Summary
Acute graft-versus-host disease (GVHD) is a leading cause of non-relapse mortality following allogeneic haematopoietic cell transplantation. Attempts to improve treatment response in clinically-established GVHD have not improved overall survival, often due to the increased risk of infectious complications. Alternative approaches to decrease GVHD-related morbidity and mortality have focused on the ability to predict GVHD prior to clinical manifestation in an effort to provide an opportunity to abort GVHD development, and to gain new insights into GVHD pathophysiology. This review outlines the research efforts to date that have identified clinical and laboratory-based factors that are predictive of acute GVHD and describes future directions in developing algorithms that will improve the ability to predict the development of clinically relevant GVHD.
Keywords: BMT, GVHD, BMT Immunology, Biomarkers, Prediction
Introduction
Allogeneic haematopoietic cell transplantation (HCT) is an increasingly used curative modality for haematological malignancies and other conditions. In recent years, improvements in infection prophylaxis and monitoring, immunosuppressive strategies, DNA-based tissue typing, and supportive care measures have improved outcomes following this procedure. Despite these advances, acute graft-versus-host disease (GVHD) remains a significant barrier to more widespread use of allo-HCT due to the morbidity and mortality from GVHD and complications resulting from its prevention and treatment.
Acute GVHD primarily affects the skin, liver and gastrointestinal (GI) tract, and typically occurs within 2 months of allo-HCT (Ferrara, et al 2009), although it may occur later (Jagasia, et al 2012). GVHD occurs when donor T cells demonstrate immunological intolerance to genetically defined proteins on host cells [Figure 1] (Ferrara, et al 2009). Human leucocyte antigens (HLAs), encoded by the major histocompatibility complex (MHC), are the most influential proteins. Class 1 HLA proteins (A, B and C) are expressed in various densities on almost all nucleated cells, while class 2 molecules (DR, DQ and DP) are primarily expressed on B cells, dendritic cells and monocytes. The likelihood of GVHD is directly related to the degree of HLA disparity between patients and donors, but moderate to severe acute GVHD occurs in roughly 40% of patients receiving HLA-identical grafts (Jagasia, et al 2012). The only proven front-line therapy for GVHD is systemic corticosteroid therapy and, despite multiple clinical trials, no agent has been found that improves overall survival for patients who fail steroid treatment. This lack of progress has been due to lack of efficacy, or in the case of increased GVHD response to treatment, an offset of increased infectious complications as a result of intensified immunosuppression (Deeg 2007).
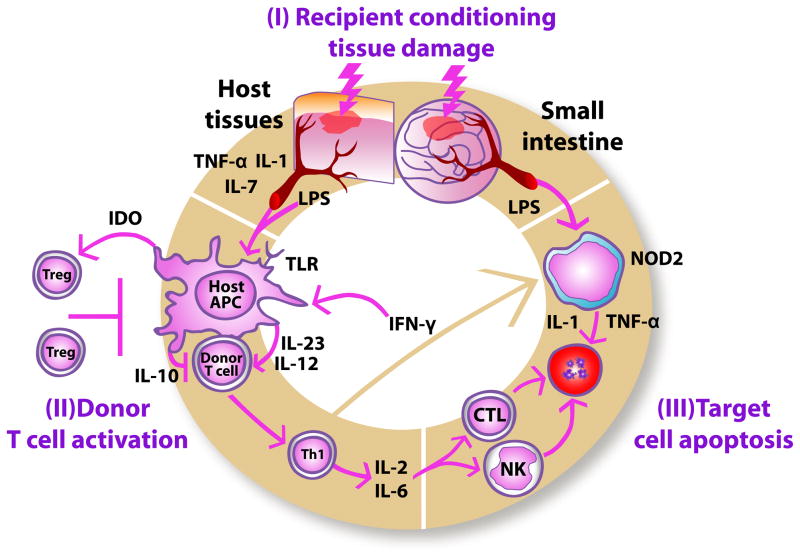
Figure 1.
Pathophysiology of acute graft-versus-host disease. During the first phase (I), recipient conditioning regimen damages patient tissues and causes release of inflammatory cytokines such as TNF-α, IL-1 and IL-7, which leads to activation of host antigen-presenting cells (APCs). In the second phase (II), host APCs activate mature donor cells through IL-12 and IL-23 to produce T helper cell type 1 (Th1) cytokines, such as IL-2, IL-6 and IFN-γ. The synthesis of inflammatory cytokines is partly inhibited by IL-10. Activated Th1 cells induce increased indoleamine 2,3-dioxygenase (IDO) secretion from host APCs through IFN-γ secretion, thus stimulating immunotolerizing Tregs. IFN-γ also stimulates mononuclear cells to secrete inflammatory cytokines, such as IL-1 and TNF-α. (III) Th1 cells promote proliferation and differentiation of activated cytotoxic T lymphocytes (CTLs) and stimulate Natural Killer (NK) cells, which, in turn, induce apoptosis. Lipopolysaccharide (LPS) and bacterial cell wall components that have leaked through the damaged intestinal mucosa stimulate mononuclear cells through interactions with NOD2 and other innate immunity proteins, thus triggering additional inflammatory cytokine production causing apoptosis.
Due to the incidence of GVHD in the setting of HLA-matched allo-HCT and lack of advances in GVHD therapy, many groups have tried to find additional risk factors that contribute to the subsequent development of acute GVHD following allo-HCT. These efforts have identified clinical factors of the patient and donor, as well as pre-transplant conditioning and GVHD prophylaxis strategies that are associated with more frequent GVHD. From a laboratory perspective, investigators have identified non-HLA genetic factors in donors and recipients that correlate with increased risk for GVHD as well as plasma protein patterns in the early post-HCT course that predict subsequent GVHD. Unfortunately, the published efforts have sometimes resulted in contradictory findings. In this review, we focus on studies of large registry cohorts that included multivariate analyses whenever possible and, when not available, studies for which large numbers of consecutive patients were enrolled. When only smaller studies were available, we highlight risk factors which were identified in more than one study. Finally, potentially important risk factors are also identified, even if the findings are not yet conclusive.
Patient and donor characteristics
Several patient characteristics have been identified that increase the risk for development of acute GVHD (Table I). The risk for acute GVHD rises with increasing patient age, (Hahn, et al 2008, Lee, et al 2007, Urbano-Ispizua, et al 2002). This effect is due to uncertain mechanisms, but thymic involution that naturally occurs with age leading to loss of negative selection of host-reactive T-cell clones has been proposed as one mechanism (Storb and Thomas 1985). Animal HCT models suggest that antigen-presenting cells of older recipients have increased allostimulatory activity (Ordemann, et al 2002). In a recently published study of over 5,500 patients, neither patient or donor age was found to contribute to GVHD risk on multivariate analysis, although both were significantly associated with transplant-related mortality (Jagasia, et al 2012).
Table I.
Recipient, Donor and Graft properties influencing aGVHD risk.
| Recipient/Donor Properties | Characteristic | Impact* | Study population (n) | Reference |
|---|---|---|---|---|
| Recipient Age | NSA | Related, Unrelated (5561) | (Jagasia, et al 2012) | |
| >30 years | HR=1.5 [1.4–1.7] | Unrelated (3857) | (Lee, et al 2007) | |
| >42 years | RR=1.7 [1.6–2.5] | Related (315) | (Urbano-Ispizua, et al 2002) | |
| >40 years | HR=1.4 [1.2–1.8] | Related (1960) | (Hahn, et al 2008) | |
|
| ||||
| Indication for transplant | CML | HR=2 [1.1–3.6] | Related (493) | (Remberger, et al 2002) |
| HR=1.4 [1.2–1.6] | Related (1960) | (Hahn, et al 2008) | ||
| OR=1.5 [1.1–2.0] (unrelated donor only) | Related, Unrelated (5561) | (Jagasia, et al 2012) | ||
|
| ||||
| Use of unrelated donor | Use of unrelated donor | 2.6 [1.8–3.7] | Related, Unrelated (1481) | (Stern, et al 2006) |
| RR=2.4 [2.1–2.8] | Related, Unrelated (4566) | (Arora, et al 2009) | ||
| HR=1.7 [1.5–1.8] | Related, Unrelated (2941) | (Flowers, et al 2011) | ||
|
| ||||
| HLA Match | Any mismatch (on best available HLA typing) | HR=2 [1.8–2.2] | Related, Unrelated (2941) | (Flowers, et al 2011) |
| HR=3.1 [1.1–8.6] (4/6 vs. 5–6/6) | Unrelated Cord Blood; Paediatric (88) | (Geyer, et al 2011) | ||
| NSA (4/6 vs. 5/6 vs. 6/6) | Unrelated Cord Blood (265) | (MacMillan, et al 2009) | ||
| Any mismatch at HLA-A, -B, -C, or DRB1 | HR=1.5 (1 mismatch); HR=1.8 (2 mismatches) | Unrelated (1874) | (Flomenberg, et al 2004) | |
| RR=2.6 [2.2–3.2] | Related, Unrelated (4566) | (Arora, et al 2009) | ||
| OR=1.3 [1.1–1.5] (unrelated donor only) | Related, Unrelated (5561) | (Jagasia, et al 2012) | ||
| NSA (Any degree of mismatch) | Unrelated, Cord Blood (803) | (Eapen, et al 2011) | ||
| C antigen mismatch; B allele or antigen mismatch | ↑ aGVHD | Unrelated (1933) | (Woolfrey, et al 2011) | |
| >1 mismatch at HLA-A, -B, -C, -DRB1 or -DQB1 | HR=2.5 [1.4–4.5] (severe GVHD) | Unrelated (334) | (Loiseau, et al 2007) | |
| Any mismatch at HLA-A, -B, -C, DRB1 or DP | HR=1.2 [1.1–1.4] (1 mismatch); | Unrelated (3857) | (Lee, et al 2007) | |
| HR=1.6 [1.5–1.8] (2 mismatches) | ||||
| DQ mismatch | NSA | Unrelated (1933) | (Woolfrey, et al 2011) | |
| DQ or DP mismatch | NSA [0.85–1.26] | Unrelated (1874) | (Flomenberg, et al 2004) | |
| DP mismatch, when adjusted for other mismatch | HR=1.3 [1.1–1.5] | Unrelated (5929) | (Shaw, et al 2007) | |
|
| ||||
| ABO Blood Group | Minor ABO mismatch | HR=2.9 [1.4–6.0] | Unrelated (154) | (Ludajic, et al 2009) |
| ABO incompatibility | NSA | Related, Unrelated (1856) | (Wang, et al 2010) | |
| NSA | Related, Unrelated (5561) | (Jagasia, et al 2012) | ||
|
| ||||
| Gender Disparity | Female donor to male recipient | RR=1.3 [1.02–1.7] | Related, Unrelated (1481) | (Stern, et al 2006) |
| HR=1.1 [1.04–1.2] | Related, Unrelated (2941) | (Flowers, et al 2011) | ||
| Alloimmunized female donor to male recipient | HR=3.6 [1.4–9.0] | Related, Unrelated; Reduced Intensity (111) | (Remberger, et al 2008) | |
|
| ||||
| Graft Properties | ||||
| Stem cell source | PBSC or BM use | NSA | Unrelated (513) | (Anasetti, et al 2011) |
| NSA | Related, Unrelated (2941) | (Flowers, et al 2011) | ||
| Use of umbilical cord blood | ||||
| Matched related cord blood vs. matched related marrow | RR=0.4 [0.2–0.7] | Related; Cord blood (2165) | (Rocha, et al 2000) | |
| Compared to matched unrelated marrow |
HR=0.45 [0.2–0.96] (Matched cord blood) NSA [0.4–1.3] (1–2 Mismatched cord) |
Unrelated, Cord Blood; Paediatric (785) | (Eapen, et al 2007) | |
| Compared to matched marrow/mismatched marrow |
NSA [0.6–1.1] (vs. matched marrow) HR=0.7 [0.4–0.99] (vs. mismatched marrow) |
Unrelated, Cord Blood (600) | (Laughlin, et al 2004) | |
| 2 vs. 1 cord blood unit | RR=2 [1.2–3.4] | Unrelated Cord Blood (265) | (MacMillan, et al 2009) | |
| Use of haplo-identical donor | ||||
| Compared to mismatched unrelated donor | NSA | Haplo-identical, Mismatched Unrelated (35) | (Cho, et al 2012) | |
| Compared to matched related, matched unrelated donors | NSA | Related, Unrelated, Haplo-identical (90) | (Burroughs, et al 2008) | |
|
| ||||
| Stem cell dose | High CD34+ cell dose | HR=2.6 [1.3–5.3] | Related (315) | (Urbano-Ispizua, et al 2002) |
| CD34+ cell dose | NSA | Unrelated (932) | (Pulsipher, et al 2009) | |
|
| ||||
| Treg | Low Treg content in graft | ↑ aGVHD | Related (32) | (Rezvani, et al 2006) |
| RR=5 [1.9–16.7] | Related (58) | (Wolf, et al 2007) | ||
|
| ||||
| Conditioning/Post-transplant management | ||||
| Conditioning intensity | Use of Reduced Intensity Conditioning | HR=0.3 [0.1–0.7] | Related (137) | (Couriel, et al 2004b) |
| OR=0.09 [0.01–0.6] | Related (110) | (Mlynarczewska, et al 2004) | ||
| ↓ aGVHD | Related, Unrelated (5561) | (Jagasia, et al 2012) | ||
| HR=0.2 [0.1–0.5] | Unrelated Cord Blood; Paediatric (88) | (Geyer, et al 2011) | ||
|
| ||||
| Conditioning-related Toxicity | No oral intake for >9 days | OR=7.7 [1.4–40.7] (severe GVHD) | Related, Unrelated (231) | (Mattsson, et al 2006) |
| Presence of diarrhoea (>5.5 days duration, >7.94 ml/kg mean (days -3 to 0), or >8.94 ml/kg max) | OR=6–12 | Related (101) | (Liu, et al 2010) | |
|
| ||||
| TBI use in conditioning | Use of TBI | HR=1.4 [1.2–1.7] | Related (1960) | (Hahn, et al 2008) |
| HR=1.5 [1.3–1.5] | Related, Unrelated (2941) | (Flowers, et al 2011) | ||
|
| ||||
| GVHD prophylaxis | Single agent GVHD prophylaxis | OR=2.5 [1.3–4.8] | Related (493) | (Remberger, et al 2002) |
| Use of calcineurin inhibitor monotherapy | OR=2 [1.1–3.7] | Related, Unrelated (304) | (Wermke, et al 2010) | |
| Use of Tacrolimus in GVHD prophylaxis | OR=0.6 [0.5–0.9] (related donor) OR=0.8 [0.7–0.9] (unrelated donor) |
Related, Unrelated (5561) | (Jagasia, et al 2012) | |
| Use of Tacrolimus and methotrexate (compared to cyclosporine and methotrexate) | RR=0.6 | Related (329) | (Ratanatharathorn, et al 1998) | |
| ↓ aGVHD | Unrelated (180) | (Nash, et al 2000) | ||
| In vivo T-cell depletion | ||||
| With anti-thymocyte globulin | HR=0.2 [0.1–0.4] | Unrelated; Reduced Intensity (111) | (Remberger, et al 2008) | |
| RR=0.5 [0.3–0.9] | Unrelated Cord Blood (265) | (MacMillan, et al 2009) | ||
| OR=0.3 [0.2–0.6] | Related, Unrelated (304) | (Wermke, et al 2010) | ||
| NSA [0.7–1.04] | Related, Unrelated (1676) | (Soiffer, et al 2011) | ||
| With alemtuzumab |
HR=0.4 [0.3–0.6] (vs. T-cell replete) HR=0.4 [0.3–0.5] (vs. ATG) |
Related, Unrelated (1676) | (Soiffer, et al 2011) | |
| NSA (vs. ATG) | Related, Unrelated (108) | (Norlin and Remberger 2011) | ||
Relative risk (RR), hazard ratio (HR) or odds ratio (OR) provided when reported, otherwise increased or decreased risk is reported. Confidence intervals, when provided, are in brackets.
aGVHD - acute graft-versus-host disease; TBI – Total body irradiation; CML – Chronic myeloid leukaemia; ATG – antithymocyte globulin; NSA – No significant association; RR – Relative risk; HR – Hazard ratio; OR – Odds ratio
The underlying diagnosis for which a patient is receiving allo-HCT has also been identified as a risk factor; receiving allo-HCT for chronic myeloid leukaemia (CML) was found to convey increased risk for GVHD when compared to other diseases on multivariate analysis in large studies (Hahn, et al 2008, Jagasia, et al 2012, Remberger, et al 2002). CML is associated with increased serum concentrations of tumour necrosis factor α (TNF-α), which may predispose patients to the development of GVHD (Hahn, et al 2008).
Related donors are more likely to share minor histocompatibility antigens, and therefore should experience less alloreactivity. Thus, it is not surprising that HCT from an unrelated donor has been shown to increase the risk for acute GVHD (Arora, et al 2009, Flowers, et al 2011, Stern, et al 2006). Especially convincing, Flowers et al (2011) reported risk factors for GVHD in 2941 patients receiving allogeneic HCT from related (n=1554) or unrelated (n=1387) donors. On multivariate analysis, the use of an unrelated donor (Hazard Ratio [HR]=1.66), HLA-mismatched donor (HR=1.74), the use of total body irradiation (TBI) in the conditioning regimen (HR=1.49), or a female donor for a male recipient (HR=1.14) were all associated with increased risk for GVHD, while patient age and the use of mobilized peripheral blood stem cells did not.
The use of an HLA-mismatched donor is widely accepted to increase the risk of acute GVHD; four reports on large patient cohorts confirm that anything less than an 8/8 matched donor (at A, B, C and DRB1 loci) conveys increased risk for GVHD in the absence of T-cell depletion (Arora, et al 2009, Flowers, et al 2011, Jagasia, et al 2012, Lee, et al 2007). When investigating which HLA-antigens contributed the greatest risk for acute GVHD, a study of 1933 patients determined increased risk with mismatches of either a C antigen or a B allele/antigen when compared to donors matched at A, B, C and DRB1 loci (Woolfrey, et al 2011). The effect of DP and DQ loci mismatches on GVHD risk is unclear. Some studies show no increased GVHD risk for DQ mismatches in multivariate analysis (Flomenberg, et al 2004, Lee, et al 2007, Woolfrey, et al 2011). In another study, HLA-DQ mismatch impacted GVHD risk only if another HLA mismatch existed (Loiseau, et al 2007). Likewise, conflicting results have been seen for DP mismatches (Flomenberg, et al 2004, Lee, et al 2007, Shaw, et al 2007). The impact of HLA-matching in the setting of umbilical cord blood transplantation on GVHD risk has not been clear-cut. The risk of GVHD when using 5/6 HLA-matched umbilical cord blood units is comparable to 6/6 matched, but reports have varied on whether the use of a 4/6 matched graft increases risk (Geyer, et al 2011, MacMillan, et al 2009). A large registry study of 803 children found that HLA-disparity did not appear to impact on acute GVHD risk, although increasing disparity (two antigen mismatch vs. one antigen mismatch vs. no mismatches), especially mismatches at HLA-C, was associated with increased transplant-related mortality (Eapen, et al 2011).
ABO antigens are expressed on a wide variety of tissues and it is common for naturally-occurring isoagglutinins to develop; thus it has been speculated that ABO antigen disparity between donor and patient may lead antibody-mediated host tissue damage causing the release of inflammatory cytokines and further immune trafficking. Minor ABO incompatibility was shown to increase the risk for acute GVHD [HR=2.92] (Ludajic, et al 2009), but this association was not confirmed in larger studies (Jagasia, et al 2012, Wang, et al 2010).
Multiple reports have shown that the selection of a female donor for a male patient increases GVHD risk [HR 1.33–1.91] (Flowers, et al 2011, Stern, et al 2006), particularly when the female is allo-immunized as a result of previous pregnancy [HR=3.55] (Remberger, et al 2008). These findings may be explained by minor histocompatibility antigens encoded on the Y chromosome (H-Y antigens) whose presence in male donors may be recognized by grafts from female donors and cause alloreactivity (Miklos, et al 2004).
Graft Properties
Graft properties have also been identified as risk factors for the development of acute GVHD (Table I). The selection of peripheral blood stem cells did not impact the incidence of GVHD in a phase III, prospective, randomized clinical trial in unrelated donors (Anasetti, et al 2011), or in a large, single centre study (Flowers, et al 2011).
When suitably HLA-matched donors are unavailable, HCT may be performed using grafts from alternative donors (umbilical cord blood units, haplo-identical related donors), although these alternative donor sources provide unique clinical challenges (Anasetti, et al 2012). Cord blood transplantation is associated with delayed cell count recovery and increased risk of graft failure, which subsequently places patients at risk for haemorrhage and/or life-threatening infection. Either in vivo/ex vivo T-cell depletion or aggressive immunosuppressive GVHD prophylaxis strategies are used to minimize the risk associated with the HLA-disparity inherent to haplo-identical transplantation. These strategies predispose recipients to delayed immune reconstitution, thus increasing risk of infection and relapse of underlying malignancy. Large studies (n=2165 and 785, respectively) have demonstrated decreased risk of GVHD when using related donor HLA-matched cord blood compared to HLA-matched marrow grafts from either related or unrelated donors (Eapen, et al 2007, Rocha, et al 2000). Likewise, unrelated donor cord blood units that are 4–6/6 HLA-matched provide comparable GVHD risk to matched unrelated donor grafts (Eapen, et al 2007, Laughlin, et al 2004). These findings highlight that when HLA-matching is similar, cord blood transplants confer less GVHD risk compared to bone marrow or peripheral blood as a stem source. Furthermore, the risk of GVHD is comparable for HLA-mismatched cord blood units compared to matched bone marrow or peripheral blood transplants. The predominance of naïve T-cells in cord blood grafts may explain these findings. However, when two unrelated umbilical cord blood units are transplanted simultaneously to overcome risk of graft rejection, the risk of GVHD is elevated compared to single cord unit grafts (MacMillan, et al 2009).
Provided that intensive GVHD prophylaxis is given, haplo-identical marrow transplantation strategies result in comparable risk of GVHD with standard graft transplantation (Burroughs, et al 2008, Cho, et al 2012). However, no prospective studies have been reported that directly compare GVHD risk between umbilical cord blood and haplo-identical transplantation.
The immunophenotypic makeup of the graft may also influence GVHD risk. High CD34+ stem cell dose was reported to increase the risk of GVHD in related donors (Urbano-Ispizua, et al 2002), although a larger study found no association in unrelated donors (Pulsipher, et al 2009). Regulatory T-cells (Treg) are a subset of T lymphocytes that suppress immune activation, thus reducing alloreactivity. High graft Treg content has been shown to decrease the risk of acute GVHD (Rezvani, et al 2006, Wolf, et al 2007). Initial trials of Treg enrichment/expansion to prevent GVHD have been promising and further research efforts are on-going (Hippen, et al 2011).
Conditioning/Post-HCT Management
Research efforts have tried to identify transplant conditioning and GVHD prophylaxis strategies that can decrease GVHD risk (Table I). GI tract damage plays an important role in GVHD pathophysiology (Hill and Ferrara 2000). Conditioning-associated damage of the GI mucosa allows increased translocation of inflammatory stimuli, such as lipopolysaccharide, an endotoxin present in the normal bacterial flora of the GI tract, which promotes the release of inflammatory cytokines and further damage to the GI tract (Figure 1). This may partly explain repeated findings with different graft sources of decreased GVHD incidence associated with the use of reduced-intensity conditioning regimens that result in lowered conditioning-related GI damage (Couriel, et al 2004a, Geyer, et al 2011, Jagasia, et al 2012, Mlynarczewska, et al 2004). The increased risk for GVHD in patients experiencing more GI damage has been supported by clinical observations of higher incidences of GVHD in patients who experienced prolonged periods without oral intake [HR 7.7, p=0.02] (Mattsson, et al 2006), or severe diarrhoea in the early post-transplant period [39% vs. 6%, p<0.001] (Liu, et al 2010). Likewise, the inclusion of TBI in the conditioning regimen has reproducibly increased the risk of acute GVHD (Flowers, et al 2011, Hahn, et al 2008, Jagasia, et al 2012), which suggests that damage caused by radiation may be immunologically-recognized differently from that of myeloablative chemotherapy.
Several studies have investigated GVHD prophylaxis strategies and development of acute GVHD. Single-agent GVHD prophylaxis with either cyclosporine or methotrexate was shown to increase risk of GVHD over multi-agent prophylaxis (Remberger, et al 2002, Wermke, et al 2010). The use of tacrolimus in the prophylaxis regimen was shown to decrease the risk for acute GVHD (Jagasia, et al 2012), particularly when used in combination with methotrexate (Nash, et al 2000, Ratanatharathorn, et al 1998). Ratanathorathorn et al (1998) and Nash et al (2000) confirmed the superior efficacy of GVHD prophylaxis using tacrolimus and methotrexate when compared to cyclosporine and methotrexate in phase III randomized clinical trials for related and unrelated donor HCT, respectively.
In vivo T-cell depletion reduces GVHD risk in a number of transplant settings (MacMillan, et al 2009, Remberger, et al 2008, Wermke, et al 2010), which is unsurprising given the central role T lymphocytes play in GVHD physiology (Figure 1). This strategy is not universally implemented however, due to increased risk for complications, such as Epstein-Barr virus post-transplant lymphoproliferative disorder (PTLD), infection and relapse (Soiffer, et al 2011). The efficacy of the anti-CD52 antibody, alemtuzumab, which targets lymphocytes, monocytes and dendritic cells, in reducing GVHD beyond that afforded by anti-thymocyte globulin is not clear. GVHD rates were comparable when receiving alemtuzumab or anti-thymocyte globulin in one study (Norlin and Remberger 2011). By contrast, a large registry-based study found a significant reduction in GVHD incidence with alemtuzumab use when compared to transplantation with T-replete grafts or use of anti-thymocyte globulin, and no decreased GVHD risk after the use of anti-thymocyte globulin when compared to T-replete graft transplantation (Soiffer, et al 2011).
Genetic Factors
There have been many reports identifying genetic predisposition for acute GVHD in both recipients and donors (Table II). Most of these studies have focused on fully HLA-matched donor/patient pairs to remove the known acute GVHD risk associated with HLA-mismatched HCT. As one might expect, a majority of the identified genetic polymorphisms are within genes encoding for innate immunity or inflammatory/immunoregulatory proteins.
Table II.
Genetic polymorphisms influencing aGVHD risk.
| Gene | Polymorphism | Impact* | Study population (n) | Reference |
|---|---|---|---|---|
| IFNG | IFNG Intron genotype | NSA | Related (100) | (Socie, et al 2001) |
| Recipient IFNG Intron 3/3 | OR=3.9 [1.2–12.4] | Related (80) | (Cavet, et al 2001) | |
| ↑ aGVHD | Related(88) | (Middleton, et al 2002) | ||
| Recipient IFNG Intron 2/2 | OR=0.2 [0.05–0.99] | Related (110) | (Mlynarczewska, et al 2004) | |
|
| ||||
| IL1A | −889(C/T) | NSA | Unrelated (90) | (MacMillan, et al 2003) |
| NSA | Unrelated (426) | (Mehta, et al 2007) | ||
|
| ||||
| IL1RN | IL1RN*2 | NSA | Related (570) | (Lin, et al 2003) |
| NSA | Unrelated (90) | (MacMillan, et al 2003) | ||
| Absence of donor IL1RN*2 | OR=6.2 | Related (99) | (Cullup, et al 2001) | |
| HR=2.1 [1.1–3.9] | Related (107) | (Rocha, et al 2002) | ||
|
| ||||
| IL1B | −511(C/T) | NSA | Related (570) | (Lin, et al 2003) |
| NSA | Unrelated (90) | (MacMillan, et al 2003) | ||
| +3953(C/T) | NSA | Related (77) | (Bertinetto, et al 2006) | |
| +3954(C/T) | NSA | Related (570) | (Lin, et al 2003) | |
|
| ||||
| IL6 | −174(C/G) | NSA | Related (570) | (Lin, et al 2003) |
| Recipient −174(G/G) | HR=4.1 | Related (108) | (Middleton, et al 2003) | |
| OR=2.2 [1.1–4.5] | Related, Unrelated (166) | (Ambruzova, et al 2009) | ||
| Donor −174(G/*) | HR=4.3 [1.5–12.1] | Related (160) | (Mullighan, et al 2004) | |
| Donor −174(G/G) | OR=3.9 [1.1–14.0] | Related (93) | (Karabon, et al 2005) | |
|
| ||||
| IL10 | −1064: Increased dinucleotide repeats (Alleles 12–15) | |||
| Recipient with Alleles 12–15 | OR=4.6 [1.2–17.8] | Related (80) | (Cavet, et al 2001) | |
| ↑ aGVHD | Related (88) | (Middleton, et al 2002) | ||
| Recipient homozygous for allele 13 | ↑ aGVHD | Related, Unrelated (196) | (Nordlander, et al 2002) | |
| −1082(A/G), −819(C/T), −592(A/C) Haplotype | NSA | Related (160) | (Mullighan, et al 2004) | |
| Recipient (GCC/GCC) | OR=0.09 [0.01–0.96] | Related (93) | (Karabon, et al 2005) | |
| HR=0.2 [0.06–0.7] (severe GVHD) | Related; Paediatric (57) | (Goussetis, et al 2011) | ||
| RR=7.9 [2.9–21.5] | Related (100) | (Socie, et al 2001) | ||
| Donor (GCC/GCC) | RR=0.3 [0.1–0.8] | Related (100) | (Socie, et al 2001) | |
| Absence of both donor and recipient (GCC) | OR=2.9 [1.1–7.7] | Related (77) | (Bertinetto, et al 2006) | |
|
| ||||
| IL23R | 1142A>G | |||
| Donor 1142(G/*) | RR=0.5 [0.3–0.97] | Related, Unrelated (407) | (Elmaagacli, et al 2008) | |
| ↓ aGVHD | Related, Unrelated, Haplo-identical; Paediatric (231) | (Gruhn, et al 2009) | ||
| OR=0.4 [0.2–0.95] | Related, Unrelated (304) | (Wermke, et al 2010) | ||
| variant SNPs | NSA [0.5–2.3] | Unrelated (390) | (Nguyen, et al 2010) | |
|
| ||||
| NOD2 | SNPs 8,12,13 | NSA | Related, Unrelated (198) | (Sairafi, et al 2008) |
| NSA | Related, Unrelated, Haplo-identical; Paediatric (231) | (Gruhn, et al 2009) | ||
| NSA [0.6–2.3] | Unrelated (390) | (Nguyen, et al 2010) | ||
| NSA [0.4–1.5] | Related, Unrelated (304) | (Wermke, et al 2010) | ||
| Donor only SNPs | RR=0.003 [0.002–0.007] (severe GVHD) | Related, Unrelated (403) | (Elmaagacli, et al 2006) | |
| Simultaneous SNPs in donor and recipient: | RR=4.3 [2.1–9.3] (severe GVHD) | Related, Unrelated (403) | (Elmaagacli, et al 2006) | |
| 1+ variant in either donor or recipient | ↑ aGVHD | Related, Unrelated (700) | (Holler, et al 2008) | |
| Donor SNP13 | ↑ aGVHD (unrelated only) | Related, Unrelated (700) | (Holler, et al 2008) | |
|
| ||||
| TNF | TNFd3 | NSA | Related (100) | (Socie, et al 2001) |
| NSA | Related, Unrelated (196) | (Nordlander, et al 2002) | ||
| NSA | Unrelated, Paediatric (180) | (Goyal, et al 2010) | ||
| Recipient TNF d3/d3 | ↑ aGVHD | Related (49) | (Middleton, et al 1998) | |
| OR=3.3 [1.1–10.2] | Related (80) | (Cavet, et al 2001) | ||
| TNFd4 | ||||
| Recipient TNF d4/d4 | HR=2.3 (severe GVHD) | Unrelated, Paediatric (180) | (Goyal, et al 2010) | |
| Recipient TNFd4 | 4.3 [1.3–14.6] (related only) | Related, Unrelated (196) | (Nordlander, et al 2002) | |
| −308(A/G) | NSA | Related (100) | (Socie, et al 2001) | |
| NSA | Related (107) | (Rocha, et al 2002) | ||
| NSA | Related, Unrelated (196) | (Nordlander, et al 2002) | ||
| NSA | Related (570) | (Lin, et al 2003) | ||
| NSA | Related (49) | (Middleton, et al 1998) | ||
|
| ||||
| VDR | ApaI(A/a) | |||
| Recipient ApaI(a/*) | ↑ aGVHD | Related (88) | (Middleton, et al 2002) | |
| Donor ApaI(A/A) | OR=7.2 [1.6–32.1] | Related, Unrelated, Haplo-identical (123) | (Bogunia-Kubik, et al 2008) | |
| Taql(T/t) | ||||
| Recipient TaqI(T/*) | HR=2.6 [1.2–5.6] (severe GVHD) | Related (107) | (Rocha, et al 2009) | |
| FokI(F/f) | ||||
| Donor FokI(F/F) | OR=4.5 [1.2–16.5] | Related, Unrelated, Haplo-identical (123) | (Bogunia-Kubik, et al 2008) | |
Relative risk (RR), hazard ratio (HR) or odds ratio (OR) provided when reported, otherwise increased or decreased risk is reported. Confidence intervals, when provided, are in brackets.
aGVHD - acute graft-versus-host disease; NSA – No significant association; SNP – Single nucleotide polymorphism; RR – Relative risk; HR – Hazard ratio; OR – Odds ratio
Innate Immunity
One of the most frequently studied genetic predictors of acute GVHD is nucleotide-binding oligomerization domain containing protein 2 (NOD2), which encodes an intracellular receptor (NOD2) that binds bacterial cell wall products resulting in upregulated nuclear factor kappa B (NFκB) activation (Figure 1). Several NOD2 variants, which can be identified by the presence of single nucleotide polymorphisms (SNPs), result in a decreased capacity to release inflammatory cytokines in response to bacterial wall products. The presence of donor and/or recipient NOD2 variants (SNPs 8, 12 and 13) were originally identified as risk factors for GVHD (Holler, et al 2004). Some groups have reported that the presence of NOD2 variants in either donors, recipients, or both, confers increased risk of GVHD (Elmaagacli, et al 2006, Holler, et al 2008), while other groups have not been able to replicate these findings (Gruhn, et al 2009, Nguyen, et al 2010, Sairafi, et al 2008, Wermke, et al 2010). The difference may be due to variability in patient populations or differences in HCT strategies, such as the use of in vivo T-cell depletion, gut decontamination, or less toxic conditioning regimens decreasing the extent of GI damage (van der Velden, et al 2011).
Inflammatory/Immunoregulatory Genes
The role of pro-inflammatory (e.g. interferon-gamma [IFN-γ], TNF, interleukin-6 [ILIL6], etc.) and anti-inflammatory proteins is well established in GVHD pathophysiology (Figure 1). Several groups have investigated how genetic polymorphisms that modulate activity and/or expression of these proteins influence GVHD risk.
Different polymorphisms of interleukin-10 (IL-10, IL10), a protein that inhibits the synthesis of inflammatory cytokines and polarizes lymphocytes to a T-helper cell type 2 (Th2) response, have been investigated as genetic risk factors for acute GVHD. The number of dinucleotide repeats in the upstream regulatory region of the IL10 gene affect IL-10 production, with patients possessing larger number of dinucleotide repeats (denoted as alleles 12–15) producing less IL-10. Recipients possessing low IL-10 producing genotypes have been found to have increased GVHD incidence following HCT (Cavet, et al 2001, Middleton, et al 2002, Nordlander, et al 2002). The influence of IL-10 promoter polymorphisms on GVHD risk, primarily -1082(A/G), -819(C/T) and -582 (A/C), has also been investigated. Three common haplotypes represent high (GCC), intermediate (ATA) and low (ACC) IL-10 production (Suarez, et al 2003). As one might expect, the presence of the high-producing (GCC) haplotype in HCT recipients correlated with decreased risk for aGVHD (Goussetis, et al 2011, Karabon, et al 2005). Contrary results were reported in one large study, however, that found the presence of recipient or absence of donor GCC haplotype increased risk for GVHD (Socie, et al 2001). Another study found no association between -1082, -819,-582 haplotype and GVHD risk (Mullighan, et al 2004).
TNF-α is an inflammatory cytokine that is involved in GVHD pathology (Figure 1). TNFd is a dinucleotide repeat sequence that is positioned downstream of the TNF gene for which different alleles have been found to alter TNF-α production. The TNF d3/d3 and d4/d4 genotypes are associated with increased TNF-α production compared to other polymorphisms. Patients who carry the d3/d3 genotype showed increased rates of GVHD in some (Cavet, et al 2001, Middleton, et al 1998), but not other studies. An increased GVHD risk was found in patients with the d4/d4 – but not d3/d3 – genotype (Goyal, et al 2010, Nordlander, et al 2002). The two studies that found a positive correlation between TNF d3/d3 and GVHD did not report findings for the d4/d4 genotypes. Despite multiple attempts, no association has been found between the polymorphism in the TNF promoter region at position -308, also associated with increased TNF-α production, and GVHD risk (Lin, et al 2003, Middleton, et al 1998, Nordlander, et al 2002, Rocha, et al 2002, Socie, et al 2001).
Polymorphisms of genes related to interleukin-1 (IL-1), IL1A, IL1B and IL1RN, have been examined for their role in acute GVHD due to the role of IL-1 in promoting GVHD pathophysiology (Figure 1). Polymorphisms of IL1A and IL1B, which encode for the IL-1α (IL1A) and IL-1β (IL1B) members of the IL-1 cytokine family, have not been associated with acute GVHD risk (Bertinetto, et al 2006, Lin, et al 2003, MacMillan, et al 2003, Mehta, et al 2007). IL1RN encodes for IL-1Ra (IL1RN), an IL-1 antagonist that competitively binds the IL-1 receptor. Allele 2 of IL1RN (IL1RN*2) is associated with increased plasma concentrations of IL-1Ra (Hurme and Santtila 1998), and was predicted to decrease GVHD incidence. The absence of donor IL1RN*2 (Cullup, et al 2001, Rocha, et al 2002), or presence of patient IL1RN*2 (Bertinetto, et al 2006) increased rates of aGVHD in some studies, although others reported no significant association (Lin, et al 2003, MacMillan, et al 2003).
Several studies have investigated interferon-gamma (IFNG) polymorphisms and their influence on GVHD. IFNG*2 is associated with a thymine at position +874 and 12 dinucleotide repeats, while alleles 3, 4, and 5 all have an adenine at +874 but possess different numbers of dinucleotide repeats(Pravica, et al 2000). IFNG*2 is associated with higher production of IFN-γ than other alleles. IFNG*3 in the recipient may increase the likelihood of aGVHD (Cavet, et al 2001, Middleton, et al 2002), while IFNG*2 may be protective (Mlynarczewska, et al 2004), although one large study did not find significant correlation between either donor or recipient IFNG +874(T/A) polymorphisms and GVHD risk (Socie, et al 2001). The association of high-IFN-γ producing phenotype with lower GVHD risk suggests that IFN-γ production in the setting of allo-HCT may have a more potent immunotolerizing effect, perhaps through induction of indoleamine 2,3-dioxygenase (IDO) secretion by host antigen-presenting cells, rather than a proinflammatory effect on mononuclear cells as expected when IFN-γ is present in high concentrations (Figure 1).
Possession of the IL6 polymorphism containing the G allele at IL6 -174 is associated with increased serum levels of the proinflammatory cytokine IL6 in normal individuals. Researchers have found that recipients (Ambruzova, et al 2009, Middleton, et al 2003) or donors (Karabon, et al 2005, Mullighan, et al 2004) homozygous for IL6 -174(G/G) correlated with higher rates of GVHD compared to non-homozygous pairs, although no association was found in a large, independent study (Lin, et al 2003).
The interleukin-23 receptor (IL-23R, IL23R) is present on many immune effector cells which, when bound by IL-23 activates inflammatory signalling through the JAK/STAT pathway leading to T-cell activation and proliferation. IL-23 has been implicated in many autoimmune conditions(Duvallet, et al 2011). An IL23R SNP at position 1142 where an adenine is substituted with a guanine (1142G>A) has recently been found to lead to selective loss of function on T cells, rendering them insensitive to IL-23 (Sarin, et al 2011). This polymorphism in the donor correlated with a lower incidence of acute GVHD (Elmaagacli, et al 2008, Gruhn, et al 2009, Wermke, et al 2010), however one study found no association of this SNP with GVHD risk (Nguyen, et al 2010).
Allelic variation of the vitamin D receptor (VDR) has also been investigated for its impact on the development of acute GVHD. Vitamin D has been shown to polarize T-cell populations towards Th2 cytokine production, blunt T-cell proliferation in response to dendritic cell activation, and to increase IDO expression leading to improved immune tolerance through Treg stimulation [Figure 1] (Rosenblatt, et al 2010). Genetic polymorphisms have been identified that affect vitamin D receptor transcription and/or activity, including alleles of the ApaI and TaqI sites, located in intron 8 and 9, respectively, which influence vitamin D receptor activity. The “A” allele of ApaI (as compared to the “a” allele) and the “t” allele of TaqI (compared to the “T” allele) are associated with higher vitamin D activity as evidenced by higher levels of vitamin D receptor responsive gene products such as osteocalcin (Uitterlinden, et al 2004). Transplant recipients with at least one “a” or “T” allele, ApaI(a/*), or TaqI(T/*), which both correspond to lower vitamin D receptor activity, have been shown be at increased risk for aGVHD (Middleton, et al 2002, Rocha, et al 2009). Donor ApaI(A/A) or FokI(F/F) has also been reported to increase risk for GVHD on multivariate analysis (Bogunia-Kubik, et al 2008). The “F” allele of FokI produces a shorter vitamin D receptor protein than the “f” allele, (424 versus 427 amino acids in length), and the shorter protein encoded by the “F” allele has been shown to be the more active vitamin D receptor variant. It is not clearly understood why increased acute GVHD risk might be associated with decreased vitamin D receptor activity polymorphisms in the recipient and conversely, with higher activity polymorphisms in the donor, but this finding may possibly be explained by differences in vitamin D effect on recipient tissues and donor alloreactive T cells.
In summary, numerous reports have identified genetic polymorphisms that correlate with increased risk for acute GVHD in large patient cohorts, but these findings are often contradictory or could not be replicated. One explanation for the inconsistent findings may be that a biased approach was commonly used in that small numbers of selected genes were evaluated. It is likely that multiple genetic factors that impact risk of GVHD are present in any given patient. The distribution of these factors may also vary in different patient populations. Large-scale, whole genome approaches are needed to better understand the interplay across different genetic factors that impact GVHD risk. Currently, genetic risk factors are not routinely evaluated prior to transplantation, although advances in technology may make genotyping readily available and cost effective. Once available, however, there may be barriers to utilizing high-risk polymorphisms to select donors due to limited availability of fully HLA-matched donors, which is the most important genetic factor to use in donor selection. More likely, genetic factors will be used to risk stratify patients and guide GVHD prevention strategies.
Plasma Proteins
Several groups have tried to identify plasma protein profiles in the pre- and early post-HCT time period that are predictive for subsequent development of acute GVHD (Table III). While pre-HCT characteristics and genetic polymorphisms may help to identify which patients have an increased risk for acute GVHD, they do not identify individual patients in whom the alloreaction that will culminate in GVHD is underway. Changes in plasma proteins associated with inflammation/alloreactivity or markers of target organ damage (GVHD biomarkers) in the early post-transplant period may offer a look into the earliest stages of GVHD pathophysiology prior to clinical manifestations, thus providing clinicians with a potential window to intervene and abort the GVHD process.
Table III.
Post-HCT protein concentrations influencing aGVHD risk.
| Plasma Protein | Characteristic | Impact | Study population (n) | Reference |
|---|---|---|---|---|
| CD8 | CD8 >129 u/ml at day 15 | ↑ aGVHD | Related, Unrelated (61) | (August, et al 2011) |
|
| ||||
| CD40L | Plasma concentrations post-transplant | NSA | Related, Unrelated (61) | (Ambruzova, et al 2009) |
|
| ||||
| IL2R | IL2R >22 ng/ml at day 15 | ↑ aGVHD | Related, Unrelated (61) | (August, et al 2011) |
|
| ||||
| IL6 | Increased IL6 levels from day +1 until day +21 | ↑ aGVHD | Related(101) | (Liu, et al 2010) |
| Plasma IL6 concentrations during conditioning | NSA | Related, Unrelated (112) | (Remberger, et al 1995) | |
|
| ||||
| IL7 | Increased day 7 and/or day 14 IL7 | ↑ aGVHD | Related (31) | (Dean, et al 2008) |
| Day 14 IL7 >11.9 pg/ml | ↑ aGVHD | Related, Unrelated; Full intensity (40) | (Thiant, et al 2010) | |
| Day 30 IL7 >5.9 pg/ml | ↑ aGVHD | Related, Unrelated; Reduced intensity (45) | (Thiant, et al 2011) | |
|
| ||||
| sBAFF | sBaff >43 pg/ml prior to conditioning and at days 0, 7 and 14 | ↓ aGVHD | Related, Unrelated; Full intensity (45) | (Cho, et al 2010) |
|
| ||||
| TNFα | Higher TNFα levels during conditioning | ↑ aGVHD | Related, Unrelated (112) | (Remberger, et al 1995) |
| Increased TNFα transcription | ↑ aGVHD; | Related, Unrelated (23) | (Ritchie, et al 2005) | |
| Day 7/Baseline TNFR1 ratio >2.5 | HR=2.4 | Related, Unrelated; Full intensity (438) | (Choi, et al 2008) | |
| ↑ aGVHD severity | Related, Unrelated; Full intensity; Paediatric (82) | (Kitko, et al 2008) | ||
| High Day 7/Baseline TNFR1 ratio | ↑ aGVHD | Related, Unrelated; Reduced intensity (106) | (Willems, et al 2010) | |
| TNFR1 >1040 pg/ml at day 15 | ↑ aGVHD | Related, Unrelated (61) | (August, et al 2011) | |
| Increased TNFα levels from day +1 until day +21 | ↑ aGVHD | Related (101) | (Liu, et al 2010) | |
Relative risk (RR), hazard ratio (HR) or odds ratio (OR) provided when reported, otherwise increased or decreased risk is reported. Confidence intervals, when provided, are in brackets.
aGVHD - acute graft-versus-host disease; NSA – No significant association; RR – Relative risk; HR – Hazard ratio; OR – Odds ratio
The most frequently reported protein elevated prior to GVHD onset is TNFα, an inflammatory cytokine, with reports showing increased concentrations during HCT conditioning as predictive for post-HCT GVHD (Remberger, et al 1995), and several reports showing elevated Day 7 post-HCT/pre-HCT ratios are predictive for acute GVHD (Choi, et al 2008, Kitko, et al 2008, Willems, et al 2010). Other groups have shown increased TNF transcription (Ritchie, et al 2005), increased concentrations at day 15 post-HCT (August, et al 2011), and increased concentrations in the first 3 weeks post-HCT are predictive of later GVHD (Liu, et al 2010). These findings have led to clinical trials of TNFα blockade as either GVHD prophylaxis (Choi, et al 2012) or treatment in combination with steroid therapy (Couriel, et al 2004b, Levine, et al 2008); these initial studies identified a potential benefit from TNFα blockade. A multicentre trial investigating the efficacy of adding an additional treatment to steroids at the onset of grade 2–4 GVHD (mycophenolate mofetil, etanercept, pentostatin, denilileukin deftitox) did not find additive benefit of etanercept to steroids at GVHD onset, however (Alousi, et al 2009). The findings of the multicentre study should be interpreted with caution due to the allowance of patients who developed grade 2–4 GVHD while on mycophenolate mofetil GVHD prophylaxis to participate on the study and be randomized to non-mycophenolate treatment arms. Patients who had already failed mycophenolate therapy and may be less responsive to treatment were assigned to the non-mycophenolate treatment arms.
Interleukin 7 (IL-7, IL7) is a growth factor that is important for homeostatic proliferation of lymphocytes (Boyman, et al 2012), particularly in periods of severe lymphopenia, such as shortly after HCT, which results in expansion of mature donor lymphocyte populations. Increased IL-7 concentrations at days 7 and 14 post-HCT correlated with later acute GVHD in matched related donor HCT (Dean, et al 2008). The significance of day 14 IL-7 concentrations was validated in patients receiving HCT following myeloablative conditioning (Thiant, et al 2010). Likewise, following reduced intensity conditioning, day 30 IL-7 concentrations correlated with the later onset of GVHD as is often seen in this setting (Thiant, et al 2011).
Other plasma protein/cytokines identified in reports include IL-6 (IL6), with increased concentrations during the first 3 weeks post-HCT predicting later GVHD (Liu, et al 2010), although concentrations during conditioning had no apparent association with subsequent GVHD development (Remberger, et al 1995). Elevated concentrations of 3 proteins found on the surface of cytotoxic T cells, CD40L, CD8 and IL-2R, at day 15 post-HCT have all been associated with subsequent development of acute GVHD (August, et al 2011), but these findings have not been repeated. In addition, increased soluble B-cell activating factor (sBAFF) concentrations in the early post-HCT period have been associated with a decreased risk of developing acute GVHD (Cho, et al 2010). The protective effect of sBAFF may be explained, in part, by BAFF-directed expansion of immunotolerant Tregs, which was described in an animal skin allograft model (Walters, et al 2009).
Diagnostic and prognostic GVHD Biomarkers
Several biomarkers diagnostic for acute GVHD have recently been identified by the University of Michigan Blood and Marrow Transplant group. The initial report identified a panel of 4 biomarkers, IL2Rα, TNFR1, hepatocyte growth factor (HGF) and IL-8 (IL8), that were elevated at the onset of GVHD when compared to plasma samples at similar time points from patients who never developed GVHD (Paczesny, et al 2009). This study did not compare plasma concentrations of patients at GVHD onset with those of patients who had similar symptoms (rash or diarrhoea) that were proven to be from other causes, however, so investigators sought organ-specific biomarkers that could differentiate GVHD rash or diarrhoea from other causes of similar symptoms. Elafin, a protease found in the skin with antimicrobial properties (Verrier, et al 2012), was discovered and validated as a plasma biomarker of skin GVHD through a large-scale, unbiased proteomic approach (Paczesny, et al 2010a). Elevated plasma concentrations of elafin help discriminate GVHD rash from rashes of other causes (e.g. medication effect). Likewise, REG3α, an antibacterial protein found in Paneth cells of the small intestine (Cash, et al 2006), was subsequently identified and validated as a lower GI GVHD biomarker using the same proteomic strategy (Ferrara, et al 2011). Plasma REG3α concentrations help discriminate GVHD from other aetiologies of post-HCT diarrhoea, such as infection and conditioning-related toxicity. REG3α was the first diagnostic GVHD biomarker that predicted treatment response; higher concentrations at the onset of lower GI GVHD correlated with steroid-refractory disease. Interestingly, both target organ-specific biomarkers are innate immunity proteins.
Elevated concentrations of the aforementioned GVHD biomarkers at GVHD onset also correlate with increased non-relapse mortality (NRM). In the REG3α study (Ferrara et al 2011), 3 high-risk parameters were identified at the onset of GVHD diarrhoea that each independently predicted 1-year NRM: onset clinical severity (>1 litre of stool per day and/or bloody stool [stages 2–4] versus <1 litre of stool per day [stage 1]), severity of histological findings on diagnostic biopsy (complete denudation [stage 4] versus less severe damage [stages 1–3]), and high REG3α concentrations (>151 ng/ml versus lower concentrations). Patients with any 2 risk factors present at onset had significantly higher NRM (66%) than those with no or any 1 onset risk factor (25% and 34%, respectively, p<0.001), and patients with all 3 risk factors present had significantly higher 1-year NRM than those with any 2 risk factors (86% versus 66%, p<0.001). If this novel scoring system at the onset of lower GI GVHD is confirmed in additional patients, it may permit better risk stratification of patients with lower GI GVHD, thus identifying patients in whom standard GVHD therapy may be insufficient.
A similar proteomics approach has been taken to identify protein fragments in the urine of post-HCT patients that identified acute GVHD at onset (Weissinger, et al 2007). A 31-peptide pattern appeared to correlate with acute GVHD, but the identity of the components remains largely unreported. The two peptides that were identified were collagen 1-α fragments, and it is unclear how these may relate to GVHD-specific pathophysiology.
Biomarker panels
The association of elevated TNFR1 ratios with subsequent development of GVHD prompted researchers at the University of Michigan to measure diagnostic biomarkers (IL2Rα, TNFR1, and elafin) at day 0, day 7 and day 14 following unrelated donor allogeneic HCT in patients who had not yet developed acute GVHD with the intent of finding biomarker patterns that predicted GVHD. Logistic regression was used to assign individual weights to each biomarker and determine which biomarkers contributed to the most sensitive and specific predictive algorithm. The peak concentrations of IL-2Rα, TNFR1 and elafin were used to categorize patients at high or low risk for acute GVHD. Day 14 concentrations of the same 3 biomarkers were used to identify additional high-risk patients from those initially categorized as low risk and who had not yet developed GVHD. A range of useful specificities and sensitivities were identified. For example, at 75% specificity (false positive rate of 25%), 57% of patients who would later develop GVHD were correctly identified (Paczesny, et al 2010b). These findings need to be replicated in multi-centre studies, but data from the following study suggests that such validation is likely.
Biomarker concentrations were measured following GVHD onset at days 0, 14 and 28 of GVHD therapy for patients enrolled on a multi-centre study to predict key GVHD outcomes, such as day-28 treatment response and day-180 mortality. When combined into a mathematical model assigning individual weights to each biomarker using logistic regression modelling, samples at all 3 time points correlated with clinical response to therapy and mortality (Levine, et al 2012). More importantly, the biomarkers were independent predictors for GVHD outcomes even after adjusting for clinical predictors, such as severity of GVHD at onset, donor type and initial response to treatment. These findings suggest that combining clinical and laboratory risk factors into a single algorithm will provide more precise GVHD risk assessment than relying on either alone.
Conclusions
Acute GVHD remains a leading cause of transplant-related mortality following allo-HCT. Limited progress has been made in the treatment of GVHD, highlighting the importance of developing more effective prediction and prevention strategies. Several clinical, genetic, and biomarker-based risk factors have been identified that correlate with acute GVHD risk. Translating these observations into clinical applications is next. Ultimately, it is possible to envision an algorithm that predicts risk of GVHD for individual patients based on established clinical predictors of GVHD (e.g., age, the selection of an unrelated or alternative donor, HLA-mismatch, the use of high-dose TBI, etc.), genetic risk factors (e.g. IL10 and NOD2 polymorphisms) and GVHD biomarker concentrations measured early enough after HCT (e.g., in the first days to weeks), so that there is a window of opportunity to pre-empt clinical GVHD from developing. The first step will be to identify the most important contributors to GVHD risk from the long list of potential clinical, genetic, and proteins involved in the pathophysiology of GVHD and, for post-HCT biomarkers, to determine the optimal timing of their measurement. Prospective clinical data and research sample collection is needed to accomplish this goal. Next, statistical models will need to be compared to each other to identify the model(s) that provide the most useful information with the least cost and complexity. Once these steps are completed, we will be well positioned to test GVHD risk stratification schemes that guide risk-adapted therapy (either through selection of prophylaxis regimen or the use of pre-emptive therapy) to improve outcomes following allo-HCT.
Acknowledgments
A.C.H., J.L.M.F. and J.E.L. drafted the figure and wrote the paper. This work was partially supported by National Institutes of Health (NIH) grants P01-CA039542 and U10-HL069330 and the Judith Devries Fund.
References
- Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, Pasquini M, Goldstein SC, Ho VT, Hayes-Lattin B, Wingard JR, Horowitz MM, Levine JE. Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute graft vs. host disease: a randomized phase II trial from the BMT CTN. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambruzova Z, Mrazek F, Raida L, Jindra P, Vidan-Jeras B, Faber E, Pretnar J, Indrak K, Petrek M. Association of IL6 and CCL2 gene polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009;44:227–235. doi: 10.1038/bmt.2009.16. [DOI] [PubMed] [Google Scholar]
- Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Johnston L, Maziarz RT, Pulsipher M, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Carter SL, Horowitz MM, Confer DL. Increased Incidence of Chronic Graft-Versus-Host Disease (GVHD) and No Survival Advantage with Filgrastim-Mobilized Peripheral Blood Stem Cells (PBSC) Compared to Bone Marrow (BM) Transplants From Unrelated Donors: Results of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Protocol 0201, a Phase III, Prospective, Randomized Trial. Blood (ASH Annual Meeting Abstracts) 2011;118:1. [Google Scholar]
- Anasetti C, Aversa F, Brunstein CG. Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant. 2012;18:S161–165. doi: 10.1016/j.bbmt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, Selby GB, Antin JH, Kernan NA, Kollman C, Nademanee A, McGlave P, Horowitz MM, Petersdorf EW. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August KJ, Chiang KY, Bostick RM, Flanders WD, Waller EK, Langston A, Worthington-White D, Rowland P, Moore KF, Khoury HJ, Horan JT. Biomarkers of immune activation to screen for severe, acute GVHD. Bone Marrow Transplant. 2011;46:601–604. doi: 10.1038/bmt.2010.165. [DOI] [PubMed] [Google Scholar]
- Bertinetto FE, Dall’Omo AM, Mazzola GA, Rendine S, Berrino M, Bertola L, Magistroni P, Caropreso P, Falda M, Locatelli F, Busca A, Amoroso A. Role of non-HLA genetic polymorphisms in graft-versus-host disease after haematopoietic stem cell transplantation. Int J Immunogenet. 2006;33:375–384. doi: 10.1111/j.1744-313X.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- Bogunia-Kubik K, Middleton P, Norden J, Dickinson A, Lange A. Association of vitamin D receptor polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Int J Immunogenet. 2008;35:207–213. doi: 10.1111/j.1744-313X.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69:1597–1608. doi: 10.1007/s00018-012-0968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, Jones RJ, Ambinder RF, Maris MB, Blume KG, Niederwieser DW, Bruno B, Maziarz RT, Pulsipher MA, Petersen FB, Storb R, Fuchs EJ, Maloney DG. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transpl. 2008;14:1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98:1594–1600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- Cho BS, Min CK, Kim HJ, Lee S, Kim YJ, Lim JY, Jeong DC, Cho B, Kim HK, Eom KS, Cho SG, Kim DW, Lee JW, Min WS, Kim CC, Chung NG. High levels of B cell activating factor during the peritransplantation period are associated with a reduced incidence of acute graft-versus-host disease following myeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:629–638. doi: 10.1016/j.bbmt.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Cho BS, Yoon JH, Shin SH, Yahng SA, Lee SE, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW, Kim HJ. Comparison of allogeneic stem cell transplantation from familial-mismatched/haploidentical donors and from unrelated donors in adults with high-risk acute myelogenous leukemia. Biol Blood Marrow Transplant. 2012;18:1552–1563. doi: 10.1016/j.bbmt.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Choi SW, Kitko CL, Braun T, Paczesny S, Yanik G, Mineishi S, Krijanovski O, Jones D, Whitfield J, Cooke K, Hutchinson RJ, Ferrara JLM, Levine JE. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112:1539–1542. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Stiff P, Cooke K, Ferrara JL, Braun T, Kitko C, Reddy P, Yanik G, Mineishi S, Paczesny S, Hanauer D, Pawarode A, Peres E, Rodriguez T, Smith S, Levine JE. TNF-Inhibition with Etanercept for Graft-versus-Host Disease Prevention in High-Risk HCT: Lower TNFR1 Levels Correlate with Better Outcomes. Biol Blood Marrow Transplant. 2012;18:1525–1532. doi: 10.1016/j.bbmt.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couriel DR, Saliba RM, Giralt S, Khouri I, Andersson BS, de Lima M, Hosing C, Anderlini P, Donato M, Cleary K, Gajewski J, Neumann J, Ippoliti C, Rondon G, Cohen A, Champlin R. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transpl. 2004a;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, Khouri I, Andersson B, Gajewski J, Donato M, Anderlini P, Kontoyiannis DP, Cohen A, Martin T, Giralt S, Champlin R. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004b;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- Cullup H, Dickinson AM, Jackson GH, Taylor PR, Cavet J, Middleton PG. Donor interleukin 1 receptor antagonist genotype associated with acute graft-versus-host disease in human leucocyte antigen-matched sibling allogeneic transplants. Br J Haematol. 2001;113:807–813. doi: 10.1046/j.1365-2141.2001.02811.x. [DOI] [PubMed] [Google Scholar]
- Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, Odom J, Foley J, Gress R, Bishop MR. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–5741. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med. 2011;43:503–511. doi: 10.3109/07853890.2011.577093. [DOI] [PubMed] [Google Scholar]
- Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, Brown M, Champlin RE, Garcia-Lopez J, Hattersely G, Koegler G, Laughlin MJ, Michel G, Nabhan SK, Smith FO, Horowitz MM, Gluckman E, Rocha V. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12:1214–1221. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmaagacli AH, Koldehoff M, Hindahl H, Steckel NK, Trenschel R, Peceny R, Ottinger H, Rath PM, Ross RS, Roggendorf M, Grosse-Wilde H, Beelen DW. Mutations in innate immune system NOD2/CARD 15 and TLR-4 (Thr399Ile) genes influence the risk for severe acute graft-versus-host disease in patients who underwent an allogeneic transplantation. Transplantation. 2006;81:247–254. doi: 10.1097/01.tp.0000188671.94646.16. [DOI] [PubMed] [Google Scholar]
- Elmaagacli AH, Koldehoff M, Landt O, Beelen DW. Relation of an interleukin-23 receptor gene polymorphism to graft-versus-host disease after hematopoietic-cell transplantation. Bone Marrow Transplant. 2008;41:821–826. doi: 10.1038/sj.bmt.1705980. [DOI] [PubMed] [Google Scholar]
- Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, Levine JE, Choi SW, Huber E, Landfried K, Akashi K, Vander Lugt M, Reddy P, Chin A, Zhang Q, Hanash S, Paczesny S. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, Hurley C, Kollman C, Anasetti C, Noreen H, Begovich A, Hildebrand W, Petersdorf E, Schmeckpeper B, Setterholm M, Trachtenberg E, Williams T, Yunis E, Weisdorf D. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, Pereira SE, Nash RA, Mielcarek M, Fero ML, Warren EH, Sanders JE, Storb RF, Appelbaum FR, Storer BE, Martin PJ. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MB, Jacobson JS, Freedman J, George D, Moore V, van de Ven C, Satwani P, Bhatia M, Garvin JH, Bradley MB, Harrison L, Morris E, Della-Latta P, Schwartz J, Baxter-Lowe LA, Cairo MS. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br J Haematol. 2011;155:218–234. doi: 10.1111/j.1365-2141.2011.08822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussetis E, Varela I, Peristeri I, Kitra V, Spanou K, Moraloglou O, Paisiou A, Karatasaki S, Soldatou A, Constantinidou N, Graphakos S. Cytokine gene polymorphisms and graft-versus-host disease in children after matched sibling hematopoietic stem cell transplantation: a single-center experience. Cell Mol Immunol. 2011;8:276–280. doi: 10.1038/cmi.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Lin Y, Schultz KR, Ferrell RE, Kim Y, Fairfull L, Livote E, Yanik G, Atlas M. Tumor necrosis factor-alpha gene polymorphisms are associated with severity of acute graft-versus-host disease following matched unrelated donor bone marrow transplantation in children: a Pediatric Blood and Marrow Transplant Consortium study. Biol Blood Marrow Transplant. 2010;16:927–936. doi: 10.1016/j.bbmt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Gruhn B, Intek J, Pfaffendorf N, Zell R, Corbacioglu S, Zintl F, Beck JF, Debatin KM, Steinbach D. Polymorphism of interleukin-23 receptor gene but not of NOD2/CARD15 is associated with graft-versus-host disease after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2009;15:1571–1577. doi: 10.1016/j.bbmt.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, Gale RP, Hale GA, Horan J, Isola L, Maziarz RT, van Rood JJ, Gupta V, Halter J, Reddy V, Tiberghien P, Litzow M, Anasetti C, Pavletic S, Ringden O. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol. 2011;23:462–468. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler E, Rogler G, Brenmoehl J, Hahn J, Greinix H, Dickinson AM, Socie G, Wolff D, Finke J, Fischer G, Jackson G, Rocha V, Hilgendorf I, Eissner G, Marienhagen J, Andreesen R. The role of genetic variants of NOD2/CARD15, a receptor of the innate immune system, in GvHD and complications following related and unrelated donor haematopoietic stem cell transplantation. Int J Immunogenet. 2008;35:381–384. doi: 10.1111/j.1744-313X.2008.00795.x. [DOI] [PubMed] [Google Scholar]
- Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Scholmerich J, Andreesen R. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–894. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ, Antin JH, Bolwell BJ, Bredeson C, Cahn JY, Cairo M, Gale RP, Gupta V, Lee SJ, Litzow M, Weisdorf DJ, Horowitz MM, Hahn T. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabon L, Wysoczanska B, Bogunia-Kubik K, Suchnicki K, Lange A. IL-6 and IL-10 promoter gene polymorphisms of patients and donors of allogeneic sibling hematopoietic stem cell transplants associate with the risk of acute graft-versus-host disease. Hum Immunol. 2005;66:700–710. doi: 10.1016/j.humimm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kitko CL, Paczesny S, Yanik G, Braun T, Jones D, Whitfield J, Choi SW, Hutchinson RJ, Ferrara JL, Levine JE. Plasma elevations of tumor necrosis factor-receptor-1 at day 7 postallogeneic transplant correlate with graft-versus-host disease severity and overall survival in pediatric patients. Biol Blood Marrow Transpl. 2008;14:759–765. doi: 10.1016/j.bbmt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK, Noreen H, Oudshoorn M, Petersdorf E, Setterholm M, Spellman S, Weisdorf D, Williams TM, Anasetti C. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, Jones D, Khaled Y, Kitko CL, Bickley D, Krijanovski O, Reddy P, Yanik G, Ferrara JLM. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Logan BR, Wu J, Alousi AM, Bolanos-Meade J, Ferrara JL, Ho VT, Weisdorf DJ, Paczesny S. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–3860. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Storer B, Martin PJ, Tseng LH, Gooley T, Chen PJ, Hansen JA. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- Liu D, Yan C, Xu L, Wang Y, Han W, Zhang X, Liu K, Huang X. Diarrhea during the conditioning regimen is correlated with the occurrence of severe acute graft-versus-host disease through systemic release of inflammatory cytokines. Biol Blood Marrow Transplant. 2010;16:1567–1575. doi: 10.1016/j.bbmt.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Loiseau P, Busson M, Balere ML, Dormoy A, Bignon JD, Gagne K, Gebuhrer L, Dubois V, Jollet I, Bois M, Perrier P, Masson D, Moine A, Absi L, Reviron D, Lepage V, Tamouza R, Toubert A, Marry E, Chir Z, Jouet JP, Blaise D, Charron D, Raffoux C. HLA association with hematopoietic stem cell transplantation outcome: the number of mismatches at HLA-A, -B, -C, -DRB1, or -DQB1 is strongly associated with overall survival. Biol Blood Marrow Transpl. 2007;13:965–974. doi: 10.1016/j.bbmt.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Ludajic K, Balavarca Y, Bickeboller H, Rosenmayr A, Fischer GF, Fae I, Kalhs P, Pohlreich D, Kouba M, Dobrovolna M, Greinix HT. Minor ABO-mismatches are risk factors for acute graft-versus-host disease in hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant. 2009;15:1400–1406. doi: 10.1016/j.bbmt.2009.07.002. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, Radloff GA, DeFor TE, Weisdorf DJ, Davies SM. Interleukin-1 genotype and outcome of unrelated donor bone marrow transplantation. Br J Haematol. 2003;121:597–604. doi: 10.1046/j.1365-2141.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, Blazar BR, Wagner JE. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant. 2006;38:629–633. doi: 10.1038/sj.bmt.1705493. [DOI] [PubMed] [Google Scholar]
- Mehta PA, Eapen M, Klein JP, Gandham S, Elliott J, Zamzow T, Combs M, Aplenc R, MacMillan ML, Weisdorf DJ, Petersdorf E, Davies SM. Interleukin-1 alpha genotype and outcome of unrelated donor haematopoietic stem cell transplantation for chronic myeloid leukaemia. Br J Haematol. 2007;137:152–157. doi: 10.1111/j.1365-2141.2007.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton PG, Taylor PR, Jackson G, Proctor SJ, Dickinson AM. Cytokine gene polymorphisms associating with severe acute graft-versus-host disease in HLA-identical sibling transplants. Blood. 1998;92:3943–3948. [PubMed] [Google Scholar]
- Middleton PG, Cullup H, Dickinson AM, Norden J, Jackson GH, Taylor PR, Cavet J. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30:223–228. doi: 10.1038/sj.bmt.1703629. [DOI] [PubMed] [Google Scholar]
- Middleton PG, Norden J, Cullup H, Cavet J, Jackson GH, Taylor PR, Dickinson AM. Oestrogen receptor alpha gene polymorphism associates with occurrence of graft-versus-host disease and reduced survival in HLA-matched sib-allo BMT. Bone Marrow Transplant. 2003;32:41–47. doi: 10.1038/sj.bmt.1704090. [DOI] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, Viatte S, Soiffer RJ, Antin JH, Ritz J. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarczewska A, Wysoczanska B, Karabon L, Bogunia-Kubik K, Lange A. Lack of IFN-gamma 2/2 homozygous genotype independently of recipient age and intensity of conditioning regimen influences the risk of aGVHD manifestation after HLA-matched sibling haematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34:339–344. doi: 10.1038/sj.bmt.1704581. [DOI] [PubMed] [Google Scholar]
- Mullighan C, Heatley S, Doherty K, Szabo F, Grigg A, Hughes T, Schwarer A, Szer J, Tait B, To B, Bardy P. Non-HLA immunogenetic polymorphisms and the risk of complications after allogeneic hemopoietic stem-cell transplantation. Transplantation. 2004;77:587–596. doi: 10.1097/01.tp.0000111769.45088.a2. [DOI] [PubMed] [Google Scholar]
- Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels P, Buell D, Fitzsimmons W, Anasetti C, Storb R, Ratanatharathorn V. Phase III study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- Nguyen Y, Al-Lehibi A, Gorbe E, Li E, Haagenson M, Wang T, Spellman S, Lee SJ, Davidson NO. Insufficient evidence for association of NOD2/CARD15 or other inflammatory bowel disease-associated markers on GVHD incidence or other adverse outcomes in T-replete, unrelated donor transplantation. Blood. 2010;115:3625–3631. doi: 10.1182/blood-2009-09-243840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlander A, Uzunel M, Mattsson J, Remberger M. The TNFd4 allele is correlated to moderate-to-severe acute graft-versus-host disease after allogeneic stem cell transplantation. Br J Haematol. 2002;119:1133–1136. doi: 10.1046/j.1365-2141.2002.03965.x. [DOI] [PubMed] [Google Scholar]
- Norlin AC, Remberger M. A comparison of Campath and Thymoglobulin as part of the conditioning before allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2011;86:57–66. doi: 10.1111/j.1600-0609.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- Ordemann R, Hutchinson R, Friedman J, Burakoff SJ, Reddy P, Duffner U, Braun TM, Liu C, Teshima T, Ferrara JL. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, Misek DE, Cooke KR, Kitko CL, Weyand A, Bickley D, Jones D, Whitfield J, Reddy P, Levine JE, Hanash SM, Ferrara JL. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczesny S, Braun T, Lugt MV, Harris A, Fiema B, Hernandez J, Choi S, Kitko C, Magenau J, Yanik G, Peres EM, Pawarode A, Mineishi S, Whitfield J, Jones D, Couriel D, Reddy P, Hanash S, Ferrara JLM, Levine JE. Three Biomarker Panel at Day 7 and 14 Can Predict Development of Grade II-IV Acute Graft-Versus-Host Disease. Blood (ASH Annual Meeting Abstracts) 2010a;116:675. [Google Scholar]
- Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, Olsen S, Choi SW, Wang H, Faca V, Pitteri S, Zhang Q, Chin A, Kitko C, Mineishi S, Yanik G, Peres E, Hanauer D, Wang Y, Reddy P, Hanash S, Ferrara JL. Elafin is a biomarker of graft-versus-host disease of the skin. Science Translational Medicine. 2010b;2:13ra12. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum Immunol. 2000;61:863–866. doi: 10.1016/s0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- Pulsipher MA, Chitphakdithai P, Logan BR, Leitman SF, Anderlini P, Klein JP, Horowitz MM, Miller JP, King RJ, Confer DL. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606–2616. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, Fay JW, Nademanee A, Antin JH, Christiansen NP, van der Jagt R, Herzig RH, Litzow MR, Wolff SN, Longo WL, Petersen FB, Karanes C, Avalos B, Storb R, Buell DN, Maher RM, Fitzsimmons WE, Wingard JR. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- Remberger M, Ringden O, Markling L. TNF alpha levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant. 1995;15:99–104. [PubMed] [Google Scholar]
- Remberger M, Persson U, Hauzenberger D, Ringden O. An association between human leucocyte antigen alleles and acute and chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2002;119:751–759. doi: 10.1046/j.1365-2141.2002.03924.x. [DOI] [PubMed] [Google Scholar]
- Remberger M, Mattsson J, Hassan Z, Karlsson N, LeBlanc K, Omazic B, Okas M, Sairafi D, Ringden O. Risk factors for acute graft-versus-host disease grades II-IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single centre study. Bone Marrow Transpl. 2008;41:399–405. doi: 10.1038/sj.bmt.1705913. [DOI] [PubMed] [Google Scholar]
- Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D, Seconi J, Wood C, Walton J, Watt V. Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2005;11:706–712. doi: 10.1016/j.bbmt.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Rocha V, Wagner JE, Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- Rocha V, Franco RF, Porcher R, Bittencourt H, Silva WA, Jr, Latouche A, Devergie A, Esperou H, Ribaud P, Socie G, Zago MA, Gluckman E. Host defense and inflammatory gene polymorphisms are associated with outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;100:3908–3918. doi: 10.1182/blood-2002-04-1033. [DOI] [PubMed] [Google Scholar]
- Rocha V, Porcher R, Fernandes JF, Filion A, Bittencourt H, Silva W, Jr, Vilela G, Zanette DL, Ferry C, Larghero J, Devergie A, Ribaud P, Skvortsova Y, Tamouza R, Gluckman E, Socie G, Zago MA. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia. 2009;23:545–556. doi: 10.1038/leu.2008.323. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Bissonnette A, Ahmad R, Wu Z, Vasir B, Stevenson K, Zarwan C, Keefe W, Glotzbecker B, Mills H, Joyce R, Levine JD, Tzachanis D, Boussiotis V, Kufe D, Avigan D. Immunomodulatory effects of vitamin D: implications for GVHD. Bone Marrow Transplant. 2010;45:1463–1468. doi: 10.1038/bmt.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairafi D, Uzunel M, Remberger M, Ringden O, Mattsson J. No impact of NOD2/CARD15 on outcome after SCT. Bone Marrow Transpl. 2008;41:961–964. doi: 10.1038/bmt.2008.9. [DOI] [PubMed] [Google Scholar]
- Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci U S A. 2011;108:9560–9565. doi: 10.1073/pnas.1017854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BE, Gooley TA, Malkki M, Madrigal JA, Begovich AB, Horowitz MM, Gratwohl A, Ringden O, Marsh SG, Petersdorf EW. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110:4560–4566. doi: 10.1182/blood-2007-06-095265. [DOI] [PubMed] [Google Scholar]
- Socie G, Loiseau P, Tamouza R, Janin A, Busson M, Gluckman E, Charron D. Both genetic and clinical factors predict the development of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transplantation. 2001;72:699–706. doi: 10.1097/00007890-200108270-00024. [DOI] [PubMed] [Google Scholar]
- Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, Devine S, Isola L, Lazarus HM, Marks DI, Porter DL, Waller EK, Horowitz MM, Eapen M. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Passweg JR, Locasciulli A, Socie G, Schrezenmeier H, Bekassy AN, Fuehrer M, Hows J, Korthof ET, McCann S, Tichelli A, Zoumbos NC, Marsh JC, Bacigalupo A, Gratwohl A. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–226. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- Storb R, Thomas ED. Graft-versus-host disease in dog and man: the Seattle experience. Immunol Rev. 1985;88:215–238. doi: 10.1111/j.1600-065x.1985.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Suarez A, Castro P, Alonso R, Mozo L, Gutierrez C. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation. 2003;75:711–717. doi: 10.1097/01.TP.0000055216.19866.9A. [DOI] [PubMed] [Google Scholar]
- Thiant S, Yakoub-Agha I, Magro L, Trauet J, Coiteux V, Jouet JP, Dessaint JP, Labalette M. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. 2010;45:1546–1552. doi: 10.1038/bmt.2010.13. [DOI] [PubMed] [Google Scholar]
- Thiant S, Labalette M, Trauet J, Coiteux V, de Berranger E, Dessaint JP, Yakoub-Agha I. Plasma levels of IL-7 and IL-15 after reduced intensity conditioned allo-SCT and relationship to acute GVHD. Bone Marrow Transplant. 2011;46:1374–1381. doi: 10.1038/bmt.2010.300. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Urbano-Ispizua A, Rozman C, Pimentel P, Solano C, de la Rubia J, Brunet S, Perez-Oteyza J, Ferra C, Zuazu J, Caballero D, Bargay J, Carvalhais A, Diez JL, Espigado I, Alegre A, Rovira M, Campilho F, Odriozola J, Sanz MA, Sierra J, Garcia-Conde J, Montserrat E. Risk factors for acute graft-versus-host disease in patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings. Blood. 2002;100:724–727. doi: 10.1182/blood-2001-11-0057. [DOI] [PubMed] [Google Scholar]
- van der Velden WJ, Donnelly JP, Blijlevens NM. Differences in T cell depletion and intestinal mucositis explain inconsistent effects of NOD2/CARD15 polymophisms in SCT. Biol Blood Marrow Transplant. 2011;17:1421–1423. doi: 10.1016/j.bbmt.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Verrier T, Solhonne B, Sallenave JM, Garcia-Verdugo I. The WAP protein Trappin-2/Elafin: A handyman in the regulation of inflammatory and immune responses. Int J Biochem Cell Biol. 2012;44:1377–1380. doi: 10.1016/j.biocel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, Marino E, Thaxton J, Weinberg A, Mackay F, Brink R, Sprent J, Grey ST. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sorror ML, Leisenring W, Schoch G, Maloney DG, Sandmaier BM, Storb R. The impact of donor type and ABO incompatibility on transfusion requirements after nonmyeloablative haematopoietic cell transplantation. Br J Haematol. 2010;149:101–110. doi: 10.1111/j.1365-2141.2009.08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissinger EM, Schiffer E, Hertenstein B, Ferrara JL, Holler E, Stadler M, Kolb HJ, Zander A, Zurbig P, Kellmann M, Ganser A. Proteomic patterns predict acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:5511–5519. doi: 10.1182/blood-2007-01-069757. [DOI] [PubMed] [Google Scholar]
- Wermke M, Maiwald S, Schmelz R, Thiede C, Schetelig J, Ehninger G, Bornhauser M, Wassmuth R. Genetic variations of interleukin-23R (1143A>G) and BPI (A645G), but not of NOD2, are associated with acute graft-versus-host disease after allogeneic transplantation. Biol Blood Marrow Transplant. 2010;16:1718–1727. doi: 10.1016/j.bbmt.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Willems E, Humblet-Baron S, Dengis O, Seidel L, Beguin Y, Baron F. Elevations of tumor necrosis factor receptor 1 at day 7 and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45:1442–1448. doi: 10.1038/bmt.2009.360. [DOI] [PubMed] [Google Scholar]
- Wolf D, Wolf AM, Fong D, Rumpold H, Strasak A, Clausen J, Nachbaur D. Regulatory T-cells in the graft and the risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2007;83:1107–1113. doi: 10.1097/01.tp.0000260140.04815.77. [DOI] [PubMed] [Google Scholar]
- Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M, Gajewski J, Hale GA, Horan J, Battiwalla M, Marino SR, Setterholm M, Ringden O, Hurley C, Flomenberg N, Anasetti C, Fernandez-Vina M, Lee SJ. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]