Abstract
Snail and Slug play critical roles in the epithelial to mesenchymal transition (EMT), the mesenchymal to epithelial transition (MET) and in the maintenance of mesenchymal morphology. In this research, we investigated the correlation of DNA methylation with the transcriptional level of these two genes during the EMT/MET process. First, we used several cell lines associated with EMT/MET processes of induced pluripotent stem cell generation and differentiation, trophoblast invasion, as well as cancer progression to examine the association between DNA methylation and transcription levels of these two genes. We found an inverse correlation between DNA methylation of first intron regions and transcription levels of Snail and Slug genes in these EMT/METs. To further verify the results, we treated two trophoblast cell line BeWo and HTR8/SVneo and one induced pluripotent stem cell line with 5-aza-2′-deoxycytidine (5-aza-dC), an inhibitor of DNA methyltransferase, which caused increased expression of these two genes. Lastly, we cloned the promoters of both Snail and Slug into pGL3-Basic vector, after in vitro DNA methylation and transfection into IMR90 and HTR8/SVneo cells; we observed the significant reduction of their promoter activity due to DNA methylation. In summary, based on these results, DNA methylation is one of the molecular mechanisms regulating Snail and Slug genes during EMT/MET process.
Keywords: Stem cell differentiation, epithelial-to-mesenchymal transition, DNA methylation
Introduction
Epithelial-mesenchymal transition (EMT) is a process whereby epithelial cells lose their polarity and adhesiveness, change into a mesenchymal phenotype and gain the capacity of increased mobility, whereas mesenchymal-epithelial transition (MET) is the opposite biological process. EMT is involved in multiple biological processes including embryogenesis, organ development, trophoblast cell invasion, and cancer progression [1]. METs are commonly associated with cancer metastasis and somatic cell reprogramming [2]. Defective EMTs or METs are often associated with failures in embryonic development and diseases [3].
The Snail family proteins, Snail (coding by SNAI1 gene) and Slug (coding by SNAI2 gene) proteins, play critical roles in initiating EMT, repressing MET and maintaining mesenchymal phenotype [4,5]. As zinc-finger transcription factors whose sequence is highly conserved among mammals, these proteins function as transcriptional repressors via binding to E-Box sites on the promoters of target genes such as E-cadherin, Claudins, MMPs, and Wnt [6]. Dys-regulation of Snail and Slug and defective EMTs have been observed in multiple diseases. For instance, in cancer biology, over-expression of Snail and Slug are often observed in highly metastatic cancer cell lines and are associated with poor therapeutic prognosis [7]. In placental trophoblast cells, dys-regulation of Snail has been associated with the decreased trophoblast invasion and shallow placentation seen in pre-eclampsia [8]. Furthermore, in an embryonic stem cell in vitro differentiation model, re-activation of Snail and Slug coincides with stem cell differentiation [9] whereas knock-down of Snail and Slug dramatically impairs the ability of embryonic stem cells to differentiate [10]. Beside their roles in EMT, Snail and Slug are also important in MET processes. Silencing of Snail and Slug is necessary for MET initiation and it has been reported that Snail knock-down facilitates the generation of induced pluripotent stem cells(iPSC) from fibroblast donor cells [2,11]. These data indicate that Slug and Snail are critical gateway genes for epithelial cells moving into or out of the mesenchymal cell state via EMT or MET. Due to their important biological roles, unraveling the transcriptional regulation mechanism of Snail and Slug genes is a key to understanding embryo development and the etiology of EMT/MET-associated diseases.
Chromatin modification and DNA methylation are important mechanisms of epigenetic gene regulation. Histone deacetylases(HDAC) and histone de-acetylation are involved in the repression of Snail gene [12]. Furthermore, in mouse cancer study, the transcription of Snail was reported to be associated with the DNA methylation of its proximal promoter [13], however, the role of DNA methylation in human Snail gene has not been established. Likewise, the role of DNA methylation in Slug gene regulation is largely unknown. In this study, we investigated the regulation of Snail and Slug transcriptional activity by DNA methylation. We further demonstrated that DNA methylation of Snail and Slug genes correlates with EMT during induced pluripotent stem cell differentiation, trophoblast invasion and cancer progression.
Materials and Methods
iPSC culture and fibroblast differentiation
IMR90 cells were purchased from the American Type Culture Collection (ATCC) and cultured in DMEM supplemented with 10% fetal bovine serum and 2 mmol/L l-glutamine. iPSC were established using IMR90 cells in our laboratory and cultured in growth media (DMEM/F12+20% FBS+ 10ng/ml FGF2) on top of mouse embryonic fibroblast cells. To differentiate into fibroblast cells, iPSC were cultured in DMEM supplemented with 10% FBS with weekly passaging. After six weeks, the homogenous fibroblast cells were collected as iPSC-derived fibroblasts.
Cell culture and 5-aza-dC treatment
Two cancer cell lines (S18 and S22) with different metastatic abilities were used [14]. These cells were cultured in DMEM supplemented with 10% FBS. Two trophoblast cell lines, BeWo, obtained from ATCC and HTR8/SVneo, a gift from Professor Christ Graham, are cultured in DMEM supplemented with 10% FBS.
For 5-aza-dC treatment, 5 × 103 BeWo, HTR8/SVneo, and iPS cells were plated in wells of 24-well dishes before treatment. 0,05, 2.5, and 5.0µM 5-aza-dC (Sigma) were added into culture media for 2 or 3 days. Medium was refreshed every other day prior to the harvesting cells for RNA analysis.
Immunocytochemistry of E-Cadherin and VIM
Cells were fixed by 4% paraformaldehyde for 20 mins. After cell membrane was penetrated by 0.5% Triton X-100 in PBS for 20 mins, blocked with PBS with 4% BSA for half hour, and then incubated with antibody against E-Cadherin and VIM (Abcam, CA) for two hours. The secondary antibody conjugated with FITC was incubated for one hour. Nuclei of Cells were stained by DAPI (Invitrogen) and examined under a Nikon microscope equipped with fluorescence optics.
Sodium bisulfite genomic sequencing
Genomic DNA from both cultured cells were extracted, and then the DNA methylation-Gold kit (Zymo Research) was used to bisulfite treat genomic DNA, followed by amplification via nested PCR (2 rounds of 35 cycles) using primers listed in table 1. PCR products were then cloned into pTopo TA vector (Invitrogen), and ten to twelve clones from each sample were selected for sequencing.
Table 1.
Primers for quantitative RT-PCR, bisulfite genomic sequencing and cloning Snail and Slug promoters.
| Gene symbol |
NCBI GI/ID | Primer sequence (5` to 3`) | position | |
|---|---|---|---|---|
| Quantitative RT-PCR primers | ||||
| Slug | GI:324072669 | F | CCTTCTCCAGAATGTCTCTC | 921–1085 |
| R | ATTTGGTTGGTCAGCACAGGAGA | |||
| MMP3 | GI:73808272 | F | ACACACACTTTGAAGAGTAACAGC | 1461–1583 |
| R | CGAACATTTCAATTCACAGAGAC | |||
| Snail | GI:301336132 | F | GGCGTGTGCTCGGACCTTCT | 801–920 |
| R | AGGCAGGGGCAGGTATGGAG | |||
| Bisulfite genomic sequencing primers | ||||
| Snail 1st | +705 | |||
| Intron | 6615 | OutF | TTTGTTTTGTGGATAATTTTTTTG | |
| InF | ATTTTTTTGATTTAATTATGTATTGA | +716 | ||
| InR | TACCACCCTAAAACTCTCCTAAAA | +1114 | ||
| OutR | CATCTAACAAAAAAATCAACTCTACC | +1136 | ||
| Slug 1st | ||||
| Intron | 6591 | OutF | TGTATATATGTATAATGAGTATGTGAAAG | +501 |
| InF | ATGTGAAAGATGAGTAATATAAGGATTGA | +521 | ||
| InR | CTTAATACACACATAAACTAACAACTAC | +803 | ||
| OutR | TAAAATATTTCATTTCCACCCTAACA | +846 | ||
| MMP3 | 4314 | OutF | GGAAATGGTTTTGTTGTTATTTGGA | −101 |
| InF | GTTATTTGGATGAAAGTAAGGATGAG | −89 | ||
| InR | TAAACAAAATTCATACTAATATCCTC | +169 | ||
| OutR | AAAAACTCCCCACCTAACCAAATCAATT | +210 | ||
| Snail | ||||
| Promoter | 6615 | OutF | GAGGAAATTTT(C/T)GTTTTTTTTTAAGTT | −442 |
| InF | GGGGTTTTATTT(C/T)GTAGAGGTTT | −300 | ||
| InR | CCAC(G/A)CCCCTTTATCACCTCC | −70 | ||
| OutR | AAAAAATAATAAAAAATAAAAAAAAC | −33 | ||
| Slug | ||||
| Promoter | 6591 | OutF | AGGTTTTTATTAATATTAGAGGTTG | −409 |
| InF | TTGGTTTGGTGTGGTGTAGGG | −387 | ||
| InR | TAAATATTTTCAAAAAAAATAACCT | −194 | ||
| OutR | ATTCAAAATAAACTATTTTTTAAAATTTC | −143 | ||
| Primers for cloning Snail and Slug promoters | Position to TSS | |||
| Snail | 6615 | F | AAGCGCTCAGACCACCGGGC | −350 |
| R | GGGAAGAGACTGAAGTAGAGGAGA | +1126 | ||
| Slug | 6591 | F | TTGTGCAAGGCAAACCTCTC | −450 |
| R | GTATGACAGGCATGGAGTAACTCTC | +1035 | ||
DNA methylation inversely correlated transcription levels of Snail and Slug genes.
5-aza-dC treatment increased expression of Snail and Slug genes.
DNA methylation decreased Snail and Slug promoter activities.
Analysis of mRNA expression by Quantitative RT-PCR
Total RNA was prepared using RNA easy kit (Qiagen, CA). Trace level DNA was eliminated with RNase-free DNase I (Qiagen, CA) pre-treatment. The total RNA was reverse-transcribed into cDNA with Superscript III First-Strand Synthesis System for quantitative RT-PCR (Invitrogen, CA).The power SYBR PCR regent (ABI, CA) was used for running qPCR. Delta Ct method was conducted to analyze results. At least three biological replicates were performed.
Luciferase reporter assays
Luciferase reporter assay was performed according to published procedure [15]. Briefly, Snail and Slug upstream regions, −350 to +84 and −450 to +164 relative to transcription start site, were cloned into pGL3-Basic vector (Promega), upstream of firefly luciferase coding sequence via PCR and subsequent ligation. Primers were listed in table 1. In vitro methylation of these pGL3-Snail and pGL3-Slug plasmids was performed by incubation with SssI methylase (New England) for 3 hrs. The resistance of these methylated plasmids to BstUI digestion was used to verify the success of in vitro methylation.
IMR90 cells plated at 4×104 cells per well in a 96-well plate were transiently transfected with 0.1 µg of methylated and non-methylated plasmids, along with 0.005µg of the internal control plasmid (pRL-TK vector, expressing Renilla luciferase using the Lipofectimine 2000 transfection reagent (In vitrogen, CA). 30 hours post-transfection, the activities of both luciferases were determined using the Dual-Luciferase Reporter System (Promega) according to the manufacturer's instructions. Assays were performed 3 times each in duplicate and promoter activity determined by the ratio of the two luciferase activities.
Statistical analysis
The students` T Test was used for the statistical analysis of qPCR and luciferase reporter assays. A P-value of less than 0.05 was considered statistically significant.
Results
The transcription levels of Snail and Slug genes were inversely associated with the DNA methylation status of first intron regions in EMT and MET during iPSC generation from fibroblast and its subsequent re-differentiation into fibroblast
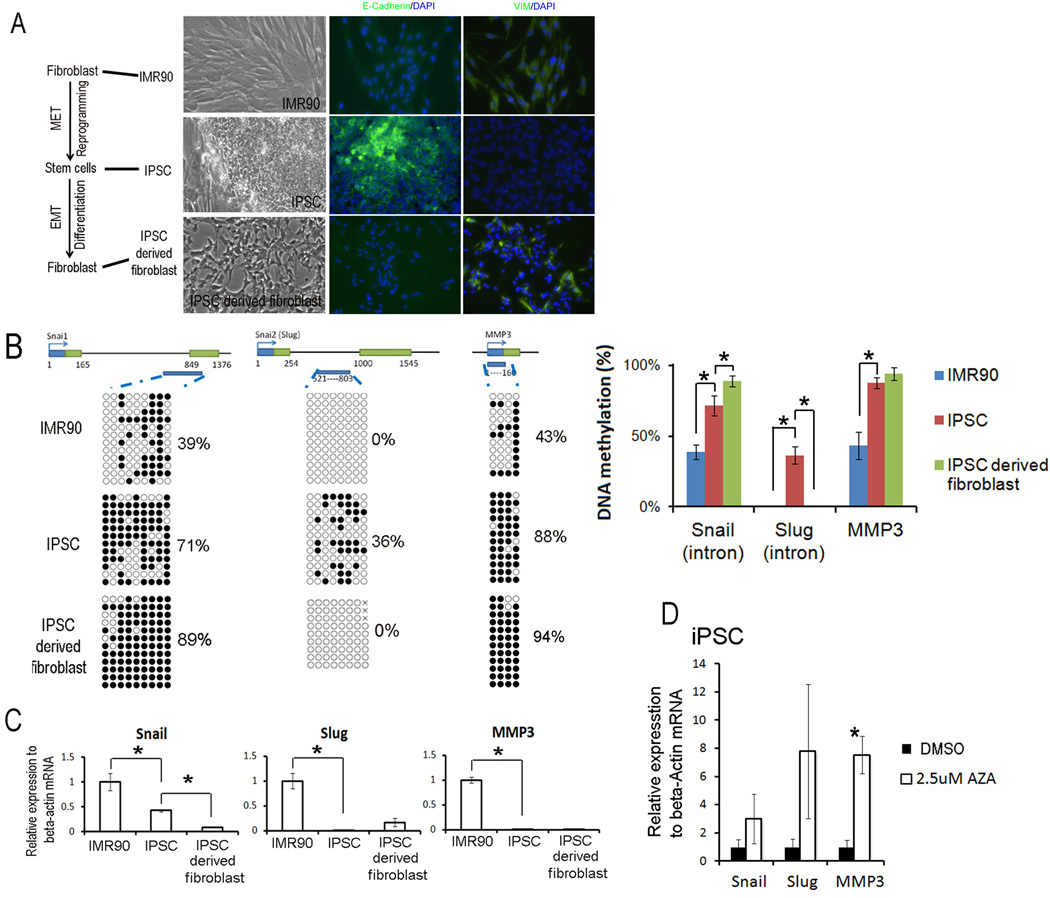
EMT/METs occur during induced pluripotent stem cells (iPSC) generation from donor cell- fibroblast and re-differentiation into fibroblast [2]. To test the relationship between DNA methylation and the transcriptional activity of Snail and Slug genes, we used the donor fibroblast cell (IMR90), the iPSC derived from IMR90, and the iPSC-differentiated fibroblast cells together as a continuous model to describe the EMT and MET events during somatic cell reprogramming and mesenchymal differentiation processes. iPSC cells showed epithelial morphology, and IMR90 and iPSC-derived fibroblasts showed mesenchymal shape. After immunocytochemical staining of epithelia marker E-Cadherin and mesenchymal marker vimentin (VIM), iPSC was E-Cadherin positive and VIM negative, and IMR90 and iPSC-derived fibroblasts showed E-Cadherin negative and VIM positive staining (Fig1a).
Fig 1. DNA methylation of first introns and mRNA level of Snail and Slug genes in EMT and MET during induced pluripotent stem cell generation from fibroblast and its re-differentiation into fibroblast.
A. The morphology and immunecytochemistrial staining of fibroblast cell IMR90, established iPSC cells from IMR90, and iPSC differentiated fibroblasts. B. Dynamic DNA methylation changes of first introns of Snail and Slug genes in the MET/EMT of induced pluripotent stem cell generation and fibroblast differentiation. The top panel showed genomic regions of Snail, Slug and MMP3 genes. The open boxes were the first and second exons. DNA methylation data was analyzed by quantification tool for methylation analysis online software (http://quma.cdb.riken.jp/). DNA methylation of CpG sites were represented by cycles, with open cycles and close cycles describing unmethylated and methylated CpG sites respectively, and the “x” decoding non-sequenced CpG sites. C. Dynamic changes in mRNA levels in the MET/EMT of induced pluripotent stem cell generation and fibroblast differentiation. Relative gene expressions in IMR90, iPSC and derived fibroblast were measured by quantitative RT-PCR and analyzed by delta Ct difference. Data was presented as the mean ± SEM. Asterisk (*) decoded statistically significant difference in IMR90 vs. iPSC or iPSC vs. iPSC derived fibroblast. D. Changes in gene expression in iPSC treated with 2.5 µM 5-aza-dC for 2 days. Asterisk decoded statistically significant difference as compared to DMSO control. Data was presented as the mean ±SEM. Each experiment was performed three biological replicates.
Because DNA methylation surrounding the transcription start site is closely associated with transcription activity [16], we performed sodium bisulfite genomic sequencing to evaluate the DNA methylation of proximal promoters (Snail −300 to −70; Slug −387 to −194 bp, relative to their transcription start sites respectively) and first intron (Snail +716 to +1114; Slug+521 to +803 bp) regions of Snail and Slug genes in this model of EMT/METs (Fig 1b). Matrix metalloproteinase 3 (MMP3) has been reported to be transcriptionally regulated by DNA methylation [17]. Here, MMP3 is used as DNA methylation-regulated method control. Furthermore, we performed qRT-PCR to monitor the dynamic changes in mRNA level and observed their relationship with the DNA methylation change in these cells.
We found that the proximal promoter regions of Snail and Slug genes were consistently hypomethylated in our checked cells and there was no dynamic change (FigS1); in contrast, the DNA methylation of first intron were dynamically altered. In the MET of somatic cell reprogramming and iPSC generation, we found that Snail, Slug and MMP3 genes all gained DNA methylation. Accordingly, the mRNA levels in all three genes were significantly down-regulated (Fig1 b and c). During the EMT of iPSC differentiation into fibroblast, Snail further gained more methylation and its mRNA level was further down-regulated in the iPSC-derived fibroblast vs iPSC. However, the Slug gene showed an opposite effect; it was de-methylated and transcriptionally reactivated (Fig1 b and c).
These results showed that, like MMP3, the transcriptional activities of Snail and Slug genes were also negatively associated with the DNA methylation status of the first intron regions. These results also indicated that the MET of iPSC generation was associated with turning down of all three genes and dynamically changing their epigenetic marks like DNA methylation; whereas, the EMT of iPSC differentiation showed only activation in Slug gene, highlighting the distinct characteristics of the MET and EMT processes.
To further evaluate the repressive role of DNA methylation on Snail and Slug gene expression, we examined the impact of 5-aza-dC treatment on these genes. 5-aza-dC is an inhibitor of DNA methyltransferases (DNMT). Treatment of cultured cells with 5-aza-dC usually results in genome-wide de-methylation and the re-activation of methylation-repressed genes. We treated iPSC for two days with 2.5uM of 5-aza-dC. Transcription levels of Snail and Slug genes, as well as MMP3 genes, were up-regulated after 5-aza-dC treatment (Fig 1 D). These results indicated that DNA methylation could repress Snail and Slug genes, as it does the MMP3 genes.
Transcription levels of Snail and Slug were inversely associated with the DNA methylation status of first intron regions in EMT of trophoblast invasion
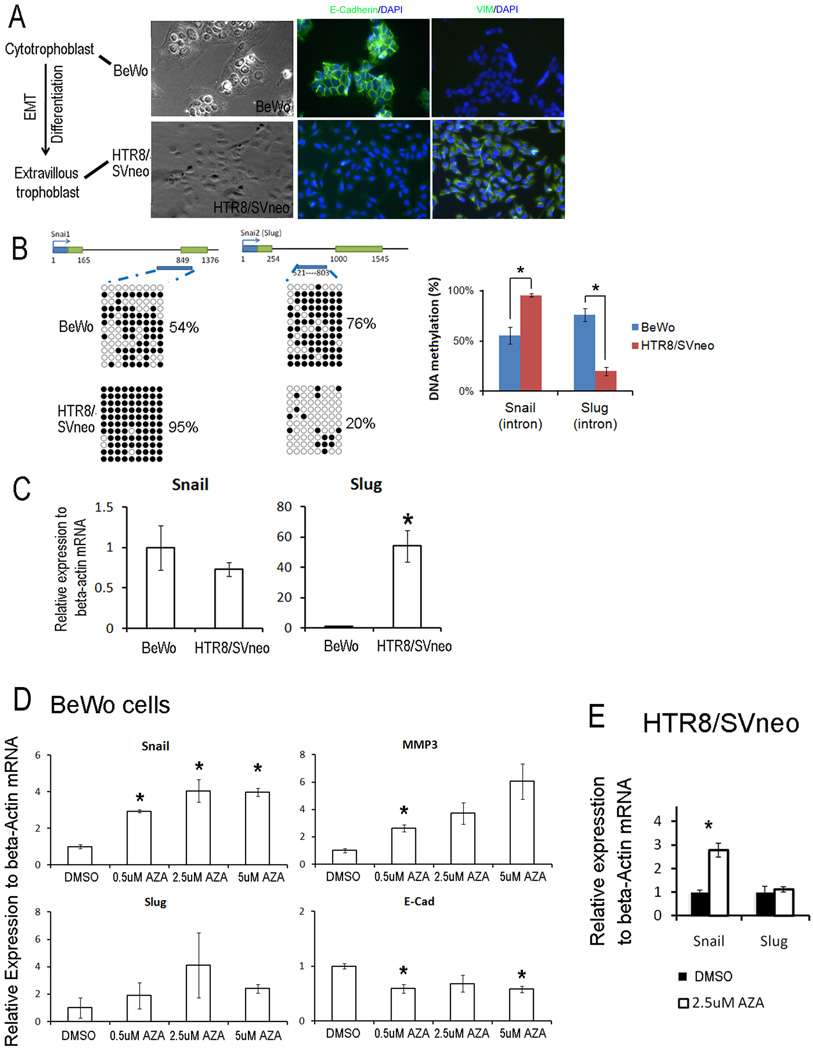
In order to test the role of DNA methylation in the EMT events occurring during trophoblast invasion, where the cytotrophoblast (epithelial-morphology) transform into extravillous trophoblast (EVT) (mesenchymal-morphology) [1], we used the human cytotrophoblast-like cell line, BeWo, and the EVT-like cell line, HTR8/SVneo, to mimic the EMT of trophoblast invasion. The morphology and immunostaining of E-Cadherin and VIM of BeWo and HTR8/SVneo cells are demonstrated in Fig2a. To test the role of DNA methylation in transcription regulation of Snail and Slug in the EMT of trophoblast invasion, we compared DNA methylation in both proximal promoters and first intron of Snail and Slug genes and their mRNA level in these two cell lines. Similar to MET/EMT of IPSC generation and differentiation, proximal promoters of Snail and Slug genes were hypomethylated in both HTR8/SVneo and BeWo cells (FigS1); however, the first intron region showed different DNA methylation levels between the two cell lines (Fig 2b). This difference suggested that DNA methylation is associated with the EMT of trophoblast invasion. Snail gene was higher methylated in HTR8/SVneo than BeWo cells (95% vs 54%), and its corresponding mRNA level was down-regulated in HTR8/SVneo cells compared with BeWo cells. However, the Slug gene was less methylated (20%) in HTR8/SVneo cells compared with the BeWo cells (76%). mRNA levels of the Slug genes were significantly higher in HTR8/SVneo cells than BeWo cells. Further, we checked the DNA methylation of EVT cells in placenta and found similar DNA methylation pattern with HTR8/SVneo cells (Fig S2).
Fig 2. DNA methylation of first introns and mRNA levels of Snail and Slug genes in EMT in trophoblast invasion.
A. The morphology and immunocytochemistrial staining of trophoblast cell lines, BeWo and HTR8/SVneo. B. Dynamic DNA methylation change of first introns in the EMT of trophoblast invasion. C. Dynamic changes in mRNA levels in the EMT of trophoblast invasion. Asterisk decoded statistically significance compared to BeWo cells. D. Changes of gene expression in trophoblast BeWo cells treated with 0.5, 1.5 and 2.5 µM 5-aza-dC for 3 days. E. Changes of gene expression in trophoblast HTR8/SVneo cells treated with 2.5 µM 5-aza-dC for 3 days. Asterisk decoded statistically significance compared to DMSO control. Gene expression is relative to housekeeping gene beta-actin and data was presented as the mean ±SEM. Each experiment was performed three biological replicates.
Next, we treated BeWo and HTR8/SVneo cells for three days with 0.5, 2.5 and 5µM of 5-aza-dC. In BeWo cells, transcription levels of Snail and Slug genes were significantly up-regulated after 5-aza-dC treatment (Fig 2d). In HTR8/SVneo cells (Fig 2e), the transcription level of Snail gene was significantly up-regulated, similar to BeWo cells. In contrast, Slug gene expression did not change; this result was consistent with the hypomethylated status of Slug gene in HTR8/SVneo cells (Fig2b). Hence, these results indicated that DNA methylation may repress Snail and Slug genes in the EMT of trophoblast invasion.
Transcription levels of Snail gene were inversely associated with DNA methylation status in EMT of cancer cell motility
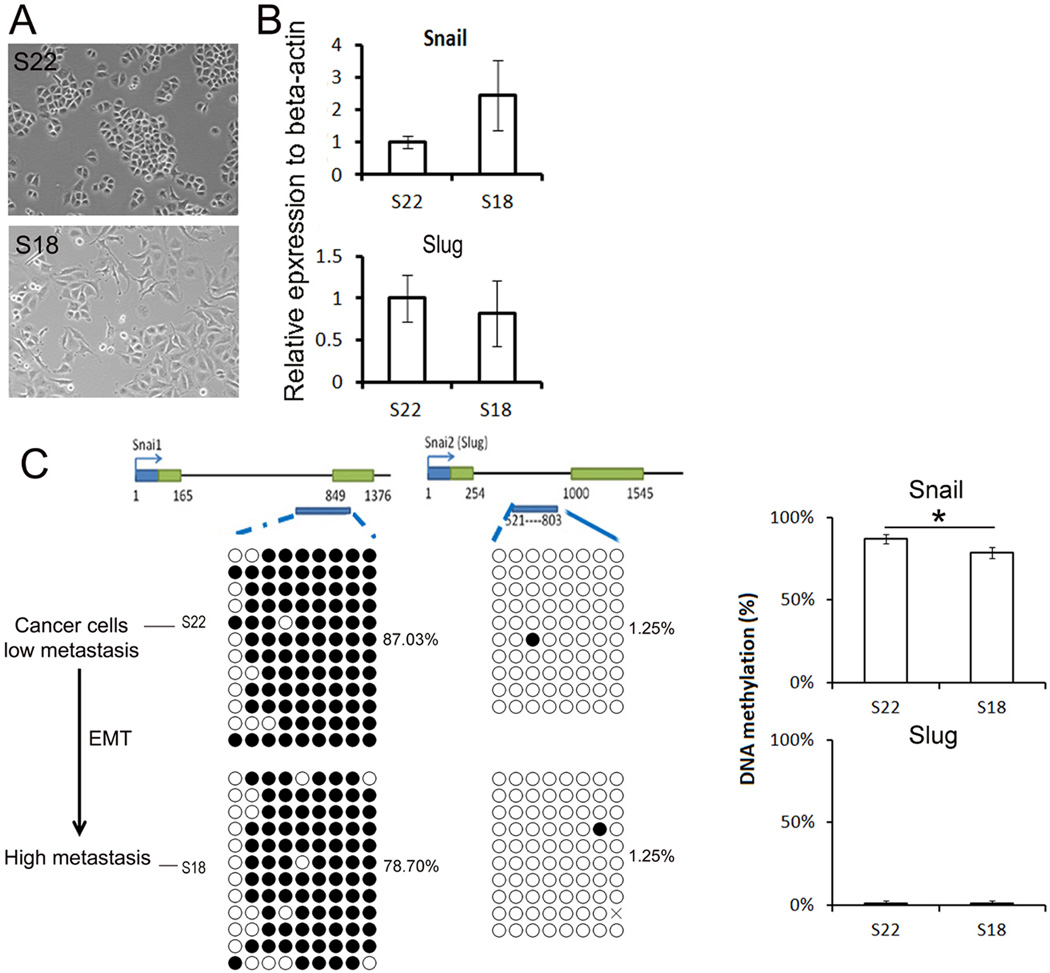
To mimic cancer EMT, we used two nasopharyngeal carcinoma cell lines, the non-metastatic cell line, S22 (epithelial morphology), and the highly metastatic cancer cell line, S18 (partial loss of epithelial morphology), both of which were derived from the same parental cell line, CNE. The S18 cell line showed significant EMT characteristics and much stronger metastasis capacity in vitro and in vivo assays compared to S22 [14].
In this cancer EMT model, we found that S18 cell line evidenced a more mesenchymal-morphology than S22 (Fig 3a). The first intron region of Snail gene was significantly less methylated in S18 than S22 (78% vs. 87%) shown in Fig 3b, and the Snail mRNA expression level in the S18 cell line was higher than in S22 cells shown in Fig 3c. The first intron of Slug gene was hypo-methylated in both cells (1.25% vs. 1.25%), and its mRNA expression levels were similar in both S18 cells and with S22 cell lines (Fig 3b and c). These results showed that the transcriptional activities of Snail genes were negatively associated with DNA methylation status in cancer cell motility.
Fig 3. DNA methylation and mRNA levels of Snail and Slug genes in EMT in cancer cell mobility.
A. Morphology of S18 and S22 cells. B. mRNA levels of Snail and Slug genes in cancer cell lines. Data was presented as the mean ± SEM. C. DNA methylation of Snail and Slug genes in cancer cell lines. DNA methylation of Snail and Slug genes was presented as the mean ± SEM. Asterisk decoded statistically significant difference.
DNA methylation in promoters repressed the promoter activity of Snail and Slug
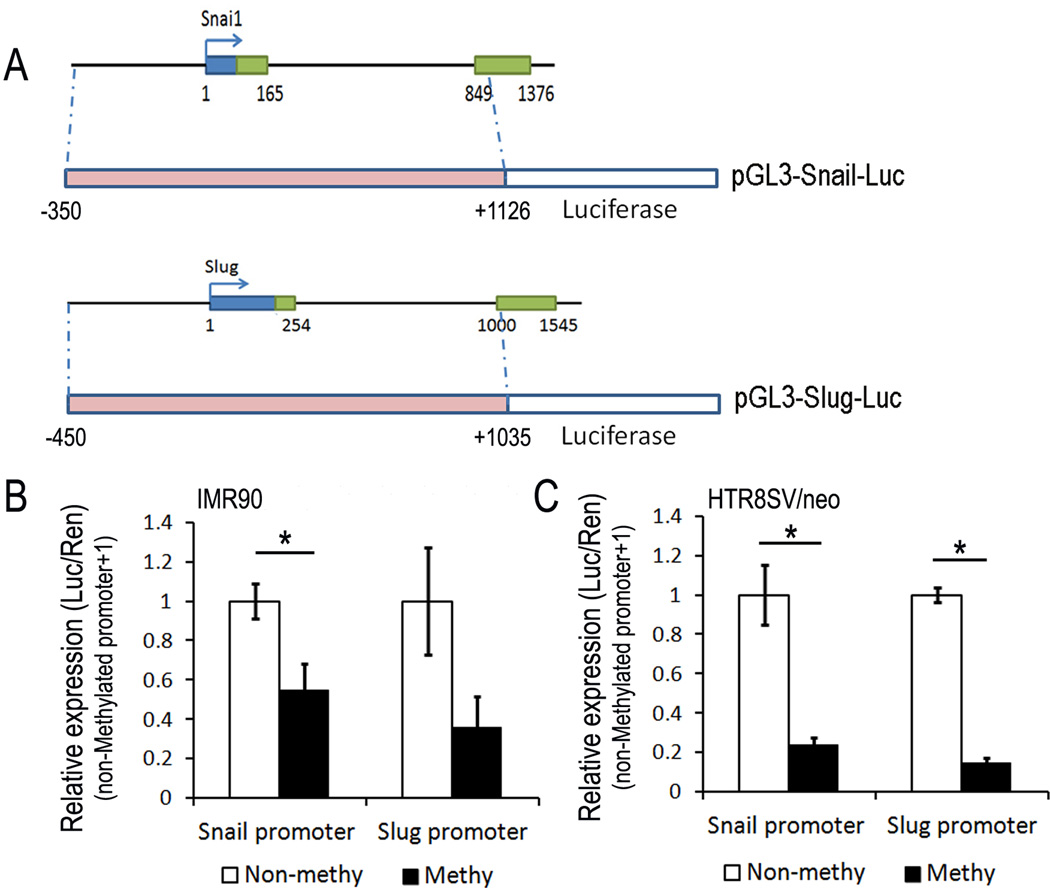
To further investigate the role of DNA methylation of promoters in an in vivo model, we cloned the Snail (−350 to +1126) and Slug (−450 to +1035) promoters into the pGL-3 basic vector (Promega) upstream of firefly luciferase coding sequence respectively (Fig 4a). Then we artificially added DNA methylation to both promoters using SssI methylase and evaluated its effect on the expression of these promoters post-transfection in IMR90 cells and HTR8/SVneo cells respectively. This In vitro DNA methylation of both Snail-Luc construct and the Slug-Luc construct caused significant suppression of promoter-mediated firefly luciferase activity in both constructs (down-regulated to 55% and 36% respectively in IMR90 cells, shown in Fig 4b; down-regulated to 24% and 15% respectively in HTR8/SVneo cells, shown in Fig 4c). These results further provided supporting evidence that transcription of the Snail and Slug genes are both repressed by DNA methylation.
Fig 4. Transcriptional repression of DNA methylation in Snail and Slug promoters.
A. Schematic diagram of pGL3-Snail-Luc and pGL3-Slug-Luc constructs used in luciferase reporter assay. B. Effect of in vitro DNA methylation on Snail and Slug promoter activities in IMR90 cells. C. Effect of in vitro DNA methylation on Snail and Slug promoter activities in HTR8/SVneo cells. Data was presented as the mean ±SEM.
Discussion
Epigenetic regulation of Snail and Slug
As EMT initiating genes, molecular mechanism of transcription regulation of Snail and Slug genes attracted the attention of researchers. Previously several groups reported the direct binding of transcription factors and chromatin remodeling proteins on Snail and Slug promoters and regulating their transcriptions. In breast cancer cell lines, metastasis associated factor 3 (MTA3) and histone deacetylase 1/2 (HDAC) has been shown to bind to Snail promoter and repress its transcription by decreasing histone acetylation levels [18]. Furthermore, Snail protein can negatively regulate itself by binding to its own promoter [19]. Snail can also bind to the Estrogen receptor 1 (ER1) gene (a MTA3 gene regulator) to regulate MTA3 expression, and subsequently MTA3 protein affects Snail transcription [20]. Although the role of histone modification in Snail gene has been demonstrated [18], the role of DNA methylation in Snail and Slug gene regulation is limited, except one report in mouse species[13]. In that report, DNA methylation of the proximal promoter of Snail gene was associated with gene repression. Current study focused on the role of DNA methylation in the transcription regulation of human Snail and Slug genes and demonstrated that DNA methylation repressed gene transcription (Fig 4). Furthermore, the different region of promoter showed distinct dynamic DNA methylation in EMTs. The first intron regions of both genes showed dynamic DNA methylation changes in these MET/EMT models of induced pluripotent stem cell generation, stem cell differentiation, trophoblast invasion and cancer progression, which is inversely associated with their transcription level change indicating a close link between DNA methylation and transcription regulation. Because defective EMTs have been linked to the etiology of diseases like cancer, we expected that improper DNA methylation of Snail and Slug first intron regions could be involved in the pathology of these diseases.
The specific characteristic of EMT and MET
EMT plays an important role in human development and cancer progression. EMTs occur in many different cell types, different tissue/organs, and at different time/developmental stages [21]. Furthermore, the outcome of each EMT is different. In some EMTs, cells could become mesenchymal cells permanently, and in other EMTs, cells just stay in mesenchymal phenotype for a while and finally turn back to epithelial phenotype again through MET. Because of the wide variety of EMT or MET events which can occur in various organ systems, the molecular mechanism of each EMT/MET is likely to be distinct. In this study, we found that Slug played critical roles in EMTs of iPSC differentiation and trophoblast invasion; however, the EMT of cancer progression implicated Snail as one of the major players.
This research also provided a chance to compare EMT with MET. We compared EMT and MET in iPSC generation and differentiation processes. Both Snail and Slug were dynamically changed during this MET process, however, only Slug had a significant change in the contiguous EMT process. This result indicated that, in our system, MET was not the simple reverse process of EMT.
DNA methylation and placental trophoblast
DNA methylation of placental trophoblast has been previously described [22]. Genome-wide DNA methylation analysis of trophoblast cells previously performed using Illumina Methylation27 array has greatly extended our knowledge of the role of DNA methylation in trophoblast function and differentiation. However, this technique has its limits, as only 2 or 3 CpG sites per gene can be detected. Therefore, for studies targeting specific genes, use of the bisulfite-sequencing technique can produce more reliable data. In this study, we used the bisulfite-sequencing method to study the Snail and Slug genes and found that Slug played a role in the EMT in trophoblast invasion. These results agreed with a previous report of Slug function in EMT process via silencing the E-cadherin gene and initiating cancer EMT and progression [23].
In summary, our research demonstrated that Snail and Slug genes are regulated by DNA methylation and the DNA methylation of first intron were associated with their transcription in EMT/MET processes.
Supplementary Material
Acknowledgement
The authors would like to thank Drs Yuwen Zhang and Trixie Smith and Ms Susan Ferguson in designing experiments and preparing manuscript.
Funding: This work was supported by 3U54HD040093-09S1 from the National-Institute-of-Children Health-and-Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta. 31:747–755. doi: 10.1016/j.placenta.2010.06.017. PMID: 20659767. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Liang J, Ni S, Zhou T, Qing X, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 7:51–63. doi: 10.1016/j.stem.2010.04.014. PMID: 20621050. [DOI] [PubMed] [Google Scholar]
- 3.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. PMID: 19487819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. PMID: 11689706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol. 1998;198:277–285. PMID: 9659933. [PubMed] [Google Scholar]
- 6.Waldmann J, Slater EP, Langer P, Buchholz M, Ramaswamy A, et al. Expression of the transcription factor snail and its target gene twist are associated with malignancy in pheochromocytomas. Ann Surg Oncol. 2009;16:1997–2005. doi: 10.1245/s10434-009-0480-y. PMID: 19412634. [DOI] [PubMed] [Google Scholar]
- 7.Tang P, Yu Z, Zhang K, Wang Y, Ma Z, et al. Slug down-regulation by RNA interference inhibits invasion growth in human esophageal squamous cell carcinoma. BMC Gastroenterol. 11:60. doi: 10.1186/1471-230X-11-60. PMID: 21599940. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Blechschmidt K, Mylonas I, Mayr D, Schiessl B, Schulze S, et al. Expression of E-cadherin and its repressor snail in placental tissue of normal, preeclamptic and HELLP pregnancies. Virchows Arch. 2007;450:195–202. doi: 10.1007/s00428-006-0343-x. PMID: 17149611. [DOI] [PubMed] [Google Scholar]
- 9.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. PMID: 18056451. [DOI] [PubMed] [Google Scholar]
- 10.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, et al. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. PMID: 18796538. [DOI] [PubMed] [Google Scholar]
- 11.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 7:64–77. doi: 10.1016/j.stem.2010.04.015. PMID: 20621051. [DOI] [PubMed] [Google Scholar]
- 12.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. PMID: 14673164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraga MF, Herranz M, Espada J, Ballestar E, Paz MF, et al. A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors. Cancer Res. 2004;64:5527–5534. doi: 10.1158/0008-5472.CAN-03-4061. PMID: 15313885. [DOI] [PubMed] [Google Scholar]
- 14.Li XJ, Ong CK, Cao Y, Xiang YQ, Shao JY, et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res. 71:3162–3172. doi: 10.1158/0008-5472.CAN-10-3557. PMID: 21289131. [DOI] [PubMed] [Google Scholar]
- 15.Hattori N, Imao Y, Nishino K, Hattori N, Ohgane J, et al. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells. 2007;12:387–396. doi: 10.1111/j.1365-2443.2007.01058.x. PMID: 17352742. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta PK, Smith EM, Kim K, Murnane MJ, Smith BD. DNA hypermethylation near the transcription start site of collagen alpha2(I) gene occurs in both cancer cell lines and primary colorectal cancers. Cancer Res. 2003;63:1789–1797. PMID: 12702564. [PubMed] [Google Scholar]
- 17.Couillard J, Demers M, Lavoie G, St-Pierre Y. The role of DNA hypomethylation in the control of stromelysin gene expression. Biochem Biophys Res Commun. 2006;342:1233–1239. doi: 10.1016/j.bbrc.2006.02.068. PMID: 16516860. [DOI] [PubMed] [Google Scholar]
- 18.Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. PMID: 12705869. [DOI] [PubMed] [Google Scholar]
- 19.Peiro S, Escriva M, Puig I, Barbera MJ, Dave N, et al. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–2084. doi: 10.1093/nar/gkl141. PMID: 16617148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21:2907–2918. doi: 10.1210/me.2007-0293. PMID: 17761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. PMID: 17645776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novakovic B, Gordon L, Wong NC, Moffett A, Manuelpillai U, et al. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: implications and opportunities for understanding trophoblast function. Mol Hum Reprod. 17:344–353. doi: 10.1093/molehr/gar005. PMID: 21289002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. PMID: 11912130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.