Abstract
Cocaine addiction affects millions of people with disastrous personal and social consequences. Cocaine is one of the most reinforcing of all drugs of abuse, and even those who undergo rehabilitation and experience long periods of abstinence have an over 80% chance of relapse. Yet there is no FDA-approved treatment to decrease the likelihood of relapse in rehabilitated addicts. Recent studies, however, have demonstrated a promising potential treatment option with the help of the serum enzyme butyrylcholinesterase (BChE), which is capable of breaking down naturally occurring (−)-cocaine before the drug can influence the reward centers of the brain or affect other areas of the body. This activity of wild-type (WT) BChE, however, is relatively low. This prompted the design of variants of BChE which exhibit significantly improved catalytic activity against (−)-cocaine. Plants are a promising means to produce large amounts of these cocaine hydrolase variants of BChE, cheaply, safely with no concerns regarding human pathogens and functionally equivalent to enzymes derived from other sources. Here, in expressing cocaine-hydrolyzing mutants of BChE in Nicotiana benthamiana using the MagnICON virus-assisted transient expression system, and in reporting their initial biochemical analysis, we provide proof-of-principle that plants can express engineered BChE proteins with desired properties.
1. Introduction
Cocaine is the second most widely abused recreational drug in the United States after marijuana [20]. Cocaine addiction is a chronic disorder addressed through prolonged, often ineffective, behavioral intervention and for which there is no approved pharmacological treatment. Similarly, acute intoxication (i.e. overdose) by cocaine is also only symptomatically treated [17, 19].
The serum enzyme butyrylcholinesterase (BChE) is a bioscavenger capable of binding several plant alkaloids [3, 11, 13, 16, 23]. BChE is likewise capable of hydrolyzing several plant secondary metabolites and their synthetic derivatives such as succinylcholine, acetylsalicylic acid (aspirin) and cocaine [12, 14]. Cocaine is hydrolyzed by serum BChE into the inactive metabolite ecgonine methyl ester and the inactive side product, benzoic acid, unlike the hepatic pathway through which the drug is converted into the bioactive metabolite norcocaine. However, due to its relatively low catalytic efficiency against the relevant enantiomer of (−)-cocaine, and despite its strategic disposition in the circulation, in situations of exposure to acutely toxic concentrations of cocaine (as in the case of cocaine overdose), the endogenous BChE is expected to be easily overwhelmed.
Several groups have created site-directed mutant variants of BChE to improve catalytic efficiency against (−)-cocaine [1, 4, 5, 10, 22, 24, 25]. In order to utilize these enzymes as a possible anti-cocaine treatment, a sustainable, cost effective supply of the protein must be established. Here we report expression of cocaine hydrolyzing mutants of BChE in the dicotyledonous plant Nicotiana benthamiana using the MagnICON virus-assisted transient expression system and their initial biochemical analysis. This work provides the proof-of-principle that plants may be an attractive means of producing cocaine-hydrolyzing variants of BChE in quantities relevant for clinical use.
2. Materials and Methods
2.1 Cloning of plant-expression optimized synthetic genes encoding BChE variants and their expression in plants
The plant-expression optimized gene encoding the WT form of human BChE, pBChE [6, 7] with C-terminal His-tag (H6) was used as template for introduction of site-directed mutations (QuickChange kit, Stratagene) to create the following sited-directed mutations: F227A/S287G/A328W/Y332A, A199S/S287G/A328W/Y332G [25], A199S/F227A/S287G/A328W/Y332G, and F227A/S287G/A328W/Y332G) [27]. The genes were transiently expressed in wild-type (WT) N. benthamiana plants using the MagnICON vector system based on deconstructed tobacco mosaic virus [TMV, 18].
2.2 Enrichment preparation of BChE variants and biochemical analyses
The proteins were partially purified following a protocol similar to one used for WT pBChE [6, 7] based on concanavalin A (ConA) chromatography.
Estimation of concentration of BChE and variants thereof was conducted using quantitative immunoblot assay with highly purified samples of plasma-derived and plant-derived BChE, whose molar concentrations were previously determined [6, 7], serving as standards. To this end, standards were resolved by SDS-PAGE on 8% polyacrylamide gels, transferred to nitrocellulose membranes, immunodecorated with rabbit polyclonal anti-hBChE antibodies (kind gift of Dr. Oksana Lockridge), and detected by anti-rabbit IgG-Horse Radish Peroxidase (HRP) antibodies followed by chemiluminescence assay. High resolution (at least 600dpi) greyscale images were used for densitometry analysis with Image J Software and data was used to plot standard curves fitted by linear-regression (GraphPad Prism). Samples of variants with unknown concentrations were resolved alongside the standards and densitometry results together with the regression equations were used to obtain concentration of the BChE variants. Several dilutions of samples were applied to make sure samples were well within the linear range of the standard curve. Results showed excellent correlation with butyrylthiocholine (BTC) hydrolysis assays (see below) by the mutants and individual specific activities could thus be calculated. In all subsequent experiments we have used these specific activities to estimate BChE variant concentration.
2.3 Enzymatic Assays
Two enzyme assays were performed. The spectrophotometric Ellman assay was used to assess basic BChE activity with BTC (Sigma) as the substrate (1 mM). Assays were run at 30°C in a Spectramax 190 spectrophotometer (Molecular Devices) as previously described [8]. To evaluate cocaine hydrolysis, a previously described radiometric assay was used with 3H cocaine as substrate over a wide range of concentrations [2]. Data were subjected to non-linear regression analysis (Sigma-Plot), and estimates of VMAX and KM were derived along with their standard errors. Turnover numbers (KCAT) could be derived, in turn, from these Vmax values and the assay’s molar concentrations of BChE variants obtained as described above.
3. Results and Discussion
We have previously described the production of a double mutant of BChE A328W/Y332A in transgenic plants [9]. This mutant has enhanced hydrolytic activity toward (−)-cocaine [15, 21, 22]. The catalytic prowess of this mutant, (which we call Variant 1) was sequentially improved by introducing additional or different site-directed changes to create Variants 2 (F227A/S287G/A328W/Y332A), 3 (A199S/S287G/A328W/Y332G), 4 (A199S/F227A/S287G/A328W/Y332G), and 5 (F227A/S287G/A328W/Y332G) [25, 27].
Using the MagnICON expression system [18], deconstructed-TMV-based vectors were introduced into WT tobacco plants by infiltration either by using needle-less syringe injection or by application of vacuum on whole plants submerged in agrobacterial suspensions (Fig 1).
Figure 1.

Transient plant expression of cocaine-hydrolase variants of BChE. Agrobacterium tumefaciens cells harboring the deconstructed TMV-vectors containing the recombinant BChE variant genes (A) were infiltrated by applying vacuum to whole-submerged N. benthamiana plants (B1) or by leaf injection with needle-less syringe into leaves (B2). Plants were harvested at 14-17 days post-infiltration when peak expression is reached (C).
Leaf samples were harvested at the indicated time points and assayed by the Ellman-assay and immunoassay to determine the expression level of the BChE enzyme variants (Fig 2). Multiple 0.2 g leaf samples from different plants were assayed per time point for BChE activity. Peak expression time was around 14 days but with some variation among the variant forms (14-17 days). Accumulation levels varied considerably between the various mutants and ranged from 16 to 100 mg per kg fresh weight leaf material (Fig 2).
Figure 2.
Variants of BChE designed for cocaine hydrolysis accumulate over time in plants infiltrated with TMV-vectors. Multiple (2 or 3) different leaf samples (0.2 g fresh weight) from different plants were harvested at the given time points. Protein levels determined from the 0.2 g-leaf sample were then extrapolated to determine estimated protein accumulation in 1 kilogram (kg) of fresh leaf material. Mean protein level values ± SEM were determined based on activity assays in conjunction with immunoassays from plants infiltrated with MagnICON vectors expressing Variant 2 (A), Variant 3 (B), Variant 4 (C) and Variant 5 (D).
Partial purification was achieved by ConA affinity chromatography as exemplified for Variant 4 (Fig 3), and Variants 3-5 were tested for cocaine hydrolysis activity in a radiometric assay [2]. Michaelis-Menten constant (KM) values for Variants 3-5 were (mean±SEM, respectively) 2.6±0.1, 2.7±0.1, and 12.4±1.2 μM compared to the reported WT BChE KM of 4.5 μM [22]. The turnover number was determined for one variant thus far (Variant 4) and was 5200±63 min−1 (mean±SEM), similar to the established value of 5700 min−1 determined for the variant derived from mammalian cell system [26]. The efficiency of catalysis (KCAT/KM) was determined for one mutant thus far (Variant 4) and was (1.91± 0.09) x109 M•min−1, a ~1500 fold increase over the established value of 1.3×106 M•min−1 for WT BChE [4]. This outcome is very similar to that reported for the original version of the same mutant expressed in mammalian cell culture [26]. However, a caveat to bear in mind is that the present experiments did not bring the isolated protein to the level of purity at which active site titrations could be performed to establish beyond all possible doubt the precise abundance of catalytic units. These findings therefore do not prove that catalytic efficiency in the plant-derived enzyme is exactly identical to that which the mutant BChE would exhibit if isolated from mammalian, or especially human cells. Nonetheless they represent powerful encouragement for a plan to produce human BChE-based cocaine hydrolases for testing in higher animals, including non-human primates. Moreover, they strongly suggest that such enzymes could exhibit the properties expected and needed for eventual therapeutic applications in humans.
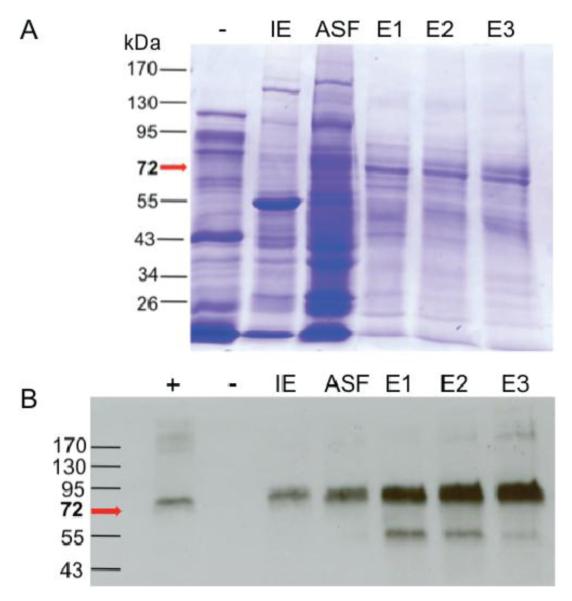
Figure 3.
ConA purified preparations of Variant 4 resolved by SDS-PAGE and subject to Coomassie Staining (A) or Western Blot (B). Lanes (+) and (−) represent a positive control of plant-derived WT BChE and negative control of WT Nicotiana benthamiana. Crude extracts were loaded based on equivalent amounts of total soluble protein. The recombinant protein was partially purified from the initial extract (IE) by 40%-70% ammonium sulfate fractionation (ASF). The protein was then subject to affinity chromatography using Con A-sepharose, eluting with increasing concentrations of methyl-α-D-gluco-pyranoside (E1-E3). E3 corresponds to an 82 fold increase in purity based on specific activity. All Variant 4 pBChE samples were loaded based on equal BChE activity at 240 mU (A) or 2.4mU (B).
In this paper we have reported for the first time that Nicotiana benthamiana can be used to express different cocaine hydrolase variants of BChE, including Variant 4 (A199S/F227A/S287G/A328W/Y332G), which is the most efficient cocaine hydrolyzing variant of BChE designed to date [25, 27]. Average peak projected yield was found to range from 16 – 100 mg BChE/kg fresh weight leaf material. Of those plant-derived variants tested thus far, all have been found to exhibit nearly identical kinetic properties to those variants derived from other sources. Although the catalytic properties of the plant-derived variants are not yet fully proven to be identical to those derived from other sources, their similarity to those reported for the corresponding variants expressed in mammalian cells is nevertheless a promising and important finding that warrants further investigation. Future work, with protein preparations of much higher purity will further characterize these plant-derived variants in terms of molecular features such as glycosylation, evaluate their stability in vivo, and test their ability to rescue animals from acute cocaine intoxication.
Acknowledgments
Work was supported in part by the National Institute for Drug Abuse Program Grant P1 DA031340 awarded to the Mayo Clinic and subcontracted to ASU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brimijoin S, Shen ML, Sun H. Radiometric solvent-partitioning assay for screening cocaine hydrolases and measuring cocaine levels in milligram tissue samples. Analytical Biochemistry. 2002;309:200–205. doi: 10.1016/s0003-2697(02)00238-5. [DOI] [PubMed] [Google Scholar]
- [3].Decker M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur J Med Chem. 2005;40:305–313. doi: 10.1016/j.ejmech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [4].Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol. 2009;75:318–323. doi: 10.1124/mol.108.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310:1046–1052. doi: 10.1124/jpet.104.068122. [DOI] [PubMed] [Google Scholar]
- [6].Geyer BC, Evron T, Soreq H, Mor TS. Organophosphate Intoxication: Molecular Consequences, Mechanisms and Solutions. In: Ramesh CG, editor. Handbook of Toxicology of Chemical Warfare Agents. Academic Press; San Diego: 2009. pp. 691–717. [Google Scholar]
- [7].Geyer BC, Kannan L, Cherni I, Woods RR, Soreq H, Mor TS. Transgenic plants as a source for the bioscavenging enzyme, human butyrylcholinesterase. Plant Biotechnol J. 2010;8:873–886. doi: 10.1111/j.1467-7652.2010.00515.x. [DOI] [PubMed] [Google Scholar]
- [8].Geyer BC, Muralidharan M, Cherni I, Doran J, Fletcher SP, Evron T, Soreq H, Mor TS. Purification of Transgenic Plant-Derived Recombinant Human Acetylcholinesterase-R. Chem Biol Interact. 2005;157-158:331–334. doi: 10.1016/j.cbi.2005.10.097. [DOI] [PubMed] [Google Scholar]
- [9].Geyer BC, Woods RR, Mor TS. Increased organophosphate scavenging in a butyrylcholinesterase mutant. Chem Biol Interact. 2008;175:376–379. doi: 10.1016/j.cbi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [10].Huang X, Gao D, Zhan CG. Computational design of a thermostable mutant of cocaine esterase via molecular dynamics simulations. Organic & biomolecular chemistry. 2011;9:4138–4143. doi: 10.1039/c0ob00972e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khan SB, Azhar ul H, Perveen S, Afza N, Malik A, Nawaz SA, Shah MR, Choudhary MI. Butyrylcholinesterase inhibitory guaianolides from Amberboa ramosa. Arch Pharm Res. 2005;28:172–176. doi: 10.1007/BF02977710. [DOI] [PubMed] [Google Scholar]
- [12].Koetzner L, Woods JH. Characterization of butyrylcholinesterase antagonism of cocaine-induced hyperactivity. Drug Metab Dispos. 2002;30:716–723. doi: 10.1124/dmd.30.6.716. [DOI] [PubMed] [Google Scholar]
- [13].Loizzo MR, Tundis R, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem. 2008;15:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- [14].Masson P, Froment MT, Fortier PL, Visicchio JE, Bartels CF, Lockridge O. Butyrylcholinesterase-catalysed hydrolysis of aspirin, a negatively charged ester, and aspirin-related neutral esters. Biochim Biophys Acta. 1998;1387:41–52. doi: 10.1016/s0167-4838(98)00104-6. [DOI] [PubMed] [Google Scholar]
- [15].Masson P, Nachon F, Bartels CF, Froment MT, Ribes F, Matthews C, Lockridge O. High activity of human butyrylcholinesterase at low pH in the presence of excess butyrylthiocholine. Eur J Biochem. 2003;270:315–324. doi: 10.1046/j.1432-1033.2003.03388.x. [DOI] [PubMed] [Google Scholar]
- [16].McGehee DS, Krasowski MD, Fung DL, Wilson B, Gronert GA, Moss J. Cholinesterase inhibition by potato glycoalkaloids slows mivacurium metabolism. Anesthesiology. 2000;93:510–519. doi: 10.1097/00000542-200008000-00031. [DOI] [PubMed] [Google Scholar]
- [17].Nnadi CU, Mimiko OA, McCurtis HL, Cadet JL. Neuropsychiatric effects of cocaine use disorders. Journal of the National Medical Association. 2005;97:1504–1515. [PMC free article] [PubMed] [Google Scholar]
- [18].Santi L, Giritch A, Roy CJ, Marillonnet S, Klimyuk V, Gleba Y, Webb R, Arntzen CJ, Mason HS. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc Natl Acad Sci U S A. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558–2569. doi: 10.1161/CIRCULATIONAHA.110.940569. [DOI] [PubMed] [Google Scholar]
- [20].Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2009. Substance Abuse and Mental Health Services Administration. (NSDUH Series H-36). [Google Scholar]
- [21].Sun H, El Yazal J, Lockridge O, Schopfer LM, Brimijoin S, Pang YP. Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J Biol Chem. 2001;276:9330–9336. doi: 10.1074/jbc.M006676200. [DOI] [PubMed] [Google Scholar]
- [22].Sun H, Pang YP, Lockridge O, Brimijoin S. Re-engineering Butyrylcholinesterase as a Cocaine Hydrolase. Mol Pharmacol. 2002;62:220–224. doi: 10.1124/mol.62.2.220. [DOI] [PubMed] [Google Scholar]
- [23].Taylor P. Chapter 8: Anticholinesterase Agents. In: Brunton LL, Goodman LS, Gilman A, Lazo JS, Parker KL, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. McGraw-Hill Medical Pub. Division; New York: 2006. [Google Scholar]
- [24].Xue L, Ko MC, Tong M, Yang W, Hou S, Fang L, Liu J, Zheng F, Woods JH, Tai HH, Zhan CG. Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Molecular pharmacology. 2011;79:290–297. doi: 10.1124/mol.110.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang W, Xue L, Fang L, Chen X, Zhan CG. Characterization of a high-activity mutant of human butyrylcholinesterase against (−)-cocaine. Chemico-biological interactions. 2010;187:148–152. doi: 10.1016/j.cbi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. Most efficient cocaine hydrolase designed by virtual screening of transition states. Journal of the American Chemical Society. 2008;130:12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zheng F, Yang W, Xue L, Hou S, Liu J, Zhan CG. Design of high-activity mutants of human butyrylcholinesterase against (−)-cocaine: structural and energetic factors affecting the catalytic efficiency. Biochemistry. 2010;49:9113–9119. doi: 10.1021/bi1011628. [DOI] [PMC free article] [PubMed] [Google Scholar]