Abstract
Ubiquitously reduced signaling via Methuselah (MTH), a G-protein coupled receptor (GPCR) required for neurosecretion, has previously been reported to extend life and enhance stress resistance in flies. Whether these effects are due to reduced MTH signaling only in specific tissue(s) and through with signaling effects reduced MTH might produce these phenotypes remains unknown. We determined that reduced expression of mth targeted only to the insulin-producing cells (IPCs) of the fly brain was sufficient to extend life and enhance oxidative stress resistance. Paradoxically, we discovered that overexpression of mth targeted to the same cells has similar phenotypic effects to reduced expression due to MTH’s interaction with β-arrestin, which uncouples GPCRs from their G-proteins. We confirmed the functional relationship between MTH and β-arrestin by finding that IPC-targeted overexpression of β-arrestin alone mimics the longevity phenotype of reduced MTH signaling. As reduced MTH signaling also inhibits insulin secretion from the IPCs, the most parsimonious mechanistic explanation for its longevity and stress resistance enhancement might be through reduced insulin/IGF signaling (IIS). However, examination of phenotypic features of long-lived IPC-mth modulated flies as well as several downstream IIS targets implicates enhanced activity of the JNK stress resistance pathway more directly than insulin signaling in the longevity and stress resistance phenotypes.
Introduction
The first single locus longevity mutation discovered in flies by a targeted forward genetic screen was in the methusaleh (mth) gene (Lin et al. 1998). A member of the Class B secretin family of G-protein-coupled receptors (GPCRs), this hypomorphic mutation (hereafter termed mth01) when ubiquitously expressed increased longevity of both sexes by 35% and enhanced survival in response to oxidative, thermal, and starvation stress. Null mutations in mth kill flies before they reach adulthood, indicating that some mth activity is essential for successful development. In addition to its longevity enhancing properties, mth01 preserves germline stem cell function with age (Wallenfang et al. 2006) and increases several aspects of sensorimotor function throughout early and midlife (Petrosyan et al. 2007). In natural populations of several Drosophila species, polymorphisms in mth experience strong positive selection and among populations of D. melanogaster ranging from Florida to New England single nucleotide polymorphisms (SNPs) in mth covary with multiple life history traits including lifespan (Schmidt et al. 2000). Under laboratory conditions, the life history effects of mth01 appear to be condition-dependent (Mockett et al. 2001; Baldal et al. 2006).
GPCRs communicate signals from extracellular peptide ligands to intracellular signaling proteins affecting a broad range of cellular processes including hormone signaling, transduction of extrinsic sensory stimuli, and neurotransmission (Rosenbaum et al. 2009). As a consequence of their extracellular activation domains and diversity of intracellular effects, GPCRs are the target of approximately half of all marketed drugs (Ja et al. 2007). MTH has to date been shown to be essential for normal neurotransmitter release in flies (Song et al. 2002), although it may have additional secretory or other functions. Specifically mth01 also decreases evoked excitatory junctional potentials (EJPs) at fly neuromuscular junctions.
One endogenous MTH ligand – SUN, the product of the stunted (sun) gene – has been identified (Cvejic et al. 2004). SUN resembles the ε-subunit of mitochondrial F1Fo-ATP synthase, which has recently been discovered to occupy extra-mitochondria locations such as the plasma membrane of multiple cell types (Cvejic et al. 2004). The endogenous source of the SUN ligand is not clear. As might be expected if SUN is a MTH agonist, reduced function mutations in sun robustly lengthen life and enhance stress resistance (Cvejic et al. 2004) as do synthetic, constitutively expressed, antagonistic MTH ligands (Ja et al. 2007). It is unclear whether there are additional MTH ligands in the fly genome, although the product of a gene closely related to sun by sequence analysis (CG31477) failed to activate MTH. Neither did SUN nor CG31477 activate other MTH-like receptors identified in the fly genome (Cvejic et al. 2004). Surprisingly given the seeming specificity of the SUN-MTH interaction, a randomly scrambled version of the synthetic MTH antagonist (R8-01) did activate MTH despite having no apparent sequence similarity to SUN (Ja et al. 2009), indicating the possibility of additional, unrelated, endogenous ligands. In fact, this scrambled synthetic peptide, termed SPAM (Serendipitous Peptide Activator of MTH), is an even more potent MTH activator than the known endogenous ligand.
Despite the accumulating knowledge about MTH and both its activating and inhibiting ligands, virtually no progress has been made in understanding the tissue specificity – if any – of its effects or the mechanism(s) by which its reduced activity extends life and enhances stress resistance. To address these questions, we investigated the potential relationship between MTH signaling other biochemical pathways known to extend life and/or enhance stress resistance in flies. Due to the known role of MTH in neurotransmitter release (Song et al. 2002), the widely-documented impact of insulin-IGF signaling (IIS) in the modulation of lifespan and stress resistance (Tatar et al. 2003), and the role of the insulin-producing cells (IPCs, also known as median neurosecretory cells or mNSCs) of the fly brain in mediating these effects via the secretion of Drosophila insulin-like peptide (DILPs), we focused our attention on how modulation of mth expression confined to the IPCs might affect longevity, stress resistance, and related traits.
Results & Discussion
Reduced mth expression targeted only to the IPCs increases longevity and enhances oxidative stress resistance
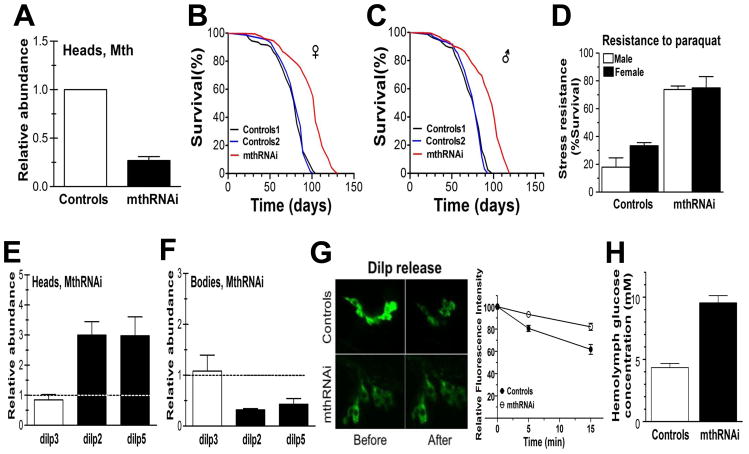
We used genomic RNAi targeted to the IPCs to knockdown mth expression locally. Transgenic flies expressing mthRNAi targeted to the IPCs were obtained from the Vienna Drosophila RNAi Center (VDRC) and backcrossed 10 times in our laboratory into a w1118 background. Flies were created according to the protocol described in Dietzl, et al., 2007 (Dietzl et al. 2007) and this RNAi construct reduced expression of mth by approximately 75% (Fig 1A). Suppression of IPC-specific MTH in this fashion increased mean fly longevity by 27% in females (Fig 1B) and 29% in males (Fig 1C) (p < 0.0001 in both cases, Mantel-Cox log-rank test). Maximum longevity was also extended as 37% of females and 50% of male IPC-mthRNAi flies were still alive when all controls had died. Both sexes also exhibited enhanced resistance to oxidative stress induced by paraquat, a potent superoxide ion generator (Frank et al. 1985) (Fig 1D). Specifically, after 24 h exposure to 20mM paraquat, more than 70% of IPC-mthRNAi flies were still alive compared with only 18% of control males and 37% of control females (p < 0.001, Student’s t-test, for both sexes). The VDRC reports that mth RNAi also suppresses the expression of one other of the 12 mth paralogs (mth2) in the fly genome. To ensure that the longevity and stress resistance effects we observed were not due to this off-target impact on mth2 expression, we also specifically reduced mth2 expression by IPC-specific RNAi and found no effect on either longevity or oxidative stress resistance (Fig. S1).
Fig 1.
RNAi knockdown of mth expression in fly IPCs significantly increases longevity and stress resistance. Both Dilp2-GAL4 and UAS-mthRNAi lines were backcrossed into w1118 for 10 generations. (A) Genomic RNAi targeted to the IPCs reduces expression of mth approximately 4-fold and data has been normalized to control that is one (B). Mean female longevity in mthRNAi flies was 104 days compared with an 82 d average in the two control lines, for males (C) analogous values were 98 days and 76 days, respectively. Longevity differences between mthRNAi and control lines were statistically significant at p < 0.0001 (Mantel-Cox log-rank test) for both sexes. (D) Resistance to 20μM of the reactive oxygen species generator paraquat is also significantly enhanced in flies with IPC-specific mthRNAi (p < 0.001, Student t-test, for both sexes). Survival shown after at 24 h exposure. (E) RT-PCR of brain-specific Dilp messages indicates that IPC targeted mthRNAi increases the abundance of Dilp2 and Dilp5 in the brain and dotted line represents the control as one but (F) reduces secretion from IPCs such that abundance in the body is lower and dotted line represents control as one There was no significant effect on Dilp3 production (G) Direct measurement of K+-stimulated DILP2 secretion shows reduced rate of disappearance of GFP-tagged DILP2 in IPC-specific mthRNAi flies compared with controls. DILP release was stimulated by high (97mM) K+ and Ca2+. Before = before K+ stimulation, After = after 15 minutes Ca++ and K+ stimulation. (H) Hemolymph glucose concentration was approximately doubled in IPC mthRNAi flies compared with controls. Control 1 = w1118 x Dilp2-GAL4; Control 2 = w1118 x UAS-mthRNAi; mthRNAi = UAS-mthRNAi x Dilp2-GAL4.
Reduced IIS is well-known to extend life in flies (Clancy et al. 2001; Tatar et al. 2001; Hwangbo et al. 2004) and signaling from the IPCs is known to be instrumental in this effect (Broughton et al. 2005). Given the known role of MTH in synaptic exocytosis, a plausible prediction for the mechanism by which IPC-specific mthRNAi extends life and enhances stress resistance is that reduced MTH inhibits secretion of DILPs from the IPCs to peripheral tissues which in turn reduces global IIS. Accordingly, we measured by quantitative RT-PCR the abundance of transcripts of the three Dilps (Dilp2, Dilp3, and Dilp5) produced by the IPCs in fly heads (where the IPCs are located) and bodies. To our surprise rather than reduced message, we found increased transcript abundance of Dilp2 and Dilp5 (but not Dilp3) in the heads of IPC-mthRNAi flies, suggesting upregulation of DILP production by the IPCs (Fig 1E). Our observations are consistent with published reports showing upregulation of some combination of DILPs by genetic or physical manipulation of IPCs or germ cells (Luong et al. 2006; Teleman et al. 2006; Broughton et al. 2008; Flatt et al. 2008; Sekine et al. 2010; Song et al. 2010). We propose that a possible explanation for increased dilp transcripts in the might be compensatory transcription in response to reduced DILP release from the IPCs. It has been suggested that both Foxo and JNK are capable of regulating Dilps at transcript level directly or by a feedback mechanism (Hwangbo et al. 2004; Wang et al. 2005). For instance, in mammals Foxo upregulates neuropeptides in the hypothalamus during stress and a similar mechanism may be operating here (Kim et al. 2006; Matsumoto et al. 2006). The same Dilp transcripts are reduced in the body also (Fig 1F).
To assess what was happening at the protein level, we directly assayed DILP2 secretion from the IPCs by standard high K+-stimulated depolarization using a DILP2-GFP reporter and indeed observed reduced DILP2 release in the IPC-mthRNAi flies (Fig. 1G). Consistent with reduced concentration of circulating DILPs, we found a nearly two-fold increase in glucose concentration in the hemolymph of our IPC-mthRNAi flies (Fig 1H). We also found as would be expected in mammals with reduced circulation insulin elevated lipid accumulation (data not shown)
Overexpression of mth targeted to the IPCs also increases longevity and enhances oxidative stress resistance
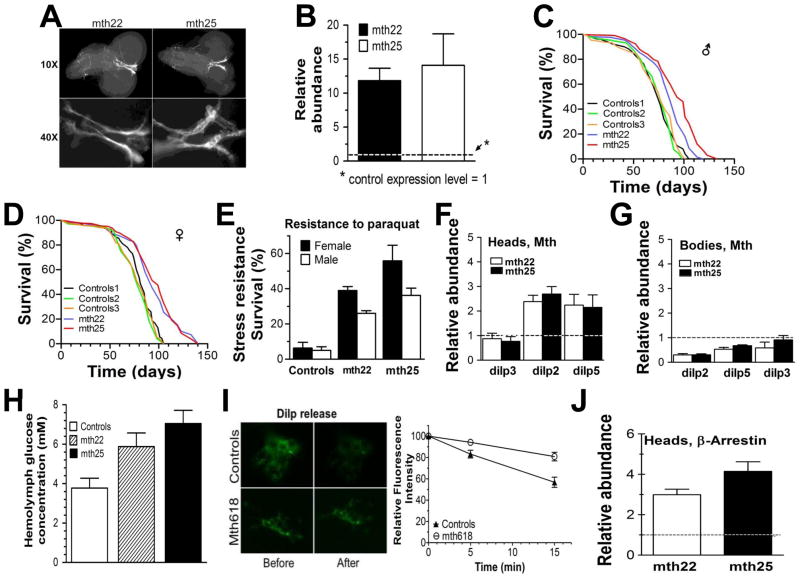
We also investigated the effect of overexpressing mth in the IPC’s on fly lifespan and oxidative stress resistance. We used mth UAS-constructs containing full length mth cDNA plus a GFP reporter in a w1118 background. Two transgenic lines were investigated in detail. The mth22 line has the transgene inserted on chromosome 2, the mth25 line on chromosome 3. As with the RNAi construct, both lines employ the IPC-specific Dilp2-GAL4 driver. We confirmed localization of MTH to the IPCs visually in fly larvae (Fig 2A) in both lines and determined by qRT-PCR that mth mRNA was increased by 12–14-fold in adults (Fig 2B) although we do not have direct evidence of DILP abundance itself. We expected that mth overexpression would shorten life and reduce oxidative stress resistance relative to controls, but in fact we observed the opposite. Mean longevity of IPC-specific mth-overexpressing males increased by 12% (mth22) and 16% (mth25) compared with the composite mean of the three control lines (Fig 2C). Female longevity showed a 14% (mth22) and 21% (mth25) increase compared similarly with controls (Fig 2D) (p < 0.0001, Mantel-Cox log-rank test in all comparisons of both transgenic lines to any of their control lines). Maximum longevity of both sexes was also extended. In males 9% (mth22) and 28% (mth25) of transgenic flies were still alive when all controls had died. For females, these values were 25% (mth22) and 33% (mth25). Notably, although mean longevity increased significantly in both transgenic lines, the quantitative longevity effect was somewhat smaller than the IPC-mthRNAi flies.
Fig 2.
Effect of tissue-specific overexpression of mth on lifespan and stress resistance (A) Localization of mth transgene in IPCs, including the axons which carry the secreted peptides to the periphery. (B) Relative mRNA abundance in the transgenic flies increased 12–14 fold compared to the mean of three control lines at 5 days of age based on real-time quantitative PCR. (C & D) Survival of transgenic flies (n = 230–330 per survival experiment) compared with controls. Flies were maintained at 25°C, 12/12 h dark/light cycle and were transferred to fresh food vials every 3–4 days. Mean longevity of males was 74, 79, and 77 days for the three control strains compared with 86 and 93 days for mth22 and mth25 lines, respectively. For females, the analogous longevities were 85, 79, and 81 days for the same control strains compared with 93 and 99 days for mth22 and mth25, respectively. (E) Stress resistance to paraquat in mth overexpressing flies. Newly eclosed flies were sex-segregated and maintained in fresh food vials for 5 days before testing. Then flies were starved for 6 h and transferred to vials containing Kimwipes wetted with 20mM paraquat in 5% sucrose solution, and monitored for survival at 25°C. Data shown are survival at 36 h post-paraquat exposure. Data for three control lines has been combined. Relative abundance of mRNAs of three Dilps produced by the IPCs in heads (F) and bodies (G) of transgenic lines compared to combined values of three control lines as measured by RT-PCR. As with the IPC-mthRNAi lines, Dilp2 and Dilp5 were significantly affected, whereas Dilp3 was not. (H) Glucose concentration in hemolymph of both transgenic lines is significantly higher than in control lines. (I) Direct observation of GFP-tagged Dilp2 release in IPCs of control (w1118 x UAS-mth618) and mth overexpressing lines (UAS-mth618 x Dilp2-GFP-Gal4) showing ~40% reduction in DILP2 release over 15 minutes in the transgenic line. DILP release was stimulated as in Figure 1. (J) Effect of IPC-specific mth overexpression on β-arrestin levels in the brains of 5 day old flies. Relative abundance of all RT-PCR is shown have been normalized to control which is shown as dotted line as one. In all other experiments: Control 1 = w1118 x Dilp2-GAL4, Control 2 = w1118 x UAS-mth22-GFP, Control 3 = w1118 x UAS-mth25-GFP; mth22 = UAS-mth22-GFP x Dilp2-GAL4; mth25 = UAS-mth25-GFP x Dilp2-GAL4.
Resistance to oxidative stress was also enhanced in these transgenic lines. In response to 20mM paraquat, controls became sluggish by 12 h and by 36 h more than 90% were dead. In contrast, both mth22 and mth25 flies were still active 24 h postexposure and survival at 36 h was enhanced 3- to 6-fold relative to controls (Fig. 2E). To verify that these results were specific to the IPCs, we also overexpressed mth specifically in the mushroom body of the fly brain and found no effect on either longevity or stress resistance (Fig S2).
Surprised at these seemingly paradoxical results, we examined how mth overexpression affected insulin production and secretion by the IPCs. As with the IPC-mthRNAi flies, we found increased transcripts of Dilp2 and Dilp5 but not Dilp3 in the heads of both transgenic lines (Fig 2F), again suggesting that these Dilp transcripts were being upregulated in the IPCs to compensate for reduced peptide release. These same Dilp transcripts were diminished relative to controls in the bodies of both transgenic lines (Fig 2G). The precise meaning of reduced Dilp transcripts in the periphery is unclear and little is known about the regulation of Dilps within and outside the IPCs which to our knowledge are the only cells in which dilp2 and dilp5 are predominately expressed. Consistent with the hypothesis of reduced circulating DILPS, we found higher glucose abundance in the hemolymph of transgenic flies relative to controls (Fig 2H). We again directly measured release of DILP2 by K+ stimulated depolarization of the IPCs. This assay was necessarily performed in another mth overexpression line (mth618) that lacked GFP-tagged mth and instead had GFP-tagged DILP2. This line (mth618) like mth22 and mth25 was also long-lived and stress resistant (Fig. S3). We observed that indeed DILP2 secretion was inhibited by IPC-specific mth overexpression (Fig 2I) as it was in the IPC-mthRNAi flies. To rule out the possibility that overexpression of mth was simply killing the IPCs, we evaluated the same Dilp transcript levels in much older (40 d) flies and it was similarly increased (Fig. S4A). In addition, we visualized GFP-labeled DILP2 and noted its presence throughout still existing IPC axons as well as distant aorta of fly heart (Fig. S4B, C). Presence of DILP2 in distant axonal projections and aorta suggests maintenance of functional transport.
Increased β-arrestin associated with mth overexpression inhibits MTH signaling
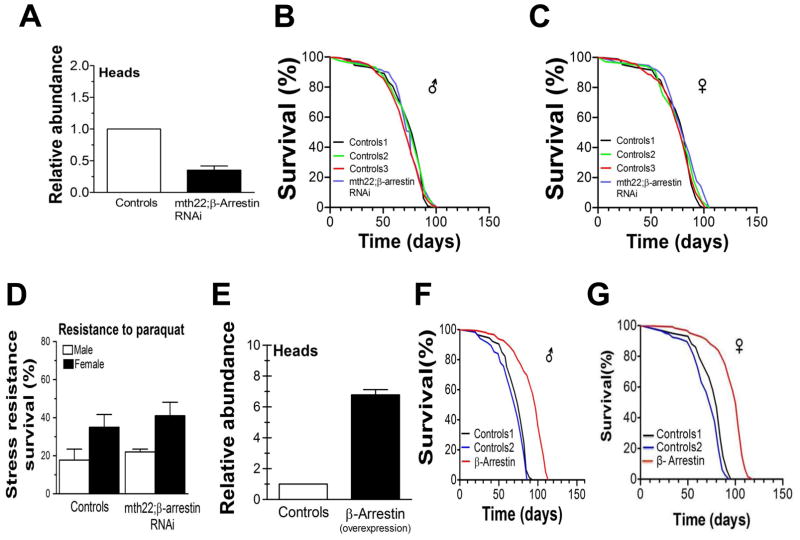
A possible mechanism by which overexpression of mth could phenocopy reduced mth expression is suggested by the potential functional relationship between MTH and β-arrestin. Arrestins, as the name implies, often act to inhibit signal transduction by any of several mechanisms, including uncoupling GPCRs from their G-proteins (Moore et al. 2007). Furthermore, β-arrestin is known to have a high affinity for secretin family GPCRs like MTH (Oakley et al. 2001). If mth overexpression in the IPCs also increased β-arrestin (called kurtz in flies) expression, conceivably as a homeostatic control to prevent MTH hyperactivation, then MTH-dependent GPCR signaling could in principle be inhibited despite increased MTH abundance. Supporting this hypothesis, we found β-arrestin expression in the heads of both mth22 and mth25 transgenic lines to be increased 3–4 fold relative to controls (Fig 2J). If this increased expression of β-arrestin indeed represents a mechanism by which mth overexpression can inhibit insulin release by the IPCs, then forced reduction of β-arrestin expression in the IPCs should increase coupling of MTH to its GPCR, thereby increasing MTH signaling and insulin release, and thus compromise the long-life and stress resistance phenotypes of mth22 and mth25 lines. To evaluate this hypothesis, we crossed flies with genomic β-arrestin RNAi targeted to the IPCs to both of our IPC-specific mth overexpressing lines. Expression of β-arrestin in these doubly manipulated lines was reduced by about two-thirds relative to both transgenic mth lines, returning its expression level to approximately that of wild-type flies (Fig 3A). This reversion of β-arrestin expression to approximately its control values in these transgenic mth lines abolished both the enhanced longevity and stress-resistance phenotypes (Fig. 3B–D). If this putative mechanism by which β-arrestin levels can regulate MTH signaling is operative, it would suggest that overexpression of β-arrestin alone in the IPCs should reduce normal MTH signaling and also extend life and enhance stress resistance We tested this hypothesis by employing the same UAS construct and Dilp2-Gal4 driver used to overexpress mth in the IPCs to overexpress β-arrestin instead. We discovered that this construct increased β-arrestin expression by 3–4 fold as shown in (Fig 3E) and resulted in a 31% increase in mean male longevity compared to both control lines (Fig 3F) and an 18% and 25% increase in female longevity compared to both controls (Fig 3G).
Fig 3.
Effect of targeted manipulation of β-arrestin abundance in the IPCs. (A) Relative suppression of β-arrestin abundance by IPC-specific β-arrestin RNAi compared with control strain (w1118 x Dilp2-GAL4). Simultaneous IPC-specific overexpression of mth and suppression of IPC-specific β-arrestin suppression by RNAi abolishes the longevity phenotype (B,C) and the stress resistance phenotype (D) in both sexes. Control 1 = w1118 x Dilp2-Gal4, Control 2 = w1118 x UAS-mth22, Control 3 = w1118 x UAS-β-arrestinRNAi, mth22;β-arrestinRNAi = Dilp-2-Gal4 x UAS-m22-β-arrestinRNAi. Overexpression of β-arrestin alone in the IPC’s (E) also extends life in both sexes (F,G). Relative abundance of all RT-PCR is shown have been normalized to control that is one. Control 1 = w1118 x Dilp2-Gal4, Control 2 = w1118 x UAS-β-arrestin, β-arrestin = Dilp2-Gal4 x UAS-β-arrestin.
IPC-specific mth longevity effects are FOXO-dependent
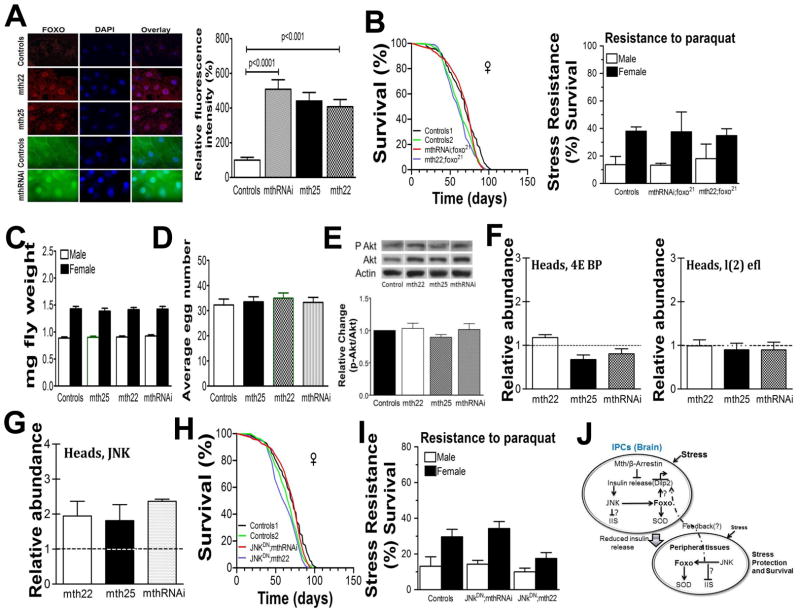
Enhanced longevity and stress resistance in C. elegans and flies are often the result of signals from several pathways integrated and coordinated by activation of the FOXO transcription factor (Hwangbo et al. 2004; Wang et al. 2005; Broughton & Partridge 2009; Hay 2011). Under conditions of abundant food and/or low stress, FOXO is bound to 14-3-3 proteins in the cytoplasm and thus inactivated, but under low food or high stress it translocates to the nucleus where it activates a symphony of genes, including cellular antioxidants and molecular chaperones, increasing survival and enhancing stress resistance (Brunet et al. 2004; Essers et al. 2004; Hwangbo et al. 2004; Murphy 2006). In order to determine whether activation of Drosophila FOXO (dFOXO) might play a key role in IPC-mth-mediated longevity and stress-resistance enhancement, we first visualized its cellular location (Junger et al. 2003) in both of our mth overexpression lines. In all cases, compared to controls, the long-lived strains displayed enhanced nuclear localization of dFOXO (Fig 4A). We also found that dFOXO localized to the nucleus in the originally published long-lived mth01 mutation (1) (Fig S5). In addition, we observed, as expected with the increased nuclear location of FOXO, that both CuZn- and MnSOD activities were increased (Fig S6). Seeking to confirm the role of dFOXO in these longevity and stress resistance phenotypes, we crossed a mutant loss-of-function FOXO variant, dfoxo21(Junger et al. 2003), into our long-lived IPC-mthRNAi and mth22 overexpression lines and found that both their longevity and stress-resistance phenotypes were abolished in both females (Fig 4B) and males (Fig S7).
Fig 4.
(A) Enhanced nuclear localization of dFOXO in all three IPC-mth mediated long-lived fly genotypes. (B) A mutant loss- of- function dFOXO variant abolishes both the longevity and stress resistance phenotypes of both IPC-mth underexpressing and overexpressing fly lines. (C) Body weight for UAS-mth25, mth22 and mthRNAi crossed with Dilp2-Gal4 in comparison to controls w1118 x Dilp2-Gal4 shows no difference (p>0.05. males [white], females [black], n = 50 flies). The flies were weighed in batches of five and 10 replicates for genotype. The sensitivity of the balance was +−/− 0.01 mg. (D) Total cumulative eggs laid per female over 15 days UAS-mth25, mth22 and mthRNAi crossed with Dilp2-Gal4 in comparison to controls w1118 x Dilp2-Gal4 shows no difference (p>0.05). (E) Akt phosphorylation was examined using anti-phospho-(Ser505) Akt and anti-Akt antibodies. Actin was used as a loading control. To assess Akt kinase activity, phosphorylation state of Akt substrates in adult lysates of the indicated genotypes was analyzed using phospho- Akt substrate antibody. The ratio of phospho-Akt to total Akt in controls was arbitrarily set to 1. We find no significant difference between controls and mth25, mth22, or mthRNAi fly lines. Experiments were performed four times. Data are mean ± SE; p>0.05 in all comparisons. (F) RT-PCR of brain-specific 4E BP and l(2) efl messages indicates that IPC targeted m22, m25 and mthRNAi showed no change in the abundance of immediate Foxo target genes. (G) JNK (=Bsk) message is increased in the heads by roughly two-fold in mth22, mth25 and mthRNAi targeted to IPCs. A mutant dominant negative JNK (= Bsk) abolishes both the longevity (H) and stress resistance (I) phenotypes of both IPC-mth overexpressing and underexpressing fly lines. (J) A working model incorporating the complex relationships among IPC, Mth, β-arrestin, JNK, insulin, and FOXO signaling. Mth and β-arrestin inhibits insulin secretion and subsequently complexes with JNK to activate Foxo via stress dependent pathway independent of classic IIS pathway. Further Foxo activates Sod to increase stress resistant and lifespan. In peripheral tissues oxidative stress affects JNK/FOXO signaling which may mediate peripheral stress response or remotely via IPCs is an open question. Relative abundance of all RT-PCR is shown have been normalized to control which is shown as dotted line as one.
So far we have shown for the first time that the critical targets for the longevity and stress resistance phenotypes associated with reduced MTH signaling are the IPCs of the fly brain. We have also determined that the lifespan and oxidative stress effects of MTH signaling are FOXO-dependent and rely not only on the abundance of MTH itself, but also on the abundance of the β-arrestin scaffold protein which interacts with MTH to inhibit its signaling in these critical cellular targets. Furthermore, we have shown that MTH activity inhibits the secretion of two of the three insulins produced in the IPCs and increases systemic levels of circulating glucose. In sum, these results are consistent with a mechanisms by which reduced MTH signaling increases longevity and enhances stress resistance by directly reducing systemic IIS as it has previously been shown in worms and mice as well as flies(Apfeld & Kenyon 1999; Clancy et al. 2001; Tatar et al. 2001; Ikeya et al. 2002; Bluher et al. 2003).
However, other observations lead us to question this direct and parsimonious link between mth expression in the IPCs and IIS. First, substantially reduced IIS typically leads to reduced adult body size and/or fecundity (Clancy et al. 2001; Tatar et al. 2001; Ikeya et al. 2002; Broughton et al. 2005). As with the original long-lived mth01 mutation (Lin et al. 1998), we find neither reduced body size nor fecundity in either our long-lived IPC-mthRNAi or IPC-mth overexpressing fly lines (Fig 4C,D). In addition, a major downstream phosphorylation target of IIS is AKT and we find no significant change in the p-AKT:AKT ratio in any of our long-lived IPC-mth modulated flies (Fig 4E). Finally, translocation of dFOXO from cytoplasm to the nucleus via IIS typically stimulates the transcription of a symphony of downstream targets including the translational regulator 4E-BP (thor in flies) and the small heat shock protein l(2)efl (Puig et al. 2003; Wang et al. 2005; Flatt et al. 2008). Transcript levels of neither of these targets are affected by IPC-mth modulation (Fig 4F). Although these are commonly known Foxo downstream targets, work from different groups has shown that Foxo signaling is complex. Subtle changes in stress conditions or with interacting signaling partners can induce novel signaling partners and targets (Salih & Brunet 2008). Other possible explanation could be redundancy of different Dilps role in IIS signaling because it has been shown that only knocking down all three dilps2,3 and 5 affect 4E-BP transcript level (Gronke et al. 2010). In case of Mth-dependent reduction in insulin release affects Dilps2 and 5 transcript levels not Dilp3. The compensatory functions of Dilp3 could be important in masking many obvious impaired IIS dependent phenotypes.
Another possibility is that MTH signaling in the IPCs engages the JNK stress-signaling pathway which itself can directly activate dFOXO and repress IIS, thus leading to increased longevity and stress-resistance (Wang et al. 2005; Karpac & Jasper 2009). There are several a priori reasons for examining the JNK pathway in this context. JNK is known to be capable of activating FOXO (Wang et al. 2005). In addition, JNK signaling specifically in the IPCs is essential for stress tolerance (Karpac et al. 2009), and perhaps most intriguingly, β-arrestin can act as a scaffold protein to bring together GPCRs and JNK (McDonald et al. 2000). We investigated this possibility by assessing JNK (called Bsk in flies) transcript levels relative to controls in the brains of our IPC-mthRNAi as well as mth22 and mth25 overexpression lines of long-lived stress-resistant flies. In all cases we observed increased JNK transcript abundance (Fig 4G). We also found JNK transcripts elevated compared with controls in the heads of the original long-lived mth01 mutant flies (Fig S6). To further explore the potential functional significance of these observations, we co-targeted a dominant negative form of JNK (BskDN) (Wang et al. 2005) to the IPCs in both our IPC-mthRNAi and mth22 flies and found that both longevity and oxidative stress phenotypes reverted to control levels in both females (Fig 4H) and males (Fig S8B). Stress resistance (Fig, 4I), FOXO subcellular localization and IPC insulin release also reverted to control levels when JNK signaling was inactivated (Fig. S8C, D). Together these observations strongly implicate local JNK signaling downstream of altered mth expression in the IPCs as a key pathway modulating both longevity and stress resistance. Our current working model of the relationship among IPC-specific mth, β-arrestin, insulin release, JNK and FOXO activity is shown in Fig 4J. We propose that mth/β-arrestin inhibits insulin secretion and complexes with JNK to activate downstream Foxo intracellularly and further Foxo activates Sod to increase stress resistance. We speculate that Mth/β-arrestin/JNK function is geared towards stress pathway which may function independent of canonical IIS pathway. Modulation of IPCs and changes in stress tolerance within neurons seem to be sufficient to protect peripheral tissues in spite of insulin resistance like phenotype. This insulin resistance phenotype may initiate a feedback response from periphery which influences the production of dilps in IPCs via Foxo or an unknown mechanism. In summary Mth/β-arrestin/JNK-mediated phenotypes are due to age-related protection of critical neurons and subsequently whole organism by activating systematic humoral response to increase stress resistance and lifespan.
Materials and Methods
Drosophila stocks
A set of methuselah transgenes, mth01, UAS-mth22-GFP, UAS-mth25-GFP and UAS mth618, with full length cDNA was gift from late Seymour Benzer. The w1118 the parental strain was also from Benzer’s laboratory. The Dilp2-Gal4, Foxo21 strains were provided by Marc Tatar (Brown University, Providence, RI). Flies transgenic for RNAi of IPC-targeted mth & mth2 RNAi and β-arrestin (kurtzRNAi) were provided by the Vienna Drosophila RNAi Stock Center. UASBskDN, UAS β-arrestin from Bloomington stock centre and ok107 was gift from Dr. Kanae lijima-Ando, Thomas Jefferson University, Philadelphia.
All the fly crosses described were generated from crosses of virgin females and males from the specified strain. All fly genotypes used in these experiments were backcrossed into w1118 for at least 10 generations.
Food medium and rearing conditions
Flies were transferred to fresh food vials ((0.45% agar, 5% dextrose, 2.5% sucrose, 8.3% corn meal and 1.5% dried yeast, all (w/v)) with phosphoric and propionic acids supplemented to prevent mold, as previously described(Lewis, E.B. A new standard food medium. Drosophila Inf. Serv.34, 117–118 (1960))every 3–4 d and scored for survival. All flies were maintained at 25°C, 60% relative humidity and 12 h light: 12 h dark.
Lifespan Analysis
Flies were raised in bottles containing Lewis medium. Groups of eclosed adults (0–3 d old) were transferred to fresh bottles and allowed to mate for 2 d. Males and females were then separated under CO2 anesthesia and randomly distributed into 1 L demography cages. These cages were adopted from Marc Tatar (Brown University) and each with a ventilated lid, a gasket-covered aperture and a 25-mm-diameter plastic tube affixed to an opening along the cage side near the floor. Food vials were attached via the tube and changed every 3–4 d. Male and female flies were aged separately and in each cage at least 150–200 flies were introduced. For each crosses 3–5 replicate demography cages were used for data analysis. All the statistical analysis was done using Graph Pad or Systat 13.0 software.
Stress Test
Flies were collected within 24 hrs post eclosion. Male and females flies were separated on ice; 200 flies in groups of 20 were aged for 5 days. For oxidative stress experiments, flies were maintained at 25C (60% humidity, 12 hrs light-dark cycle) and kept in 50 ml vials with filter papers discs moistened with either 5% sucrose alone or 20mM paraquat with 5% sucrose for 24 to 40 hrs after being starved for 6 hrs.
Immunostaining
Larval brain, salivary gland and fat body were dissected in Standard saline for NMJ dissection (without calcium). Subsequently specimen were fixed in 4% formaldehyde in PBT for 30 minutes at RT and the primary antibody Foxo (1:200), and anti-GFP (1:200) were used to incubate O/N at 4°C and DAPI at working concentration of 300nM were used at RT for double staining. These images were analyzed using confocal fluorescence microscopy. Antibody against Anti-Foxo Rabbit polyclonal antibody was bought from Cell Signaling and Puig Foxo antibody gift from Marc Tatar, Brown University. Rat Anti-GFP and DAPI were bought commercially available.
Quantitative RT-PCR
Semi-quantitative reverse transcriptase PCR experiments
Transcript levels of Drosophila methuselah and other components were measured with quantitative PCR. Live flies were frozen in liquid nitrogen and stored at −80 °C. Heads were separated using a funnel with fine mesh. Total RNA was isolated from at least 50 to 75 heads of 5 and 40 day old flies, using RNeasy kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. The RNA purity and amount were measured spectrophotometrically. Single stranded cDNA was generated with 0.5 μg of total RNA, using the reverse transcriptase kit from Applied Biosystems. Real-time PCR was performed using the Taq SYBR Green Supermixon an ABI prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). CT values for the detected mRNA levels for each gene was normalized by two independent internal controls: the housekeeping genes actin and RP49. Both internal control yielded similar results. The relative change in mRNA from control and experimental conditions was estimated using the 2−ΔCT method (LivakandSchmittgen,2001). Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta DeltaC (T)) Method. Methods. 2001 Dec;25(4):402–8.
The primers were:
mth F ‘TTTTTTGGCTTTCCGTCATCA’, R ‘TTGTGGGAGGAGCCTCTGAA’
Dilp2 F ‘AGCAAGCCTTTGTCCTTCATCTC’, R ‘ACACCATACTCAGCACCTCGTTG’
Dilp3 F ‘AGAGAACTTTGGACCCCGRGAA’ R ‘TGAACCGAACTATCACTCAACAGTCT’
Dilp5 F ‘GAGGCACCTTGGGCCTATTC’ R ‘CATGTGGTGAGATTCGGAGCTA’
JNK (Bsk) F ‘TTCACAGAGACTTAAAGCCA’ R ‘CATAGGGAGTCATCATAAAGGT’
Arrestin (Kurtz) F ‘TACCACCACGGCGAAAAAA’ R ‘ACCGTCCGATTTGAGTTGTTG’
Thor (4E-BP) F ‘TCAAGCCATCACCCAGG’ R ‘TAAGTTTGGTGCCTCCAGG’
I(2)efl F ‘AGGGACGATGTGACCGTGTC’ R ‘CGAAGCAGACGCGTTTATCC’
Methods for the mthRNAi
UAS-RNAi strains were generated at VDRC. For details please see ref. Dietzl et al [Nature 448,151–156,12 Jul 2007]. We will like to acknowledge the VDRC for sharing the reagents.
Insulin Release assay
For the first time direct assay of insulin secretion was performed at IPCs by using UAS-Dilp2-GFP; Dilp2-Gal4 (Flies were gift from Ed. Levitan from University of Pittsburg) expressing to IPCs. This reporter flies were crossed to UAS-mth RNAi and UAS-mth618 transgenes. The rationale behind using this strain was that it does not have GFP tag which will complicates the change of fluorescence measurement in case of UAS-mth22 or 25-GFP flies. Depolarization of the preparation with the high K+ caused a decrease in the fluorescence of the IPCs as the insulin peptide released by exocytosis. We used the saline normally used to depolarize NMJ (Neuromuscular synapses). Standard saline 128 mM NaCl, 2 mM KCl, 1.8 mM Ca, 4 Mg, 35.5mM sucrose, 5 mM sodium HEPES, pH 7.2 without Ca for dissection and subsequently solution was changed to high-potassium standard saline Same as standard saline except replace 85 mM NaCl with KCl. In brief, preparation was stimulated by high-potassium for 15 minutes and to avoid any photo-bleaching picture were taken at 5 minutes and 15 minutes. Insulin peptides are released in two phases and 5 minutes represent early release and 15 minutes is delayed release. We have successfully employed this method earlier and methods presented here is a modified version of Ranjan et al., J. Neuroscience 1998. We have quantified the fluorescence on a fluorescence microscope, using ImageJ software freely available from the NIH.
Glucose Measurement
For the determination of glucose levels in hemolymph, the Sigma hexokinase assay kit was used. Manufactures instructions as well as Broughton S J et al PNAS: 102 (8) 3105 reference suggestions were followed. In brief, Hemolymph was pooled from females after 5-h starvation. Flies were decapitated, and hemolymph was collected from the thorax by capillary action and subsequently glucose was measured.
SOD Measurement
SOD activity for Mn-SOD and CuZN-SOD in 5 and 40 day flies were measured by using Superoxide Dismutase Assay Kit II from Calbiochem Cat. No. 574601.
Supplementary Material
Acknowledgments
We wish to thank Marc Tatar, Brown University, for Foxo and Dilp2 antibodies, Foxo mutants and Dilp2-Gal4 lines, Edwin Levitan, University of Pittsburgh, for UAS-Dilp2-GFP and Dilp2-Gal4 lines, and the Vienna Drosophila RNAi Center for the mth and β-arrestin RNAi flies. This research was supported by an Ellison Medical Foundation New Scholar Award to R.R and an NIH National Institute on Aging grant (R01 AG037962) to S.N.A.
References
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Baldal EA, Baktawar W, Brakefield PM, Zwaan BJ. Methuselah life history in a variety of conditions, implications for the use of mutants in longevity research. Exp Gerontol. 2006;41:1126–1135. doi: 10.1016/j.exger.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nature cell biology. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. Embo J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D’Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci U S A. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R, Rasper J, Braun HE, Ashton G. Disappearance of organochlorine residues from abdominal and egg fats of chickens, Ontario, Canada, 1969–1982. J Assoc Off Anal Chem. 1985;68:124–129. [PubMed] [Google Scholar]
- Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS genetics. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. Interplay between FOXO, TOR, and Akt. Biochimica et biophysica acta. 2011;1813:1965–1970. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current biology: CB. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Madrigal M, Roberts RW, Benzer S. The Drosophila G protein-coupled receptor, Methuselah, exhibits a promiscuous response to peptides. Protein Sci. 2009;18:2203–2208. doi: 10.1002/pro.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, West AP, Jr, Delker SL, Bjorkman PJ, Benzer S, Roberts RW. Extension of Drosophila melanogaster life span with a GPCR peptide inhibitor. Nature chemical biology. 2007;3:415–419. doi: 10.1038/nchembio.2007.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Hull-Thompson J, Falleur M, Jasper H. JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell. 2009;8:288–295. doi: 10.1111/j.1474-9726.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Jasper H. Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab. 2009;20:100–106. doi: 10.1016/j.tem.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nature neuroscience. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell metabolism. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Orr WC, Rahmandar JJ, Sohal BH, Sohal RS. Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp Gerontol. 2001;36:441–463. doi: 10.1016/s0531-5565(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol. 2006;41:910–921. doi: 10.1016/j.exger.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Petrosyan A, Hsieh IH, Saberi K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes & development. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PS, Duvernell DD, Eanes WF. Adaptive evolution of a candidate gene for aging in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10861–10865. doi: 10.1073/pnas.190338897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine O, Love DC, Rubenstein DS, Hanover JA. Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. The Journal of biological chemistry. 2010;285:38684–38691. doi: 10.1074/jbc.M110.155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Ranjan R, Dawson-Scully K, Bronk P, Marin L, Seroude L, Lin YJ, Nie Z, Atwood HL, Benzer S, Zinsmaier KE. Presynaptic regulation of neurotransmission in Drosophila by the g protein-coupled receptor methuselah. Neuron. 2002;36:105–119. doi: 10.1016/s0896-6273(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Song W, Ren D, Li W, Jiang L, Cho KW, Huang P, Fan C, Song Y, Liu Y, Rui L. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell metabolism. 2010;11:427–437. doi: 10.1016/j.cmet.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes & development. 2006;20:417–422. doi: 10.1101/gad.374406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.