray-matter, although at a more central location (x = 11, y = −42, z = −31; t = 5.87; Cluster size = 55 voxels; p = 0.03 corrected for multiple comparisons across the whole brain, N = 19). Figure 5B includes a scatter plot of VBM white-matter concentration at 7 months in this region and receptive language at 12 months.
Figure 5.
(A). Coronal (y = −42), sagittal (x = 11), and axial (z = −31) view of the right inferior cerebellar peduncle at 7 months. (B) VBM WM concentration at 7 months, measured as cubic millimeters of WM per voxel in the right inferior cerebellar peduncle, as a function of receptive language skills at 12 months.
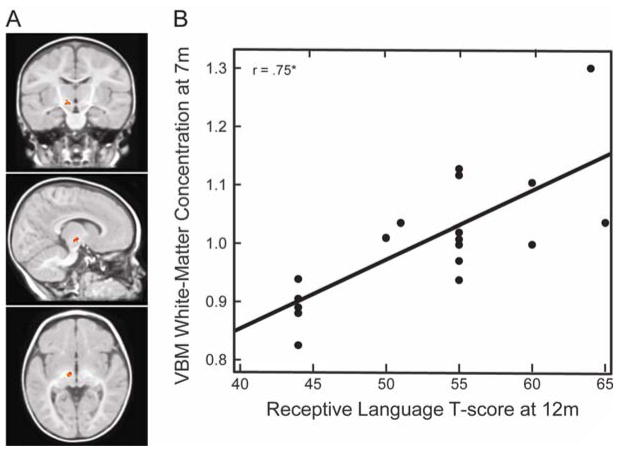
In addition, white-matter concentration in a cluster located in the left posterior limb of the internal capsule (PLIC)/cerebral peduncle at 7 months (Figure 6A) was positively and strongly associated with receptive language skills at 12 months (x = −9, y = −12, z = −4; t = 5.61; Cluster size = 70 voxels; p = 0.02 corrected for multiple comparisons across the whole brain; N = 19). Figure 6B displays a scatter plot of VBM white-matter concentration in this region at 7 months and receptive language at 12 months.
Figure 6.
(A). Coronal (y = −12), sagittal (x = −9), and axial (z = −4) view of the left internal capsule/cerebral peduncle at 7 months. (B) VBM WM concentration at 7 months, measured as cubic millimeters of WM per voxel in the left internal capsule/cerebral peduncle, as a function of receptive language skills at 12 months.
4. Discussion
The purpose of our study was to examine the whole brain and explore areas associated with infants’ language development. The findings support the view that individual differences in gray-matter and white-matter, as early as at 7 months, are associated with language skills (i.e., expressive and receptive) at 12 months. The significant correlations between early gray-matter concentration and early white-matter concentration (p < .05, corrected for multiple comparisons) in specific locations (i.e., right cerebellum, right hippocampus, and left PLIC/cerebral peduncle), and later language functioning in infants is consistent with earlier findings of associations with language performance in adults, children, and adolescents (Breitenstein et al., 2005; Murdoch, 2010; Konczak & Timmann, 2007; Geng et al., 2012). Our findings extend previous research that explored the relationships between these regions and language, by a) specifically focusing on typically developing infants as opposed to clinical child populations or adults, b) examining the whole brain without a priori hypotheses utilizing MRI and VBM, and c) providing information regarding right hippocampal, right cerebellar, and left PLIC/cerebral peduncle influences on language.
4.1. Cerebellum and language
The cerebellum, located in the hindbrain, is a distinct subdivision of the brain (Glickstein, Strata, & Voogd, 2009). Its topographic organization has been extensively studied and mapped (Schmahmann et al., 1999; Stoodley & Schmahmann, 2010). The results of recent neuroanatomical, clinical, and neuroimaging studies have demonstrated that the cerebellum, in addition to its traditional role in motor functions, contributes to the modulation of a broad spectrum of linguistic functions in older children and adults (e.g., verbal fluency, word retrieval, syntax, reading, writing, and metalinguistic activities), as well as the direct organization, construction, and execution of linguistic processes (see Murdoch, 2010 for review).
The cerebellum interacts with brain structures including the frontal lobe via three cerebellar peduncles (i.e., inferior, superior, middle) which connect the cerebellum to the pons (Fabbro, 2000; Murdoch, 2010). These connections provide the neural substrate allowing the cerebellar involvement in linguistic function. Dubois and colleagues (Dubois, Hertz-Pannier, Dehaene-Lambertz, Cointepas, & Le Bihan, 2006) examined early maturation of white-matter in 1-to-4 month old infants, and reported that cerebellar peduncles exhibited increased maturation early in development, along with other tracts including the corpus callosum, capsules, and cortico-spinal tract. Cerebellar peduncles have also been shown to have implications for communication skills in adulthood (Catani et al. 2008). For example, the left superior cerebellar peduncle was linked to social impairment within an adult population with Asperger’s syndrome (Catani et al., 2008). Our findings are consistent with cerebellar contributions to linguistic functions, and indicate relationships with local concentrations of both gray-matter and white-matter very early in life, as early as 7 months.
While the cerebellum appears to be important for many aspects of language development, the issue of cerebellar laterality in relation to language functioning is unclear and research has produced conflicting findings. The majority of evidence from adult lesion/pathology, fMRI, and positron-emission tomography (PET) studies indicates that the right cerebellar hemisphere is associated with language, while the left cerebellar hemisphere is associated with visuospatial functions (Schmahmann, 2004). Activation in the right cerebellum has been demonstrated in healthy adult subjects during verb generation tasks (e.g., retrieval of verbs semantically related to nouns), as well as story listening tasks (e.g., accuracy of responses to questions asked from stories) (Frings et al., 2006; Papathanassiou et al., 2000). Functional brain imaging, including magnetoencephalography (MEG), revealed that the right cerebellum was activated during verbal working memory tasks (Ioannides & Fenwick, 2005), while verbal memory defects were associated with right-sided cerebellar lesions (Hokkanen, Kauranen, Roine, Salonen, & Kotila, 2006). Cerebellar patients often failed tasks linked to prefrontal cortex such as verbal fluency, verb generation, planning, and tasks requiring the learning and memory of arbitrary associations (Diamond, 2000). Although these findings suggest that the right cerebellar hemisphere has a role in cognitive and linguistic functioning and that the language comprehension and/or production conjunction network includes the right hemisphere regions (Papathanassiou et al., 2000), other studies show conflicting results concerning the laterality of verb/word generation skills in cerebellar patients (Arasanz, Staines, Roy, & Schweizer, 2012). For example, adult patients with lesions affecting the right cerebellar hemisphere did not differ from those with lesions affecting the left cerebellar hemisphere during verb generation performance (Richter et al., 2007). In addition, recent studies have identified functional brain regions sensitive to high-level linguistic processing in individual adults, including regions in the left and right cerebellum (Fedorenko et al., 2010; Fedorenko, Behr, & Kanwisher, 2011). The research literature concerning the laterality of cerebellar involvement in language functioning of diverse subjects (e.g., those with or without clinical diagnoses such as lesions) necessitates more research to uncover specific relationships between cerebellum and language performance (Glickstein, 2006).
Research that explored cerebellar functioning in children and adolescents also produced mixed results (Aarsen, Van Dongen, Paquier, Van Mourik, & Catsman-Berrevoets, 2004; Richter et al., 2005). In a group of children with chronic cerebellar lesions, one study observed no signs of aphasia (i.e., loss of ability to understand or express speech) (Richter et al., 2005), while another reported several expressive language problems, including word finding difficulties and non-fluent speech (Aarsen et al., 2004).
No previous study has examined cerebellar functioning in infants in relation to behavioral outcomes. Thus, our findings contribute to the existing research in the field by revealing strong relationships between gray-matter concentrations (regions VIIB and 8) and white-matter concentrations (inferior cerebellar peduncle) in the right cerebellum at 7 months and receptive language skills at 12 months in typically developing infants. From the standpoint of localization, our finding are consistent with research that found posterior cerebellum (lobules 6-to-9) activation during language tasks and designated the posterior regions as language centers within the cerebellum (Stoodley & Schmahmann, 2009, 2010). Our findings of associations between both gray-matter and white-matter concentrations in the right cerebellum and later receptive language increase the overall evidence that speaks to the importance of cerebellum for early language development.
4.2. Hippocampus and language
While it has been demonstrated that hippocampus reaches adult volume by around 7–10 months (Herschkowitz, 2000), not much is known regarding the associations between hippocampus and language development. The hippocampus is part of the limbic system located in the medial temporal lobe, and is critically important for associative memory and learning, which in turn contribute to language acquisition (Bartha et al., 2003; Breitenstein et al., 2005; Henke, Weber, Kneifel, Wieser, & Buck, 1999). In studies conducted with adults, evidence linked hippocampus activation with learning new vocabulary, new semantic facts, making semantic decisions, and semantically associating unrelated words in memory. For example, functional magnetic resonance imaging (fMRI) studies in adults have shown that increasing vocabulary proficiency for novel words was related to modulations of activity within the left hippocampus (Breitenstein et al., 2005), and successful semantic encoding (i.e., the acquisition of new and true general factual knowledge about the world) activated the left cortical network that included the left hippocampus (Maguire & Frith, 2004). In an fMRI study by Bartha et al. (2003), a semantic language processing task (i.e., listening to nouns and deciding whether they belong to a category) was associated with bilateral activation in both the anterior and posterior parts of the hippocampal formation and the parahippocampal gyrus. Henke et al. (1999) measured regional cerebral blood flow (rCBF) during semantic decision paradigms and reported that associative word learning (i.e., deciding whether or not word pairs fit in meaning) activated the two hippocampi (i.e., left and right anterior HF).
While such fMRI and rCBF studies were conducted to explore hippocampal influences in language (Bartha, et al., 2003; Breitenstein et al., 2005; Henke, et al., 1999), to our knowledge there are no whole brain MRI studies in adults that suggest relationships between hippocampal gray-matter concentration and language outcomes. Moreover, no specific data exist in infants that examine or demonstrate a relationship between hippocampus and language development. Our findings add to the body of research on infants by a) demonstrating a strong correlation between very early hippocampal gray-matter concentration and expressive language skills within the first year of life, and b) reporting a relationship between language and hippocampus, interestingly confined to the right hemisphere, in contrast to previous research studies in adults which reported left-dominated (Breitenstein et al., 2005) or bilateral (Bartha et al., 2003, Henke et al. 1999) hippocampal relationships to language.
4.3. Posterior limb of the internal capsule (PLIC)/cerebral peduncle and language
Cerebral white-matter largely consists of densely packed myelinated neuronal axons, and the integrity of these pathways is associated with efficiency in cognitive processing (Westlye et al., 2010). The internal capsule is an extensive fiber system that conveys information from motor areas (primary and supplementary), frontopontine peduncles, and thalamic peduncles to the brain stem and cerebellar regions; as well as linking the thalamus to prefrontal cortex (Sullivan, Zahr, Rohlfing, & Pfefferbaum, 2010). Early studies of the internal capsule with adults demonstrated links between subcortical lesions in the internal capsule and aphasia, characterized by non-fluent speech, disturbances in articulation, poor repetition, and poor auditory comprehension (Damasio & Geschwind, 1984; Metter et al., 1988). In adult patients with lesions of the internal capsule, direct relations were identified between verbal fluency and the posterior limb of the internal capsule (Metter et al., 1988).
There is strong evidence that the most significant period of white-matter myelination occurs between midgestation and the second year of life (see Geng et al., 2012). The characteristics of white-matter growth in the early years of life has been associated with neuronal plasticity (Lee et al., 2010), and cognitive functions across the life-span (Brauer, Anwander, & Friederici, 2011). The specific white-matter regions associated with later measures of receptive language in the present study (i.e., inferior cerebellar peduncle and PLIC/cerebral peduncle) can be unambiguously characterized as early myelinated fiber tracts as demonstrated by developmental studies of white-matter maturation within 0-to-2 year olds (Geng et al., 2012), and 7-to-87 year olds (Brickman et al., 2012). Furthermore, the PLIC has been shown to have a higher degree of maturation at birth in comparison to other areas of the brain (Geng et al., 2012). Therefore, both the PLIC and the cerebellar peduncles are among brain areas more likely to yield significant relationships with language in infants via VBM analysis.
4.4. Implications for brain interactions that support cognition and language
The traditional view that two brain structures are primarily responsible for language functions (i.e., Wernicke’s area for analyzing acoustic signals and Broca’s area for motor planning and articulation of speech) has changed (Atallah et al., 2004; Doya, 1999, 2000; Opitz & Friederici, 2003; Kuhl & Damasio, 2012). Imaging and lesion studies have provided evidence that language processing is a product of complex and distributed neural networks, including three association areas involved in cognitive behavior (i.e., prefrontal, parietal temporal occipital, and limbic areas) (Kuhl & Damasio, 2012; Hickok & Poeppel, 2007). In addition, there is increasing evidence that language processes are distributed across the two hemispheres (Kuhl & Damasio, 2012). For example, results of functional imaging studies have indicated bilateral organization of speech processing and speech recognition (Hickok & Poeppel, 2007).
Recent research also suggests that cortical regions sensitive to language can be functionally defined in individual adults (Fedorenko et al., 2010, 2011). Federenko and colleagues (2011) found a high degree of functional specificity in these language-sensitive regions identified in individual participants. These individually and functionally defined language regions exhibited strong language activation with little or no response to nonlinguistic tasks (e.g., arithmetic, music, general cognitive control). It will be of theoretical interest to examine the specificity of brain activation in response to linguistic and nonlinguistic stimuli in infants to understand the developmental time course of these specializations.
The current findings that gray-matter and white-matter concentrations in the right cerebellum, gray-matter concentration in the right hippocampus, and white-matter concentration in the left PLIC/cerebral peduncle are associated with infants’ language skills support the view that a distributed neural network may contribute to language development. In adults, co-activation of dorsolateral prefrontal cortex and the contralateral neocerebellum has been found during several tasks such as verb generation (i.e., producing verbs related to specific nouns) and verbal fluency (i.e., producing different words that begin with a specified letter within a given time limit) (Raichle et al., 1994; Schlosser et al., 1998). Our findings of significant relationships between gray-matter and white-matter concentrations in the right cerebellum and later receptive language are theoretically interesting in light of motor theories of speech perception (Galantucci, Fowler, & Turvey, 2006) and action-based language learning theory (ABL), incorporating the potential contributions of mirror neurons (Glenberg & Gallese, 2012).
Interactions between the hippocampus and the prefrontal cortex have also been demonstrated (Opitz & Friederici, 2003). Although there is a broad agreement that the primary function of the hippocampus is the encoding of episodic or spatial memories (Vargha-Khadem et al., 1997), other functions of the hippocampus have been studied. For example, the hippocampal-prefrontal interaction has been empirically tested with adults using functional magnetic resonance imaging (fMRI), and it was found that both the hippocampal system and the prefrontal cortex were involved in learning language-like rules (Opitz & Friederici, 2003). Future research in infants will explore the brain areas associated with language function in adults, as well as examine connectivity for language-related areas in the infant brain using methodologies such as Diffusion Tensor Imaging (DTI). This work will benefit from direct comparisons to adult data on brain structure and function.
Our whole brain VBM analyses did not reveal significant associations between traditional language areas of the brain (i.e., Broca’s and Wernicke’s) and language development, although fMRI studies with 2-to-4 month-old infants (Dehaene-Lambertz et al., 2010; Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; Dehaene-Lambertz, Hertz-Pannier, & Dubois, 2006) and an MEG study in 6-month and 12-month-old infants (Imada et al., 2006) provide evidence of functional activity in these areas during speech processing. These authors report left-lateralized activations in response to speech within traditional language areas in infants (e.g., superior temporal and angular gyri, left-inferior frontal regions). The lack of significant associations between traditional language areas of the brain and later language development may be due to incomplete maturation and myelination in these areas at 7 months (Leroy et al., 2011; Pujol et al., 2006). However, we do see relationships between early myelinating white-matter tracts in the left hemisphere (i.e., left PLIC/cerebral peduncle) and later receptive language.
Pujol et al. (2006) reported that the exponential increase in toddlers’ vocabulary coincides with the end of rapid myelination at about 18 months, and argued that progress in language acquisition may correlate with maturation of the whole temporofrontal language network. The relationship between myelin deposition and language performance does not imply causation, however, and the developmental pattern of myelination in multiple areas of the brain (not just temporal and frontal regions) is a topic of continued research (Pujol et al. 2006). In general, myelination proceeds from caudal to cephalad (Barkovich, 2005). More specifically, myelination begins in the cerebellum and the internal capsule prior to 3 months, and proceeds to the corpus callosum, the occipital and parietal lobes, and lastly, the frontal and temporal lobes (Deoni et al., in press). Thus, it may be that our results are influenced by early myelination of the cerebellum and the PLIC/cerebral peduncle, located in the posterior portion of the brain, and the hippocampus, located in the medial temporal lobe. Future research is needed to examine gray-matter and white-matter concentration, and the myelination patterns, across the entire infant brain in relation to longitudinal child language outcomes.
4.5. Methodological Limitations
While we are confident in the unbiased and objective nature of the whole brain VBM analysis, we acknowledge that the interpretation of the VBM results obtained in this study has inherent limitations (see Mechelli, Price, Priston, & Ashburner, 2005). For example, accuracy of localization is critical (Mechelli et al. 2005) and may be particularly difficult to accomplish in the infant brain. In addition, VBM is not a surrogate for multivariate volumetric analysis, and thus, it does not measure inter-regional dependencies in the brain (Mechelli et al., 2005) that might be associated with certain developmental skills such as language.
Our modest sample may limit the generalizability of the findings to larger and more diverse populations. Future research can explore the implications of gray-matter and white-matter concentration at 7 months for longer-term language development. Moreover, future research can benefit from other methodologies such as development of cortical maturation indices to estimate relative maturation of language-related areas in the brain (Leroy et al., 2011), and examination of the degree of white-matter myelination in language-related as well as non-language related areas in the infant brain (Pujol et al., 2006; Deoni et al., in press) to further investigate the predictors of language development.
In addition, it is important to note that inter-individual variability in gray- and white-matter concentrations found in this study may be due to varied volumes, differences in cortical maturation or myelination, or simply due to segmentation challenges. However, all infant brains were segmented in the same reproducible manner and the effects of development on automatic tissue segmentation should be comparable across subjects yielding a consistent dataset. There is recent work developing automatic brain MRI segmentation tools to use with newborns as young as 38–44 weeks of gestational age, and this improved technology may increase researchers’ ability to more reliably segment MR images of infant brains (Gui et al., in press).
4.6. Conclusions
The results of this whole brain VBM study show, for the first time, that early gray-matter and/or white-matter concentration in right cerebellum, right hippocampus, and left PLIC/cerebral peduncles predict later language performance in infants. Our findings contribute in three ways. First, the results are consistent with adult neuroimaging studies that report posterior cerebellar (Stoodley & Schmahmann, 2009, 2010) and PLIC (Damasio & Geschwind, 1984; Metter et al., 1988; Sullivan et al., 2010) involvement in language. Second, we extend previous research by reporting an association between right cerebellar gray-matter, right cerebellar white-matter, and left PLIC/cerebral peduncle white-matter at 7 months and receptive language performance at 12 months. Third, our results reveal an early relationship between hippocampal gray-matter concentration at 7 months, confined to the right hemisphere, and later expressive language skills.
VBM identified links between brain anatomy at 7m and language skills at 12m
WM concentration in the cerebellum and PLIC correlated with receptive language
GM concentration in the cerebellum correlated with receptive language
GM concentration in the hippocampus correlated with expressive language
The cerebellum, hippocampus, and PLIC may contribute to early language development
Acknowledgments
Funding for the research was provided by the Santa Fe Institute Consortium, a grant to P.K.K. from the National Institutes of Health (HD37954), and funding for the University of Washington’s Science of Learning Center (The LIFE Center, SMA-0835854, PI, P. K. Kuhl). This work was also supported by an NIH UW Research Core Grant, University of Washington P30 DC04661.
Footnotes
The coordinates presented in this paper are specific to our template and only generally comparable to other templates. We present the MNI-like coordinates to aid the reader in the location of our significant brain regions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62(8):1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation (FMRIB Technical Report No TR07JA1) 2007a Retrieved from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation (FMRIB Technical Report No TR07JA2) 2007b Retrieved from www.fmrib.ox.ac.uk/analysis/techrep.
- Arasanz CP, Staines WR, Roy EA, Schweizer TA. The cerebellum and its role in word generation: A cTBS study. Cortex. 2012;48:718–724. doi: 10.1016/j.cortex.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Frank MJ, O’Reilly RC. Hippocampus, cortex, and basal ganglia: Insights from computational models of complementary learning systems. Neurobiology of Learning and Memory. 2004;82:253–267. doi: 10.1016/j.nlm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee GC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee GC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. Magnetic resonance techniques in the assessment of myelin and myelination. Journal of Inherited Metabolic Disease. 2005;28:311–343. doi: 10.1007/s10545-005-5952-z. [DOI] [PubMed] [Google Scholar]
- Bartha L, Brenneis C, Schocke M, Trinka E, Koylu B, Trieb T, Benke T. Medial temporal lobe activation during semantic language processing: fMRI findings in healthy left- and right-handers. Cognitive Brain Research. 2003;17:339–346. doi: 10.1016/S0926-6410(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Research. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster A, Sommer J, Wolbers T, Knecht S. Hippocampus activity differentiates good from poor learners of a novel lexicon. Neuroimage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Zimmerman ME. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of Aging. 2012;33:1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo GC. Doctoral dissertation. University of Washington; 2010. Predicting the predictors: Individual differences in longitudinal relationships between infant phonetic perception, toddler vocabulary, and preschooler language and phonological awareness. [Google Scholar]
- Cardillo Lebedeva GC, Kuhl PK. Individual differences in infant speech perception predict language and pre-reading skills through age 5 years. Paper presented at the Annual Meeting of the Society for Developmental & Behavioral Pediatrics; Portland, OR. 2009. [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Murphy DGM. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Damasio A, Geschwind N. The neural basis of language. Annual Review of Neuroscience. 1984;7:127–147. doi: 10.1146/annurev.ne.07.030184.001015. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. TRENDS in Neurosciences. 2006;29:367–373. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, Dehaene S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain & Language. 2010;114:53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC, III, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white-matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. doi: 10.1016/j.neuroimage.2012.07.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia, and the cerebral cortex? Neural Networks. 1999;12(7–8):961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Current Opinion in Neurobiology. 2000;10:732–739. doi: 10.1016/S0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white-matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Fabbro F. Introduction to language and cerebellum. Journal of Neurolinguistics. 2000;13:83–94. doi: 10.1016/S0911-6044(00)00005-1. [DOI] [Google Scholar]
- Fedorenko E, Hsieh P, Nieto-Castañón A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: Defining ROIs functionally in individual subjects. Journal of Neurophysiology. 2010;104:1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. PNAS. 2011;108:16428–16433. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood [Supplemental Material] NeuroImage. 2009;47:S102. doi: 10.1016/S1053-8119(09)70884-5. [DOI] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles H, Hein-Kropp C, Gizewski ER, Timmann D. Cerebellar involvement in verb generation: An fMRI study. Neuroscience Letters. 2006;409:19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Galantucci B, Fowler CA, Turvey MT. The motor theory of speech perception reviewed. Psychonomic Bulletin and Review. 2006;13:361–377. doi: 10.3758/BF03193857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Gouttard S, Sharma A, Gu H, Styner M, Lin W, Gilmore JH. Quantitative tract-based white matter development from birth to age 2 years. NeuroImage. 2012;61:542–557. doi: 10.1016/j.neuroimage.2012.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Gallese V. Action-based language: A theory of language acquisition, comprehension, and production. Cortex. 2012;48:905–922. doi: 10.1016/j.cortex.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Glickstein M. Thinking about the cerebellum: The cerebellum- motor control or more? Brain. 2006;129:288–290. doi: 10.1093/brain/awh728. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Strata P, Voogd J. Cerebellum: History. Neuroscience. 2009;162:549–559. doi: 10.1016/j.neuroscience.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cerebral Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Gordon N. The cerebellum and cognition. European Journal of Paediatric Neurology. 2007;11:232–234. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Groeschel S, Vollmer B, King MD, Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. International Journal of Developmental Neuroscience. 2010;28:481–489. doi: 10.1016/j.ijdevneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Gui L, Lisowski R, Faundez T, Huppi PS, Lazeyras F, Kocher M. Morphology-driven automatic segmentation of MR images of the neonatal brain. Medical Image Analysis. doi: 10.1016/j.media.2012.07.006. in press. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23:425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci USA. 1999;96(10):5884–5889. doi: 10.1073/pnas.96.10.5884. Retrieved from http://www.jstor.org/stable/48212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz N. Neurological bases of behavioral development in infancy. Brain & Development. 2000;22(7):411–416. doi: 10.1016/s0387-7604(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews: Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hokkanen LS, Kauranen V, Roine RO, Salonen O, Kotila M. Subtle cognitive deficits after cerebellar infarcts. European Journal of Neurology. 2006;13:161–170. doi: 10.1111/j.1468-1331.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. The Four-Factor Index of Social Status. Department of Sociology, Yale University; New Haven: 1975. [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca’s area: a developmental magnetoencephalography study. Brain Imaging. 2006;17(10):957–962. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Fenwick PB. Imaging cerebellum activity in real time with magnetoencephalographic data. Progress in Brain Research. 2005;148:139–150. doi: 10.1016/S0079-6123(04)48012-1. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. The Journal of Neuroscience. 2008;19:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konczak J, Timmann D. The effect of damage to the cerebellum on sensorimotor and cognitive function in children and adolescents. Neuroscience and Biobehavioral Reviews. 2007;31:1101–1113. doi: 10.1016/j.neubiorev.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Kuhl P, Rivera-Gaxiola M. Neural substrates of language acquisition. The Annual Review of Neuroscience. 2008;31:511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- Kuhl P, Conboy B, Padden D, Nelson T, Pruitt J. Early speech perception and later language development: Implications for the critical period. Language Learning and Development. 2005;1(3&4):237–264. [Google Scholar]
- Kuhl P, Conboy B, Coffey-Corina S, Padden D, Rivera-Gaxiola M, Nelson T. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e) Phil Trans R Soc B. 2008;363:979–2000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P, Damasio A. Language. In: Kandel Eric, Schwartz James, Jessell Thomas, Siegelbaum Steven, Hudspeth AJ., editors. Principles of Neural Science. McGraw-Hill Professional Publishing; 2012. pp. 1353–1372. [Google Scholar]
- Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, Kwon JS. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. NeuroImage. 2010;52:9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Leroy F, Glasel H, Dubois J, Hertz-Pannier L, Thirion B, Mangin J, Dehaene-Lambertz G. Early maturation of the linguistic dorsal pathway in human infants. The Journal of Neuroscience. 2011;31:1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. The brain network associated with acquiring semantic knowledge. Neuroimage. 2004;22:171–178. doi: 10.1016/j.neuroimage.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Marchman VA, Fernald A. Speed of word recognition and vocabulary knowledge in infancy predict cognitive and language outcomes in later childhood. Developmental Science. 2008;11:F9–F16. doi: 10.1111/j.1467-7687.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe M, Lalonde E, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis and visualization in clinical research. Proceedings of the 14th IEEE symposium on computer-based medical systems (CBMS2001),; 2001. pp. 381–386. [DOI] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Structural plasticity in the bilingual brain: Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1(1):1–9. [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service, Inc; Circle Pines: 1995. [Google Scholar]
- Metter EJ, Riege WH, Hanson WR, Jackson CA, Kempler D, Van Lancker D. Subcortical structures in aphasia. An analysis based on (F-18)-fluorodeoxyglucose, positron emission tomography, and computed tomography. Archives of Neurology. 1988;45:1229–1234. doi: 10.1001/archneur.1988.00520350067018. [DOI] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain and Language. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Molfese V. Discrimination of language skills at five years of age using event-related potentials recorded at birth. Developmental Neuropsychology. 1997;13:135–156. doi: 10.1080/87565649709540674. [DOI] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service, Inc; Circle Pines: 1995. [Google Scholar]
- Murdoch BE. The cerebellum and language: Historical perspective and review. Cortex. 2010;46:858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- National Institute of Child Health, Human Development Early Child Care Research Network . Pathways to Reading: The role of oral language in the transition to reading. Developmental Psychology. 2005;41:428–442. doi: 10.1037/0012-1649.41.2.428. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyul LG, Udupa JK. On standardizing the MR image intensity scale. Magnetic Resonance in Medicine. 1999;42:1072–1081. doi: 10.1002/(SICI)1522-2594(199912). [DOI] [PubMed] [Google Scholar]
- Opitz B, Friederici AD. Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. Neuroimage. 2003;19:1730–1737. doi: 10.1016/S1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choe MS, Flax J, Grant PE, Benasich AA. Association between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. NeuroImage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: A contribution to the definition of language epicenters with PET. NeuroImage. 2000;11:347–357. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin E, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Medical Image Analysis. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, Macleod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, Timmann D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. Journal of Neurology. 2007;254:1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- Richter S, Schoch B, Kaiser O, Groetschel H, Hein-Kropp C, Maschke M, Timmann D. Children and adolescents with chronic cerebellar lesions show no clinically relevant signs of aphasia or neglect. Journal of Neurophysiology. 2005;94:4108–4120. doi: 10.1152/jn.00611.2005. [DOI] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Klarman L, Garcia-Sierra A, Kuhl PK. Neural patterns to speech and vocabulary growth in American infants. NeuroReport. 2005;16:495–498. doi: 10.1097/00001756-200504040-00015. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Non-rigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10(3):233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Shi F, Fan Y, Tang S, Gilmore JH, Lin W, Shen D. Neonatal brain image segmentation in longitudinal MRI studies. Neuroimage. 2010;49:391–400. doi: 10.1016/j.neuroimage.2009.07.066.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Elison JT, Goldman BD, Styner M, Gu H, Connelly M, Gilmore JH. Associations between white matter microstructure and infants’ working memory. Neuroimage. doi: 10.1016/j.neuroimage.2012.09.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich W, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao F, Liu H, Kuhl P. Speech perception in infancy predicts language development in the second year of life: A longitudinal study. Child Development. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. Retrieved from http://www.jstor.org/stable/2892865. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Fjell AM. Life-span changes of the human brain white matter: Diffusion Tensor Imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Whitwell JL. Voxel-based morphometry: An automated technique for assessing structural changes in the brain. The Journal of Neuroscience. 2009;29:9661–9664. doi: 10.1523/JNEUROSCI.2160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]