Abstract
FCMR, a Fc receptor specific for pentameric IgM, is expressed at high levels by B cells. Although circulating IgM has profound effects on responses to pathogens, autoimmunity and B cell homeostasis, the biologic consequences of its binding to FCMR are poorly understood. We interrogated FCMR contributions to B cell function by studying mice lacking FCMR. FCMR transcripts are expressed at different levels by various B cell subsets. FCMR-deficient mice have reduced numbers of developing B cells, splenic FO and peritoneal B-2 cells, but increased levels of peritoneal B-1a cells and autoantibodies. Following immunization, germinal center B cell and plasma cell numbers are increased. FCMR-deficient B cells are sensitive to apoptosis induced by BCR ligation. Our studies demonstrate that FCMR is required for B cell differentiation and homeostasis, the prevention of autoreactive B cells and responsiveness to antigenic challenge.
Introduction
IgM is the first antibody isotype produced by all vertebrates after initial antigen encounter (1). It is present as a membrane-bound form on the surface of B cells and as a secreted form (sIgM) that is mainly found in the blood. sIgM is comprised of two classes, natural and immune IgM. Natural IgM, characterized by polyreactivity and low affinity, is found in normal quantities in mice raised under germ-free or specific pathogen-free conditions (2, 3). Immune IgM is secreted following exposure to specific pathogens.
The study of mice deficient in sIgM (Sμ−/−) has provided unexpected insights into its role in diverse processes, ranging from B cell survival to atherosclerosis (3, 4), as well as in autoimmunity and protection against infection (5). In addition, Sμ−/− mice also show increased levels of serum IgA, elevated humoral immunity to T-dependent (TD) Ag, an increased propensity to develop IgG autoantibodies and autoimmune disease, and have an expanded population of B-1a cells (6–9).
Peritoneal B-1a cells and, to a lesser extent, marginal zone (MZ) B cells, have been identified as the major sources of natural IgM with spleen and bone marrow being the major sites of natural IgM production by B-1 cells (10, 11). Interestingly, Sμ−/− mice have increased numbers of both B-1a and MZ B cells, suggesting that B cells sense the presence of sIgM (12). The mechanisms governing expansion of these populations could be related either to modulation of the antigenic environment by natural IgM or its interaction with specific Fc receptors on the B cell itself. Indeed, it was recently reported that sIgM enhances BCR signaling and regulates B cell homeostasis in different peripheral compartments (13).
Although several ligands and receptors for IgM have been characterized - C1q (14); mannose-binding lectin (15); polymeric Ig receptor (pIgR) (16); and Fcα/μR (17) - a long-postulated receptor specific for IgM, the FCMR (18, 19), had proven to be remarkably elusive. Nonetheless, recent elegant studies have provided definitive evidence for the presence of FCMR on human and mouse lymphocytes and have characterized the genes encoding the proteins (20–22). It should be noted, however, that other studies have suggested that this molecule does not bind IgM but instead confers resistance to cell death mediated by TNFR1 and CD95 signaling (23–25). A clear definition of the function of the receptor in the biology of normal B cells has not been developed.
Here, we took advantage of FCMR-deficient (Fcmr−/−) mice to understand the contributions of the receptor to B cell development, differentiation and function. These experiments identified essential roles played by FCMR in B lymphopoiesis in the BM, the population of mature B cell subsets, responses to TD and T-independent (TI) antigens, signaling through the B cell receptor (BCR) and inhibition of autoreactivity. We determine that FCMR is critically involved in multiple aspects of B cell differentiation, homeostasis and function.
Materials and Methods
Mice and cell line
Fcmr−/− mice on a C57BL/6 (B6) genetic background were provided by the University Health Network, Toronto, Canada. Briefly, to generate these mice, exons 2–8 of the Fcmr gene were replaced by a neomycin resistance gene cassette which was assembled using a 7.5 kbp fragment found within an intron located in the 5′ leader sequence of the gene and a 0.65 kbp fragment that was synthesized a downstream of the last methionine codon in the gene by PCR (Supplemental Fig. 1). After electroporating this construct into ES cells, homologous recombinant cells were injected into blastocysts and implanted into pseudopregnant mice. The chimeras produced were bred until germ line transmission occurred in the progeny. Mice were analyzed for heterozygosity of the rearranged allele and then heterozygous mice were bred together to obtain homozygosity of the rearranged allele. Sμ−/− mice (7) were provided by Dr. Troy Randall (University of Rochester). Wild type (+/+) controls were littermates generated by crosses of mutant heterozygotes. Mice were used in this study under protocol LIG-5E approved by the NIAID IACUC. The human YTS NK cell line and methods used for infection with a control lentivirus (LV) or a LV expressing full-length mouse Fcmr (mFCMR-LV) were described previously (20).
Flow cytometry (FACS)
Single-cell suspensions were prepared from bone marrow (BM) of the tibia and femur from one leg, spleen, and peritoneum using standard procedures. After red cell lysis, cells were blocked with anti-CD16/32 Ab (2.4G2), and stained for FACS in 0.5% FBS in PBS. Abs used were to CD3 (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8 (53-6.7), CD11b (M1/70), CD11c (N418), CD19 (MB19-1), CD21 (eBio8D9), CD23 (B3B4), CD24 (30-F1), CD43 (eBioR2/60), CD138 (281-2), B220 (RA3-6B2), Gr-1 (RB6-8C5), Ly-77 (GL7), NK1.1 (PK136), CD25 (7D4), pre-BCR (SL156), Igκ (187.1), BP-1 (6C3), AA4.1 (AA4.1), IgMb (AF6-78) and IgDb (217-170). Data were collected on a FACSort or LSRII (BD Biosciences) and analyzed with FlowJo software (Tree Star). Ab-stained cells were also sorted by a FACSAria (BD Biosciences) with a purity ≥95%.
RNA isolation and quantitative real time RT-PCR (qPCR)
Splenic CD4+ T, CD8+ T, NK, and B cells were purified by negative selection using MACS MicroBeads kits. Splenic Gr-1+, CD11b+, CD11c+ cells and BM c-kit+ cells were purified by positive selection using MACS MicroBeads kits. B cell subsets were purified by FACS. Total RNA isolated from purified cells using the RNAquous-4PCR kit (Ambion) was treated with DNase I according to the manufacturer’s recommendations. Complementary DNA was synthesized with Qscript™ cDNA synthesis kit (Quanta Biosciences). qPCR reactions were performed using Lightcycler® 480 SYBR green I master supermix, according to the manufacturer’s instructions (Roche Diagnostics). Oligonucleotide primers for amplifying the mouse Fcmr and 18S rRNA were purchased from Qiagen. All reactions were done in triplicate and the average value of triplicates was used for calculating the relative levels of each mRNA species. Relative quantification of Fcmr transcripts was made with the 2nd derivative maximum method using the Roche Lightcycler software and calculating the fold change over 18S rRNA levels.
Testing for antinuclear Ab (ANA)
Serum ANAs were detected using 12-well microscope slides covered with HEp-2 cells (Netherlands Foundation for Biological Research). Slides were incubated with mouse serum at the 1/200, 1/500 and 1/1,000 dilutions for 30 min at room temperature. After two washes of 5 min with PBS, goat anti-mouse IgG-FITC (Sigma-Aldrich) was added at 1:250 dilutions for 30 min at room temperature and washed with PBS for 5 min at least three times. Fluorescence was detected by fluorescence microscopy at x200 magnification using a Zeiss Axio Imager Upright microscope (Carl Zeiss).
Immunohistochemistry
Splenic paraffin sections were stained for GCs using biotinylated PNA, streptavidin-HRP and the DAB method. Immunostained sections were scanned at original magnification x20 using a ScanScope XT (Aperio Technologies). Aperio ImageScope (version 11) software was used for digital slide image viewing and analysis. The total area (mm2) was measured for each spleen, and the number of positive pixels for PNA reactive areas was determined. Specific immunoreactivity (IR) was calculated as the number of positive pixels per mm2 of spleen tissue.
Immunization and Ab detection
For TI or TD responses, 6–8 wk-old mice were immunized i.p. with 20 μg of NP-Ficoll, 50 μg of NP-LPS or 100 μg NP-KLH (Biosearch Technologies) in alum. Blood samples were taken from each group at 2 wk after TD immunization (NP-KLH) or 1 wk after TI immunization (NP-Ficoll or NP-LPS). For recall responses, mice were challenged with NP-KLH at 12 wk post primary immunization. Sera were collected at 2 and 13 wk after primary immunization. NP-specific Abs were measured by ELISA using NP(27)-BSA (Biosearch Technology) pre-coated plates, followed by incubation with diluted serum samples and developed with HRP-conjugated mouse-isotype specific Ab (Southern Biotech) and p-nitrophenyl phosphate substrate (MP Biomedical). Serum anti-dsDNA IgG levels were analyzed with a mouse anti-dsDNA IgG-specific ELISA kit (Alphadiagnostic International). The plates were read at 450 nm using an ELISA plate reader.
ELISPOT
Spleen and BM Ab-secreting cells were quantified by an NP-specific Ig ELISPOT assay. Aliquots of 1.25–5.0 × 105 spleen and BM cells were plated in duplicate in NP-BSA pre-coated 96-well PVDF membrane plates (Millipore) and were incubated for 1 day at 37°C in 5% CO2. The plates were washed with PBS containing 0.05% Tween-20 and incubated with HRP-conjugated anti-mouse IgM or IgG1 (Jackson ImmunoResearch Laboratory), followed by reaction with FAST 5-bromo-4-chlor-3-indolyl phosphate/NBT chromogen substrate (Sigma). The spots were detected with a CTL-ImmunoSpot® S5 Core Analyzer (Cellular Technology) and analyzed by ImmunoSpot® Software 4.0 (Cellular Technology).
Reconstitution and serum transfer
Lethally irradiated (5 Gy) Rag1−/− mice were reconstituted i.v. one day later with 2×105 HSCs purified from BM cells of 2 month old +/+ and Fcmr−/− mice using lineage cell depletion kits (Miltenyi Biotec). After two wk, 100 μl of sera collected from 2 month old B6 and Sμ−/− mice were injected i.v. three times per wk for two wk. BM cells were harvested and B cell subpopulations were analyzed by FACS.
Assays for cell proliferation and apoptosis
Splenic B cells were purified by negative selection using a MACS MicroBeads kit (Miltenyi Biotec) according to the manufacturer’s instructions. For in vitro B cell proliferation assays, cells seeded at 2 × 105 cells/well were cultured in 96-well flat-bottom plates with LPS (1 μg/ml, Sigma), CpG-DNA (2.5 μg/ml, InvivoGen), anti-CD40 mAb (1 μg/ml, Alexis), recombinant mouse IL-4 (10 ng/ml, R&D Systems) or F(ab′)2 anti-mouse IgM Ab (10 μg/ml, Jackson ImmunoResearch Laboratory) for 3 days. Cells were pulsed with one μCi of 3H-thymidine for the last 16 h of culture and incorporation was measured by liquid scintillation counting.
For analyses of apoptosis, purified B cells seeded at 5 × 105 cells/well in 24-well plates were stimulated with LPS, anti-CD40 mAb or F(ab′)2 anti-mouse IgM Ab for 24 h. Cells were then stained with annexin V (BD Biosciences) and 7-AAD (eBioscience) for FACS analysis.
Statistical analysis
Significance of differences between groups was evaluated by Student’s t test. A P value < 0.05 was considered significant.
Results
Fcmr is highly expressed by B lineage cells
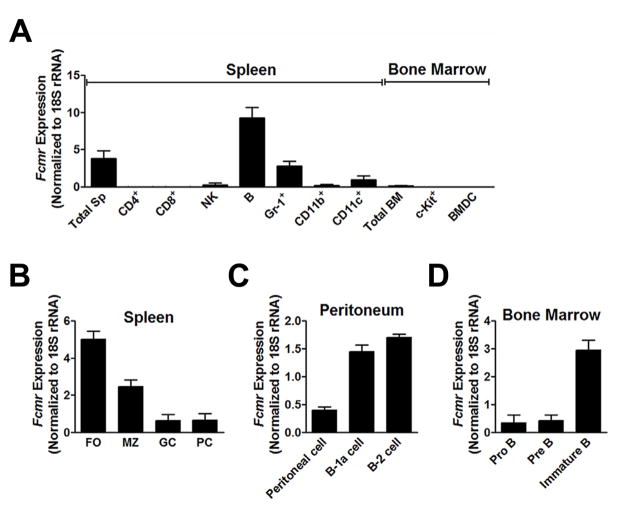
Previous studies using RT-PCR and flow cytometry indicated that Fcmr, the gene encoding FCMR, is expressed primarily by cells of the adaptive immune system (20, 21, 24). Since there is no commercially available source of antibodies to FCMR to study cell surface protein expression, we extended these analyses by using qPCR to quantify Fcmr transcript levels in purified subpopulations of hematopoietic cells from spleen, peritoneum and BM. Splenic CD4+ and CD8+ T cells expressed very low levels of Fcmr transcripts with slightly higher levels in NK cells, while expression in B cells was 500-fold higher than in T cells. Transcripts in follicular (FO) B cells were 2-fold higher than in MZ B cells, while expression in germinal center (GC) B cells and plasma cells (PCs) was markedly lower (Fig. 1B). Transcript levels in peritoneal B-1 and B-2 cells were comparable to those of MZ B cells (Fig. 1C). These findings indicate that FCMR is differentially expressed in distinct subsets of mature peripheral B cells and may differentially affect their functions.
Figure 1.
Fcmr expression by various cell types. Fcmr transcript levels were determined by real time PCR. (A) CD4+ T, CD8+ T, NK, B, Gr-1+, CD11b+ and CD11c+ cells were purified from spleen cells, c-kit+ cells were purified from BM cells, and BMDC were generated in vitro by culturing BM cells with GM-CSF and IL4 for 7 days. (B) FO (B220+CD21loCD23+) and MZ (B220+CD21hiCD23−) B cells were sort-purified from splenocytes; GC B cells (B220+GL7+PNA+) and PCs (B220−7-AAD−CD138+) were sort-purified from spleens of NP-KLH-immunized mice. (C) Peritoneal B-1a (CD19+CD23−CD5+) and B-2 (CD19+CD23+CD5−/low) cells were sort-purified by FACS. Data represent the mean ± SD of two experiments. (D) BM pro-B (B220+CD43+IgM−), pre-B (B220+CD43−/loIgM−) and immature B (B220loHSA+IgM+) cells were sort-purified by FACS. All data represent the mean ± SD of two to three experiments.
The finding of high levels of Fcmr transcripts in peripheral B cells prompted us to investigate whether differing levels of expression were associated with progression of B cell development in the BM. Studies of sort-purified B lineage subsets demonstrated that transcript levels in pro-B and pre-B cells were low but were significantly higher in more differentiated immature B cells (Fig. 1D) suggesting that FCMR influences early B cell development.
Studies of splenic myeloid cells revealed low levels of transcripts in CD11b+ macrophages with higher levels in CD11c+ dendritic cells (DCs) and Gr-1+ granulocytes (Fig. 1A). Parallel studies of BM cells (Fig. 1A) revealed only very low levels of transcripts among BM-derived dendritic cells (BMDC) and by c-kit+ cells that include hematopoietic stem cells (HSCs), multipotent progenitors, common myeloid progenitors and common lymphoid progenitors.
Fcmr−/− mice exhibit altered B cell differentiation in the BM and distribution in peripheral compartments
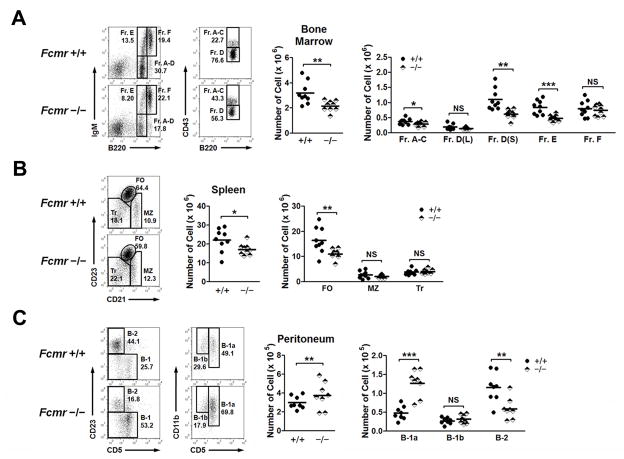
To determine if differential expression of Fcmr by subsets of developing B lineage cells and peripheral B cell subsets might affect B cell differentiation and function, we first quantified B cell subsets in the BM, spleen, and peritoneum of 3 month old mice (Fig. 2). Using the gating parameters shown in the left panels of Fig. 2A, FACS analyses of BM cells from wild type (+/+) and Fcmr−/− mice showed that mutant mice had modestly reduced numbers of pro-B (Hardy Fraction (Fr.) A–C) and large pre-B cells (Fr. D, determined by size) but more significant reductions in small pre-B cells (Fr. D, determined by size) and immature B cells (Fr. E). The numbers of mature recirculating B cells (Fr. F) were similar for mice of both genotypes (Fig. 2A, right panel).
Figure 2.
Abnormal B cell development in Fcmr−/− mice. Cells from BM (A), spleen (B) and peritoneum (C) of 3 month old Fcmr+/+ and Fcmr−/− mice were stained and analyzed by FACS. Left panels, FACS profiles of (A) were gated on lymphocytes, (B) on B220+ cells and (C) on CD19+ cells. Pre-B cells were further subdivided into large and small subsets based on FSC and SSC (not shown). The numbers indicate percentages of cells falling in each gate. Right panels show graphs of the absolute numbers of each B cell population. Each symbol represents a mouse. *p< 0.05, **p< 0.01, ***p< 0.005, NS, not significant.
Parallel studies of splenic B cell subsets showed that the total numbers of MZ and transitional (Tr) B cells were the same for mice of both genotypes, but that the numbers of FO B cells were significantly lower for Fcmr−/− mice (Fig. 2B). Analyses of peritoneal B cell subsets (Fig. 2C) showed that the total numbers of B cells were significantly higher for Fcmr−/− mice. Although numbers of B-1b cells were similar for both sets of mice, the numbers of B-1a cells were significantly higher and the numbers of B-2 cells were significantly lower for Fcmr−/− mice.
Interestingly, the patterns of B cell development and distribution of peripheral B cell subsets seen with Fcmr−/− mice had many similarities to those reported for two strains of mice deficient in sIgM (Sμ−/− mice) (6, 7, 9). The fact that both knockout strains were generated using 129 ES cells and were studied at different ages as F2 (7, 9) or N6 (6) mice in crosses with B6 mice complicates reliable comparisons with our Fcmr−/− mice. To avoid problems associated with relating data on mice on different genetic backgrounds and in different environmental conditions, we elected to perform direct comparisons of mice bred in our colony that included Fcmr−/− and Sμ−/− knockouts serially backcrossed onto the B6 background using +/+ littermate mice as controls (Supplemental Fig. 2). These studies demonstrated that the numbers of cells in bone marrow Fr. A C, D and E and of peritoneal B-1a and B2 cells were not significant different for the two mutant strains. However, the numbers of peritoneal B-1b cells were significantly higher for Sμ−/− than Fcmr−/− mice, and in spleen, the follicular B cell numbers for Sμ−/− mice were significantly lower than Fcmr−/− mice and both were significant lowerlythan for +/+ mice. Finally, the numbers and proportions of both MZ and Tr B cells were significantly higher for Fcmr−/− than +/+ mice (Supplemental Fig. 2). These results suggest that much of the Sμ−/− B cell phenotype can be ascribed to the absence of FCMR-sIgM receptor-ligand interactions. We conclude that expression of FCMR significantly influences pre-B and immature B cell development in the BM and the distribution of mature B cells into distinct subsets in the spleen and peritoneum, and suggest that these effects are mediated in large part by binding of sIgM.
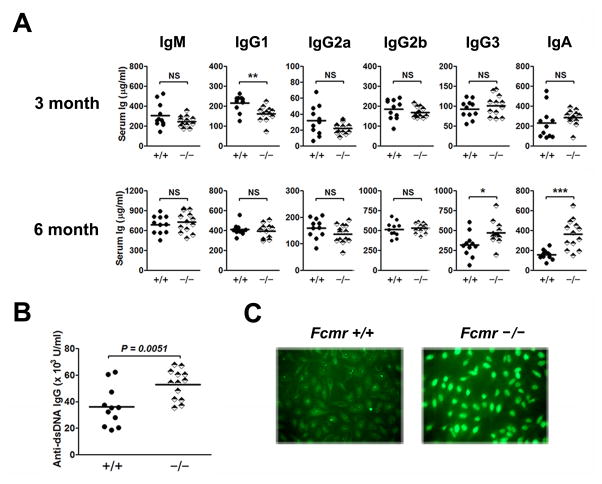
We also quantified basal levels of serum Ig isotypes of 3 and 6 month old +/+ and Fcmr−/−mice (Fig. 3A). For 3 month old mice, the levels of serum IgM, IgA and all IgG isotypes except IgG1 (lower in Fcmr−/− mice) were similar for both sets of mice. For 6 month old mice, the levels of both IgG3 and IgA were increased in sera from Fcmr−/− mice while levels of the other isotypes were comparable between the cohorts. The increased levels of IgG3 and IgA in sera of 6 month-old Fcmr−/− mice could reflect the increased numbers of B-1a cells that characterize the strain (Fig. 2C). The elevated serum levels of IgG3 in Fcmr−/− mice and the prior demonstration of elevated levels of class switched anti-DNA Ab in sera of Sμ−/− mice (6) prompted us to test sera from 6 month old Fcmr−/− mice for autoantibodies. Levels of IgG anti-dsDNA Ab were significantly increased in Fcmr−/− mice (Fig. 3B). Serum ANAs giving a diffuse staining pattern were present in sera from 3 out of 7 six month old Fcmr−/− mice (Fig. 3C) but none of 7 +/+ age-matched controls. We conclude that FCMR may negatively regulate autoimmunity by functioning as a receptor for sIgM.
Figure 3.
Basal levels of serum Ig in Fcmr−/− mice. (A) Serum Ig levels of 3- and 6-month old +/+ and Fcmr−/− mice were determined by ELISA. (B) The serum levels of IgG anti-dsDNA in 6-month old mice were measured by ELISA. Each symbol represents a mouse. (C) Representative patterns of cellular staining of HEp-2 cells by sera diluted at 1:200 prepared from 6-month old +/+ (n = 7) and Fcmr−/− mice (n = 7). *p< 0.05, **p< 0.01, ***p< 0.005. NS, not significant.
Early B cell development is affected by FCMR and sIgM
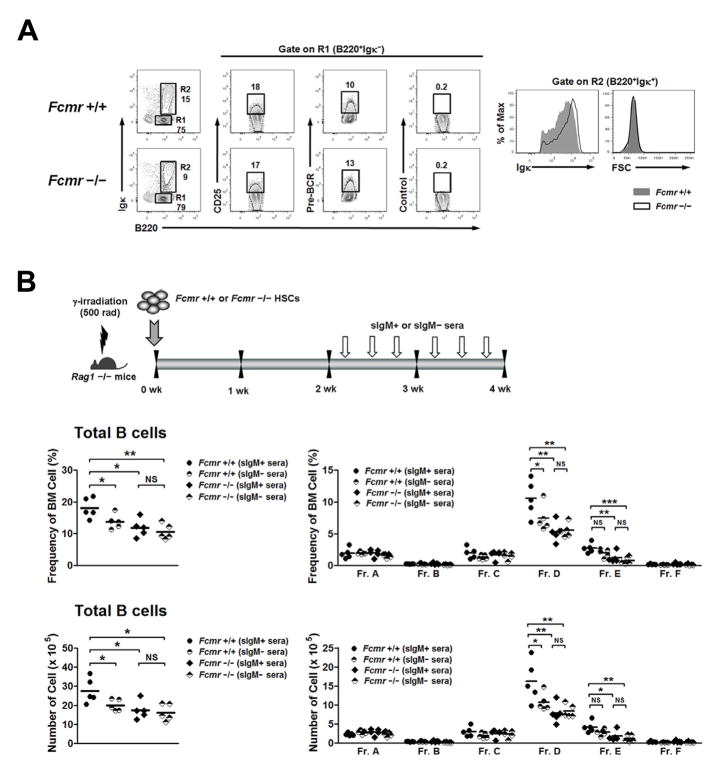
The reduced numbers of pre-B cells in Fcmr−/− mice prompted us to examine whether certain B cell intrinsic or extrinsic mechanisms might contribute to this effect. The transcription factor PAX5 was selected as one candidate since it regulates the expression of a number of genes encoding proteins that promote B cell differentiation (26). However, qPCR analyses of Pax5 transcript levels showed that they were equivalent for both cohorts in sorted early B cell populations of increasing maturity (data not shown). We then asked if early B cell development of Fcmr−/− mice could be rescued by IL7 in vitro since IL7 acts in concert with the pre-BCR to promote expansion of pre-B cells. BM cells of +/+ and Fcmr−/− mice were cultured with IL7 for 6 days with +/+ BM cells yielding slightly higher frequencies of B220+ cells than Fcmr−/− BM cells (Fig. 4A). The frequencies of pre-B cells expressing CD25 or the pre-BCR were also equivalent between +/+ and Fcmr−/− mice (Fig. 4A). These data argue that heightened IL7 signaling may compensate for deficient FCMR signaling resulting in the generation of normal frequencies of pre-B cells in Fcmr−/− cultures. Surprisingly, IL7 failed to rescue immature B cell development; 36% fewer immature B cells were generated from cultures of Fcmr−/− as compared to +/+ cells (Fig. 4A). Interestingly, the levels of cell surface Ig expressed by Fcmr−/− immature B cells were slightly higher than for cells from +/+ mice even though the sizes of cells in both populations were essentially identical (Fig. 4A, right panel). This finding suggested that FCMR might influence the levels of BCR expression during B cell development in the BM, thereby affecting positive and negative selection events.
Figure 4.
FCMR and sIgM affect pre-B and immature B cell differentiation. (A) BM cells were cultured with 10 ng/ml of IL7 for 6 days and analyzed by FACS. All cells are gated on 7-AAD− single cells. The numbers are percentages of cells falling in each gate. (B) Irradiated Rag1−/− mice were transplanted with HSC-enriched lin− cells purified from BM cells of +/+ and Fcmr−/− mice, followed by injections with sIgM+ or sIgM− sera prepared from young +/+ and Sμ−/− mice, respectively. The injection schedule was based on the understanding that the half-life of serum IgM is approximately 2 days and the amount on the demonstration that the biologic readout of arthritis could regularly be induced in B cell-deficient K/BxN mice with as little as 100 μl of arthritic K/BxN serum (48). BM cells of recipient mice were analyzed by FACS. Top diagram shows injection frequencies. The middle panel shows the frequencies of total B cells (left side) and B cell subpopulations (right side). The bottom panel shows the absolute cell numbers per femur of total B cells (left side) and B cell subpopulations (right side). Each symbol represents a mouse. *p< 0.05, **p< 0.01, ***p< 0.005. NS, not significant.
To further evaluate the function of FCMR in pre-B cell development, we reconstituted sublethally irradiated Rag1−/− mice using HSC-enriched lin− BM cells isolated from +/+ and Fcmr−/− mice. Beginning 2 wks after transplantation, the recipients were injected 3 times a wk for 2 wks with sIgM-containing (sIgM+) sera from young B6 mice or sIgM-deficient (sIgM−) sera from young Sμ−/− mice. This allowed us to evaluate the effect of sIgM on early B cell development. As shown in Fig. 4B, recipients of +/+ HSCs and sIgM+ sera generated significantly more pre-B cells than recipients of +/+ HSCs and sIgM− sera. The numbers of immature B cells also tended to be higher in recipients of sIgM+ sera than those receiving sIgM− sera. As expected, the presence or absence of sIgM in sera given to recipients of Fcmr−/− HSCs had no effect on the generation of pre-B cells. From this, we conclude that FCMR functions as a gate keeper controlling pre-B and immature B cell development, and that binding of sIgM to the receptor has a positive effect on development of these cells. These results are fully consistent with the observations that natural IgM influences B cell development and survival (3, 6, 13).
To more rigorously examine the suggested associations between sIgM and FCMR, we incubated the human YTS cell line expressing full-length mouse FCMR with pentameric mouse IgM in serum free medium. The results showed clear binding of IgM to the transfected line (Supplemental Fig. 3) strongly supporting earlier reports of receptor-ligand interactions between FCMR and IgM on both mouse and human cells (20, 22).
Fcmr−/− mice respond to TD and TI antigens with increased GC formation and generation of Ag-specific PCs
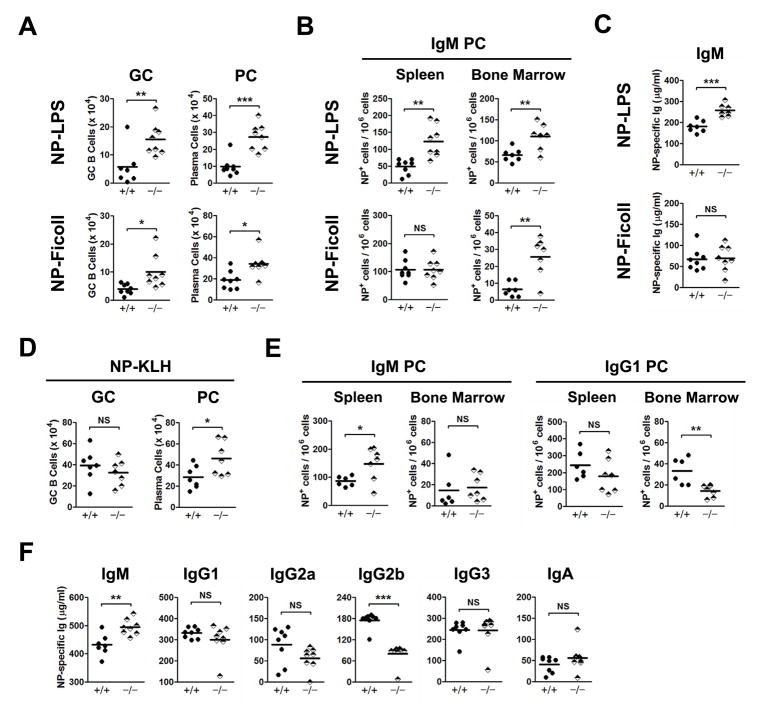
We next asked whether FCMR might influence the development of humoral immune responses to either TI or TD antigens by assessing GC development, the generation of total plasma cells, as well as Ag-specific cells in spleen and BM, and the levels of Ag-specific IgM in sera of immunized mice. We first studied responses to the TI type I Ag, NP-LPS, and the TI type II Ag, NP-Ficoll. TI Ag have been shown to generate short-lived GC that fail to engage in somatic hypermutation and affinity maturation (27, 28) while still leading to the generation of long lived plasma cells (PC) in spleen and BM (29). At one wk after immunization with either TI Ag, the total numbers of splenic GC B cells (PNAhiGL7hi) were higher for Fcmr−/− than for +/+ mice (Fig. 5A). In addition, the numbers of splenic PCs, including fully mature CD138+B220− PCs and CD138+B220+ plasmablasts, were significantly higher for Fcmr−/− mice than their +/+ counterparts (Fig. 5A). Ag-specific IgM-secreting cells in the spleen and BM were analyzed by ELISPOT and serum NP-specific IgM antibodies were measured by ELISA. Following immunization with NP-LPS, the numbers of NP-specific IgM-secreting cells in both the spleen and BM and the levels of NP-specific serum IgM were significantly higher for Fcmr−/− than +/+ mice (Fig. 5B and C). This result is in keeping with the increased numbers of B-1a cells in Fcmr−/− mice that dominate responses to TI type I Ags. Parallel studies of mice immunized with NP-Ficoll showed that the numbers of Ag-specific splenic PCs and levels of NP-specific serum IgM were similar for mice of both genotypes, although the frequencies of Ag-specific PCs were significantly higher in the BM of Fcmr−/− than +/+ mice (Fig. 5B and C). These data are consistent with the observation that the numbers of MZ B cells, which are primarily responsible for responses to TI type II Ags, are comparable in FCMR-deficient and +/+ mice.
Figure 5.
Ab responses to TI or TD antigens by Fcmr−/− mice. (A) Splenic GC B cells (B220+GL7highPNA+) and PCs (B220+/−7-AAD−CD138+) were analyzed by FACS one wk after immunization with NP-Ficoll or NP-LPS. FACS gating schemes are shown in Fig. 6A and B. Data are absolute cell numbers. (B) IgM secreting cells in the spleen and BM were determined by ELISPOT one wk after immunization with NP-Ficoll or NP-LPS. (C) Serum Ab titers of NP-specific IgM were determined by ELISA one wk after immunization with NP-Ficoll or NP-LPS. (D) GC B cells and PCs were analyzed by FACS 2 wks after immunization with NP-KLH in alum (refer to Fig. 6 for gating schemes). (E) IgG or IgM secreting cells in spleen and BM were analyzed by ELISPOT 2 wks after immunization. (F) The serum levels of NP-specific Ab were measured by ELISA 2 wks following immunization. Each symbol represents a mouse. *p< 0.05, **p< 0.01, ***p< 0.005. All data are pooled from 2 experiments.
We next examined the responses of +/+ and Fcmr−/− mice to immunization with the TD Ag, NP-KLH, in alum. Two wk after immunization, the total numbers of splenic PCs were significantly higher in Fcmr−/− than +/+ mice, even though the numbers of GC B cells were comparable for mice of both genotypes (Fig. 5D). ELISPOT analyses showed that the numbers of Ag-specific IgM-secreting PCs were about 40% higher in spleens of Fcmr−/− mice but comparable in the BM. However, studies of NP-specific IgG1-secreting cells showed that their numbers were comparable in spleens of mice of both genotypes but were lower in the BMs of Fcmr−/− mice (Fig. 5E). Analyses of NP-specific serum Igs showed that the levels of IgM Ab were higher and the levels of IgG2b were lower for Fcmr−/− mice while the levels of the other isotypes were comparable (Fig. 5F). Taken together, these results suggest that FCMR negatively regulates humoral immune responses against both TI and TD Ags.
Secondary responses to TD antigens by Fcmr−/− mice
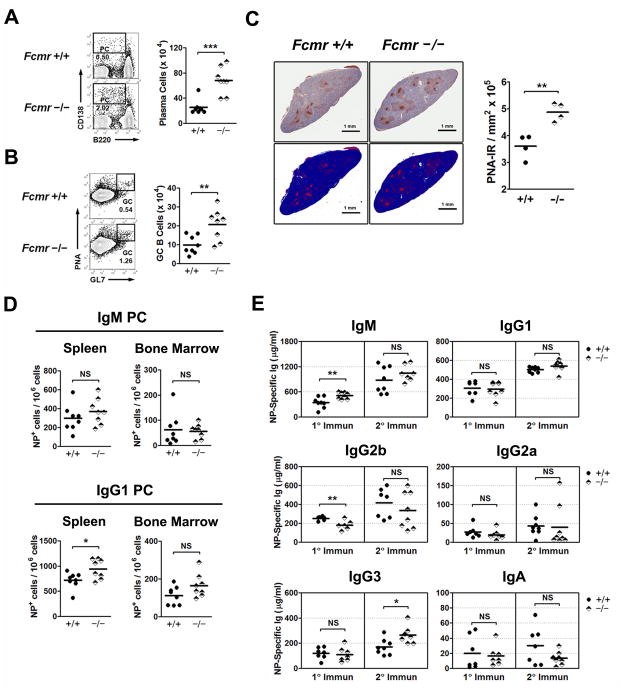
We next examined the secondary responses of +/+ and Fcmr−/− mice to NP-KLH. The mice were boosted 12 wk after a primary immunization and assayed 1 wk later for PCs and serum Abs. The numbers of splenic PCs detected by flow cytometry were significantly higher for Fcmr−/− than +/+ mice (Fig. 6A), similar to what was observed in the primary response. In contrast to the primary response, however, the numbers of GC B cells assessed by FACS (Fig. 6B) or by quantitative immunohistochemistry (Fig. 6C) were significantly higher for FCMR-deficient than +/+ mice. ELISPOT analyses of cells secreting NP-specific Ab showed that the numbers of cells producing IgM in spleen or BM were similar for mice of both genotypes. However, the numbers of IgG1-producing cells were increased in the spleen and somewhat in the BM of Fcmr−/− mice (Fig. 6D) suggesting that FCMR had a negative influence on class switch recombination (CSR) in this secondary response.
Figure 6.
Secondary immune responses to TD antigens by Fcmr−/− mice. +/+ and Fcmr−/− mice were immunized i.p. with NP-KLH in alum and then boosted with same amount of NP-KLH in alum 12 wk later. (A–B) Splenocytes were analyzed by FACS for GC B cells and PCs 10 days following the second immunization. The left panel shows FACS profiles with the numbers indicating the percentages of cells falling in each gate. The right panel shows absolute cell numbers. (C) Formalin-fixed paraffin-embedded-sections of spleen were stained with PNA to show GCs (dark brown). The first column shows the original images of spleen and second column shows the corresponding markup images with immunoreactivity (IR, positive pixels; red). The computerized morphometric analysis shows the mean values of PNA-IR per mm2. A representative image of one of 8 mice per group is shown, and the graph is representative of 2 independent experiments. (D) IgG1 or IgM secreting cells in spleen and BM were analyzed by ELISPOT 10 days after the second immunization. (E) The serum levels of NP-specific Abs were determined by ELISA 2 wks after the primary immunization and 1 wk after boosting. Each symbol represents a mouse. *p< 0.05, **p< 0.01, ***p< 0.005, NS, not significant. All data are pooled from 2 experiments.
Analyses of NP-specific serum Abs revealed that except for IgG3, the levels for other isotypes were similar for mice of both genotypes at 1 wk after the boost. However, similar to the previous study (Fig. 5F), there were small but significant differences in IgM and IgG2b levels following primary immunization (Fig. 6E). Thus, although the numbers of GC B cells and PCs were increased in Fcmr−/− mice following a secondary challenge with NP-KLH, the production of Ag-specific Ab was not significantly increased, arguing against a major role for FCMR during a recall response.
FCMR influences B cell proliferation and survival but not BCR ligation-induced signaling
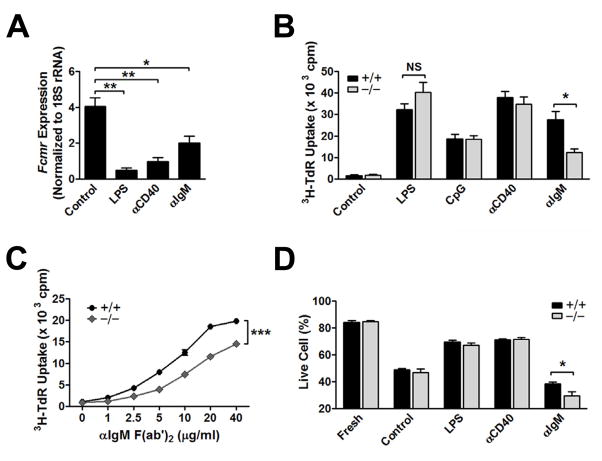
To examine the biological functions of FCMR, we tested splenic B cells from +/+ and Fcmr−/− mice for their ability to proliferate and undergo cell death upon activation in vitro. We found that transcript levels for Fcmr were markedly reduced following stimulation of purified B cells with LPS or anti-CD40, as well as following BCR ligation with anti-IgM Ab (Fig. 7A). Proliferative responses to stimulation by LPS, CpG and anti-CD40 Ab by B cells from both cohorts of mice were similar (Fig. 7B). In contrast, the response of cells from Fcmr−/− mice to BCR ligation with F(ab′)2 anti-IgM Ab was significantly reduced in a dose-dependent manner (Fig. 7B, C). Parallel studies of cell viability showed that survival of B cells from Fcmr−/− and +/+ mice was the same following treatment with LPS or anti-CD40 Ab, but that the viability of cells from Fcmr−/− mice following BCR crosslinking was significantly reduced (Fig. 7D). Thus, FCMR appears to promote B cell proliferation as well as protect against apoptosis following BCR ligation.
Figure 7.
FCMR inhibits BCR-mediated B cell apoptosis. (A) qPCR analysis of Fcmr transcripts in splenic B cells stimulated with LPS, anti-CD40 Ab, or F(ab′)2 anti-IgM for 24 h. (B) Proliferative responses of splenic B cells stimulated as in (A) for 3 days. (C) Dose-dependent proliferative responses to BCR ligation induced by F(ab′)2 anti-IgM. Splenic B cells were cultured for 3 days. (D) Freshly isolated splenic B cells stimulated for 16 h were stained with 7-AAD and Annexin-V and analyzed by FACS. Live cells were gated as 7-AAD− Annexin V−. *p< 0.05, **p< 0.01, ***p< 0.005, NS, not significant. MFI, mean fluorescence intensity.
Discussion
The results of this study demonstrate that FCMR plays a role in B cell differentiation, function and homeostasis. Mice deficient in FCMR exhibit multiple alterations in early B cell development, the distribution of mature B cell subsets, responses to antigenic challenge and are predisposed to autoimmunity. The fact that many of these changes mirror those observed in mice lacking sIgM indicates that FCMR is a critical sensor of circulating IgM and provides a basis for understanding a number of its functions in health and disease. Understanding the phenotypes that distinguish FCMR- from sIgM-deficient mice may be helpful in defining the activities of the other IgM receptor, Fcα/μ receptor (FCAMR), expressed on the surface of B cells. FCMR is but one of three receptors capable of binding IgM. The polymeric Ig receptor (pIgR) is expressed primarily by epithelial cells and mediates the transcytosis of J chain-associated polymeric IgM and IgA across mucosal surfaces (16). The FCAMR is expressed by B cells, macrophages and follicular dendritic cells but not by granulocytes, T or NK cells. FCAMR does not require the presence of the J chain to bind and endocytose IgM or IgA. Interestingly, mice deficient in FCAMR resemble Fcmr−/− mice in exhibiting enhanced germinal center responses to TI Ags (30). It has been suggested that FCAMR negatively regulates responses to TI antigens by inhibiting antigen retention by MZ B cells and follicular dendritic cells, a concept worthy of further investigation for Fcmr−/− mice. It is important to note that since FCMR is expressed to some extent by neutrophils, NK cells, macrophages and dendritic cells, not all the biologic changes associated with deficient expression of the receptor can be attributed to changes in FCRM-sIgM interactions on B cells.
The impaired B cell development in Fcmr−/− mice occurred as early as the pre-B cell stage. How FCMR affects pre-B cell survival and development remains unclear. The generation of pre-B cells is marked by clonal proliferation for ~6 divisions driven by signaling through the pre-BCR and the IL7 receptor (31) followed by cell cycle arrest and differentiation to small pre-B cells. Deficiencies in pre-BCR signaling caused either by poor expression of the pre-BCR at the cell surface or by disruptions of the signaling machinery downstream of the pre-BCR cause severe defects in pre-B and subsequent immature B cell development (32–34). While the numbers of proB cells in Fcmr−/− mice were moderately reduced, the subsequent development of large pre-B cells was not significantly affected, indicating that the pre-BCR functions normally in these cells. In fact, pre-BCR expression levels measured by FACS in IL7-cultured BM pro-B/pre-B cells revealed comparable levels between +/+ and Fcmr−/− mice. There are two issues that remain to be determined. First, the levels of FCMR protein expressed on the surface of pre-B cells are not known due to a lack of commercially available anti-FCMR specific Abs. Second, it is unclear if FCMR-triggered signaling requires engagement with soluble IgM. However, our analyses of Sμ−/− mice that lack soluble IgM but express FCMR revealed deficiencies in pre-B cell development similar to those seen in Fcmr−/− mice. This argues that pre-B cells are very sensitive to FCMR signaling and that a certain type of interaction between sIgM and FCMR is required to generate a signaling cascade of major importance for development of pre-B and immature B cells. This view is further supported by the observation that administration of sIgM enhanced production of pre-B cells in Rag1−/− mice reconstituted with Fcmr−/− BM cells (Fig. 4B). Since a previous study of Sμ−/− mice (7) did not identify defects in early B cells like those seen in our analyses of Fcmr−/− mice, the nature of FCMR-mediated signaling requires further investigation.
Analyses of the effects of FCMR deficiency on subsets of mature B cells revealed two contrasting pictures with the numbers of splenic FO B cells being significantly reduced while the numbers of peritoneal B-1a cells were significantly increased. Remarkably, these observations again mirror those made in sIgM-deficient mice (13) implicating FCMR-sIgM interactions as critical to both phenotypes. FO B cells, similar to resting small pre-B cells, are not in cell cycle and they differentiate with minimal signaling from the BCR (35). The lack of FCMR-augmented tonic signaling might reduce the level of FO BCR signaling below the threshold required for survival while also enhancing their apoptotic response to BCR ligation. This concept is in keeping with the known importance of tonic signaling for B cell survival demonstrated in studies of Cre-mediated ablation of BCRs in mature B cells (36, 37) and in mice expressing engineered BCRs without extracellular domains (35, 38). It would be also consistent with the observation that Sμ−/−mice, which are unable to activate the FCMR, have more apoptotic splenic B cells (13).
The contrasting increase in peritoneal B-1a cells in FCMR-deficient mice may relate to the greater intensity of BCR signaling associated with their selection and survival than occurs with FO B cells (39, 40) and is associated with their anergic phenotype (41). As suggested by Ehrenstein and Notley from studies of sIgM-deficient mice (3), this strength of signaling may poise B-1a B cells at the threshold for apoptosis such that a reduction in tonic signaling would promote their survival and the observed increases in cell numbers.
The known contribution of natural IgM to complement-dependent clearance of apoptotic cells (42) provides a basis for understanding how expanded populations of B-1a cells, as a source of sIgM, could predispose mice lacking FCMR to autoimmunity. Sera from 6 month-old Fcmr−/− mice had significantly increased levels of IgG anti-DNA antibodies and sera from nearly half of these mice were positive for ANAs with staining patterns characteristic for antibodies to dsDNA or histones. Previous studies of Sμ−/− mice documented spontaneous production of IgG anti-dsDNA antibodies and glomerular deposition of IgG and C3 in about a third of 12 to 18 month old mice (9). Additional studies of autoimmune MRL-Fas−/− mice deficient in sIgM documented accelerated appearance of IgG Ab to dsDNA and histones, greater renal pathology and shortened survival (8); IgG3 anti-dsDNA antibodies were also produced by most Sμ−/− mice but only when 12 months old. Taken together, these results indicate that interactions of FCMR with natural IgM act to suppress the development of IgG secreting autoreactive B cells most likely by promoting the clearance of apoptotic cells (42).
Previous studies of Sμ−/− mice revealed that CSR was impaired in primary immune responses to TD antigens (7, 43), a phenotype that was explained by the lack of an adjuvant effect afforded by IgM-immunogen complexes (44). Interestingly, our studies of Fcmr−/− mice identified enhanced IgM responses to TD antigens (Fig. 5), arguing for a negative rather than a positive regulatory effect on TD immune responses. Nonetheless, IgG responses to TD antigens were not similarly enhanced; indeed, IgG2b production was significantly decreased in Fcmr−/− mice. The lack of enhanced class switching in Fcmr−/− mice is associated with reduced expression of Fcmr by GC B cells and PCs (Fig. 1). It is worth noting that the observed changes in TD immune responses by FCMR-deficient mice could be influenced by other cell types lacking FCMR and/or expression of the FCAMR that was previously shown to influence responses to different antigens (30).
Given the abundance of natural IgM antibodies and the broad expression of FCMR, we envision that this receptor could play important roles in innate immunity. IgM immune complexes aggregated by FCMR at the cell surface are readily internalized and transported through the endocytic pathway to lysosomes where they are degraded (45). Engagement of this pathway to dispose of IgM-bound pathogens and extracellular debris could result in synergistic activation of B cells stimulated through their BCRs (46). Both our study and previous studies of Sμ−/− mice demonstrated the critical roles of sIgM and FCMR in the development of innate-like B-1a and MZ B cells that are critical to responses to fungal and parasitic infections. This role is exemplified by the finding that Sμ−/− mice respond poorly to infection with Cryptococcus neoformans (5). Interestingly, natural IgM is reported to bind malarial parasites and FCMR is implicated in innate immunity against malaria infection (47).
The identification of FCMR as a receptor for sIgM, supported by results presented here, need not be an either or proposition regarding its role in apoptosis. Indeed, our studies suggest a protective role against cell death following BCR ligation of splenic B cells and possibly during early B cell development as well. Understanding how these differing potentials might play out in the biology of individual cells will certainly be a focus of future studies.
In conclusion, the development and function of B cells in FCMR deficient mice is remarkably similar to that of Sμ−/− mice, highlighting critical roles for the sIgM-FCMR axis in regulating normal B cell differentiation, modulating innate and adaptive immunity and protecting against the development of humoral autoimmunity. Further studies are required to characterize the molecular features of FCMR-triggered signaling and its contributions to these differing phenotypes.
Supplementary Material
Acknowledgments
We appreciate the work of Mirna Pena and Alfonso Macias for handling our animal colony and of Abshari Mehrnoosh for cell sorting. We appreciate the generosity of Dr. Tak Mak and the University Health Network in providing us with the Fcmr−/− mouse strain generated by his group. We also thank Dr. Troy Randall for providing Sμ−/− mice.
This work was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Abbreviations used in this article
- BCR
B cell receptor
- ERK
extracellular signal-regulated kinase
- FO
follicular
- GC
germinal center
- MZ
marginal zone
- NP-KLH
4-hydroxy-3-nitrophenyl acetyl-keyhole limpet hemocyanin
- NP-LPS
4-hydroxy-3-nitrophenyl acetyl-lipopolysaccharides
- PC
plasma cell
- PLCγ2
phosphoinositide phospholipase C gamma 2
- sIgM
secreted IgM
- Syk
spleen tyrosine kinase
- TD
T-dependent
- TI
T-independent
- Tr
transitional
Footnotes
Disclosures
The authors declare no conflict of interest.
S.-C.C., H.W., H.C.M. and J.E.C. designed research; S.-C.C., H.W., L.T., Y.M. and D.-M.S. performed research; S.-C.C., H.W., F.B., H.C.M. and J.E.C. analyzed data; and S.-C.C., H.W., H.C.M. and J.E.C. wrote the paper.
The online version of this article contains supplemental material.
References
- 1.Fellah JS, Wiles MV, Charlemagne J, Schwager J. Evolution of vertebrate IgM: complete amino acid sequence of the constant region of Ambystoma mexicanum mu chain deduced from cDNA sequence. Eur J Immunol. 1992;22:2595–2601. doi: 10.1002/eji.1830221019. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker N, Ehrenstein MR. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J Immunol. 2002;169:6686–6690. doi: 10.4049/jimmunol.169.12.6686. [DOI] [PubMed] [Google Scholar]
- 7.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 8.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med. 2000;191:1253–1258. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurnheer MC, Zuercher AW, Cebra JJ, Bos NA. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J Immunol. 2003;170:4564–4571. doi: 10.4049/jimmunol.170.9.4564. [DOI] [PubMed] [Google Scholar]
- 13.Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. 2010;184:3386–3393. doi: 10.4049/jimmunol.0902640. [DOI] [PubMed] [Google Scholar]
- 14.Chen FH, Arya SK, Rinfret A, Isenman DE, Shulman MJ, Painter RH. Domain-switched mouse IgM/IgG2b hybrids indicate individual roles for C mu 2, C mu 3, and C mu 4 domains in the regulation of the interaction of IgM with complement C1q. J Immunol. 1997;159:3354–3363. [PubMed] [Google Scholar]
- 15.Arnold JN, Wormald MR, Suter DM, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM, Sim RB. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J Biol Chem. 2005;280:29080–29087. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 16.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre HJ, Sutherland GR, Endo Y, Fujita T, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Phillips JH, Lanier LL, Nakauchi H. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 18.Basten A, Warner NL, Mandel T. A receptor for antibody on B lymphocytes. II. Immunochemical and electron microscopy characteristics. J Exp Med. 1972;135:627–642. doi: 10.1084/jem.135.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamon EW, Andersson B, Whitten HD, Hurst MM, Ghanta V. IgM complex receptors on subpopulations of murine lymphocytes. J Immunol. 1976;116:1199–1203. [PubMed] [Google Scholar]
- 20.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, Gartland GL, Bertoli LF, Mori H, Takatsu H, Kitamura T, Ohno H, Wang JY. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. 2009;206:2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, Wang JY, Ohno H. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- 22.Murakami Y, Narayanan S, Su S, Childs R, Krzewski K, Borrego F, Weck J, Coligan JE. Toso, a Functional IgM Receptor, Is Regulated by IL-2 in T and NK Cells. J Immunol. 2012;189:587–597. doi: 10.4049/jimmunol.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kinoshita S, Nolan GP. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Foger N, Kamal MA, Prilla P, Mathieu S, Wagner C, Mak T, Chan AC, Lee KH. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 2011;118:598–608. doi: 10.1182/blood-2010-10-313643. [DOI] [PubMed] [Google Scholar]
- 25.Song Y, Jacob CO. The mouse cell surface protein TOSO regulates Fas/Fas ligand-induced apoptosis through its binding to Fas-associated death domain. J Biol Chem. 2005;280:9618–9626. doi: 10.1074/jbc.M413609200. [DOI] [PubMed] [Google Scholar]
- 26.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 27.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lentz VM, Manser T. Cutting edge: germinal centers can be induced in the absence of T cells. J Immunol. 2001;167:15–20. doi: 10.4049/jimmunol.167.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Bortnick A, Chernova I, Quinn WJ, 3rd, Mugnier M, Cancro MP, Allman D. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol. 2012;188:5389–5396. doi: 10.4049/jimmunol.1102808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, Takahashi S, Yasui T, Kikutani H, Kinoshita T, Fujita T, Tahara-Hanaoka S, Shibuya K, Shibuya A. Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci U S A. 2009;106:11230–11235. doi: 10.1073/pnas.0809917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- 33.Kawano Y, Yoshikawa S, Minegishi Y, Karasuyama H. Pre-B cell receptor assesses the quality of IgH chains and tunes the pre-B cell repertoire by delivering differential signals. J Immunol. 2006;177:2242–2249. doi: 10.4049/jimmunol.177.4.2242. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Clarke SH. Association of the pre-B cell receptor (BCR) expression level with the quality of pre-BII cell differentiation reveals hierarchical pre-BCR function. Molecular immunology. 2007;44:1765–1774. doi: 10.1016/j.molimm.2006.07.301. [DOI] [PubMed] [Google Scholar]
- 35.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 36.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 38.Fuentes-Panana EM, Bannish G, Monroe JG. Basal B-cell receptor signaling in B lymphocytes: mechanisms of regulation and role in positive selection, differentiation, and peripheral survival. Immunol Rev. 2004;197:26–40. doi: 10.1111/j.0105-2896.2004.0105.x. [DOI] [PubMed] [Google Scholar]
- 39.Dal Porto JM, Burke K, Cambier JC. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity. 2004;21:443–453. doi: 10.1016/j.immuni.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–353. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 41.Wong SC, Chew WK, Tan JE, Melendez AJ, Francis F, Lam KP. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J Biol Chem. 2002;277:30707–30715. doi: 10.1074/jbc.M202460200. [DOI] [PubMed] [Google Scholar]
- 42.Peng Y, Kowalewski R, Kim S, Elkon KB. The role of IgM antibodies in the recognition and clearance of apoptotic cells. Molecular immunology. 2005;42:781–787. doi: 10.1016/j.molimm.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci U S A. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youd ME, Ferguson AR, Corley RB. Synergistic roles of IgM and complement in antigen trapping and follicular localization. Eur J Immunol. 2002;32:2328–2337. doi: 10.1002/1521-4141(200208)32:8<2328::AID-IMMU2328>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Vire B, David A, Wiestner A. TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol. 2011;187:4040–4050. doi: 10.4049/jimmunol.1100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanden Bush TJ, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. 2008;38:400–409. doi: 10.1002/eji.200737602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czajkowsky DM, Salanti A, Ditlev SB, Shao Z, Ghumra A, Rowe JA, Pleass RJ. IgM, Fc mu Rs, and malarial immune evasion. J Immunol. 2010;184:4597–4603. doi: 10.4049/jimmunol.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.