Abstract
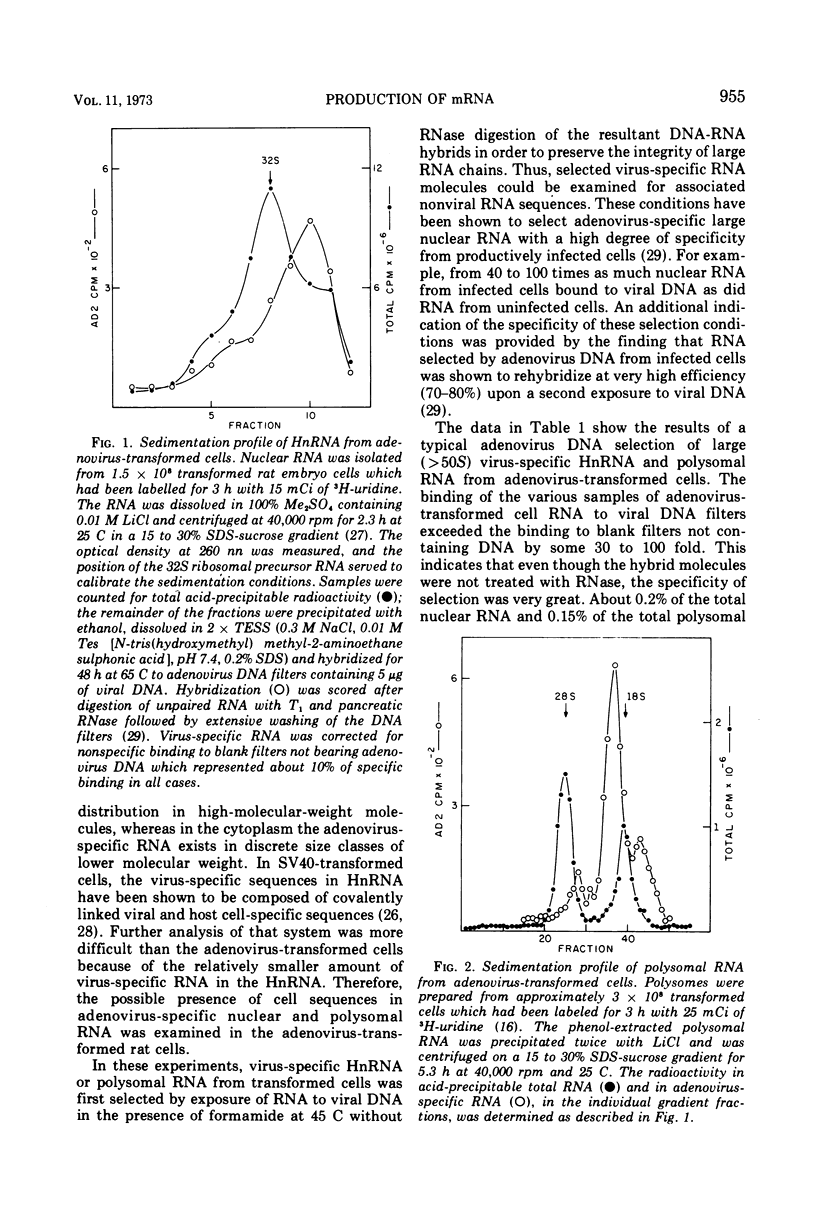
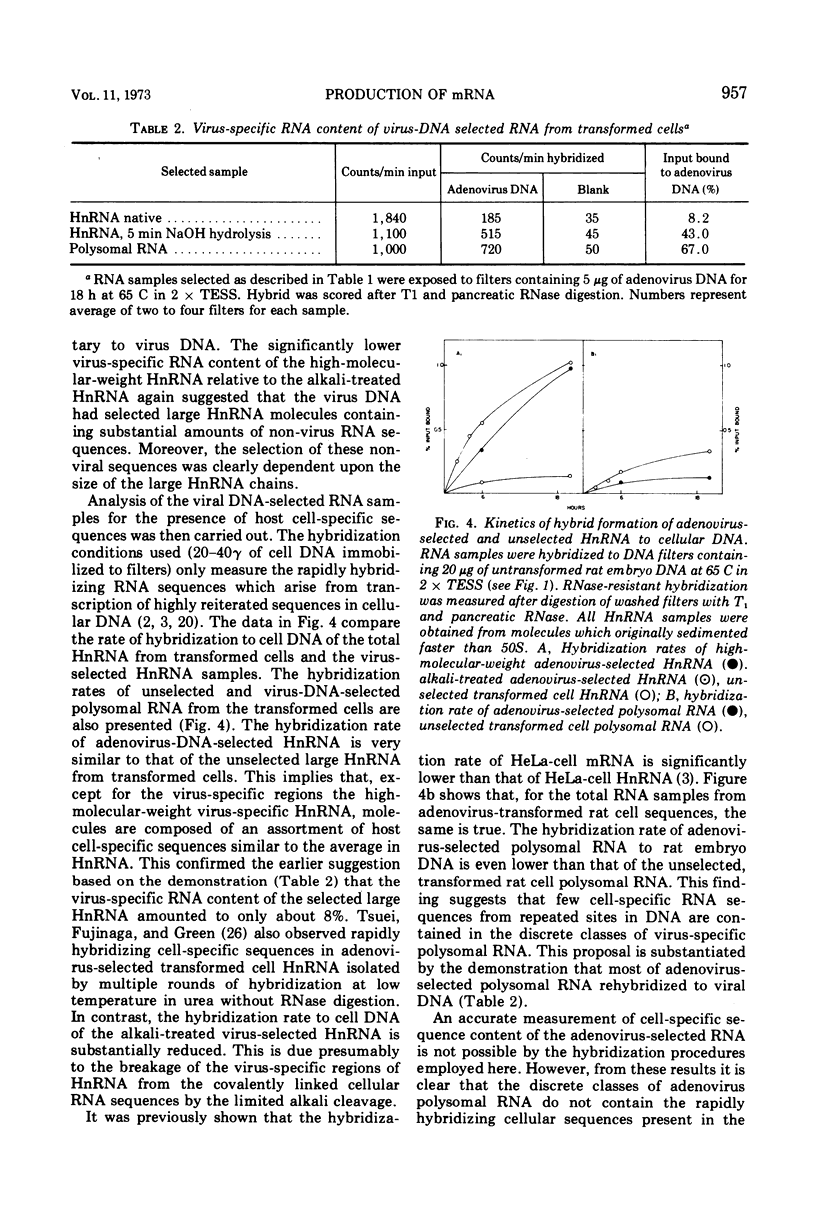
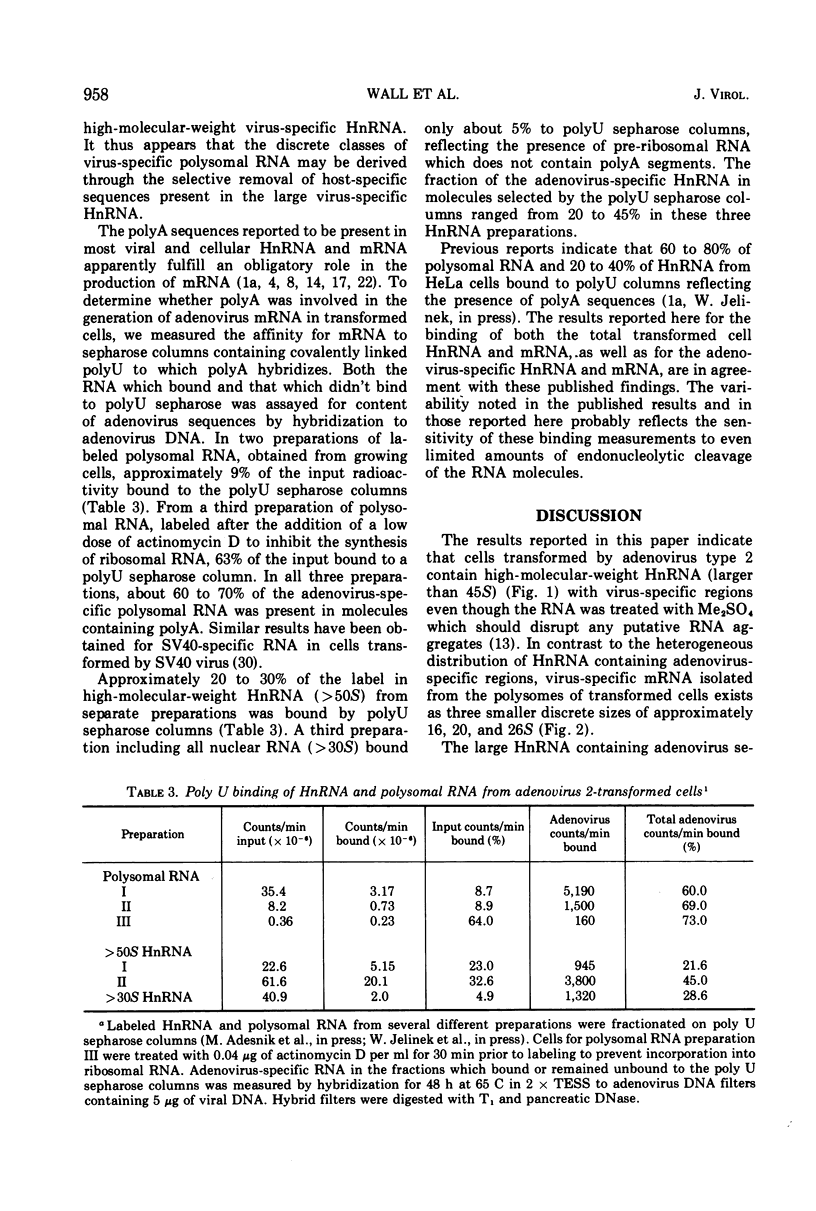
Adenovirus 2-transformed cells contain virus-specific sequences which are covalently linked to cell-specific RNA sequences in heterogeneous nuclear RNA (HnRNA) molecules larger than 45S. Virus sequences are identified by hybridization to viral DNA, and the cell sequences are detected by hybridization to cellular DNA under conditions where hybridization only occurs to reiterated sites in cell DNA. Such large composite viral-cell HnRNA molecules presumably arise through the uninterrupted transcription of host sequences and integrated viral DNA. Adenovirus-specific polysomal RNA from these cells sediments as three discrete species at 16, 20, and 26S. These specific classes of viral mRNA do not contain rapidly hybridizing host-specific RNA sequences. Both virus-specific HnRNA and mRNA contain polyadenylic acid sequences since they bind to polyU columns at levels characteristics of other polyA-terminated HnRNA and mRNA. Thus, the discrete species of virus-specific mRNA in adenovirus 2 transformed cells appear to be derived from high-molecular-weight virus-specific HnRNA through a series of post-transcriptional modifications involving polyA addition. Subsequently the HnRNA is cleaved so that the cell-specific RNA sequences that originate from the reiterated sites in cell DNA do not accompany the adenovirus mRNA to the cytoplasm. These events for the adenovirus-specific mRNA appear, therefore, to be similar to the stages in the biogenesis of the majority of mRNA in eukaryotic cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Balint R. The distribution of rapidly hybridizing RNA sequences in heterogeneous nuclear RNA and mRNA from HeLa cells. J Cell Physiol. 1970 Dec;76(3):349–356. doi: 10.1002/jcp.1040760312. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Piña M., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses. VI. A new class of virus-specific RNA molecules in cells transformed by group C human adenoviruses. Proc Natl Acad Sci U S A. 1969 Sep;64(1):255–262. doi: 10.1073/pnas.64.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Darnell J. E. SV40-specific RNA in the nucleus and polyribosomes of transformed cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1089–1096. doi: 10.1073/pnas.65.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Sporn M. B., Kelley D. E., Perry R. P. Localization of polyadenylic acid sequences in messenger ribonucleic acid of mammalian cells. Biochemistry. 1972 Aug 15;11(17):3256–3260. doi: 10.1021/bi00767a020. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Thomas W. L., Darnell J. E. Occurrence of uridylate-rich oligonucleotide regions in heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3684–3688. doi: 10.1073/pnas.69.12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagoulatos G. N., Darnell J. E., Jr Fractionation of heterogeneous nuclear RNA: rates of hybridization and chromosomal distribution of reiterated sequences. J Mol Biol. 1970 Dec 28;54(3):517–535. doi: 10.1016/0022-2836(70)90123-3. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- Tsuei D., Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: RNA transcripts containing viral and highly reiterated cellular base sequences in adenovirus-transformed cells (DNA-RNA hybridization-viral-cell mRNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):427–430. doi: 10.1073/pnas.69.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. F., Bugianesi R. L., Shen T. Y. Preparation of sepharose-bound poly (rI:rC). Biochem Biophys Res Commun. 1971 Oct 1;45(1):184–189. doi: 10.1016/0006-291x(71)90067-2. [DOI] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Poly A associated with SV40 messenger RNA. Nat New Biol. 1972 Jul 26;238(82):111–113. doi: 10.1038/newbio238111a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]



