Abstract
Background
The 12-item Stroke-Specific Quality of Life Scale (SSQOL), a shortened version of the original SSQOL, was developed to be an efficient and valid outcome in stroke research. We aimed to assess the validity of this scale in a bi-ethnic ischemic stroke population.
Methods
From a population-based study, the Brain Attack Surveillance in Corpus Christi Project, validated ischemic stroke patients who completed the 49-item SSQOL at 90 days post-stroke were identified. Cronbach’s alpha was used to assess internal consistency of the scales. Intraclass correlation coefficient (ICC) and linear regression were used to assess agreement between the two scales.
Results
Of the 45 ischemic stroke patients, mean age was 66.0 years (SD, 11.3). Fifty-six percent were female and 51% were Mexican American. The mean score of the 49-item scale was 3.33 (SD, 0.84) compared with 3.31 (SD, 0.95) from the 12-item scale. Internal consistency was 0.96 for the 49-item scale and 0.88 for the 12-item scale. The two scales were highly correlated (ICC= 0.98, R2 =0.97).
Conclusions
This study in ischemic stroke patients from diverse race-ethnic backgrounds found that the more efficient 12-item SSQOL is a valid alternative to the full scale for the assessment of health-related quality of life.
Keywords: Stroke, Quality of Life, Clinical Outcomes, Ischemia
Introduction
The Stroke-Specific Quality of Life scale (SSQOL) was developed in 1999 as a patient-centered outcome in stroke research to replace the use of more generic quality of life (QOL) measures.(1) It includes 12 domains that cover traditional health-related QOL topics (e.g., social and psychological measures) and stroke specific topics (e.g., language, mobility, vision, and upper extremity function).(1) The scale has been shown to have high internal reliability and construct validity in patients with different stroke types.(1–4)
A disadvantage to the original SSQOL is subject burden. For this reason, a 12-item version was developed and validated among patients in the Netherlands with mixed cerebrovascular disorders.(5) In this study, the 12-item SSQOL explained 91–93% of the variance of the 49-item SSQOL in the validation samples. Lacking however is further validation of the shortened version specifically in ischemic stroke patients from other race-ethnic backgrounds. In this study, we assessed the validity of the 12-item SSQOL in ischemic stroke patients identified in a bi-ethnic community from a population-based stroke surveillance study.
Methods
The Brain Attack Surveillance in Corpus Christi project is a population-based stroke surveillance study conducted in Nueces County, Texas. The methods of this project have been published previously.(6) Briefly, cases of potential stroke among patients ≥45 years of age were captured by active and passive surveillance of all hospitals in the county. Cases were ascertained actively by searching admission logs for a set of validated screening terms, and passively via discharge records using International Classification of Diseases, Ninth Revision (ICD-9) discharge codes for stroke (codes 430–438, excluding codes 433.x0 and 434.x0, where x = 1 to 9, 437.0, 437.2, 437.3, 437.4, 437.5, 437.7, 437.8, and 438). Validation of potential stroke cases was performed by board-certified neurologists who reviewed source documentation and applied international criteria.(7) Institutional Review Boards of the University of Michigan and the Corpus Christi hospitals approved this study.
Demographic and clinical information was abstracted from the medical chart. Race/ethnicity was self-reported. Stroke cases completed a baseline interview and a 90-day post-stroke assessment. The SSQOL was added to the 90-day assessment in May 2010. For the 90-day assessment, research staff visited the subject and collected data including the SSQOL and the modified Rankin scale. Assessments were conducted in English or Spanish. Content for the Spanish version was translated and back translated by our bilingual research team.
The original SSQOL consists of 49 items encompassing 12 domains.(1) Each item is ranked on a 5-point scale, with higher scores indicating better function. Domain scores are calculated by averaging the relevant items’ scores. The total score is calculated by averaging the domain scores. Post et al. developed the 12-item SSQOL by selecting the one question from each of the SSQOL domains with the highest item total correlation.(5) The 12-item SSQOL is calculated by averaging the scores of these 12 questions.
Included in the analysis were patients with validated ischemic stroke and self-reported assessments (i.e. non-proxy interviews). Only one subject had the assessment performed in Spanish and thus this patient was excluded. Subjects with more than 5 missing items on the SSQOL were excluded. For included subjects who had missing items, the denominators of the scoring systems were adjusted accordingly.
Statistical analysis
Baseline characteristics and 90-day measures were summarized using descriptive statistics. Cronbach’s alpha was used to assess internal consistency. With the 49-item SSQOL as the dependent variable and the 12-item SSQOL as the independent variable, linear regression analysis was used to determine the squared value of the correlation coefficient (i.e., R2). The intraclass correlation coefficient (ICC) was calculated by fitting a linear mixed model on test scores only with the random intercept within subjects. The Bland-Altman “difference against the mean” plot was used visually to inspect for bias in the 12-item scale across the range of scores.(8) To explore differences by race-ethnicity, models were also run separately for Mexican-American (MA) patients and non-Hispanic White (NHW) patients. All analyses were performed using Stata, version 11.0 (StataCorp, College Station, TX).
Results
From August 16, 2010 to December 13, 2010, 60 patients with ischemic stroke completed a 90-day outcome. Excluded were 12 subjects with proxy interviews, 2 with incomplete surveys, and also the 1 subject whose assessment was performed in Spanish. Thus, the final study population was 45 subjects.
Characteristics of the study population are presented in the Table. Mean age was 66.0 years (SD, 11.3) and 56% were female. Fifty-one percent were Mexican American and 42% were non-Hispanic White. The median National Institutes of Health Stroke Scale (NIHSS) at the index event was 5 (IQR, 2–8). At the 90-day outcome assessment, median modified Rankin scale score was 3 (IQR, 2–3).
Table.
Demographic and other characteristics of the subjects
| Number, %, unless otherwise specified N= 45 |
|
|---|---|
| Baseline characteristics | |
| Age, mean (SD) | 66.0 (11.3) |
| Female | 25 (55.6%) |
| Ethnicity | |
| Mexican-American | 23 (51.1%) |
| Non-Hispanic White | 19 (42.2%) |
| Other | 3 (6.7%) |
| NIHSS, median (IQR) | 5 (2–8) |
| Past medical history | |
| Hypertension | 37 (82.2%) |
| Diabetes mellitus | 25 (55.6%) |
| High cholesterol | 22 (48.9%) |
| Stroke/Transient ischemic attack | 16 (35.6%) |
| Current smoker | 13 (28.9%) |
| Coronary artery disease | 10 (22.2%) |
| Atrial fibrillation | 6 (13.3%) |
| Myocardial infarction | 1 (2.2%) |
| 90-day outcome measures | |
| SSQOL | |
| 49-item, mean (SD) | 3.33 (0.84) |
| 12-item, mean (SD) | 3.31 (0.95) |
| Modified Rankin Scale, median (IQR) | 3 (2–3) |
SSQOL = Stroke-Specific Quality of Life.
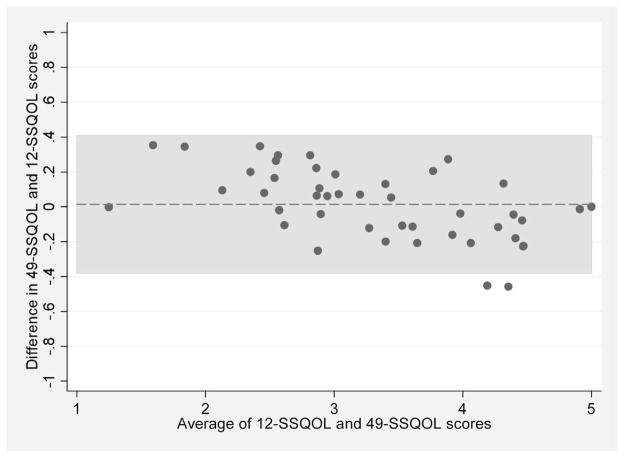
The internal consistency of the 12-item SSQOL was 0.88 and for the 49-item scale was 0.96. The mean score of the 49-item SSQOL was 3.33 (SD, 0.84) compared with 3.31 (SD, 0.95) from the 12-item scale. From the linear regression analysis, the R2 was 0.97, with an intercept of 0.45 (95% confidence interval [CI], 0.28–0.63) and coefficient of 0.87 (95% CI, 0.82–0.92). The correlation of the tests within subjects was very high (ICC from multi-level model = 0.98). These findings were similar when MA and NHW patients were analyzed separately (R2, 0.97 for MAs compared to 0.95 for NHWs; ICC, 0.98 for MAs compared to 0.96 for NHWs). The Bland-Altman plot is presented in the Figure. The mean difference comparing the 49-item score to the 12-item score was 0.01 (SD, 0.20). In patients with a low mean score (i.e. the average of the 12-item and 49-item scores), the 12-item score was slightly lower than 49-item score. Conversely, patients with a high mean score tended to have a slightly higher 12-item score compared with the 49-item score. In only 2 of the 45 patients (4.4%) was this difference more than 2 standard deviations from the mean difference.
Figure.
Bland-Altman plot of the differences between the 49-item Stroke Specific Quality of Life (SSQOL) scale and the 12-item SSQOL. Each circle represents an individual patient. The dashed horizontal line represents the mean difference and the shaded region represents the mean difference ± 2 standard deviations.
Discussion
This is the first study to assess the validity of the 12-item SSQOL in a bi-ethic ischemic stroke population. We found that the more efficient 12-item scale is highly correlated with the 49-item scale, and thus this shorter scale provides an accurate stroke-related quality of life outcome measure.
Compared to the Dutch study,(5) our sample demonstrated slightly more explained variance (>96%, versus 91–93% for Dutch study) of the 49-item scale by the 12-item scale. This difference may be due to the inclusion of mixed cerebrovascular disorders compared to our study of only ischemic stroke patients. Differences in populations and language versions of the scales may also have impacted the 12-item scale performance.
Despite the high overall agreement between the 12-item and 49-item scales, both our study and the Dutch study found that the relationship of the 12-item scale to the mean value (mean of 49-item and 12-item scores) differs somewhat at high versus low mean values.(5) The significance of these small differences is likely not meaningful, but future studies should consider this relationship if focusing on the extremes of the scale.
This study was limited by the small sample size. Because of our small sample size, we did not explore the impact of demographic factors (other than MA to NHW comparison), language, or other clinical factors may have on the relationship between scales. Our results from MAs fluent in English may not generalize to MAs not fluent in English. Further, these results do not generalize to more severe strokes with persistent language dysfunction and may not generalize to other populations with different sociodemographic characteristics.
Acknowledgments
Sources of Funding: NIHR01HL098065, NIHR01NS038916
Footnotes
Conflicts of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams LS, Weinberger M, Harris LE, et al. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 2.Williams LS, Weinberger M, Harris LE, et al. Measuring quality of life in a way that is meaningful to stroke patients. Neurology. 1999;53:1839–1843. doi: 10.1212/wnl.53.8.1839. [DOI] [PubMed] [Google Scholar]
- 3.Muus I, Williams LS, Ringsberg KC. Validation of the Stroke Specific Quality of Life Scale (SS-QOL): test of reliability and validity of the Danish version (SS-QOL-DK) Clin Rehabil. 2007;21:620–627. doi: 10.1177/0269215507075504. [DOI] [PubMed] [Google Scholar]
- 4.Boosman H, Passier PE, Visser-Meily JM, et al. Validation of the Stroke Specific Quality of Life scale in patients with aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2010;81:485–489. doi: 10.1136/jnnp.2009.184960. [DOI] [PubMed] [Google Scholar]
- 5.Post MW, Boosman H, van Zandvoort MM, et al. Development and validation of a short version of the Stroke Specific Quality of Life Scale. J Neurol Neurosurg Psychiatry. 2011;82:283–286. doi: 10.1136/jnnp.2009.196394. [DOI] [PubMed] [Google Scholar]
- 6.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asplund K, Tuomilehto J, Stegmayr B, et al. Diagnostic criteria and quality control of the registration of stroke events in the MONICA project. Acta Med Scand Suppl. 1988;728:26–39. doi: 10.1111/j.0954-6820.1988.tb05550.x. [DOI] [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]