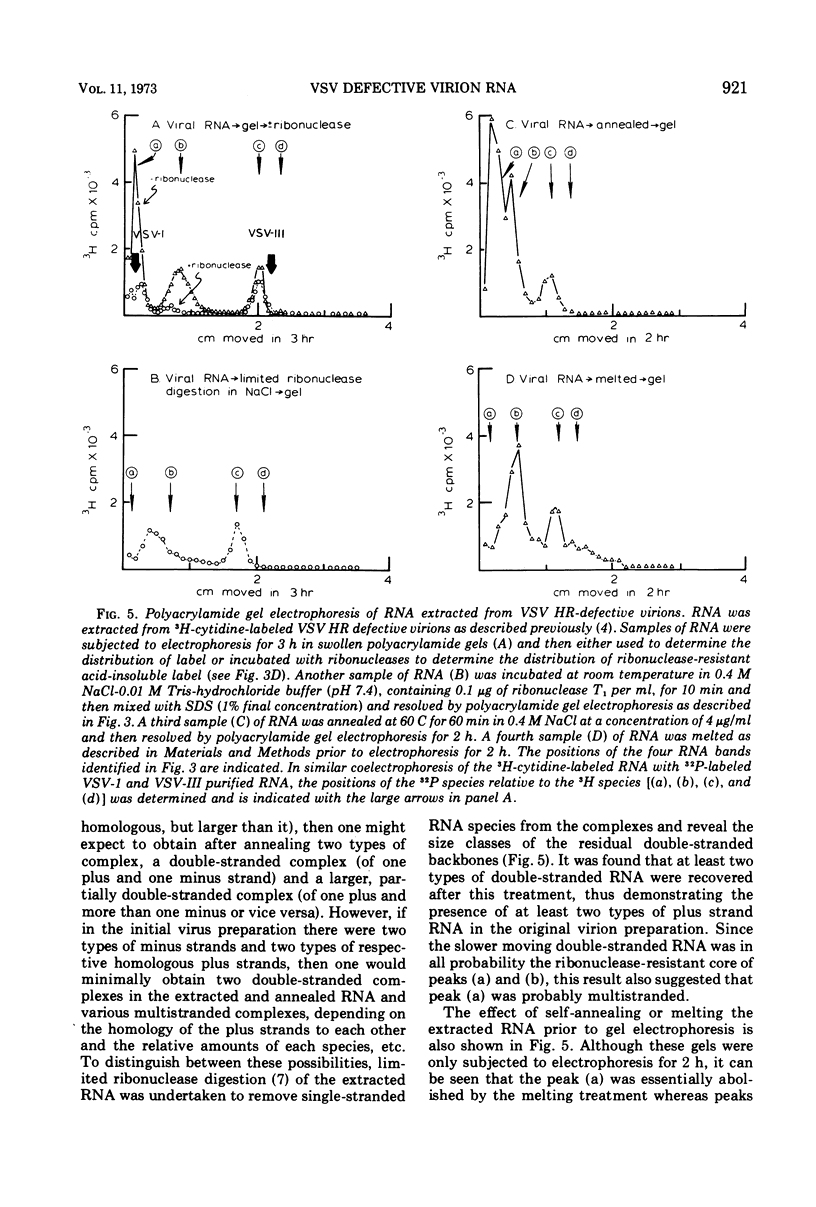
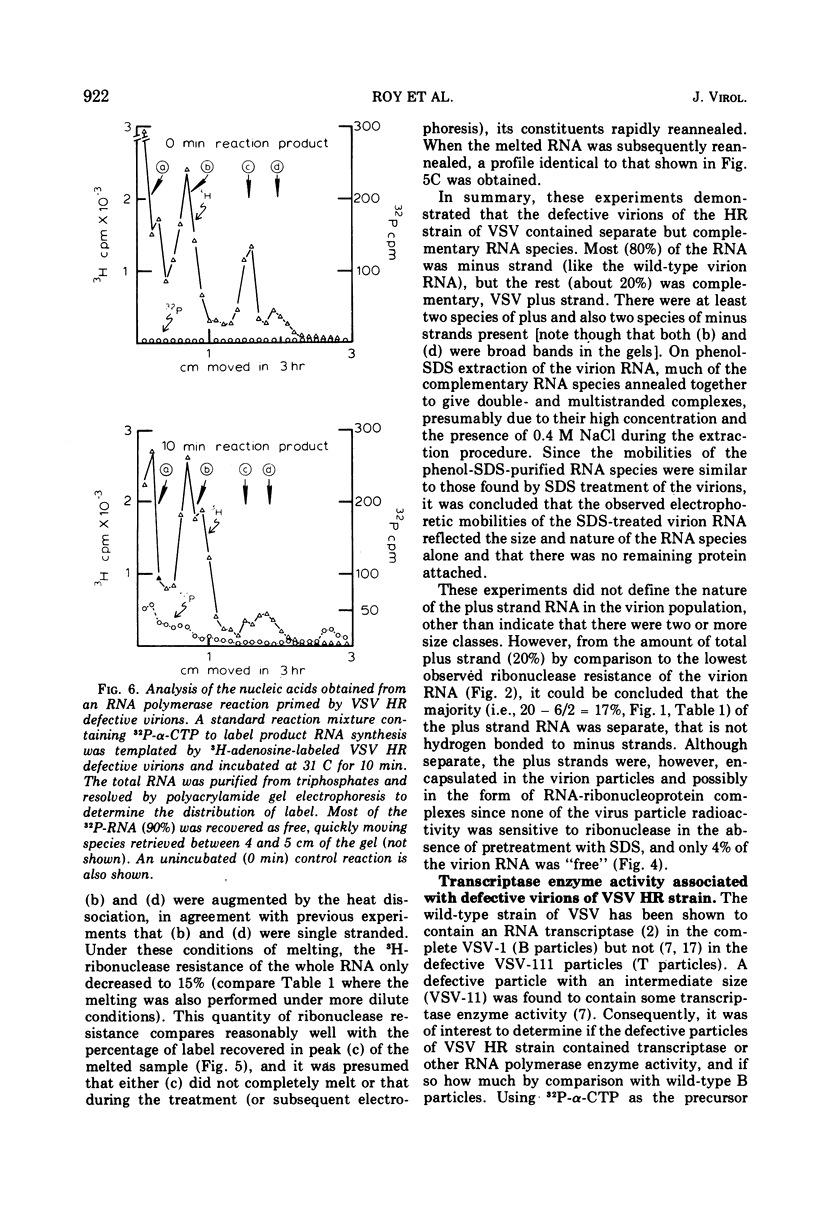
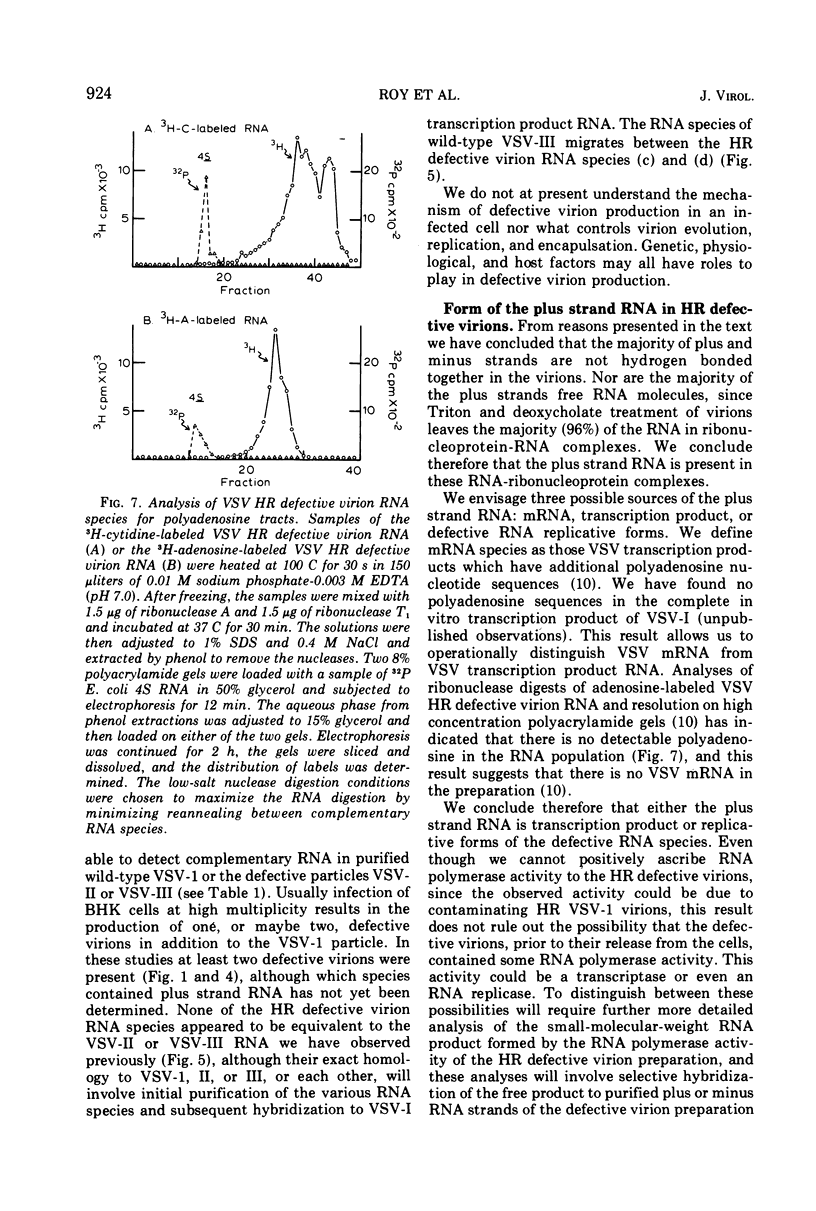
Abstract
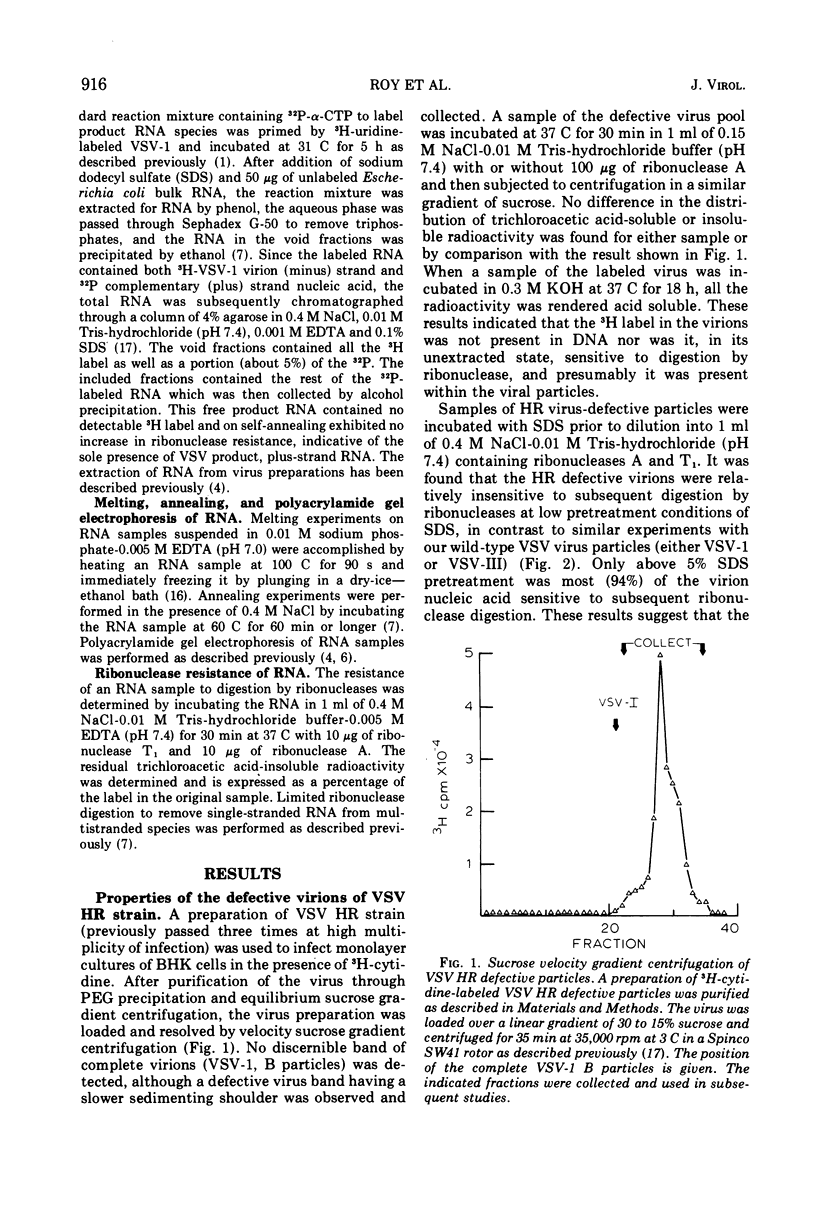
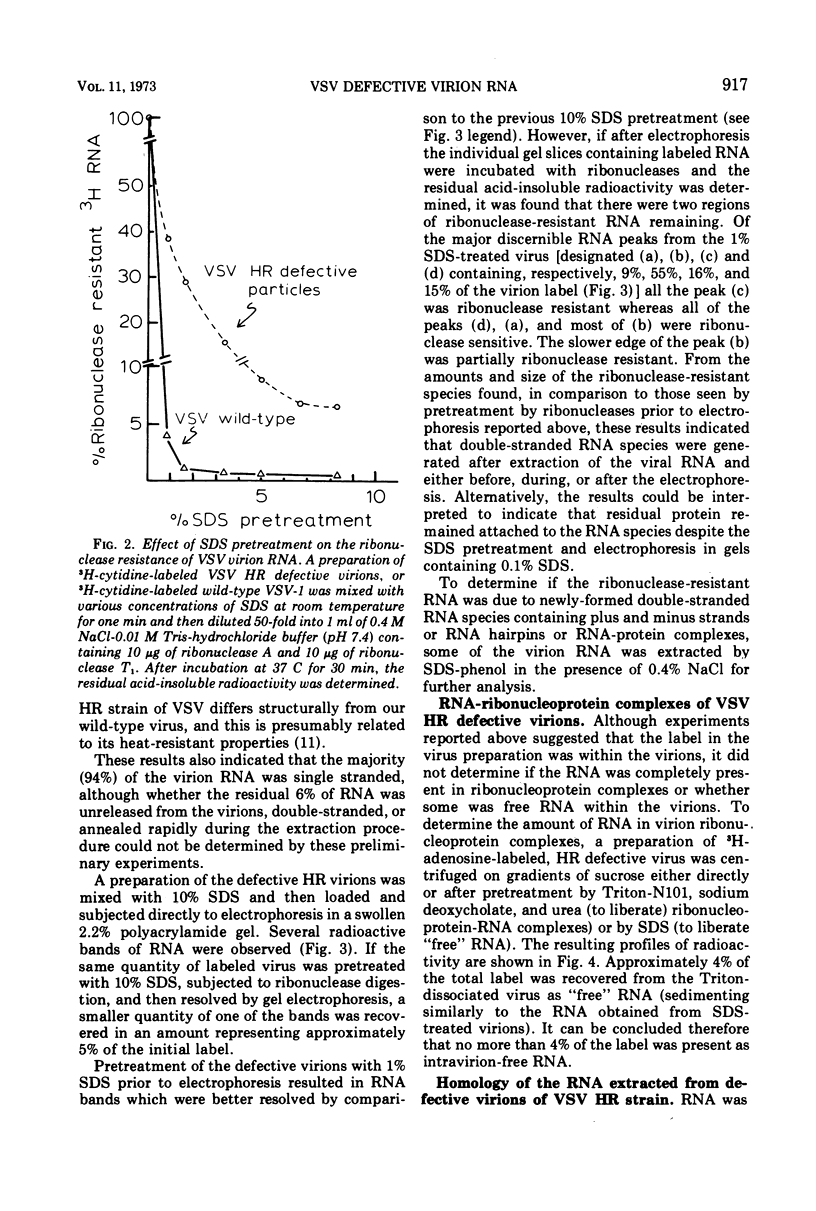
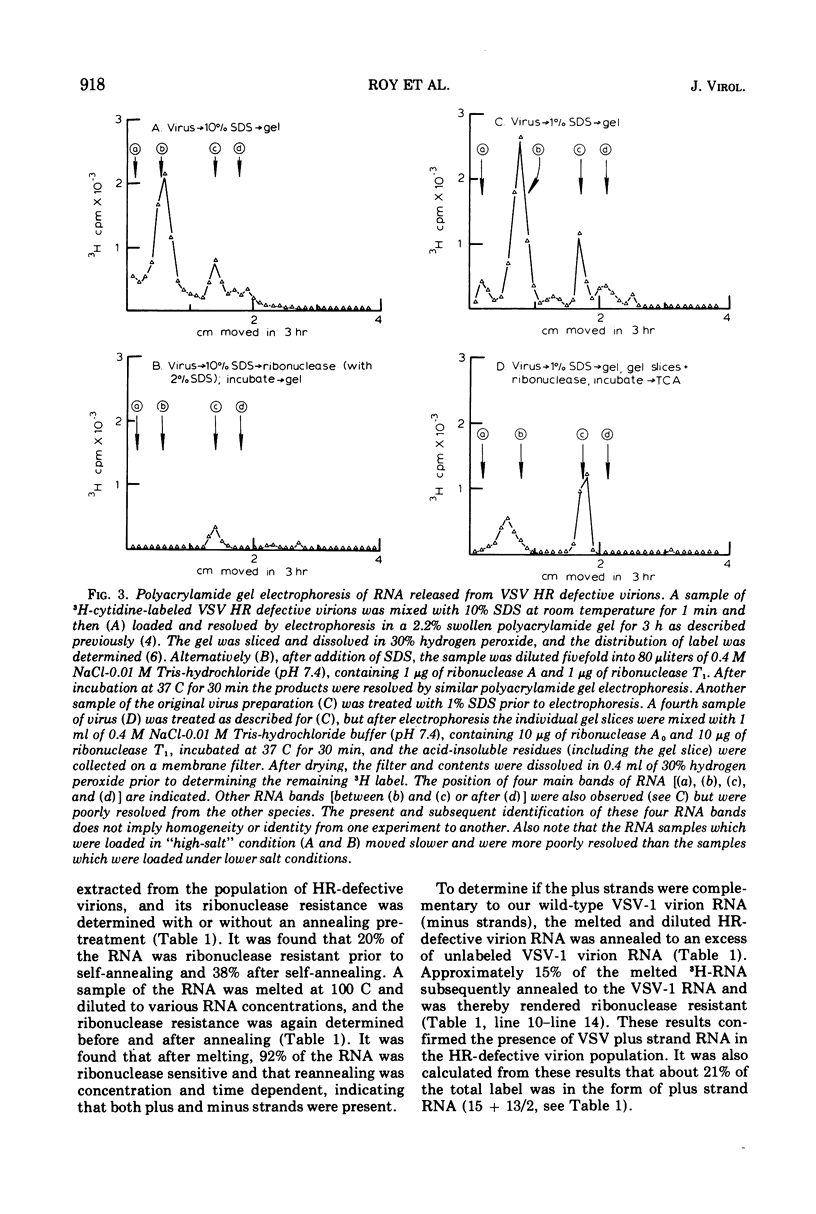
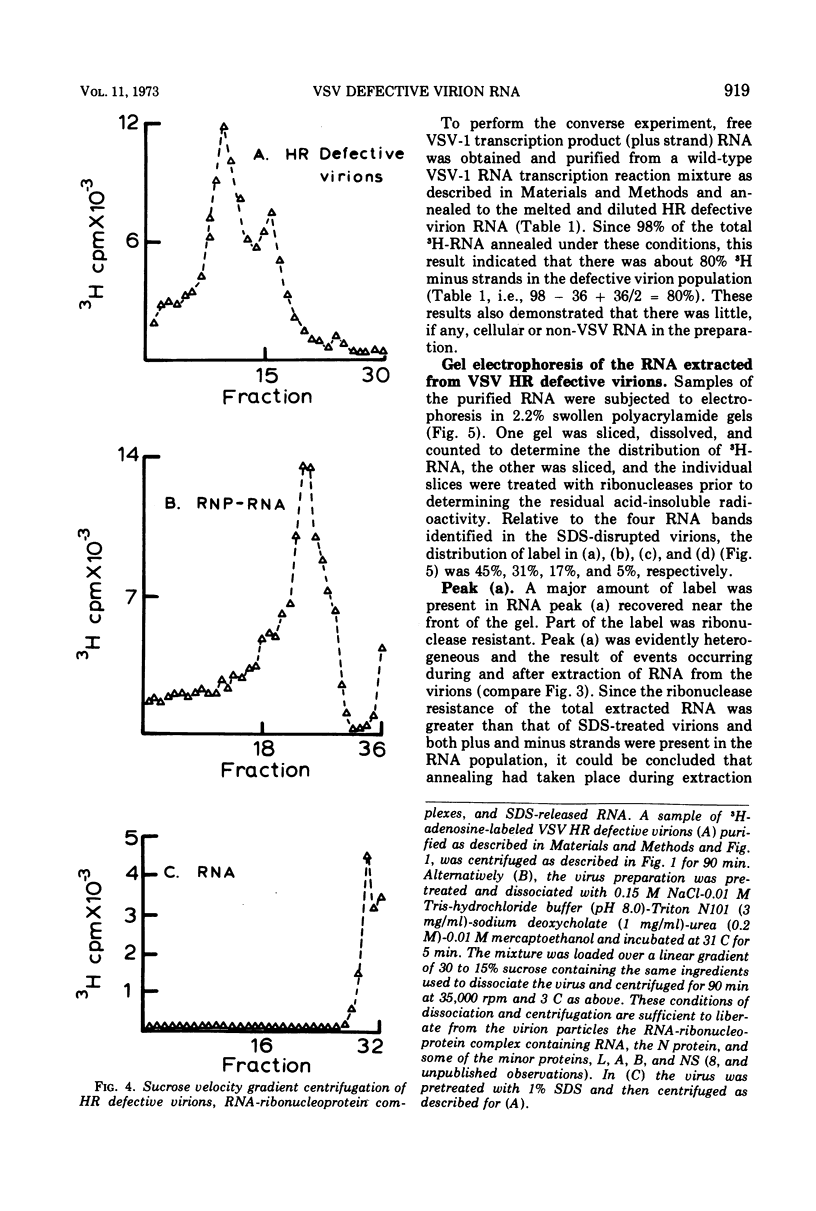
The wild-type strain of vesicular stomatitis virus (VSV) contains in its complete virion (VSV-1, B particles) a minus strand RNA. The principle defective particle of the wild-type strain (VSV-111, T particles) contains a shorter minus strand, homologous to part of the VSV-1 genome. Neither virion contains any detectable complementary (plus) strand RNA. In contrast, a preparation of a heat-resistant (HR) strain of VSV containing defective virions was found to contain both plus (21%) and minus strand RNA, present in several distinct size classes. It was found that the RNA in the HR virion preparation was at least 94% single-stranded and principally (96%) in ribonucleoprotein complexes. On extraction the plus and minus strand RNA species partially annealed to give a population of double- and multistranded RNA species. A small amount of RNA polymerase activity was associated with the HR defective virus preparation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Clark H. F., Bishop D. H., Koprowski H. Comparison of the ribonucleic acid polymerases of two rhabdoviruses, Kern Canyon virus and vesicular stomatitis virus. J Virol. 1971 Jun;7(6):726–735. doi: 10.1128/jvi.7.6.726-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bishop D. H. Complete transcription by the transcriptase of vesicular stomatitis virus. J Virol. 1971 Apr;7(4):486–490. doi: 10.1128/jvi.7.4.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J Mol Biol. 1971 May 14;57(3):513–527. doi: 10.1016/0022-2836(71)90106-9. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Properties of the product synthesized by vesicular stomatitis virus particles. J Mol Biol. 1971 Jun 28;58(3):799–814. doi: 10.1016/0022-2836(71)90041-6. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Summers D. F. Adenylate-rich sequences in vesicular stomatitis virus messenger ribonucleic acid. J Virol. 1972 Oct;10(4):683–688. doi: 10.1128/jvi.10.4.683-688.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway A. F., Wong P. K., Cormack D. V. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus. Virology. 1970 Dec;42(4):917–926. doi: 10.1016/0042-6822(70)90340-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Petric M., Prevec L. Vesicular stomatitis virus--a new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970 Aug;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Genome homology of vesicular stomatitis virus and defective T particles and evidence for the sequential transcription of the virion ribonucleic acid. J Virol. 1972 Jun;9(6):946–955. doi: 10.1128/jvi.9.6.946-955.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Isolation and properties of poliovirus minus strand ribonucleic acid. J Virol. 1970 Nov;6(5):604–609. doi: 10.1128/jvi.6.5.604-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



