Abstract
The present study examined the effect of insulin-mediated activation of the mammalian target of rapamycin complex 1 (MTORC1) signaling network on the proliferation of primary culture of theca-interstitial (T-I) cells. Our results show that insulin treatment increased proliferation of the T-I cells through the MTORC1-dependent signaling pathway by increasing cell cycle regulatory proteins. Inhibition of ERK1/2 signaling caused partial reduction of insulin-induced phosphorylation of RPS6KB1 and RPS6 whereas inhibition of PI3-kinase signaling completely blocked the insulin response. Pharmacological inhibition of MTORC1 with rapamycin abrogated the insulin-induced phosphorylation of EIF4EBP1, RPS6KB1 and its downstream effector, RPS6. These results were further confirmed by demonstrating that knockdown of Mtor using siRNA reduced the insulin-stimulated MTORC1 signaling. Furthermore, insulin-stimulated T-I cell proliferation and the expression of cell cycle regulatory proteins CDK4, CCND3 and PCNA were also blocked by rapamycin. Taken together, the present studies show that insulin stimulates cell proliferation and cell cycle regulatory proteins in T-I cells via activation of the MTORC1 signaling pathway.
Keywords: Insulin, Theca-interstitial cells, MTORC1, RPS6KB1, Cell cycle regulatory proteins
1. INTRODUCTION
In the ovary, theca-interstitial (T-I) cells play a central role in androgen production. These androgens also provide substrates for estrogen synthesis in granulosa cells (Magoffin, 2002; Magoffin, 2005; Young and McNeilly, 2010). It is well documented that luteinizing hormone (LH) is a primary regulator of T-I cell function (Palaniappan and Menon, 2009; Wood and Strauss, 2002). Our recent studies show that LH-mediated activation of PI3-kinase/AKT/MTORC1 signaling enhances the expression of genes involved in T-I cell proliferation and androgen synthesis (Palaniappan and Menon, 2010; Palaniappan and Menon, 2012). Additionally, insulin, IGF1 and other growth factors are also key players in T-I cell growth, proliferation and function (Duleba et al., 1999a; Duleba et al., 1997; Duleba et al., 1999b; Kwintkiewicz et al., 2006; Spicer et al., 2008).
Insulin acts primarily through the insulin receptor, a receptor tyrosine-kinase, signaling through the PI3-kinase and mitogen activated protein kinase (MAPK) pathways (Diamanti-Kandarakis and Papavassiliou, 2006; Myers and White, 1993). PI3-kinase propagates intracellular signaling cascades regulating a wide range of cellular processes including cell growth and proliferation by activating downstream molecules such as AKT/PKB and MTORC1 (Fingar and Blenis, 2004; Ma and Blenis, 2009; Palaniappan and Menon, 2010; Zheng et al., 2012). A recent study suggests that RAS/MAPK signaling cascade also promotes MTORC1 signaling and cell growth (Carriere et al., 2011). MTOR exists as two different complexes within the cell, MTORC1 and MTORC2, but only MTORC1 is sensitive to inhibition by rapamycin (Guertin and Sabatini, 2007). MTORC1 is a master controller of protein synthesis, integrating signals from growth factors within the context of the energy and nutritional conditions of the cell. Activated MTORC1 regulates protein synthesis by directly phosphorylating EIF4EBP1 and RPS6KB1 (Fingar et al., 2004; Fingar et al., 2002; Manning and Cantley, 2003; Palaniappan and Menon, 2010). Since insulin has been known to regulate MTORC1 signaling in target cells (Li et al., 2010; Rapley et al., 2011) in the present study we examined whether the proliferative effect of insulin on T-I cells is mediated by activating this pathway. Our results show that insulin activates T-I cell proliferation and the expression of cell cycle regulatory components (CDK4, CCND3 and PCNA) by triggering the MTORC1-dependent pathway.
2. MATERIALS AND METHODS
2.1. Materials
Medium 199, McCoy’s 5A medium, L-glutamine and HEPES buffer were purchased from Invitrogen/GIBCO (Carlsbad, CA). Penicillin–streptomycin was purchased from Roche Diagnostics (Indianapolis, IN). Collagenase (CLS I) and deoxyribonuclease I were obtained from Worthington Biochemical Corp. (Freehold, NJ). Bovine insulin, bovine serum albumin (BSA) and TUBB (β-tubulin) antibody were purchased from Sigma Chemical Co. (St. Louis, MO). MTORC1 inhibitor, rapamycin and antibodies against phosphorylated RPS6KB1 (Thr389), phospho- RPS6KB1 (Thr421/Ser424), phospho-EIF4EBP1 (Thr37/46), phosphorylated ribosomal protein S6 (Ser235/236), total RPS6KB1, total RPS6, total EIF4EBP1, CCND3, CDK4 and the Mtor siRNA kit were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against Phospho ERK1/2, total ERK and PCNA were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was obtained from Chemicon (Temecula, CA). Anti-mouse, anti-rabbit IgG horseradish peroxidase conjugates, enhanced chemiluminescence kit, the Femto Supersignal Substrate System and Restore Western blot stripping buffer were purchased from Pierce (Rockford, IL). Reagents as well as the primers and probes for the cyclin D1 (Ccnd1) and cyclin D3 (Ccnd1) real-time PCR were from Applied Biosystems (Foster City, CA). All other reagents used were conventional commercial products.
2.2. Animals
Sprague-Dawley female rats (25 days old) were purchased from Charles River Laboratories (Wilmington, MA). All the experimental protocols used in this study were approved by the University Committee on the Use and Care of Animals. Animals were housed in a temperature-controlled room with proper dark-light cycles as per the guidelines provided by the University Committee on the Use and Care of Animals. The animals were euthanized by CO2 asphyxiation. The ovaries were removed under sterile conditions and were processed immediately for the isolation of T-I cells.
2.3. Isolation and culture of theca-interstitial cells
The T-I cells were isolated, dispersed and cultured following a protocol previously published from our laboratory (Palaniappan and Menon, 2009; Palaniappan and Menon, 2010). Briefly, freshly collected ovaries were placed in Medium 199 containing 25mM Hepes (pH 7.4), 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin. The ovaries were then freed from adhering fat and actively punctured with a 27-gauge needle under a dissecting microscope to release the granulosa and blood cells. The remaining ovarian tissue was then washed three times with medium to release any remaining granulosa cells. The tissue was then minced and incubated for 30 min at 37 ° C in the same medium, supplemented with 0.65 mg/ml collagenase type 1 plus 10 μg/ml deoxyribonuclease. The dispersion was encouraged by mechanically pipetting the ovarian tissue suspension with a 10 ml pipette. The theca-interstitial cells released by this digestion were centrifuged at 250g for 5 min and washed in medium two times to eliminate remaining collagenase. The dispersed cells were then resuspended in McCoy’s 5A medium containing 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin and 100 μg/ml streptomycin and subjected to unit gravity sedimentation for 5 min to eliminate small fragments of undispersed ovarian tissue. Cell viability was assessed by trypan blue exclusion and averaged above 90%. The dispersed cells were seeded in 60 mm plates (3×106 viable cells). The plated cells were maintained overnight in McCoy’s 5A medium containing 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere of 95% air–5% CO2 at 37°C. After allowing cells to attach, they were treated with insulin for different time intervals, and inhibitor was used as indicated in the figure legends. Insulin and inhibitor concentrations were selected on the basis on our previous studies (Palaniappan and Menon, 2009; Palaniappan and Menon, 2010; Rice et al., 2005; Will et al., 2012).
2.4. Cell viability, cell number and proliferation assay
T-I cells were seeded into 96-well plates and cultured overnight with McCoy’s medium containing 0.1 % BSA. After attachment, cells were pretreated with rapamycin for 1h followed by insulin (1μg/ml) for 24 h. After the treatment periods, cell viability was determined by MTT assay as previously described (Mosmann, 1983). To test whether insulin increases T-I cell number, cells were seeded into 24 well plates and cultured overnight with McCoy’s medium containing 0.1 % BSA. After allowing to attach, the cells were treated with insulin (1μg/ml) for 24h and 48h and cell number was determined by using Countless Automated Cell Counter (Invitrogen). For BrdU cell proliferation assay, cells were labeled with BrdU followed by insulin treatment for 24h. Cell proliferation was assayed by measuring the incorporation of BrdU using BrdU immunoassay kits (Calbiochem, La Jolla, CA) as previously described (Palaniappan and Menon, 2010).
2.5. Real-Time PCR
The role of MTORC1 in insulin -mediated Ccnd1 and Ccnd3 mRNA expression were examined by pretreating the cells with or without rapamycin (20 nM) for 1 h, followed by insulin for 4 h. At the end of incubation, the cells were harvested, and total RNA extracted using TRIzol reagent following the manufacturer’s instructions. (Life Technologies). Ccnd1 and Ccnd3 mRNA expression were analyzed by real-time PCR as previously described (Palaniappan and Menon, 2010). The changes in Ccnd1 and Ccnd3 expression were calculated using the Ct method (Livak and Schmittgen, 2001) with 18S rRNA as the internal control.
2.6. Western blot analysis
After various treatments as described in the respective figure legends, cell monolayers were washed with PBS, and then solubilized using radioimmunoprecipitation assay (RIPA) buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS). Cell lysates were then sonicated and centrifuged for 10 min at 13,000 X g. The protein content of the supernatants was determined using BCA reagent (Pierce). Proteins (30–50μg/lane) were separated by electrophoresis using 10% or 4–20% gradient SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad) before immunoblot analysis as previously described (Palaniappan and Menon, 2010). Protein loading was monitored by reprobing the same blots with appropriate antibodies as indicated in the figure legends.
2.7. siRNA-mediated silencing of mTOR
The protocol for siRNA-mediated knockdown of Mtor in T-I cells was previously published from our laboratory (Palaniappan and Menon, 2010). The control and Mtor siRNA sequences are as follows: control sense siRNA 5′CGUACGCGGAAUACUUCGA 3′; antisense 5′UCGAAGUAUUCCGCGUACG 3′ and Mtor sense siRNA 5′ UGAACCCUGCCUUUGUCAUGC 3′; antisense 5′GCAUGACAAAGGCAGGGUUCA 3′. Briefly, T-I cells were transfected with control siRNA (non-targeted) or Mtor siRNA (targeted) using a Nucleofector transfection reagent (Amaxa), as per the manufacturer’s instructions. After transfection, cells were resuspended in 5% FBS/McCoy’s medium and plated. Forty-eight hours later media was replaced with serum free medium for overnight culture and then treated without or with insulin for an additional 30 min. MTOR, phospho-specific RPS6KB1 Thr389, RPS6KB1 Thr421/Ser424, RPS6 Ser235/236 and EIF4EBP1 Thr37/46 were examined by Western blot analysis using specific antibodies.
2.8. Statistical analysis
Statistical analysis was carried out using one-way ANOVA followed by the Tukey multiple comparison test using Prism software (GraphPad Prism, version 3.0; GraphPad Inc., San Diego, CA). Values were considered statistically significant at P < 0.05. Each experiment was repeated at least three times, with similar results. Blots are representative of one experiment, and graphs represent the mean ± SE of three replicates.
3. RESULTS
3.1. Insulin-induced T-I cell proliferation is rapamycin sensitive
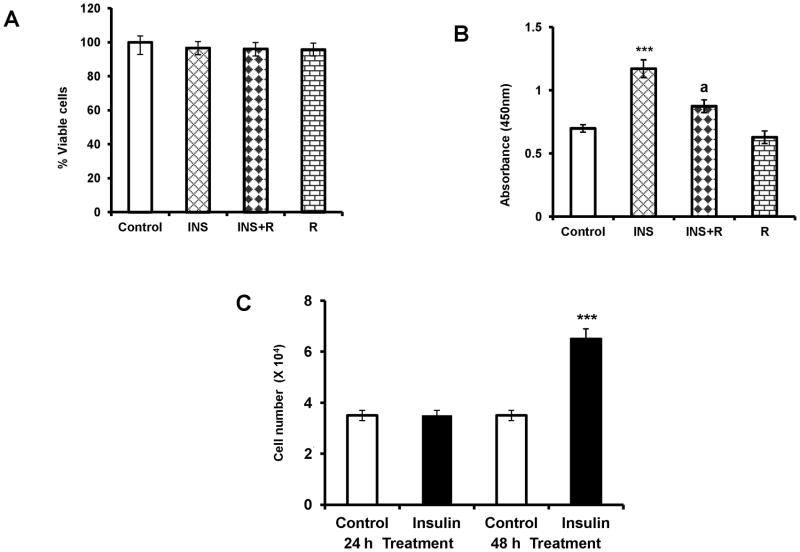
The initial experiments examined toxicity of rapamycin treatment in cultured T-I cells using cell viability assay. To test this, cultured T-I cells were preincubated with or without rapamycin (20 nM) for 1h followed by treatment with insulin for 24 h. Cell viability was analyzed by MTT assay. The results presented in Figure 1A show that repamycin treatment at 20 nM concentration did not reduce cell viability compared with control or insulin treatment group. To determine, whether inhibition of MTORC1 signaling reduces insulin-induced T-I cell proliferation, cells were treated with rapamycin for 1 h followed by insulin for 24 h in the presence of BrdU. The results indicate that insulin treatment augmented BrdU incorporation, and this stimulatory effect was abolished by the addition of the MTORC1 inhibitor, rapamycin (Fig. 1B). To provide further evidence to show that insulin increases T-I cell number, cells were treated with insulin for 24 h and 48 h and cell number was assessed. As shown in Figure 1C, insulin treatment significantly increased T-I cell number at 48 h. Collectively, these results suggest that insulin-stimulated T-I cell proliferation occurs through the MTORC1-dependent signaling pathway.
FIG. 1. Effect of MTORC1 inhibition on insulin-stimulated cell proliferation.
(A), Ovaries were collected from 25 day old Sprague-Dawley rats. T-I cells were isolated by collagenase digestion and cells were plated with McCoy’s medium. After 24 h attachment, cells were pretreated without or with rapamycin (20 nM) for 1 h prior to treatment for 24 h with insulin (1μg/ml). Control groups received vehicle (DMSO). The viability of the cells was assessed by MTT assay. Results are expressed as the percentage of viable cells compared with control. (B), Cells were incubated with MTORC1 inhibitor (rapamycin, 20 nM) for 1 h followed by insulin (1μg/ml) treatment for 24 h. Cells were labeled with BrdU and cell proliferation was assessed by BrdU incorporation as described in Materials and Methods. (C), Cells were treated with insulin (1μg/ml) for 24 h and 48 h and cell number was quantified by Cell Counter as described in Materials and Methods. All experiments were carried out three times with triplicates in each experiment. Error bars represent mean ± SE. ***, P < 0.001 vs. control. a, Significant differences (P < 0.05) compared with insulin treatment. INS= insulin; R= rapamycin
3.2. Insulin stimulates activation of RPS6KB1, RPS6 and EIF4EBP1
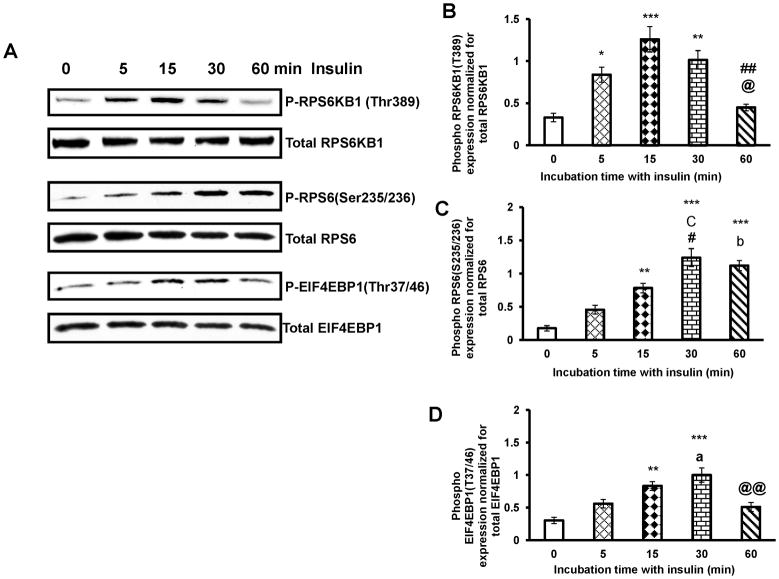
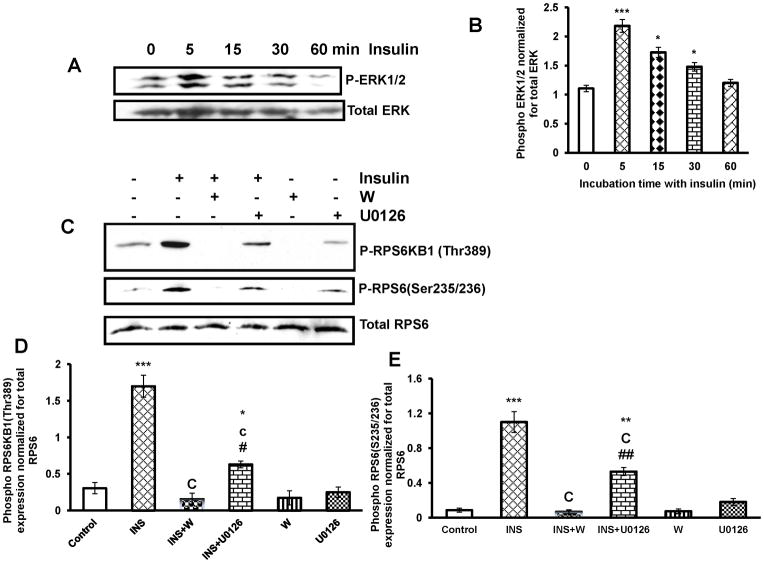
MTORC1 is a master regulator of cell growth and proliferation that is deregulated in pathological conditions. Because insulin is known to increase T- I cell proliferation, we explored whether insulin would alter RPS6KB1 and EIF4EBP1 phosphorylation in cultured T-I cells, since RPS6KB1 and EIF4EBP1 are downstream targets of MTORC1 signaling. To test this, T-I cells were cultured with insulin for different time intervals, the phosphorylation of RPS6KB1, and its target RPS6 and EIF4EBP1 were examined by Western blot analysis using phospho-specific antibodies. The results (Fig. 2A) show that within five minutes of insulin treatment, RPS6KB1 was phosphorylated at Thr389 reaching maximal phosphorylation at 15 min (Fig. 2B) whereas RPS6 (Ser235/236) and EIF4EBP1(Thr37/46) phosphorylation increased by 15 min and was further augmented at 30 min. At 60 min, the extent of RPS6KB1 and EIF4EBP1 phosphorylation was lower than that seen at 30 min (Fig. 2C and D). These results show that MTORC1 signaling is responsive to insulin in T-I cells.
FIG. 2. Time course study of insulin effect on phosphorylation of RPS6KB1, RPS6 and EIF4EBP1.
Cells were treated with insulin (1μg/ml) for different time periods. Cells were lysed using RIPA buffer and subjected to Western blot analysis using phospho-specific antibodies for RPS6KB1(Thr389), RPS6(Ser235/236) and EIF4EBP1(Thr37/46). Protein loading was monitored by stripping and reprobing the same blot with RPS6KB1, RPS6 and EIF4EBP1 antibodies (A). The graphs (B, C and D) represent densitrometric scans of phospho RPS6KB1(Thr389), phospho RPS6 (Ser235/236) and phospho EIF4EBP1(Thr37/46) protein expression normalized for total RPS6KB1, RPS6 and EIF4EBP1, respectively. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. *, P < 0.05; **, P < 0.01; and ***, P< 0.001 vs. 0 min insulin treatment. a, b and c represent significant differences compared with 5min insulin treatment (a, P < 0.05; b, P < 0.01 and c, P < 0.001, respectively). # and ## represent significant differences compared with 15min insulin treatment (#, P <0.05 and ##, P < 0.01, respectively). @ and @@ represent significant differences compared with 30 min insulin treatment (@, P < 0.05 and @@, P < 0.01, respectively).
3.3. Insulin stimulated RPS6KB1, RPS6 and EIF4EBP1 phosphorylation is sensitive to rapamycin
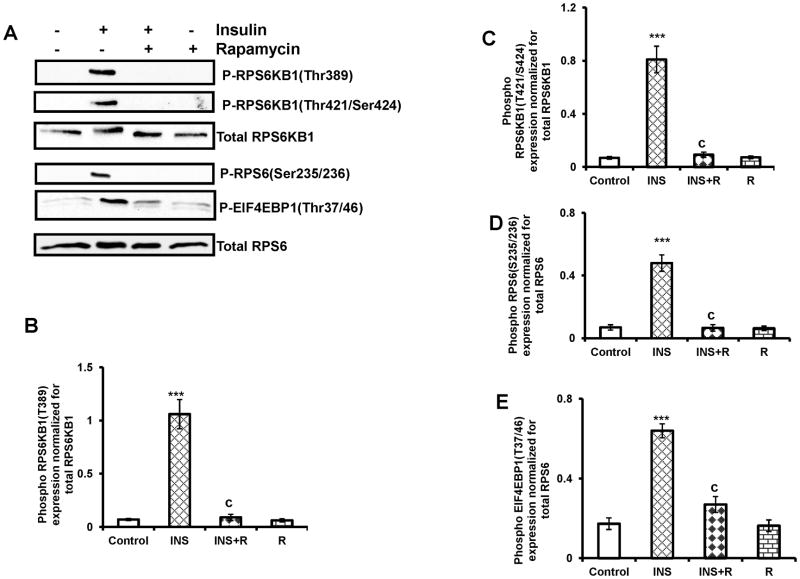
We then tested the effect of MTORC1 inhibitor, rapamycin, to further confirm that phosphorylation of RPS6KB1, RPS6 and EIF4EBP1 occurs downstream of MTORC1 activation in response to insulin. T-I cells were pretreated with rapamycin for 1 h followed by insulin treatment for 30 min. Immunoblot analysis was performed using phospho-specific RPS6KB1 Thr389, RPS6KB1 Thr421/Ser424, RPS6 Ser235/236 and EIF4EBP1 Thr37/46 antibodies. The results (Fig. 3A) clearly show that insulin-induced phosphorylation of RPS6KB1 (Fig. 3B and C), RPS6 (Fig. 3D) and EIF4EBP1 (Fig. 3E) was prevented by treatment with rapamycin.
FIG. 3. Effect of rapamycin on insulin-stimulated phosphorylation of RPS6KB1, RPS6 and EIF4EBP1.
T-I cells were pretreated without or with rapamycin (20 nM) for 1 h prior to treatment for 30 min with insulin (1μg/ml). Control groups were treated with vehicle (DMSO). Cells were lysed and subjected to Western blot analysis using phosphorylation site-specific antibodies for RPS6KB1(Thr389), RPS6KB1(Thr421/Ser424), RPS6 (Ser235/236) and EIF4EBP1(Thr37/46). The levels of RPS6KB1 or RPS6 protein were used as internal controls. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. ***, P< 0.001 vs. control. c represent significant differences compared with insulin (c, P < 0.001). INS= insulin; R= rapamycin
3.4. siRNA-mediated silencing of Mtor and its effect on RPS6KB1, RPS6 and EIF4EBP1 phosphorylation in response to insulin
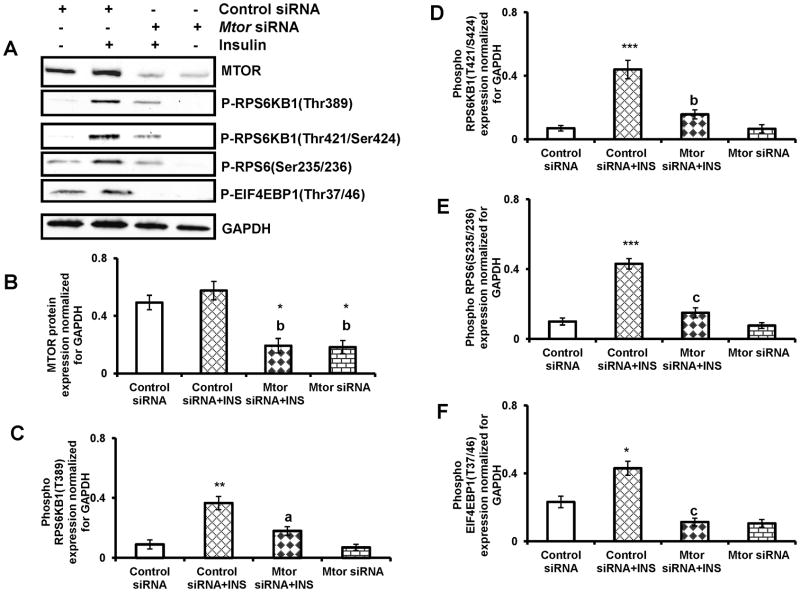
To provide further evidence of MTORC1-mediated phosphorylation of RPS6KB1, RPS6 and EIF4EBP1, T-I cells were transfected with Mtor siRNA to knockdown Mtor expression using the protocol that we recently reported (Palaniappan and Menon, 2010). Cells were transfected with control siRNA or Mtor siRNA for 48 h followed by insulin treatment for 30 min. Cell lysates were analyzed for MTOR and phospho form of RPS6KB1 Thr389, RPS6KB1 Thr421/Ser424, RPS6 Ser235/236 and EIF4EBP1 Thr37/46 by Western blot analysis. The results (Fig. 4A) show that as expected, MTOR protein expression was blocked by the siRNA targeting Mtor, compared to non-targeting siRNA (Fig. 4B). Furthermore, the knockdown of Mtor by Mtor siRNA reduced insulin- induced phosphorylation of RPS6KB1 Thr389, RPS6KB1 Thr421/Ser424, RPS6 Ser235/236 and EIF4EBP1 Thr37/46 (Fig. 4C–F). These results conclusively show that insulin-mediated activation of MTORC1 is required for RPS6KB1 Thr389, RPS6KB1 Thr421/Ser424, RPS6 Ser235/236 and EIF4EBP1 Thr37/46 phosphorylation.
FIG. 4. Mtor siRNA inhibits insulin-induced phosphorylation of RPS6KB1, RPS6 and EIF4EBP1.
T-I cells were transfected with 100 nM of control siRNA or Mtor siRNA for 48 h and then treated without or with insulin (1μg/ml) for 30 min. Proteins (30 μg) were separated by SDS-PAGE (4–20%), and then immunoblotted with MTOR, phospho-specific RPS6KB1 (Thr389), RPS6KB1 (Thr421/Ser424), RPS6 (Ser235/236) and EIF4EBP1(Thr37/46) antibodies. Protein loading was monitored by stripping and reprobing the same blot with antibody for GAPDH. The graphs (B, C, D and E) represent densitrometric scans of MTOR, phospho RPS6KB1(Thr389), phospho RPS6 (Ser235/236) and phospho EIF4EBP1(Thr37/46) protein expression normalized for GAPDH, respectively. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. *, P < 0.05; **, P < 0.01; and ***, P< 0.001 vs. control siRNA. a, b and c represent significant differences compared with control siRNA plus insulin treatment (a, P < 0.05; b, P < 0.01 and c, P < 0.001, respectively). INS= insulin
3.5. Insulin stimulates RPS6KB1 and RPS6 phosphorylation through PI3-kinase dependent pathway
Initial experiments examined whether insulin activates ERK1/2 phosphorylation in T-I cells. To test this, cells were cultured with insulin for different time intervals, and the phosphorylation of ERK1/2 was examined by immunoblot analysis. The results show that within 5 min of insulin addition ERK1/2 is robustly phosphorylated (Fig 5A and B). We have previously shown that insulin increases AKT phosphorylation at Ser 473. Furthermore, pretreatment with PI3-kinase inhibitor, Wortmannin completely blocked insulin-induced phosphorylation of AKT (Palaniappan and Menon, 2009).
FIG. 5. Effect of PI3-kinase and MEK1 inhibition on insulin-stimulated phosphorylation of RPS6KB1 and RPS6.
Cells were pretreated without or with the PI3-kinase inhibitor, Wortmannin (100 nM) for 30 min or the MEK inhibitor, U0126 (10μM) for 1 h, followed by insulin (1μg/ml) treatment for 30 min. Control groups were treated with vehicle (DMSO). The cell lysates were examined for phospho-specific RPS6KB1(Thr389) and RPS6 (Ser235/236) by Western blot. Protein loading was monitored by stripping and reprobing the same blot with RPS6 antibody. The blot is representative of one experiment, and the graph represents the mean of three experiments. Error bars represent mean ± SE. *, P < 0.05; P **, P < 0.01; and ***, P< 0.001 vs. control. b and c represent significant differences compared with insulin (b, P < 0.01 and c, P < 0.001 respectively). # and ## represent significant differences compared with INS + W (#, P < 0.05 and ##, P < 0.01, respectively). INS= insulin; W= Wortmannin
Because insulin is known to regulate both PI3-kinase/AKT pathway and ERK1/2 pathway, inhibitors were employed to determine the signaling pathway involved in insulin-induced phosphorylation of RPS6KB1 and its downstream effectors, RPS6. To test this, T-I cells were preincubated with PI3-kinase inhibitor, Wortmannin or MEK inhibitor for 30 min and 1 h, respectively followed by stimulation with insulin for 30 min. The cell lysates were then assayed for the phosphorylation of RPS6KB1 (Thr 389) and RPS6 (Ser 253/236) by immunoblot analysis. As expected, insulin treatment increased phosphorylation of RPS6KB1 and RPS6, whereas pretreatment with MEK inhibitor caused partial inhibition of RPS6KB1 and RPS6 phosphorylation. By contrast, pretreatment with Wortmannin produced complete inhibition of RPS6KB1 and RPS6 phosphorylation in response to insulin (Fig. 5C–E). Taken together, these results suggest that insulin-mediated MTORC1 activation is primarily regulated by PI3-kinase dependent signaling pathways in T-I cells.
3.6. Insulin stimulates cell cycle regulatory proteins through the MTORC1-dependent pathway
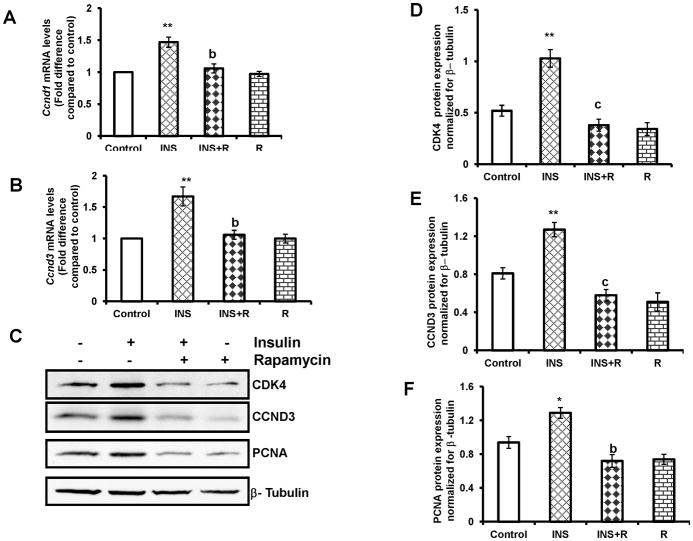
Cell cycle progression from G1 to the S phase is regulated by D-type cyclins, which modulate the activities of the cyclin-dependent kinases, a process sensitive to MTORC1 inhibitor, rapamycin (Fingar et al., 2004; Fingar et al., 2002). To determine the effect of rapamycin on Ccnd1and Ccnd3 mRNA expression, T-I cells were preincubated with rapamycin (20 nM) for 1 h followed by the addition of insulin for 4 h. Total RNA was extracted and the mRNA levels of Ccnd1and Ccnd3 were determined by real-time PCR. The results indicate that insulin -stimulated Ccnd1and Ccnd3 mRNA expression was blocked by MTORC1 inhibitor, rapamycin (Fig. 6A and B). The effect of rapamycin on insulin-induced cell cycle regulatory protein expression was then examined. Cells were preincubated with the MTORC1 inhibitor, rapamycin for 1 h, followed by insulin treatment for 24 h. The cell lysates were examined for the cell cycle regulatory proteins such as CDK4, CCND3, and PCNA by Western blot analysis. The results showed that while insulin treatment alone caused significant increases in the expression of the cell cycle regulatory proteins, the addition of the MTORC1 inhibitor, rapamycin, strongly prevented these increases, suggesting that insulin-induced MTORC1 signal is necessary for CDK4, CCND3 and PCNA protein expression (Fig. 6 C–F).
FIG. 6. Effect of rapamycin on insulin-stimulated cell cycle regulatory protein expression.
Cells were pretreated without or with rapamycin (20 nM) for 1 h followed by insulin (1 μg/ml) treatment for 4 h (Ccnd1and Ccnd3 mRNA expression) or 24 h (CDK4, CCND3 and PCNA protein expression). Control groups were treated with vehicle (DMSO). (A) and (B), Total RNA was reverse-transcribed and the resulting cDNA was subjected to real–time PCR using predesigned primers and probes for rat Ccnd1and Ccnd3 as described in Materials and Methods. The graph (A) shows changes in Ccnd1 mRNA expression normalized for 18S rRNA. The graph (B) shows changes in Ccnd3 mRNA expression normalized for 18S rRNA. (C) The cell lysates were examined for CDK4, CCND3 and PCNA protein expression by Western blot analysis. The level of β-tubulin was used as loading control. The graphs (D, E and F) represent densitrometric scans of CDK4, CCND3 and PCNA protein expression, respectively, normalized for β-tubulin. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean + SE. *, P < 0.05 and **, P < 0.01 vs. control. b and c represent significant differences compared with insulin treatment (b, P < 0.01 and c, P < 0.001, respectively). INS= insulin; R= rapamycin
4. DISCUSSION
Present study provides new insights into how insulin-mediated activation of MTORC1 signal regulates T-I cell proliferation. We show that insulin activates the downstream targets of MTORC1, RPS6KB1 and EIF4EBP1. Furthermore, inhibition of MTORC1 activation by rapamycin or siRNA-mediated knockdown of Mtor abolished the insulin-induced phosphorylation of RPS6KB1 and its target RPS6, and EIF4EBP1 phosphorylation. In addition, insulin-stimulated T-I cell proliferation and expression of cell cycle regulatory proteins were prevented by the MTORC1 inhibitor, rapamycin.
The serine/threonine kinase MTORC1 plays a key role in regulating cellular processes including transcription, protein synthesis, cell growth and proliferation (Ma and Blenis, 2009; Palaniappan and Menon, 2010). It is well established that insulin regulates MTORC1 signaling through TSC1-TSC2 protein complex (Inoki et al., 2005). This protein complex normally acts as a negative regulator of MTORC1 signaling. In response to mitogenic signals, the TSC2 protein complex is inactivated by phosphorylation at Thr 1462 which leads to activation of the MTORC1 signaling cascade (Hou et al., 2010; Inoki et al., 2005). Recently, we and others have also demonstrated that gonadotropin inhibits TSC2, leading to activation of MTORC1 signaling and augmenting the phosphorylation of downstream targets, RPS6KB1 and EIF4EBP1 (Alam et al., 2004; Kayampilly and Menon, 2007; Palaniappan and Menon, 2010). In the present study, we show that treatment with insulin induces phosphorylation of RPS6KB1 and EIF4EBP1. Furthermore, insulin-stimulated phosphorylation of RPS6KB1 and EIF4EBP1 is abrogated by MTORC1 inhibitor, rapamycin, or by siRNA-mediated knockdown of Mtor, suggesting that insulin-mediated activation of MTORC1 signal is essential for T-I cell proliferation. Studies using molecular approaches have identified RPS6KB1 as an important molecule for cell proliferation and growth (Chou and Blenis, 1995; Lane et al., 1993; Price et al., 1992; Reinhard et al., 1994; Shima et al., 1998). Furthermore, RPS6KB1 is a key regulator of mRNA translation and plays a central role in cell cycle progression through the G1 phase of proliferating cells (Bandi et al., 1993; Chou and Blenis, 1995; Ruvinsky and Meyuhas, 2006; Tee et al., 2005; Wullschleger et al., 2006). Since RPS6 is a substrate for RPS6KB1, its phosphorylation is also correlated with protein synthesis. In the present study, rapamycin treatment blocks insulin-induced phosphorylation of RPS6KB1 and RPS6 and cell cycle regulatory protein expression, suggesting that activation of MTORC1 is required for cell cycle regulatory protein expression. Therefore, based on these findings, we suggest that the increase in theca cell number seen under pathological conditions such as PCOS may be due to hyperactivation of mTORCI signaling pathway by insulin and LH. This is further supported by our recent finding that LH/hCG induces theca cell proliferation and cell cycle regulatory proteins by cAMP-mediated activation of PI3-kinase/AKT/MTORC1 pathways (Palaniappan and Menon, 2010).
Recently, it has been reported that prenatal exposure to excess androgen alters the intraovarian insulin signaling (Ortega et al., 2010). In addition to insulin, intraovarian growth factors such as IGF-1 have been known to regulate theca cell growth and proliferation (Duleba et al., 1997; Kwintkiewicz and Giudice, 2009). It is important to mention that a high level of insulin has been shown to activate IGF-1 receptors. Indeed, insulin and IGF-1 have common intracellular signal transduction pathways (PI3-kinase and MAPK) after ligand-induced receptor activation (Gallagher and LeRoith, 2010). Because of this reason, the insulin concentration used in the present study may also activate IGF-1 receptor. Recent studies have shown that IGF-1 also activates MTORC1 signaling in various cell types (Arvisais et al., 2010; Ning and Clemmons, 2010; Wahdan-Alaswad et al., 2010). MTORC1 activation promotes cellular anabolic processes such as protein and lipid synthesis, cell growth, and cell cycle progression, driving cell proliferation via its downstream targets RPS6KB1 and EIF4EBP1 (Dowling et al., 2010; Ekim et al., 2011; Fingar et al., 2004). In this study, we clearly showed the MTORC1-dependent phosphorylation of RPS6KB1 and EIF4EBP1 in response to insulin. Inactivation of MTORC1 by a pharmacological inhibitor or by siRNA-mediated knockdown of MTORC1 suppresses the insulin-induced phosphorylation of RPS6KB1 and EIF4EBP1, leading to inhibition of T-I cell proliferation and cell cycle regulatory protein expression. Therefore, it is suggested that activation of MTORC1 regulates T-I cell proliferation at least in part by up regulating the cell cycle machinery.
In summary, the findings reported here support the notion that insulin-mediated activation of MTORC1 signal controls T-I cell proliferation by increasing the expression of cell cycle regulatory proteins. Furthermore, it is suggested that in hyperthecosis seen in pathological conditions, activation of MTORC1 signaling by insulin might be responsible for the proliferation of T-I cells.
Research Highlights.
Insulin activates the MTORC1 signaling cascade in T-I cells.
Pharmacological inhibitor or siRNA-mediated knockdown of Mtor prevents insulin- induced RPS6KB1 and EIF4EBP1, phosphorylation.
Rapamycin blocks insulin-stimulated T-I cell proliferation and the expression of cell cycle regulatory proteins.
Acknowledgments
This work was supported by NIH grant HD 38424
We express our appreciation to Helle Peegel for critical reading of the manuscript and valuable comments. We also thank Dr. Diane C. Fingar, Department of Cell and Developmental Biology at this institute for generously providing EIF4EBP1 antibody.
Abbreviations
- BrdU
bromodeoxyuridine
- CCND3
Cyclin D3
- CDK
cyclin-dependent kinase
- EIF4EBP1
eukaryotic initiation factor 4E binding protein 1
- ERK
extracellular signal-regulated kinase
- IGF-1
Insulin like growth factor 1
- MAP kinase
mitogen-activated protein kinase
- MEK
MAPK kinase
- MTOR
mammalian target of rapamycin
- PCNA
proliferating cell nuclear antigen
- PI3 kinase
phosphatidylinositol -3-kinase
- RPS6
ribosomal protein S6
- RPS6KB1
ribosomal protein S6 kinase 1
- siRNA
small interfering RNA
Footnotes
Disclosure Summary: The authors have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004;279:19431–19440. doi: 10.1074/jbc.M401235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvisais E, Hou X, Wyatt TA, Shirasuna K, Bollwein H, Miyamoto A, Hansen TR, Rueda BR, Davis JS. Prostaglandin F2alpha represses IGF-I-stimulated IRS1/phosphatidylinositol-3-kinase/AKT signaling in the corpus luteum: role of ERK and P70 ribosomal S6 kinase. Mol Endocrinol. 2010;24:632–643. doi: 10.1210/me.2009-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi HR, Ferrari S, Krieg J, Meyer HE, Thomas G. Identification of 40 S ribosomal protein S6 phosphorylation sites in Swiss mouse 3T3 fibroblasts stimulated with serum. J Biol Chem. 1993;268:4530–4533. [PubMed] [Google Scholar]
- Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MM, Blenis J. The 70 kDa S6 kinase: regulation of a kinase with multiple roles in mitogenic signalling. Curr Opin Cell Biol. 1995;7:806–814. doi: 10.1016/0955-0674(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12:324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Arici A, Carbone R, Behrman HR. Proliferation and differentiation of rat theca-interstitial cells: comparison of effects induced by platelet-derived growth factor and insulin-like growth factor-I. Biol Reprod. 1999a;60:546–550. doi: 10.1095/biolreprod60.3.546. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Divergent mechanisms regulate proliferation/survival and steroidogenesis of theca-interstitial cells. Mol Hum Reprod. 1999b;5:193–198. doi: 10.1093/molehr/5.3.193. [DOI] [PubMed] [Google Scholar]
- Ekim B, Magnuson B, Acosta-Jaquez HA, Keller JA, Feener EP, Fingar DC. mTOR kinase domain phosphorylation promotes mTORC1 signaling, cell growth, and cell cycle progression. Mol Cell Biol. 2011;31:2787–2801. doi: 10.1128/MCB.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hou X, Arvisais EW, Davis JS. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology. 2010;151:2846–2857. doi: 10.1210/en.2009-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148:3950–3957. doi: 10.1210/en.2007-0202. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ. Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod. 2006;74:1034–1040. doi: 10.1095/biolreprod.105.049908. [DOI] [PubMed] [Google Scholar]
- Lane HA, Fernandez A, Lamb NJ, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Magoffin DA. The ovarian androgen-producing cells: a 2001 perspective. Rev Endocr Metab Disord. 2002;3:47–53. doi: 10.1023/a:1012700802220. [DOI] [PubMed] [Google Scholar]
- Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–1349. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- Ning J, Clemmons DR. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol. 2010;24:1218–1229. doi: 10.1210/me.2009-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82:1065–1075. doi: 10.1095/biolreprod.109.082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan M, Menon KM. Regulation of sterol regulatory element-binding transcription factor 1a by human chorionic gonadotropin and insulin in cultured rat theca-interstitial cells. Biol Reprod. 2009;81:284–292. doi: 10.1095/biolreprod.108.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol. 2010;24:1782–1793. doi: 10.1210/me.2010-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan M, Menon KM. Luteinizing Hormone/Human Chorionic Gonadotropin-Mediated Activation of mTORC1 Signaling Is Required for Androgen Synthesis by Theca-Interstitial Cells. Mol Endocrinol. 2012;26:1732–1742. doi: 10.1210/me.2012-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Rapley J, Oshiro N, Ortiz-Vega S, Avruch J. The mechanism of insulin-stimulated 4E-BP protein binding to mammalian target of rapamycin (mTOR) complex 1 and its contribution to mTOR complex 1 signaling. J Biol Chem. 2011;286:38043–38053. doi: 10.1074/jbc.M111.245449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Fernandez A, Lamb NJ, Thomas G. Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J. 1994;13:1557–1565. doi: 10.1002/j.1460-2075.1994.tb06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373–381. doi: 10.1093/humrep/deh609. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod. 2008;78:243–253. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- Tee AR, Blenis J, Proud CG. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005;579:4763–4768. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Wahdan-Alaswad RS, Song K, Krebs TL, Shola DT, Gomez JA, Matsuyama S, Danielpour D. Insulin-like growth factor I suppresses bone morphogenetic protein signaling in prostate cancer cells by activating mTOR signaling. Cancer Res. 2010;70:9106–9117. doi: 10.1158/0008-5472.CAN-10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Will MA, Palaniappan M, Peegel H, Kayampilly P, Menon KM. Metformin: direct inhibition of rat ovarian theca-interstitial cell proliferation. Fertil Steril. 2012;98:207–214. doi: 10.1016/j.fertnstert.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Strauss JF., 3rd Multiple signal transduction pathways regulate ovarian steroidogenesis. Rev Endocr Metab Disord. 2002;3:33–46. doi: 10.1023/a:1012748718150. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356:24–30. doi: 10.1016/j.mce.2011.05.027. [DOI] [PubMed] [Google Scholar]