Abstract
PARP inhibitors show promise as combination and single agents in cancer chemotherapy. Here, we evaluate results obtained with mouse fibroblasts and the common laboratory PARP inhibitor, 4-amino-1,8-naphthalimide (4-AN), and analyze the potential for enhanced cytotoxicity following the combination of a DNA damaging agent and a PARP inhibitor. Methylated DNA bases are repaired by the monofunctional glycosylase-initiated single-nucleotide base excision repair (BER) pathway. An intermediate of this process has a single-nucleotide gap in double-stranded DNA containing the 5′-deoxyribose phosphate (dRP) group atone margin. This 5′-dRP group is removed by the lyase activity of pol β prior to gap filling, then completion of repair is by DNA ligation. PARP-1 binds to and is activated by the 5′-dRP group-containing intermediate, and poly(ADP-ribos)ylation is important for efficient repair. 4-AN-mediated sensitization to the methylating chemotherapeutic agent temozolomide is extreme, producing a level of cytotoxicity not seen with either agent alone. In contrast, with agents producing oxidative DNA damage repaired by bifunctional glycosylase-initiated BER, there is only weak sensitization by co-treatment with PARP inhibitor. Other clinically utilized DNA-damaging agents repaired by different DNA repair pathways also reveal minimal 4-AN-mediated sensitization. This information has potentially important implications for strategic use of PARP inhibitors in chemotherapy.
Keywords: PARP inhibitor, mouse fibroblasts, base excision repair
Introduction
Results from clinical trials indicate that PARP inhibitors may allow selective killing of cancer cells, because they target deficiencies in DNA repair that are unique to individual types of cancer vs. normal tissues (1). However, information on PARP molecular biology necessary to predict PARP inhibitor effects is not yet clear in the literature and is not well recognized. Understanding PARP inhibitor mechanisms in model systems, such as human and mouse cells in culture, has the potential for informing strategies on cancer chemotherapy. Studies of DNA repair and cell signaling in model systems have identified types of DNA damage that result in PARP activation and this information is key to enhancing predictions on the outcome of therapeutic approaches with PARP inhibitors. Here, we discuss our perspective on the roles of PARP in mammalian cells and how the presence of the inhibited PARP-1 protein during base excision DNA repair impacts cell killing.
I) Background on DNA base excision DNA repair
The predominant repair pathway for removal of a single base lesion in double-stranded DNA is base excision repair (BER). Single base lesions occur through endogenous events including spontaneous base loss and deamination, or uracil incorporation during replication. Damage can also arise through base oxidation and alkylation from endogenous and exogenous sources. For example, methyl methanesulfonate (MMS) is a directly acting DNA methylating agent causing alkylation of base nitrogens (e.g., 7-methylguanine), whereas the oxidizing agent peroxynitrite produces reactive oxygen species (ROS) and oxidized base damage(e.g., 8-oxoguanine).
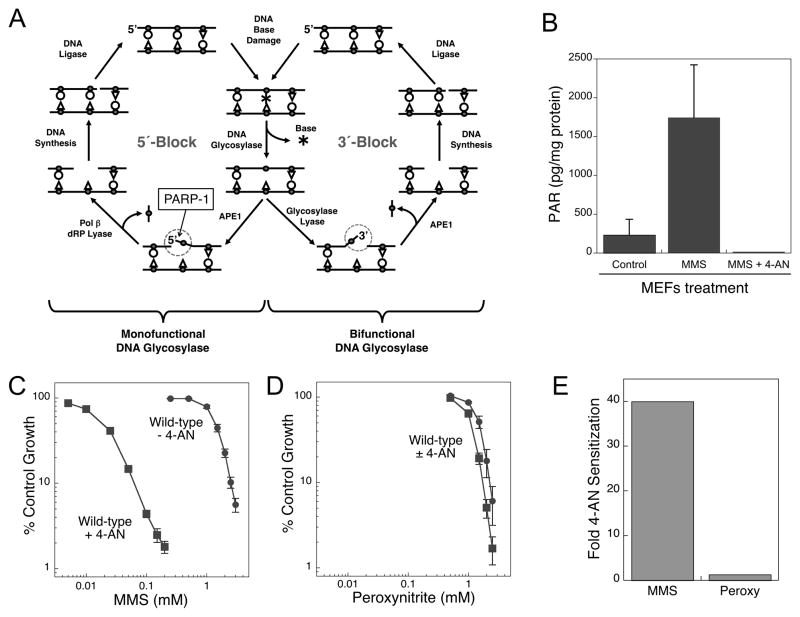
There are several known sub-pathways of BER differentiated by the enzymes involved and the size of the repair patch (reviewed in (2)). In the simplest single-nucleotide BER sub-pathway, repair is initiated by a lesion-specific monofunctional DNA glycosylase (i.e., N-methylpurine DNA glycosylase (MPG) in the case of a methylated base) that removes the damaged base leaving the toxic abasic (AP) site. The DNA backbone is incised 5′ of the AP site by AP endonuclease 1 (APE1) producing a 1-nt gap with 3′-OH and 5′-dRP groups at the margins (Fig. 1A, left side). DNA polymerase β (pol β) removes the 5′-dRP blocking group and performs single-nucleotide gap filling synthesis. In contrast, many of the glycosylases specific for oxidative DNA damage are bifunctional with an associated AP lyase activity that cleaves the DNA backbone 3′ to the abasic site leaving 3′-dRP and 5′-PO4 termini(Fig. 1A, right side). Now, APE1 cleaves the 3′ blocking group leaving a substrate suitable for DNA synthesis and ligation. In this case, formation of a 5′-dRP blocking group does not occur and there is no requirement for pol β 5′-dRP lyase gap tailoring activity (3). The 5′ and 3′ intermediates of repair are identified (Fig. 1A).
Figure 1.
A, Scheme of pol β-dependent BER initiated by a monofunctional (left side) or bifunctional (right side) DNA glycosylase showing formation of 5′-or 3′-blocking groups (circled), respectively, in repair intermediates. The specific interaction between PARP-1 and the5′-dRP-containing intermediate is indicated. B, Inhibitory effect of 4-AN (10 μM) on PAR synthesis in MMS-treated MEFs (10 mM for 20 min at 4 °C) as measured by a commercial ELISA assay (Trevigen). C, Sensitivity of wild-type MEFs to a 1 h exposure to MMS (circles) and the sensitization obtained by co-treatment with 4-AN (10 μM for 24 h; squares). D, Sensitivity of wild-type mouse fibroblasts to a 1 h exposure to peroxynitrite (circles) and the absence of sensitization provided by co-treatment with 4-AN(10 μM for 24 h; squares). The plot was drawn to the same scale as in C. Cell sensitivity was obtained by growth inhibition assays as described previously (12). E, Comparison of 4-AN-mediated fold-sensitization of wild-type MEFs to MMS (40-fold) and peroxynitrite (negligible).
II) Binding of PARP-1 to intermediates of BER
PARP-1 plays a role in DNA damage recognition and repair. It binds to nicks and strand-breaks in DNA, including the 5′-dRP containing intermediate of BER. When bound to such DNA, PARP-1 becomes catalytically activated synthesizing poly(ADP-ribose) (PAR) polymers from NAD+, and resulting in poly(ADP-ribosyl)ation of itself as well as other proteins involved in DNA repair and chromatin remodeling. Following auto-modification, PARP-1 is able to complex with other BER proteins such as pol β and XRCC1 enabling their recruitment to the damage site. With loss of the abasic site sugar by pol β lyase activity and completion of repair by pol β gap filling and DNA ligation, PARP-1 dissociates from DNA and the PAR is rapidly cleaved, primarily by poly ADP-ribose glycohydrolase (4).
In previous photoaffinity labeling studies with mouse fibroblast cell extracts, PARP-1 was found to be the predominant protein factor binding to the BER intermediate (5). Use of various alternate BER intermediates as model binding ligands revealed binding specificity for an analog of the natural 5′-dRP intermediate with much less binding using a BER intermediate with a 5′-PO4 (6). These results are consistent with a biological role for the interaction between PARP-1 and the 5′-dRP-containing intermediate (Fig. 1A, left side). The molecular mechanism of this specificity is not yet understood.
III) PARP inhibition and cellular hypersensitivity to DNA damage
Enhanced cytotoxicity associated with PARP inhibition correlates closely with PARP-1 binding to the 5′-dRP group-containing BER intermediate (Fig. 1A, left side). In the presence of DNA alkylation, and an inhibitor of its catalytic activity, PARP-1 still binds to sites of DNA damage, but auto-ribosylation is prevented (7). It is proposed that in its inhibited, inactivated state, PARP-1 binding to DNA is persistent, hindering the BER process. It is also proposed that the cytotoxicity of DNA-bound and inhibited PARP-1 is linked to formation of replication-dependent double-strand breaks.
Analysis of MMS-treated mouse embryonic fibroblasts shows that PAR synthesis is completely inhibited by the PARP inhibitor 4-amino-1,8-naphthalimide (4-AN) (8)(Fig. 1B). As expected, wild-type fibroblasts are highly (40-fold) sensitized by 4-AN to methylating agents, e.g., MMS (Fig. 1C). In contrast, combination of 4-AN and the oxidant peroxynitrite has a negligible effect on cytotoxicity (Fig. 1D) (9). The predominant DNA modifications induced by peroxynitrite include 8-oxoguanine (10), where repair is initiated by the bifunctional 8-oxoguanine DNA glycosylase (OGG1), producing a 3′-blocked repair intermediate (circled, Fig. 1A, right side). The difference in BER following treatment with the alkylating agent, MMS and the oxidizing agent, peroxynitrite is the initiation step by a monofunctional vs. a bifunctional glycosylase, respectively (Fig. 1A). Only in the former case (repair of alkylated base damage) will there be an intermediate with the 5′-dRP blocking group (circled, Fig. 1A, left side) and strong 4-AN-mediated sensitization (Fig. 1E). If the repair intermediate does not have a 5′-dRP group, PARP-1 binding is expected to be relatively weak, and the effect of inhibiting DNA-bound PARP-1 will be diminished (Fig. 1E). These observations suggest the 5′-dRP blocking group is critical for binding PARP-1 and for 4-AN-mediated extreme sensitization to DNA damage.
IV) Influence of pol β expression on the effect of PARP inhibition
The hallmark phenotype of pol β null cells is hypersensitivity to SN2 alkylating agents such as MMS, and SN1 alkylating agents such as methyl nitrosourea (MNU) and the chemotherapeutic methylating agent, TMZ (Fig. 2A) (11, 12). Pol β null cells are similarly hypersensitive to exposure to the thymidine analog 5-hydroxymethyl-2′-deoxyuridine (hmdUrd), an agent that is incorporated into cellular DNA and removed by a monofunctional DNA glycosylase, SMUG1 (12). Hypersensitivity to methylating agents in pol β null mouse fibroblasts can be reversed by deleting the DNA glycosylase responsible for initiating repair (13) or by expression of either the full-length pol β protein or the 8 kDa dRP lyase domain having 5′-dRP gap tailoring activity (14).
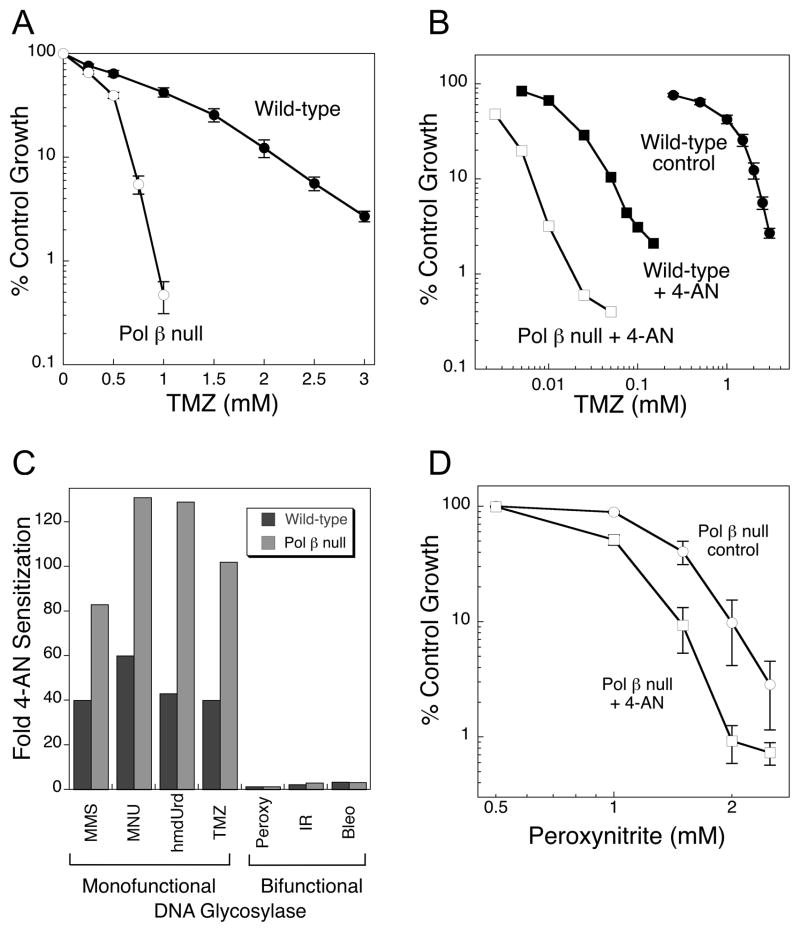
Figure 2.
A, Hypersensitivity (3.2-fold) of pol β null mouse fibroblasts (open symbols) to TMZ (4 h exposure). B, Sensitization to TMZ cytotoxicity by 24 h exposure to the PARP inhibitor, 4-AN (squares). C, Comparison of 4-AN mediated sensitization in wild-type and pol β null MEFs to agents resulting in damage repaired by monofunctional and bifunctional BER pathways. D, Low level sensitization by 4-AN of pol β null cells to peroxynitrite.
As already noted, 4-AN is a potent inhibitor of PARP-1 catalytic activity in methylating agent-treated cells, and when cells are treated with the combination of TMZ plus 4-AN, there is a very strong sensitization of both wild-type and pol β-null variants (Fig. 2B) (9). But, importantly, pol β-null cells are more sensitized than are wild-type cells (i.e., 100-and 40-fold, respectively; Fig. 2B and C). Positive TMZ/PARP inhibitor data have been reported in a number of other cellular systems, e.g., human tumor cell lines and xenografts (15, 16). Similar pol β-dependent sensitization results were obtained with other similar DNA damaging agents (MMS, MNU and hmdUrd) (Fig. 2C). It appears that through its role in removing the 5′-dRP group, pol β reduces PARP inhibitor-mediated sensitization. In the absence of pol β, the cell will be deficient in 5′-dRP lyase activity, allowing for enhanced binding of PARP-1 at the intermediate and greater 4-AN-mediated sensitization.
In the case of peroxynitrite treatment, the minimal 4-AN sensitization observed in wild-type cells (Fig. 1D), was also seen under conditions of pol β-deficiency (Fig. 2D) (9). Similar data were obtained in both cell types for clinically utilized IR and bleomycin (Fig. 2C). Bleomycin is a radiomimetic agent and requires a redox active metal ion and molecular oxygen to form reactive oxygen species resulting in oxidized sugars and abasic sites with 3′-blocking groups such as 3′-phosphoglycolate (17). Repair may involve pol β, but 5′-blocking groups are not abundantly formed, and pol β null cells are only minimally hypersensitive to peroxynitrite and bleomycin, also to IR (3, 18). Again, the results suggest a requirement for a 5′-dRP blocking group intermediate for cellular hypersensitivity in pol β-deficient cells and strong 4-AN sensitization.
V) Summary of 4-AN-mediated sensitization to other agents
As discussed above, cells are highly sensitized to TMZ by PARP inactivation, especially in the absence of pol β (Fig. 2B). In contrast, PARP inhibition has a considerably lesser effect when combined with an oxidant chemotherapy drug (bleomycin) or IR (Fig. 2C). The chemistry of DNA damage and repair, therefore regulates inhibitor effects, since in the absence of the 5′-dRP group-containing repair intermediate there is minimal PARP inhibitor-mediated sensitization.
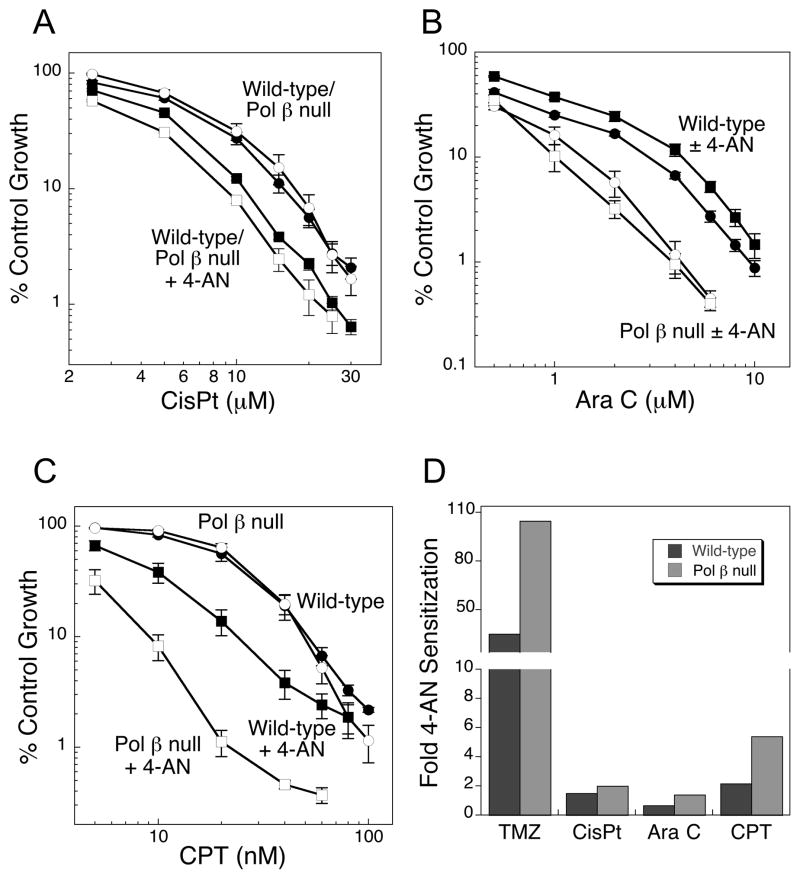
We also considered other chemotherapeutic DNA damaging agents; first we evaluated cisplatin (cisPt), a DNA crosslinking agent. The platinum of cisPt binds covalently to the N7 position of purines, mainly guanine, then forms intra-and inter-strand crosslinks (19). Intra-strand crosslinks are bulky adducts on only one DNA strand and can be repaired by nucleotide excision repair (NER). The platinum inter-strand lesion can undergo NER, but is also processed by mismatch repair, homologous recombination (HR) and translesion synthesis (TLS). Pol β null cells show no hypersensitivity to this agent, and combination with 4-AN results in minimal sensitization in both cell types (Fig. 3A) (5). Cytosine arabinoside (Ara C) is an example of a nucleoside analog (20) where cytotoxicity occurs as a result of DNA synthesis chain termination (21). Pol β null cells are only slightly hypersensitive to this agent, and 4-AN co-treatment again results in only low-level sensitization (Fig. 3B). Camptothecin (CPT) is a topoisomerase 1 (Top1) inhibitor resulting in trapping of the Top1 cleavage complex and formation of protein-linked single-strand breaks (SSBs). Repair by tyrosyl-DNA phosphodiesterase, polynucleotide kinase and SSB repair may involve pol β gap-filling synthesis, but it does not involve a 5′-blocked repair intermediate. Interestingly, 4-AN treatment resulted in some sensitization to CPT, especially in pol β null cells (4.6-fold) (Fig. 3C), but still this was extremely modest compared with the methylating agent TMZ (Fig. 3D).
Figure 3.
Level of hypersensitivity of pol β null MEFs (open circles) to exposure to A, cisPt (1 h); B, Ara C (24 h) or C, CPT (24 h) compared with wild-type MEFs (closed circles). A–C, Sensitization of pol β null (open squares) and wild-type MEFs (closed squares) by 24 h exposure to 4-AN (10 μM). D, Comparison of 4-AN mediated sensitization to TMZ and other types of clinically-utilized DNA damaging agents in wild-type and pol β null mouse fibroblasts.
Concluding remarks – Perspective on predicting PARP inhibitor sensitization
PARP inhibitors are increasingly utilized in chemotherapy as part of a combination regime or as monotherapy agents. Studies in mouse fibroblasts indicate that the magnitude of the effect of a PARP inhibitor in combination with a DNA damaging agent is dependent on the precise nature of the DNA repair process. Inhibiting PARP bound to a 5′-dRP-containing intermediate results in a dramatic cell sensitization effect, whereas in the absence of a 5′-dRP group, both PARP-1 binding and inhibitor-mediated sensitization is minimal. We hypothesize that an excess of 5′-dRP group-containing intermediates accumulate in a pol β-deficient cell, such that PARP-1 binding and PARP inhibitor sensitization are increased. This information may be useful in predicting the effect of a DNA damaging agent and PARP inhibitor combination with other cell types and agents, and possibly even in a clinical setting.
Acknowledgments
Financial support: This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (project number Z01ES050159).
We thank Natalie Gassman and Donna Stefanick for providing unpublished PAR analysis data, William Beard for help with figure preparation and Bonnie Earnhardt for editorial assistance.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Anders CK, Winer EP, Ford JM, Dent R, Silver DP, Sledge GW, et al. Poly(ADP-ribose) polymerase inhibition: “Targeted” therapy for triple-negative breast cancer. Clin Cancer Res. 2010;16:4702–10. doi: 10.1158/1078-0432.CCR-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard WA, Wilson SH. Structure and mechanism of DNA polymerase β. Chem Rev. 2006;106:361–82. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- 3.Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase β in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18:48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vos M, Schreiber V, Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: Current state of the art. Biochem Pharmacol. 2012;84:137–46. doi: 10.1016/j.bcp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Lavrik OI, Prasad R, Sobol RW, Horton JK, Ackerman EJ, Wilson SH. Photoaffinity labeling of mouse fibroblast enzymes by a base excision repair intermediate. Evidence for the role of poly(ADP-ribose)polymerase-1 in DNA repair. J Biol Chem. 2001;276:25541–8. doi: 10.1074/jbc.M102125200. [DOI] [PubMed] [Google Scholar]
- 6.Cistulli C, Lavrik OI, Prasad R, Hou E, Wilson SH. AP endonuclease and poly(ADP-ribose) polymerase-1 interact with the same base excision repair intermediate. DNA Repair (Amst) 2004;3:581–91. doi: 10.1016/j.dnarep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers AJ. The potential role and application of PARP inhibitors in cancer treatment. Br Med Bull. 2009;89:23–40. doi: 10.1093/bmb/ldp005. [DOI] [PubMed] [Google Scholar]
- 8.Ruf A, de Murcia G, Schulz GE. Inhibitor and NAD+ binding to poly(ADP-ribose) polymerase as derived from crystal structures and homology modeling. Biochemistry. 1998;37:3893–900. doi: 10.1021/bi972383s. [DOI] [PubMed] [Google Scholar]
- 9.Horton JK, Stefanick DF, Naron JM, Kedar PS, Wilson SH. Poly(ADP-ribose) polymerase activity prevents signaling pathways for cell cycle arrest following DNA methylating agent exposure. J Biol Chem. 2005;280:15773–85. doi: 10.1074/jbc.M413841200. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R, Rasheed Z, Ahsan H. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol. 2009;31:388–96. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 11.Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, et al. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature. 1996;379:183–6. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 12.Horton JK, Joyce-Gray DF, Pachkowski BF, Swenberg JA, Wilson SH. Hypersensitivity of DNA polymerase β null mouse fibroblasts reflects accumulation of cytotoxicrepair intermediates from site-specific alkyl DNA lesions. DNA Repair (Amst) 2003;2:27–48. doi: 10.1016/s1568-7864(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 13.Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, et al. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem. 2003;278:39951–9. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- 14.Sobol RW, Prasad R, Evenski A, Baker A, Yang X-P, Horton JK, et al. The lyase activity of the DNA repair protein β-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 2000;405:807–10. doi: 10.1038/35015598. [DOI] [PubMed] [Google Scholar]
- 15.Delaney CA, Wang L-Z, Kyle S, White AW, Calvert AH, Curtin NJ, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6:2860–7. [PubMed] [Google Scholar]
- 16.Daniel RA, Rozanska AL, Thomas HD, Mulligan EA, Drew Y, Castelbuono DJ, et al. Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity against childhood neuroblastoma. Clin Cancer Res. 2009;15:1241–9. doi: 10.1158/1078-0432.CCR-08-1095. [DOI] [PubMed] [Google Scholar]
- 17.Hecht SM. Bleomycin: New perspectives on the mechanism of action. J Nat Products. 1999;63:158–68. doi: 10.1021/np990549f. [DOI] [PubMed] [Google Scholar]
- 18.Horton JK, Baker A, Vande Berg BJ, Sobol RW, Wilson SH. Involvement of DNA polymerase β in protection against the cytotoxicity of oxidative damage. DNA Repair (Amst) 2002;1:317–33. doi: 10.1016/s1568-7864(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 19.Ho TV, Schärer OD. Translesion DNA synthesis polymerases in DNA interstrand crosslink repair. Environ Molec Mutagen. 2010;51:552–66. doi: 10.1002/em.20573. [DOI] [PubMed] [Google Scholar]
- 20.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: Molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–37. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 21.Cavanaugh NA, Beard WA, Wilson SH. DNA polymerase β ribonucleotide discrimination. J Biol Chem. 2010;285:24457–65. doi: 10.1074/jbc.M110.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]