Abstract
Background
Among patients with type 2 diabetes, it is not known whether risk factor control is better or worse for those who also have heart disease, depression, multiple other co-morbidities and associated management challenges.
Objective
To examine the relationship between this complex constellation of multi-morbidities, adherence to treatment and risk factor control among patients with type 2 diabetes, independent of regimen intensity.
Research Design
Observational cross-sectional study.
Subjects
1314 patients with diabetes from the Reducing Racial Disparities in Diabetes Coached Care (R2D2C2) Study.
Measures
A composite cardiometabolic risk factor profile (CMRP) was the dependent variable. Independent variables included a composite measure of patient complexity, patient-reported adherence to treatment, history of coronary heart disease (CHD), and intensity of medication regimen.
Results
A higher proportion of the most complex patients reported problems with adherence compared to the least complex patients (83.5% vs. 43.3%, p<.001). Compared to those without a history of CHD, fewer patients with CHD reported problems with medication adherence (59.3% vs. 69.3%, p<.01) and had better risk factor control, independent of complexity and regimen intensity. Better risk factor control was independently associated with less patient complexity (p=.003) and to history of CHD (p=.01).
Conclusions
The presence of a complex illness profile was associated with poorer control of risk factors. Those with CHD were more adherent to treatment and had better risk factor control. The occurrence of CHD may present an opportunity for physicians to emphasize risk factor management. Absent such a dramatic event, diabetes patients with a complex illness profile may be at highest risk for cardiovascular events and in greatest need of prevention of cardiac disease.
Trial Registration
Clinicaltrial.gov identifier: NCT01123239
Keywords: Risk factors, diabetes, patient complexity, comorbidity, cardiovascular disease
Introduction
Diabetes is a complex chronic disease affecting multiple organ systems, often accompanied by other co-morbid conditions and associated disease management burden for patients. Coronary heart disease (CHD) is a frequent complication of diabetes and its presence can create added complexity for an already burdensome regimen. Patients with type 2 diabetes also experience added burden from other, non-cardiovascular co-morbidities (e.g. osteoarthritis, chronic obstructive pulmonary disease)1-2. Complex medication regimens that accompany a greater number of, and more severe co-morbidities may overwhelm these patients, reducing adherence and resulting in poorer cardiovascular risk factor control3.
Previous studies of medication adherence among patients with cardiac disease or its risk factors have documented the relationship between co-morbidity burden and poor adherence to cardiovascular medications4-7. Since cardiovascular risk factor control is a major contributor to mortality for diabetes patients, these findings are especially troubling. A recent study among patients with diabetes who also had other non-diabetes related co-morbidities, showed that these “complex” patients gave lower priority to diabetes self-management2. Not yet known is whether and to what extent the presence of cardiovascular disease may further contribute to the management burden of diabetes patients in ways that worsen control of cardiometabolic risk factors.
Using data from the on-going Reducing Racial Disparities in Diabetes Coached Care (R2D2C2) Study8-9, this paper focused on the relationship between patient complexity, regimen intensity, problems with adherence to medications and control of cardiometabolic risk factors among type 2 diabetes patients with and without coronary heart disease. This is the first study to examine the specific contribution of burden from coronary heart disease versus that from complex non-cardiovascular multi-morbidity on simultaneous consideration of three markers of cardiometabolic risk.
Methods
Study Sample and Data Collection
The data for this paper derive from the Reducing Racial Disparities in Diabetes Coached Care (R2D2C2) Study8-9, which included patients with diabetes at six primary care clinics affiliated with an academic health system. Laboratory, administrative and medical records data were abstracted for study measures.
Study Measures
Dependent Variables
We created composite measure of risk factor control, the cardiometabolic risk management profile (CRMP), to assess the most common clinical targets for prevention of cardiovascular events for patients with diabetes: systolic blood pressure, LDL cholesterol and hemoglobin A1c. These important risk factors are typically evaluated individually and not in combination, although recent research has considered these and other risk factors as composite predictors of cardiac morbidity and mortality.11-12
The CRMP was computed using equal weighting for the proportional distance above or below clinical targets for hemoglobin A1c level (≤7%), systolic blood pressure (≤130 mm Hg) and LDL cholesterol (≤100 mg/dL), expressed as a percentage of each target value and averaged across all three indicators for each participant. If all three risk factors were exactly at target values, the CMRP score would be zero. Lower CRMP scores reflected better control of risk factors.
Independent Variables
To measure patient complexity, we used the “Potential for Benefit Scale” (PBS) previously tested8 multidimensional composite of five pre-existing measures of patients’ health status and attitudes toward healthcare: comorbidity, physical function, mental health, diabetes burden and a passive approach to healthcare. The PBS was scored as a weighted mean of the five measures, with weights determined by factor loadings on a single factor in principal components analysis.8 Comorbidity was measured using a 38-item version of the Total Illness Burden Index (TIBI),13-15 a summary measure of the presence and severity of the patient’s diseases and symptoms comorbid to diabetes and heart disease. TIBI scores ranged from 0-10. To measure physical function, we used the 10-item physical function scale (PFI-10) of the Short Form 36, for which scores reversed and converted to range from 0-10 with higher scores indicating poorer functioning.16 To represent depressive symptomatology, we used a modified version of the Center for Epidemiologic Studies Depression Scale (CES-D)17, rescaled to the range of 0 to 10. To evaluate the disease-specific burden of diabetes, we used the 8-item Diabetes Burden Scale from the Type 2 Diabetes Patient Outcomes Research Team, converted to range from 0-10.18-19 To measure passivity, we used the Provider-Dependent Health Care Orientation scale (PDHCO); scores were converted to range from 0-10 and higher scores indicated greater passivity.20
Taken together, these measures can be used to generate a profile of the “complex” patient—one with substantial disease burden coupled with a passive approach to disease management. Such a profile has been proposed in other research to represent patients’ potential for benefit from treatment and was referred to as the Potential for Benefit Scale (PBS).8
We also measured problems with adherence to treatment, using a 13-item measure of patient adherence to provider recommendations for medication regimens in the face of specific barriers.21 Other independent variables included history of coronary heart disease, which was abstracted from medical charts and included prior myocardial infarction, angina, coronary revascularization, and heart failure. We also measured regimen complexity as the simple sum of the number of different classes of medications noted in the medical record. Demographic variables including age, gender, ethnicity, time since diagnosis and years of education were patient-reported.
Statistical Analysis
We used IBM SPSS Statistics v. 20.0 (IBM, Armonk, New York) for all analyses. Univariate and distributional analysis included measures of central tendency, kurtosis and skew. Bivariate comparisons, were made using Pearson chi squared tests for dichotomous outcomes, and with t-tests or one-way analysis of variance for continuous outcomes. Multivariable ordinary least squares regression models adjusting for age of diagnosis of type 2 diabetes, duration of diabetes, ethnicity, sex and education were used to evaluate the associations of each independent variable (PBS score, history of CHD, regimen complexity, and problems with adherence) with the CRMP score.
Results
There was no statistically significant relationship between age and patient complexity (see Table 1). The most complex patients were less well educated, had had diabetes longer, fewer were male and fewer were white. Patients with a history of coronary heart disease were older, more were male, were slightly better educated, had had diabetes longer and more were white compared with those with no history of CHD (see Table 1). Greater complexity was associated with greater regimen intensity as measured by number of classes of medications prescribed (5.1 in the least complex patient versus 6.7 in the most complex patients, p<.001) and the proportion of patients on insulin (18.4% of the least complex versus 39.0% of the most complex patients, p<.001). Compared with those without a history of CHD, those with CHD were on more intensive regimens reflected in the number of classes of medications for risk factor control (7.6 vs. 5.4, p<.001). More patients with CHD vs. without CHD were on insulin (37.0% vs. 26.7%, p=.001) and more were on a statin (84.7% vs. 75.9% for those with and without CHD respectively, p=.004).
Table 1.
Comparison of patient characteristics by level of complexity as measured by the Potential for Benefit Scale and by history of coronary heart disease (n= 1314)1
| Total Sample (N=1314) | Low (N=327) | Patient Complexity2 (PBS Score) Moderate (N=654) | High (N=333) | p-value3 | CHD4 (N=235) | History of Coronary Heart Disease No CHD5 (N=1079) | p-value6 | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 58.8 (11.4) | 59.3 (11.1) | 58.5 (11.6) | 59.0 (11.1) | .521 | 64.6 (9.0) | 57.5 (11.4) | <.001 |
| Gender (% male) | 40.6 | 51.1 | 39.5 | 32.4 | <.001 | 57.9 | 36.8 | <.001 |
| Education (years) | 9.5 (5.0) | 11.3 (5.1) | 9.1 (5.0) | 8.7 (4.6) | <.001 | 10.6 (5.2) | 9.3 (4.9) | <.001 |
| Duration of diabetes (years) | 9.2 (7.5) | 7.3 (6.2) | 9.3 (7.6) | 10.7 (7.8) | <.001 | 10.7 (8.1) | 8.9 (7.3) | .002 |
| Ethnicity | <.001 | <.001 | ||||||
| % White | 26.5 | 41.3 | 23.4 | 18.6 | 40.9 | 23.5 | ||
| %Hispanic | 54.4 | 46.8 | 57.7 | 59.2 | 40.4 | 58.6 | ||
| % Vietnamese | 19.0 | 11.9 | 19.0 | 22.2 | 18.7 | 17.9 | ||
| Regimen intensity6 | ||||||||
| Medication classes prescribed (count) | 5.8 (2.2) | 5.1 (2.1) | 5.7 (2.2) | 6.7 (2.6) | <.001 | 7.6 (2.6) | 5.4 (2.2) | <.001 |
| On insulin (%) | 28.5 | 18.4 | 28.3 | 39.0 | <.001 | 37.0 | 26.7 | .001 |
| On a statin (%) | 77.5 | 77.1 | 79.5 | 73.9 | .13 | 84.7 | 75.9 | .004 |
Table entries are mean values (SD) for continuous variables or proportions for categorical variables.
Measured using the 51-item Potential for Benefit Scale (PBS); categories reflect lowest quartile (low), middle 50% (moderate) and highest quartile (high).
p-value computed from one-way analysis of variance for continuous variables and chi-squared test for categorical variables
Patients with a history of coronary heart disease (CHD) noted in the medical record.
Patients with no history of CHD noted in the medical record.
Data on medication regimen intensity were abstracted from medical records.
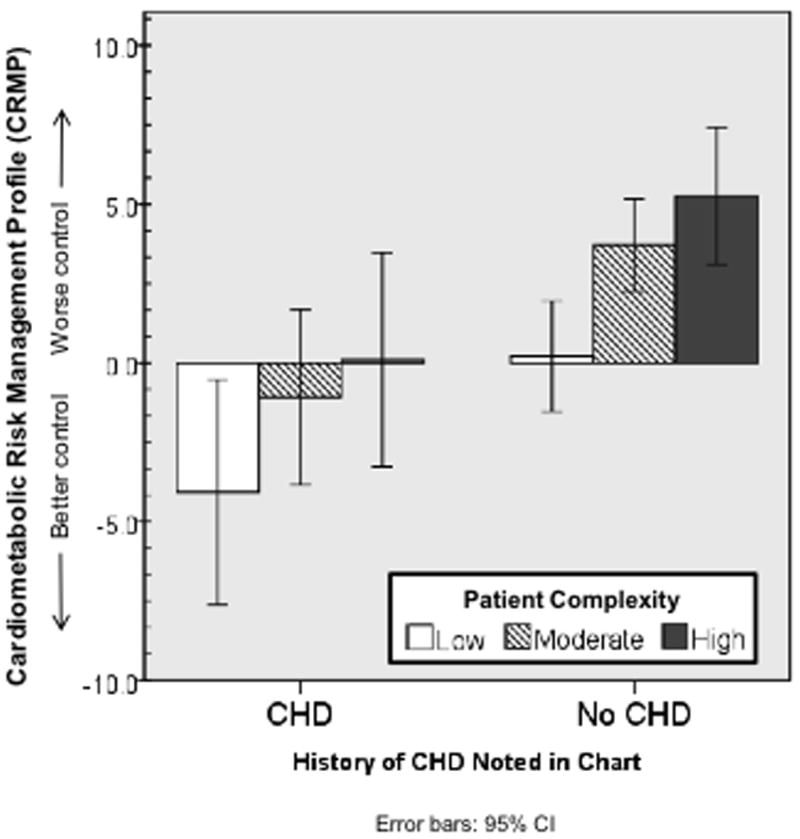
Sample-wide, the average CMRP score was 2.3 (SD=16.7) which reflects cardiometabolic risk factor control that was, on average, 2.3% above recommended targets. CMRP values ranged from -65.0 to 107.2, and were approximately normally distributed with a median value of -0.1 and negligible skewness and kurtosis. Among the least complex patients, those who also had a history of CHD had better cardiometabolic risk factor control, compared to those without CHD (CRMP scores -4.1 vs. 0.2 respectively, p<.05; see Figure 1). The most complex patients with a history of CHD also had lower CMRP scores (better risk factor control) compared to those without CHD (CRMP scores 0.1 vs. 5.2 respectively, p<.05, see Figure 1).
Figure 1.
Cardiometabolic Risk Management Profile (CRMP) scores, by level of patient complexity1 and history of coronary heart disease (CHD)
1Measured using the 51-item Potential for Benefit Scale (PBS); categories reflect lowest quartile (low), middle 50% (moderate) and highest quartile (high).
Those with heart disease had higher depressive symptom scores (4.1 vs. 3.7, p<.05), lower physical function scores (5.7 vs. 6.6, p<.001), high Total Illness Burden Index (TIBI) scores but lower scores on the passivity measure (5.1 vs. 5.4, p<.05), compared to those without CHD. There were no significant differences between the two groups in diabetes burden.
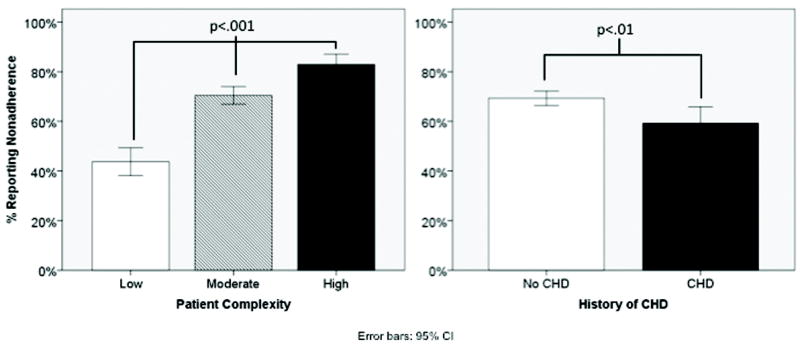
A greater proportion of the most complex patients reported problems with medication adherence compared to the least complex patients (83.5% vs. 43.3%, respectively, p<.001, see Figure 2). However, fewer patients with a history of CHD reported problems with adherence compared to those without (59.3% vs. 69.3%, respectively, p<.01, see Figure 2). These associations persisted in logistic regression models adjusted for gender, education, ethnicity, age at diagnosis of diabetes, regimen intensity and diabetes duration (data not shown). A one-point increase in PBS score was associated with a greater than two-fold increase in the odds of reporting problems with adherence (aOR 2.2, 95%CI 1.9,2.6; p<.001). Patients with a history of CHD were significantly less likely to report nonadherence (aOR 0.7, 95%CI 0.5, 1.0; p=.038).
Figure 2.
Comparison of proportion of patients reporting problems with adherence, by level of patient complexity1 and by history of CHD (n=1314)
1 Measured using the 51-item Potential for Benefit Scale (PBS); categories reflect lowest quartile (low), middle 50% (moderate) and highest quartile (high).
The most complex patients, as measured by the PBS, had worse control of risk factors (higher CRMP score) adjusted for gender, education, ethnicity, age at diagnosis of diabetes, and diabetes duration (Table 3, Model 1; p=.003). Patients with history of CHD had better risk factor control (lower CRMP score) compared to those without a history of CHD, independent of regimen intensity (p=.01). Regimen intensity was not related to risk factor control. When adherence was entered into the model (Table 3, Model 2), problems with adherence were associated with worse risk factor control (higher CRMP; p=.002). History of CHD (p=.017), but not patient complexity (p=.061), remained significantly associated with risk factor control in Model 2.
Table 3.
Association of Cardiometabolic Risk Profile (CMRP)1 with patient complexity, history of coronary heart disease, regimen intensity and medication problems with adherence, results from multiple regression analysis2
| Model 1: Patient complexity + History of CHD + Regimen Complexity + Adjustors | Model 2: Model 1 + Problems with adherence | |||
|---|---|---|---|---|
| Beta estimate (± 95% CI) | p-value | Beta estimate (±95% CI) | p-value | |
| Patient complexity3 | 1.4 (0.5, 2.4) | .003 | 0.9 (-0.4, 2.0) | .061 |
| History of CHD4 | -3.4 (-6.1,-0.8) | .010 | -3.2 (-5.8,-0.6) | .017 |
| Regimen intensity5 | 0.2 (-0.2, 0.7) | .32 | 0.2 (-0.2, 0.7) | .31 |
| Problems with adherence to medication regimen6 | -- | 3.3 (1.2, 5.4) | .002 | |
| R2 | .098 | <.001 | .106 | .002 |
Cardiometabolic Risk Profile (CMRP) is a composite measure of cardiovascular event risk computed as the mean proportional distance ([measured value/target value]-1) above or below clinical targets for hemoglobin A1c level (<7%), systolic blood pressure (<130 mm Hg) and LDL cholesterol (<100 mg/dL). A score of zero was equivalent to all three risk factors being at target values. Lower CMRP scores reflected better control of risk factors. Laboratory values, regimen intensity and history of coronary heart disease were abstracted from medical records.
Results from regression models adjusted for sex, education, ethnicity, age at diagnosis of diabetes and diabetes duration. Model 2 includes all the independent variables and covariates included in Model 1 plus problems with adherence to medication regimen.
Measured using the 51-item Potential for Benefit Scale (PBS).
Myocardial infarction or coronary heart disease noted in the medical chart
The number of classes of medications for which the patient had a prescription.
Problems with adherence to medication regimen was based on responses to a 13-item scale assessing patient-initiated deviations from the prescribed treatment plan.
Discussion
It is both intuitive and consistent with existing literature that for patients with chronic disease, managing a complicated treatment regimen is challenging and often leads to suboptimal adherence3, 22-23. Findings from this study build on this literature to suggest that, independent of the number of medications prescribed, other aspects of patient complexity are associated with poor adherence and poor risk factor control. Contrary to the predictions made in the proposed conceptual model, only patient complexity but not regimen intensity was independently associated with adherence and risk factor control.
The consequences of poor adherence are serious. Up to 50% of treatment failures in chronic disease, resulting in disease progression, functional impairments, hospitalizations and ultimately mortality, may be related to poor adherence.24-25 Among patients with chronic diseases that are largely asymptomatic, such as diabetes, hyperlipidemia and hypertension, studies show that adherence may be a disturbingly prevalent problem. Roughly one-half of patients with such diseases discontinue treatments within 1-2 years of intiation.26-27
Being able to identify patients prospectively who are less likely to adhere to medications could help to guide effective strategies tailored to patients’ specific needs and circumstances. Such tailored strategies would have greater potential for successful and sustained implementation. It could further allow the physician and patient to consider reducing management burden by modifying or reducing the medication regimen according to the patient’s risk for cardiovascular events or future mortality, as guided by risk estimators such as the UKPDS28 or the Framingham Risk Score29. However, direct measurement of patient adherence is fraught with well-documented methodologic challenges30-32. An approach that more accurately identifies complex, already burdened patients (e.g. the Potential for Benefit Scale) that, in turn, is associated both with adherence and with intermediate outcomes, could be useful in identifying those patients with greater and lesser potential for benefit from a new or added treatment.
We also observed that patients with a history of CHD reported better adherence to treatment and better risk factor control, independent of complexity and management burden. Previous studies have suggested that the occurrence of a sentinel cardiovascular event may motivate patients to improve control of their cholesterol and blood pressure by enhancing medication adherence.33-35 Consistent with this finding, patients with CHD in this study reported a less passive approach to healthcare, suggesting that they managed their diabetes more aggressively in the face of added complexity.
Our study has a number of limitations. First, the cross-sectional design does not allow for causal assertions regarding the relationships between complexity, adherence and risk factor control. Second, although the majority of our study population consisted of poor, underserved minority patients at greatest risk for complications from diabetes, we did not include other at risk racial/ethnic groups (e.g. African-Americans). Our findings may not therefore be generalizable to a more diverse ethnic or geographic population. Finally, although we had some measures of socioeconomic status, such as education, we did not have information on other indicators of compromised access to care, such as prescription drug benefits that may have affected medication adherence. Therefore, we could not measure the degree of economic stress associated with adherence.
In summary, the presence of a complex health and illness profile was associated with worse control of cardiometabolic risk factors independent of regimen intensity and history of CHD. Those with a history of CHD were more adherent to treatment and had better risk factor control. The occurrence of CHD may increase patient motivation to adhere to their regimens, or present an opportunity for physicians to emphasize effective risk factor management. However, absent such a dramatic event, diabetes patients with a complex illness profile may be at highest risk and in greatest need for effective preventive measures for cardiac disease.
Table 2.
Comparison of dimensions of patient complexity, as measured by the Potential for Benefit Scale among those with and without prior history of CHD (N=1314)1
| CHD2 (N=235) | No CHD3 (N=1079) | Total Sample (N=1314) | Mean difference4 (±95% CI) | p-value5 | |
|---|---|---|---|---|---|
| Depressive Symptoms6 | 4.1 (2.5) | 3.7 (2.4) | 3.8 (2.5) | 0.4 (0.0,0.7) | .044 |
| Diabetes Burden7 | 3.9 (2.8) | 4.1 (2.9) | 4.0 (2.9) | 0.2 (-1.8,6.4) | .276 |
| Physical Function8 | 4.3 (3.0) | 3.4 (2.9) | 3.6 (3.0) | 0.9 (0.5,1.4) | <.001 |
| TIBI9 | 3.2 (1.9) | 2.4 (1.7) | 2.6 (1.7) | 0.8 (0.5,1.0) | <.001 |
| Passivity10 | 5.1 (1.8) | 5.4 (1.8) | 5.3 (1.8) | 0.3 (0.0,0.6) | .021 |
| Total PBS Score11 | 2.6 (1.1) | 2.4 (1.0) | 2.4 (1.1) | 0.2 (0.1,0.4) | .003 |
Table entries are means (SD). All variables rescaled to a range of 0 to 10 for presentation purposes.
Patients with a history of coronary heart disease (CHD) noted in the medical record.
Patients with no history of CHD noted in the medical record.
Difference between CHD and no CHD groups with 95% confidence interval of the difference.
p-value computed from independent samples t-test
Center for Epidemiology Studies Depression Scale, 11-item version; higher scores indicating the presence of more depressive symptoms.17
Diabetes burden scale measures impact of diabetes on health, social activities, lifestyle and finances; higher scores indicate a greater degree of diabetes burden.18-19
Physical Function scale of the Medical Outcomes Study Short-Form 36; Reverse-coded so that higher scores indicate poorer physical functioning.16
Total Illness Burden Index, a measure of the presence and severity of conditions comorbid to diabetes and/or heart disease; 7 disease-specific scale scores computed from 38 individual questionnaire items; higher TIBI scores indicate greater comorbidity.13-15
Provider-Dependent Health Care Orientation scale (PDHCO), measuring patient passivity; higher scores indicate greater passivity.20
Potential for Benefit Scale (PBS), a composite measure of patient complexity scored as a weighted mean of five measures (depressive symptoms, diabetes burden, physical function, TIBI and passivity), with weights determined by factor loadings on a single factor in principal components analysis
Acknowledgments
This work was supported by the National Institute of Diabetes, Digestive and Kidney Diseases (R18DK69846 and K01DK078939), the Robert Wood Johnson Foundation (Generalist Physician Faculty Award #1051084 and Finding Answers: Disparities Research for Change #59758), the NovoNordisk Foundation, and Lilly Research Laboratories. Drs. Malik, Billimek, Greenfield, Sorkin, Ngo-Metzger, and Kaplan had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Disclosures
Drs Greenfield and Kaplan received funding for research grants from Lilly Research Laboratories and NovoNordisk Foundation (modest). Dr. Malik received funding for research grant from Lilly Research Laboratories.
References
- 1.Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity Affects the Relationship Between Glycemic Control and Cardiovascular Outcomes in Diabetes: A Cohort Study. Ann Intern Med. 2009;151:854–860. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhry NK, Fischer MA, Avorn J, et al. The Implications of Therapeutic Complexity on Adherence to Cardiovascular Medications. Arch Intern Med. 2011;17:814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 4.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 5.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26(4):483–492. doi: 10.1592/phco.26.4.483. [DOI] [PubMed] [Google Scholar]
- 6.Romanelli J, Fauerbach JA, Bush DE, Ziegelstein RC. The significance of depression in older patients after myocardial infarction. J Am Geriatr Soc. 2002;50(5):817–822. doi: 10.1046/j.1532-5415.2002.50205.x. [DOI] [PubMed] [Google Scholar]
- 7.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120(1):26–32. doi: 10.1016/j.amjmed.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan SH, Billimek J, Sorkin DH, Ngo-Metzger Q, Greenfield S. Who Can Respond to Treatment? Identifying Patient Characteristics Related to Heterofeneity of Treatment Effects. Medical Care. 2010;48(6):S9–S16. doi: 10.1097/MLR.0b013e3181d99161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorkin DH, Ngo-Metzger Q, Billimek J, August KJ, Greenfield S, Kaplan SH. Underdiagnosed and Undertreated Depression Among Racially/Ethnically Diverse Patients With Type 2 Diabetes. Diabetes Care. 2011;34(3):598–600. doi: 10.2337/dc10-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Kebbi IM, Ziemer DC, Cook CB, Miller CD, Gallina DL, Phillips LS. Comorbidity and glycemic control in patients with type 2 diabetes. Arch Intern Med. 2001;161(10):1295–1300. doi: 10.1001/archinte.161.10.1295. [DOI] [PubMed] [Google Scholar]
- 11.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Steffen LM, Jacobs DR, et al. Trends in Cardiovascular Risk Factor Levels in the Minnesota Heart Survey (1980-2002) as Compared With the National Health and Nutrition Examination Survey (1976-2002): A Partial Explanation for Minnesota’s Low Cardiovascular Disease Mortality? Am J Epidemiol. 2011;173(5):526–538. doi: 10.1093/aje/kwq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenfield S, Sullivan L, Dukes KA, Silliman R, D’Agostino R, Kaplan SH. Development and testing of a new measure of case mix for use in office practice. Med Care. 1995;33(4 Suppl):AS47–55. [PubMed] [Google Scholar]
- 14.Litwin M, Greenfield S, Elkin E, Lubeck D, Broering J, Kaplan S. Assessment of prognosis with the total illness burden index for prostate cancer: Aiding clinicians in treatment choice. Cancer. 2007;109(9):1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]
- 15.Stier DM, Greenfield S, Lubeck DP, et al. Quantifying comorbidity in a disease-specific cohort: adaptation of the total illness burden index to prostate cancer. Urology. 1999;54(3):424–429. doi: 10.1016/s0090-4295(99)00203-4. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, A R. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Medical Care. 1995;33(4):AS264–279. [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 18.Greenfield S, Kaplan SH, Silliman RA, et al. The uses of outcomes research for medical effectiveness, quality of care and reimbursement in type II diabetes. Diabetes Care. 1994;17(Supp 1):32–39. [PubMed] [Google Scholar]
- 19.Silliman RA, Bhatti S, Knan A, Dukes KA, Sullivan LM. The care of older persons with diabetes mellitus: Families and primary care physicians. Journal of the American Geriatric Society. 1996;44(11):1314–1321. doi: 10.1111/j.1532-5415.1996.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan SH, Dukes KA, Sullivan LM, Tripp TJ, Greenfield S. Is passivity a risk factor for poor health outcomes? J Gen Int Med. 1996;11(Supp 1):76. [Google Scholar]
- 21.Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: Results from the Medical Outcomes Study. J Behav Med. 1992;15(5):447–468. doi: 10.1007/BF00844941. [DOI] [PubMed] [Google Scholar]
- 22.Grant RW, O’Leary KM, Weilburg JB, Singer DE, Meigs JB. Impact of concurrent medication use on statin adherence and refill persistence. Arch Intern Med. 2004;164(21):2343–2348. doi: 10.1001/archinte.164.21.2343. [DOI] [PubMed] [Google Scholar]
- 23.Lamb DA, Eurich DT, McAlister FA, et al. Changes in adherence to evidence-based medications in the first year after initial hospitalization for heart failure: observational cohort study from 1994 to 2003. Circ Cardiovasc Qual Outcomes. 2009;2(3):228–235. doi: 10.1161/CIRCOUTCOMES.108.813600. [DOI] [PubMed] [Google Scholar]
- 24.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 25.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 26.Chodick G, Shalev V, Gerber Y, et al. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin Ther. 2008;30(11):2167–2179. doi: 10.1016/j.clinthera.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101(6):671–679. [PubMed] [Google Scholar]
- 29.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 30.Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc (2003) 2011;51(1):90–94. doi: 10.1331/JAPhA.2011.09154. [DOI] [PubMed] [Google Scholar]
- 31.Dobbels F, Berben L, De Geest S, et al. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. 2010;90(2):205–219. doi: 10.1097/TP.0b013e3181e346cd. [DOI] [PubMed] [Google Scholar]
- 32.Voils CI, Hoyle RH, Thorpe CT, Maciejewski ML, Yancy WS., Jr Improving the measurement of self-reported medication nonadherence. J Clin Epidemiol. 2011;64(3):250–254. doi: 10.1016/j.jclinepi.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 34.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165(10):1147–1152. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 35.Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population-based study in the elderly. Am J Hypertens. 1997;10(7 Pt 1):697–704. doi: 10.1016/s0895-7061(97)00056-3. [DOI] [PubMed] [Google Scholar]


