Abstract
Due to the biphasic viscoelastic nature of cartilage, joint loading may result in deformations that require times on the order of hours to fully recover. Thus, cartilaginous tissues may exhibit cumulative strain over the course of each day. The goal of this study was to assess the magnitude and spatial distribution of strain in the articular cartilage of the knee with daily activity. Magnetic resonance (MR) images of ten asymptomatic subjects (six males, four females) with mean age of 29 years were obtained at 8:00AM and 4:00PM on the same day using a 3T magnet. These images were used to create 3D models of the femur, tibia, and patella from which cartilage thickness distributions were quantified. Cartilage thickness generally decreased from AM to PM in all areas except the patellofemoral groove and was associated with significant compressive strains in the medial condyle and tibial plateau. From AM to PM, cartilage of the medial tibial plateau exhibited a compressive strain of −5.1 ± 1.0% (mean ± SEM) averaged over all locations, while strains in the lateral plateau were slightly lower (−3.1 ± 0.6%). Femoral cartilage showed an average strain of −1.9 ± 0.6%. The findings of this study show that human knee cartilage undergoes diurnal changes in strain that vary with site in the joint. Since abnormal joint loading can be detrimental to cartilage homeostasis, these data provide a baseline for future studies investigating the effects of altered biomechanics on diurnal cartilage strains and cartilage physiology.
Keywords: Magnetic Resonance Imaging, Biomechanics, Cartilage deformation
1. Introduction
Articular cartilage is an avascular and aneural connective tissue that provides a nearly frictionless surface to distribute loads across diarthrodial joints (Mow et al., 1992). During activities of daily living, articular cartilage experiences numerous cycles of relatively high levels of load. For example, the knee joint transfers forces of several times body weight during activities such as gait and stair climbing (Kutzner et al., 2010). Due to the viscoelastic properties of cartilage (Mow et al., 1980), the resulting deformation may not completely recover following each cycle of loading (Eckstein et al., 2006). Thus, cartilaginous tissues may exhibit cumulative strain throughout the day that recovers with prolonged periods of unloading (Paajanen et al., 1994; Sitoci et al., 2012).
In vivo studies have demonstrated that repeated joint loading has been shown to cause reversible decreases in the thickness and volume of articular cartilage (Eckstein et al., 2006; Van Ginckel et al., 2011b). For example, Eckstein et al. reported decreases in patellar cartilage volumes after performing activities such as knee bends, running, squatting, and walking (Eckstein et al., 2005b). Other studies have reported decreases in ankle cartilage volumes after landing from a jump (Van Ginckel et al., 2011a; Van Ginckel et al., 2011b). Furthermore, activities of daily living have been shown to decrease femoral cartilage thickness by up to 0.6mm between morning and evening MR imaging scans (Waterton et al., 2000). Similarly, decreases in height of the intervertebral discs of the lumbar spine have been observed between morning and night, with changes in disc height of approximately 1mm (Paajanen et al., 1994).
These studies suggest that cartilage might experience significant strains due to diurnal changes in cartilage thickness. However, there is limited data on the diurnal strains induced by the thickness changes experienced in the femoral, patellar, and tibial cartilage. These diurnal strains potentially play an important role in understanding normal cartilage function, as mechanical strains affect the osmotic environment of chondrocytes (Guilak et al., 1995; Wang et al., 2002). Osmotic stresses, secondary to mechanical loading of the cartilage extracellular matrix, are believed to play an important role in regulating the metabolic activity of chondrocytes (Browning et al., 2004; Phan et al., 2009), and thus, in maintaining normal joint physiology (Guilak, 2011). Furthermore, quantifying diurnal strains in healthy subjects may provide baseline data for future studies evaluating potential alterations in cartilage loading in populations at high risk for the development of OA. For example, altered cartilage loading has been thought to play a role in the development of osteoarthritis in both obese patients and patients with knee ligament injuries (Andriacchi et al., 2004; Griffin and Guilak, 2005; Van de Velde et al., 2009). Thus, the objective of this study was to assess the magnitude and distribution of diurnal strains in the articular cartilage of the knee with daily activity using magnetic resonance (MR) imaging and 3D modeling techniques. Our hypothesis was that activities of daily living would result in significant diurnal compressive strains in the cartilage of all three compartments of the knee. We also examined how these parameters were correlated to body mass index (BMI) and daily activity level.
2. Methods
MR Imaging
Following Institutional Review Board approval, healthy volunteers were recruited to participate in the study, and informed consent was obtained. A total of ten subjects (six male and four female, mean age: 29 years, range: 22–46 years) with normal body mass index (BMI, mean: 22.6, range: 21.1–24.4) were included (Table I). All participants reported having asymptomatic knees at the time of the study and denied any history of musculoskeletal injury. The right knees of all subjects were imaged with a 3 Tesla MR scanner (Trio Tim, Siemens Medical Solutions USA, Malvern, Pennsylvania) at the Center for Advanced Magnetic Resonance Development at 8:00 AM. Subjects were instructed not to exercise or perform any strenuous activity prior to the AM scan. Subjects were scanned using an eight channel receive-only knee coil and a double106 echo steady state sequence (DESS, flip angle: 25°, TR: 17 ms, TE: 6 ms, field of view: 16 ×16 cm, resolution: 512 ×512 pixels, contiguous slice thickness:1 mm) (Taylor et al., 2011). Scan time was approximately 9 minutes. During the day, the subjects were asked to perform normal daily activities. All participants wore a pedometer to quantify the number of steps taken throughout the day. For the afternoon scan, patients returned to the same facility at 4:00 PM and were immediately imaged upon arrival.
Table 1.
| Sex | Age | Height (m) | BMI | Steps |
|---|---|---|---|---|
| F | 25 | 1.65 | 21.6 | 10151 |
| F | 23 | 1.55 | 22.7 | 11005 |
| F | 22 | 1.73 | 22.8 | 8277 |
| F | 39 | 1.57 | 23.2 | 8525 |
| M | 24 | 1.85 | 21.1 | 12522 |
| M | 28 | 1.78 | 21.5 | 7067 |
| M | 24 | 1.93 | 22.5 | 5987 |
| M | 46 | 1.85 | 23.1 | 5668 |
| M | 25 | 1.65 | 23.3 | 6909 |
| M | 37 | 1.75 | 24.4 | 4460 |
| Mean | 29.3 | 1.73 | 22.6 | 8057 |
| SEM | 2.6 | 0.04 | 0.3 | 806 |
Creation of 3D joint models
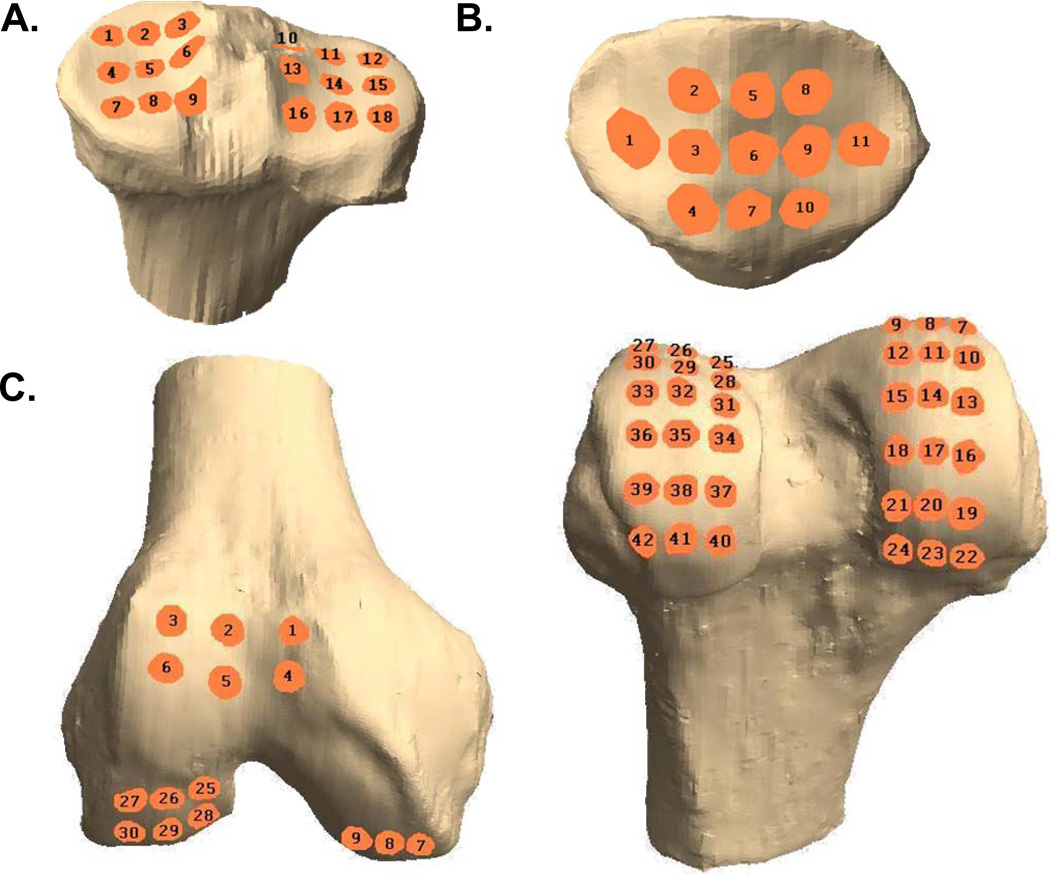
In each image, the bony and articular cartilage surfaces were segmented (Figure 1) in solid modeling software (Rhinoceros 4.0, Robert McNeel and Associates, Seattle, WA) (Bischof et al., 2010). These lines were then used to create 3D polygonal mesh models of the femur, tibia, and patella as well as the corresponding articular surfaces of cartilage (Geomagic Studio, Geomagic Inc., Raleigh, NC). The AM and PM models of the femur, tibia, and patella were then individually aligned using the iterative closest point technique (Abebe et al., 2009) to compare thickness measurements on the same regions of the AM and PM models. A grid sampling system was created on each osseous surface to quantify the changes in cartilage thickness at each sampling site: each tibial plateau was covered by a 3×3 grid, the patella was covered by a grid of 11 points, and the femoral grid consisted of 6 equidistant points localized to the subpatellar region and a total of 36 points across the medial and lateral condyles (Figure 2). All vertices on the mesh model within a 2.5 mm radius of the grid sampling point were then averaged to calculate the mean thickness at each site. Cartilage thickness was measured as the distance from each vertex on the articular surface of the cartilage to the nearest point on the surface of the bone (Figure 3) (Van de Velde et al., 2009). For each grid sampling point, diurnal strain was calculated using the difference of AM and PM thickness divided by the AM thickness at the same location.
Figure 1.
A sagittal view of an asymptomatic right human knee using a 3T MRI scanner. The cartilaginous and osseous boundaries of the femur (F), tibia (T), and patella (P) were segmented on each slice and stacked to create 3D models of the joint.
Figure 2.
3D models of the right tibia (A), patella (B), and femur (C) showing grid scheme for computing thickness changes and strains. The patella is shown from a posterior view. Right femur is shown from an inferior view (left) and a posterior view (right).
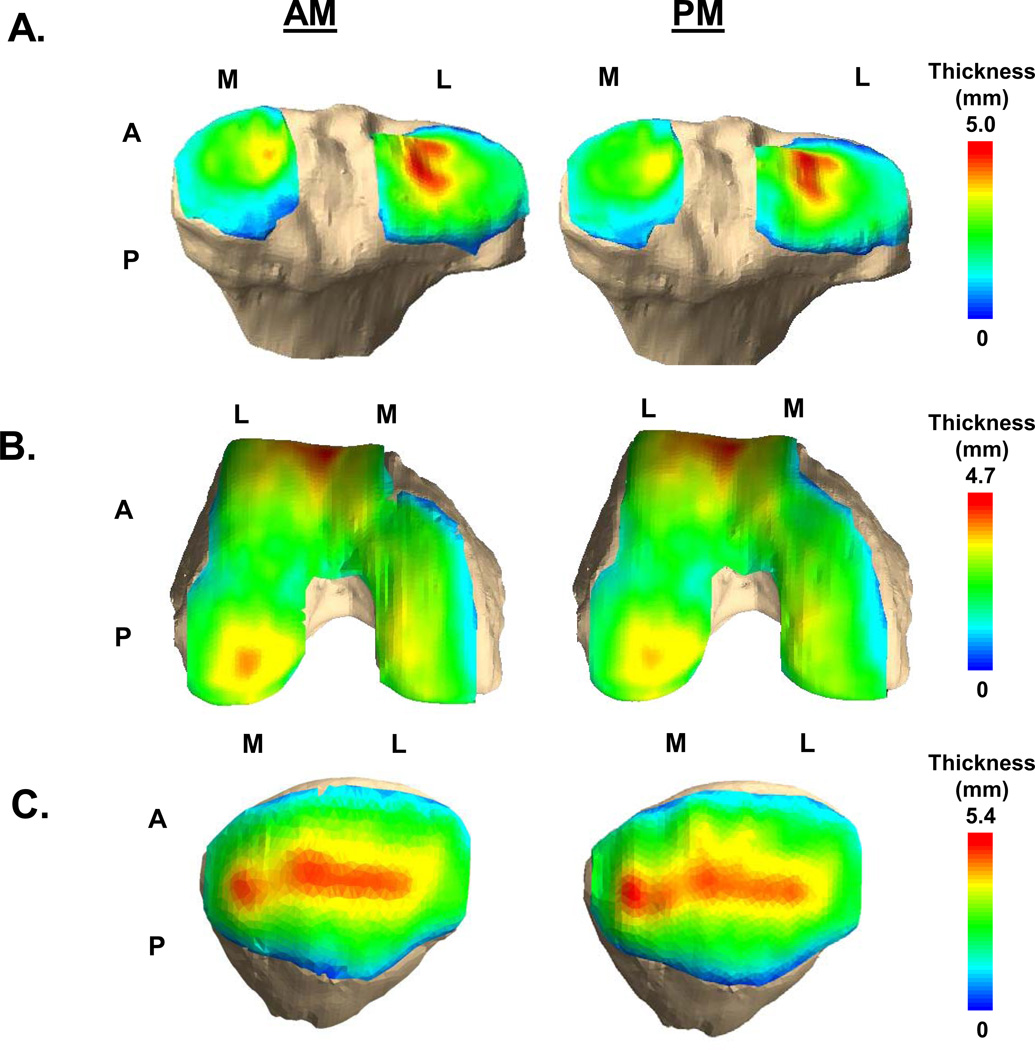
Figure 3.
Cartilage thickness maps of the tibia (A), femur (B), and patella (C) measured in the AM (left) and PM (right). (M = Medial, L=Lateral, A=Anterior, P=Posterior)
Repeatability Study
A number of previous studies have used MR imaging to measure articular cartilage volume and thickness in human subjects (Bowers et al., 2008a; Cohen et al., 1999; Eckstein et al., 2005a; Van Ginckel et al., 2011b; Waterton et al., 2000). The methodology used in the present study has been previously validated to measure cartilage thickness distributions in the tibiofemoral joint (Van de Velde et al., 2009). However, to assess the repeatability of the thickness measurements of the femoral, tibial, and patellar cartilage used in this study, the coefficient of repeatability (Bland and Altman, 1986) was calculated for four repeated trials of image segmentation at 11 different sites on the femoral, tibial, and patellar cartilage. The coefficient of repeatability was 0.03mm, which corresponds to a difference of 1.2% in cartilage thickness; thus differences less 0.03mm or 1.2% are within the noise of this technique and unlikely to represent meaningful changes in thickness.
Statistical Analysis
All analyses were performed with STATISTICA (StatSoft, Inc., Tulsa, Oklahoma). The effects of location and sex on AM cartilage thickness were tested with an ANOVA with LSD post-hoc tests. AM and PM thicknesses were compared with paired t-tests. Strains were assessed for difference from zero with t-tests. Strains were compared across compartments and sexes with an ANOVA with LSD post-hoc tests. Bonferroni corrections were applied as appropriate. Simple linear regressions were used to determine whether variation in BMI, number of steps taken, or age were significant explanatory variables in determining the amount of strain in each compartment.
3. Results
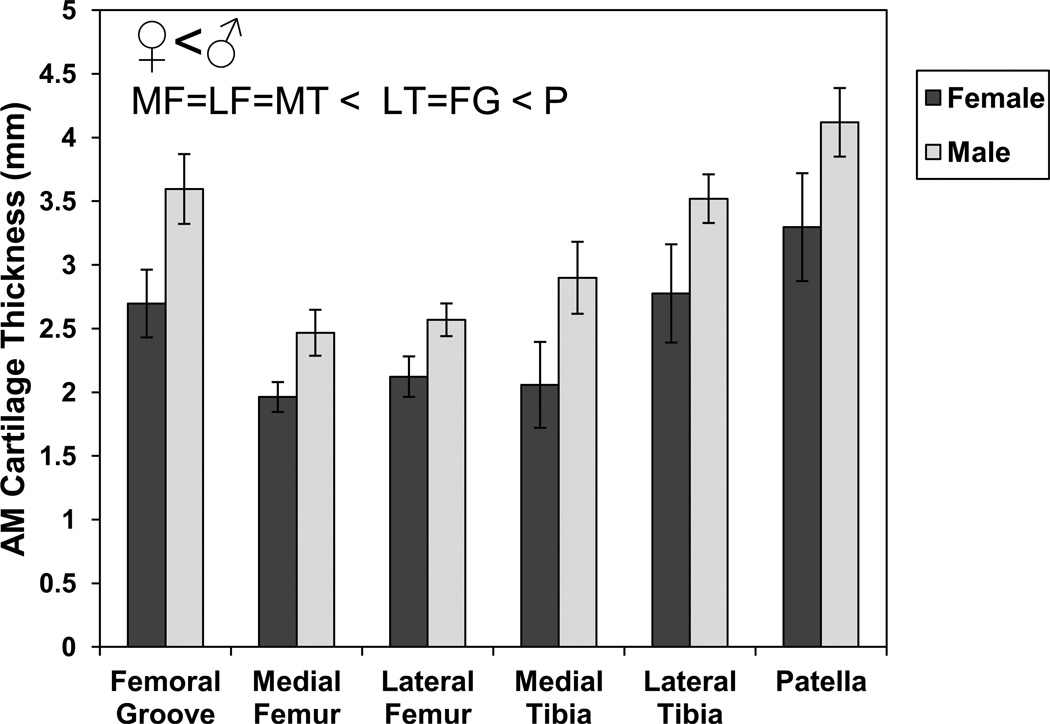
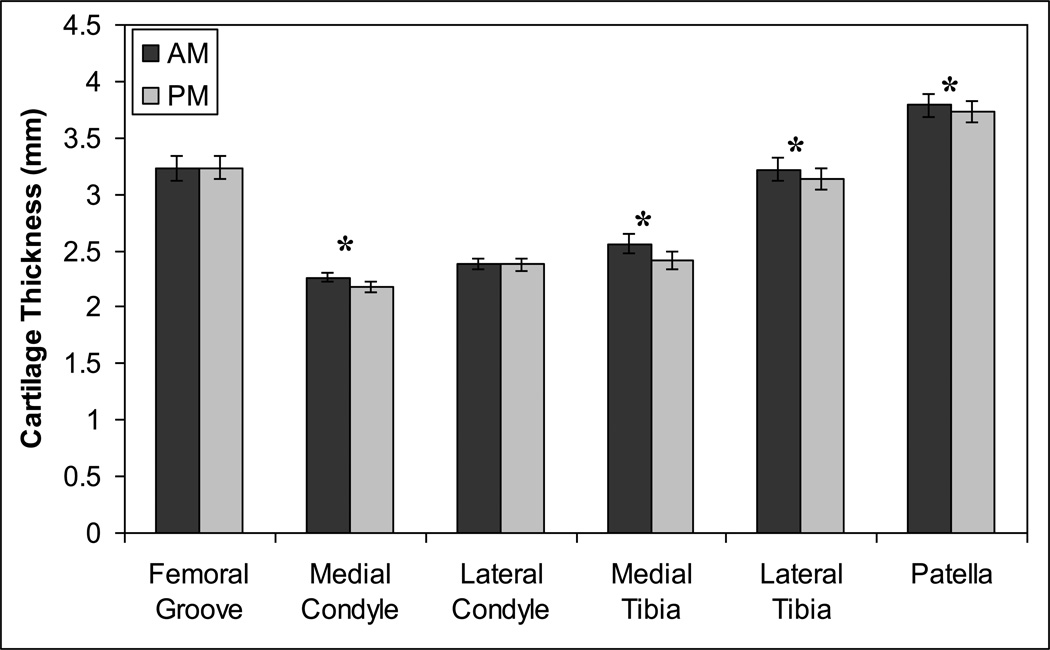
Subjects took an average of 8057 steps (Table 1) during the course of the day. AM cartilage thickness, which represents the undeformed (or minimally deformed) state, varied significantly by location and by sex with no significant sex-location interaction (Figure 4, ANOVA, location p<0.00001, sex p<0.00001, location*sex p=0.79). Cartilage was thickest on the patella, followed by the femoral groove and lateral tibia, which were thicker than the medial femur, lateral femur, and lateral tibia. Males had significantly thicker cartilage than females by an average of 28%. Cartilage thickness generally decreased from AM to PM in all areas except the patellofemoral groove and the lateral femoral condyle (Figure 5).
Figure 4.
Mean (± sem) AM cartilage thickness varies significantly between compartments and with sex (ANOVA, location p<0.0001, sex p<0.0001, location*sex p=0.80). Symbols show significant differences from post-hoc tests for each factor (sex, location) (LSD post-hoc test with Bonferroni correction, p<0.025).
Figure 5.
Mean (± sem) cartilage thickness decreases from AM to PM in most major knee compartments. *AM thickness significantly different from PM (paired t-test with Bonferroni correction, p<0.025).
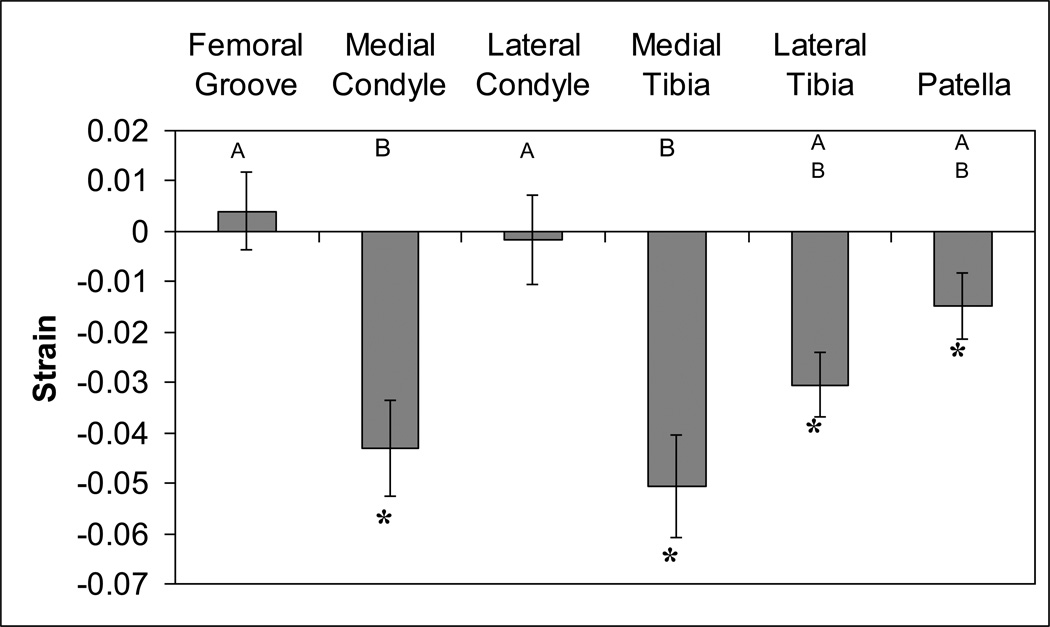
The tibial cartilage experienced significant compressive strains on both the lateral and medial sides (Figure 6). In the undeformed state (AM), the lateral plateau was significantly thicker than the medial (Figure 4). On the medial plateau, regions bordering the medial intercondylar tubercle were thickest, with the most anterior of these experiencing the greatest strain (site 3: −11.7 %). However, on the lateral tibial plateau, greatest strain was experienced on the lateral border (site 18: −5.0%, site 12: −4.8%).
Figure 6.
Cartilage in most knee compartments undergoes significant diurnal compressive strain. Bars are mean (± sem). *strains significantly different from zero (t-test with Bonferroni correction, p<0.025). Bars with different letters are significantly different from one another (ANOVA, LSD post-hoc test with Bonferroni correction, p<0.025).
The undeformed patellar cartilage was thicker than any other location (Figure 4). Patellar cartilage also experienced significant compressive strain (Figure 6). The highest strain occurred in the antero-lateral corner of the patella (site 8: −4.2%). The cartilage in the patellofemoral groove did not significantly change thickness from AM to PM; nor did it undergo significant strain.
The cartilage in the medial femoral condyle also experienced significant compressive strains (Figure 6). The lateral femoral condyle cartilage, on the other hand, did not change thickness or experience significant strain from AM to PM. The highest diurnal strains in the medial condyle were seen in the anteromedial region (site 7: −8.5%, site 8: −9.2%).
The cartilage in the medial compartment, on both the tibia and condyle, experienced the largest compressive strains during the course of the day. These strains were significantly greater than the lateral condyle and the patellofemoral groove. The lateral tibia and patella experienced intermediate strains.
The number of steps was inversely correlated with the subject’s BMI (#Steps=47261-1733*BMI, r2=0.45, p=0.03). Compressive strain in the lateral and medial tibia significantly decreased with increasing number of steps taken (Lateral Tibia: strain=−0.79+5.99*10−6*#steps, r2=0.05, p=0.03; Medial Tibia: strain=−0.18+1.65*10−5*#steps, r2=0.17, p=0.0005), whereas compressive strain in the lateral femur and medial tibia significantly increased with increasing BMI (Lateral Femur: strain=0.067–0.03*BMI, r2=0.05, p=0.05; Medial Tibia: strain=0.66–0.031*BMI, r2=0.09, p=0.003). Age did not significantly correlate with any of the strains measured.
4. Discussion
Mechanical stresses and strains alter the biophysical environment of cartilage and potentially play an important role in normal cartilage homeostasis (Guilak and Hung, 2005). Thus, an improved understanding of the effects of daily activity and joint loading on cartilage deformation is important to understanding normal cartilage function and may provide critical insights into the mechanisms leading to cartilage degeneration (Halloran et al., 2012). The findings of this study show that, with daily activity, human knee cartilage undergoes diurnal changes in thickness and significant compressive strains. The magnitude of compressive strain varied as a function of location within the joint, with average compressive strains reaching values as high as 5% in the medial compartment of the tibia.
The compressive strains observed in the present study are consistent with previous studies reporting changes in the diurnal thickness changes in knee cartilage (Waterton et al., 2000) and the intervertebral disc of the spine (Paajanen et al., 1994). These diurnal changes are likely due in large part to fluid flow resulting from compressive loading of the cartilage (Roberts et al., 1998; Setton et al., 1993). While the individual components of cartilage are effectively incompressible (Bachrach et al., 1998), mechanical loading of cartilaginous tissues results in the time-dependent exudation of water (O'Connell et al., 2011; Torzilli et al., 1983), and, due to the low permeability of cartilage, recovery of water and thickness may require times on the order of hours (Eckstein et al., 2006; Mow and Guo, 2002). For example, a recent series of studies using MR imaging demonstrated that patellar cartilage volume decreases by 5% after performing 50 deep knee bends (Eckstein et al., 2006). After 45 minutes of rest, approximately 50% of the volume loss was recovered. In another study, more than 90 minutes was required for the patellar cartilage to fully recover from 100 knee bends (Eckstein et al., 1999). In the present study, a similar mechanism is likely causing the diurnal cartilage strains observed in response to daily activity. Although we asked subjects to refrain from exercising or performing any strenuous activity prior to the morning scan, their normal activity prior to the morning scan is likely to have resulted in some cartilage deformation that may not have been measured. Thus, the measurements reported in the present study may be an underestimate of the diurnal strains experienced throughout an entire day of activity.
MR imaging techniques have been widely used to measure cartilage volume and thickness in the knee (Bowers et al., 2008b; Cohen et al., 1999; Eckstein et al., 2001; Li et al., 2005; Raynauld et al., 2003). The images used in this study were acquired in approximately 9 minutes to minimize motion artifacts (Eckstein et al., 2006; Tieschky et al., 1997). In our study, the average morning thicknesses were 2.9mm for the tibia and 2.5mm for the femur. These data were similar to thickness measurements previously reported in the literature (Ateshian et al., 1991; Bingham et al., 2008; DeFrate et al., 2004; Liu et al., 2010). For example, Ateshian et al. reported average thickness measurements of 2.9mm for the tibia and 2.0mm for the femur. Since data in the present study were collected from selected sample locations, the thinner regions near the periphery of the cartilage were not included in this calculation. Thus, the numbers reported in this paper may be slightly higher than average thickness values obtained from volumetric measurements of cartilage. Furthermore, differences in cartilage thickness measurements between studies could also be related to variations in age (Hudelmaier et al., 2001), activity (Eckstein et al., 2006), BMI (Anandacoomarasamy et al., 2012),or, based on the findings of the present study, the time of day that the images were acquired. Thus, our findings emphasize the importance of precisely controlling the loading history of the joint in clinical trials utilizing measurements of cartilage thickness or joint space narrowing.
Our results demonstrated a statistically significant but relatively weak correlation between increasing compressive strain and BMI. These findings are consistent with the hypothesis that increased body weight, as represented by BMI, may result in higher loads at the knee joint (Aaboe et al., 2011; Messier et al., 2005), thus inducing greater cumulative deformation of the cartilage. However, as the BMIs of subjects in this study were selected within a narrow range of normal values, it is not possible to form strong conclusions about the role of BMI. Further study of individuals with BMIs covering a greater range may yield additional information in this regard.
Of interest was the finding that the number of steps taken correlated inversely (albeit weakly) with increasing compressive strain. Under physiologic loading rates, the mechanical response of cartilage is characterized by rapid pressurization of the interstitial fluid, which results in little tissue dilatation at short times (Ateshian, 2009), although instantaneous normal strains can exceed 10–15% (Guterl et al., 2009). However, sustained or static loading leads to increased cartilage deformation as fluid is exuded from the cartilage (Butz et al., 2011; Chan and Neu, 2012; Cotofana et al., 2011). Our findings suggest that cyclic daily activity may in fact lead to decreased cartilage strains due to the continuous loading and unloading of the joint which allows repressurization, and therefore little deformation (Caligaris and Ateshian, 2008). More comprehensive measurements of joint motion and loading throughout the day (as opposed to the total number of steps taken) may provide additional insight into these relationships. In particular, measurements characterizing the time course of activity may be useful to the interpretation of this data, since activities performed immediately prior to the afternoon scan could have a greater effect on the deformation of the cartilage than activities performed earlier in the day.
Both the tibial and femoral cartilage showed significantly higher strain on the medial side. This observation is consistent with previous studies that have shown increased acute cartilage contact strain in the medial compartment during gait (Liu et al., 2010) or during a static lunge (Bingham et al., 2008). These data are also in agreement with predictions of higher medial compartment loads based on gait analysis techniques (Hurwitz et al., 1998; Schipplein and Andriacchi, 1991). In addition, a recent MR-based study demonstrated higher strains in the medial compartment, lower strains in the lateral tibial cartilage, and very small strains in the lateral femoral cartilage in response to compressive loads applied to the tibia (Cotofana et al., 2011). Thus, in healthy knees, the medial compartment appears to experience higher strains than the lateral compartment under different loading conditions, including gait, lunging, and compressive loading. These findings are consistent with our observations of greater diurnal strains in the medial compartment relative to the lateral compartment in response to activities of daily living.
While normal loading conditions do not necessarily lead to osteoarthritis, these regions of high strain coincide with regions where osteoarthritis lesions are likely to occur. For example, The National Health and Nutrition Examination Survey has shown a greater prevalence of osteophytic changes in the medial tibial and femoral cartilage (Dillon et al., 2006). Other epidemiologic studies have reported a higher prevalence of medial compartment osteoarthritis compared to lateral compartment osteoarthritis (McAlindon et al., 1992; Wise et al., 2012). Increases in loading in the medial compartment, as measured by the adduction moment using gait analysis techniques, are believed to be associated with a higher prevalence and a faster rate of progression of medial compartment osteoarthritis (Andriacchi et al., 2000; Vincent et al., 2012). Although many factors are likely to play a role in the development of osteoarthritis, the elevated medial compartment cartilage strains relative to the lateral side could potentially make the medial side more susceptible to degenerative changes.
An important consideration in the interpretation of these findings is that the mechanical properties of articular cartilage exhibits significant inhomogeneity with depth (Guilak et al., 1995; Wong and Sah, 2010), and thus strain measurements based on total change in thickness cannot account for zone-specific differences in strain at tissue and cellular levels. The difference in compressive modulus and local strain magnitude has been shown to vary up to an order of magnitude, potentially resulting in highly nonuniform strain fields under load (Choi et al., 2007; Guterl et al., 2009). The development of novel MR imaging modalities that allow for high-resolution imaging of cartilage strains may provide further insight into measurement of zone-specific changes in the mechanical environment of the tissue (Chan and Neu, 2012).
The observed diurnal strains reported in this study are likely due to water exudation from cartilage as a result of repeated loading throughout the day and are recovered overnight (Eckstein et al., 1999; Sitoci et al., 2012). This loss of water is associated with diurnal osmotic changes that may influence and potentially regulate chondrocyte biology (Browning et al., 2004; Peffers et al., 2010). Chondrocytes show high osmotic sensitivity to both hyper-osmotic (Erickson et al., 2001) and hypo-osmotic (Erickson et al., 2003) conditions, and the sensitivity to these changes appears to depend on the initial osmotic stress (Leddy et al., 2010). Thus, diurnal cartilage strains would be expected to expose chondrocytes to daily cycles of hyper- and hypo-osmotic conditions that may be responsible for regulating cartilage metabolism and physiology (Chao et al., 2006; Clark et al., 2010). Similarly, in intervertebral disc cells, osmotic changes have been shown to not only affect gene expression (Boyd et al., 2005), but also to influence the response to mechanical loading (Wuertz et al., 2007). Therefore, alterations to normal patterns of joint loading, and therefore osmotic signaling, could potentially disrupt normal cartilage homeostasis.
In conclusion, we found that articular cartilage experiences significant compressive strain during the course of the day, presumably resulting from loss of interstitial water due to joint loading. This diurnal strain cycle may provide significant mechanoregulatory cues for chondrocytes, and changes to these normal strain distributions, such as those that may occur with weight gain or injury, may be detrimental to normal cartilage homeostasis. These novel data provide an important baseline for investigating the effects of altered biomechanics on diurnal cartilage strains.
Acknowledgments
Supported in part by NIH grants AR055659, AR50245, AR48182, AG15768, and AR48852 and a grant from the National Football League Charities. The authors thank Libby Pennington and Wandra Davis for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: No conflicts to disclose.
References
- Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19:822–828. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Abebe ES, Moorman CT, 3rd, Dziedzic TS, Spritzer CE, Cothran RL, Taylor DC, Garrett WE, Jr, DeFrate LE. Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med. 2009;37:1904–1911. doi: 10.1177/0363546509340768. [DOI] [PubMed] [Google Scholar]
- Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, Sambrook PN, March L. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Annals of the rheumatic diseases. 2012;71:26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Lang PL, Alexander EJ, Hurwitz DE. Methods for evaluating the progression of osteoarthritis. J Rehabil Res Dev. 2000;37:163–170. [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Annals of biomedical engineering. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. Journal of biomechanics. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. Journal of biomechanics. 1991;24:761–776. doi: 10.1016/0021-9290(91)90340-s. [DOI] [PubMed] [Google Scholar]
- Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. Journal of biomechanics. 1998;31:445–451. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Bingham JT, Papannagari R, Van de Velde SK, Gross C, Gill TJ, Felson DT, Rubash HE, Li G. In vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology. 2008;47:1622–1627. doi: 10.1093/rheumatology/ken345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof JE, Spritzer CE, Caputo AM, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo cartilage contact strains in patients with lateral ankle instability. Journal of biomechanics. 2010;43:2561–2566. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bowers ME, Trinh N, Tung GA, Crisco JJ, Kimia BB, Fleming BC. Quantitative MR imaging using "LiveWire" to measure tibiofemoral articular cartilage thickness. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008a;16:1167–1173. doi: 10.1016/j.joca.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, Tung GA, Trinh N, Leventhal E, Crisco JJ, Kimia B, Fleming BC. Effects of ACL interference screws on articular cartilage volume and thickness measurements with 1.5 T and 3 T MRI. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008b;16:572–578. doi: 10.1016/j.joca.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LM, Richardson WJ, Chen J, Kraus VB, Tewari A, Setton LA. Osmolarity regulates gene expression in intervertebral disc cells determined by gene array and real-time quantitative RT-PCR. Annals of biomedical engineering. 2005;33:1071–1077. doi: 10.1007/s10439-005-5775-y. [DOI] [PubMed] [Google Scholar]
- Browning JA, Saunders K, Urban JP, Wilkins RJ. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology. 2004;41:299–308. [PubMed] [Google Scholar]
- Butz KD, Chan DD, Nauman EA, Neu CP. Stress distributions and material properties determined in articular cartilage from MRI-based finite strains. Journal of biomechanics. 2011;44:2667–2672. doi: 10.1016/j.jbiomech.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:1220–1227. doi: 10.1016/j.joca.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DD, Neu CP. Transient and microscale deformations and strains measured under exogenous loading by noninvasive magnetic resonance. PLoS One. 2012;7:e33463. doi: 10.1371/journal.pone.0033463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. Journal of biomechanics. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis and rheumatism. 2010;62:2973–2983. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, Ateshian GA. Knee cartilage topography, thickness, and contact areas from MRI: in-vitro calibration and in-vivo measurements. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7:95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- Cotofana S, Eckstein F, Wirth W, Souza RB, Li X, Wyman B, Hellio-Le Graverand MP, Link T, Majumdar S. In vivo measures of cartilage deformation: patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur Radiol. 2011;21:1127–1135. doi: 10.1007/s00330-011-2057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrate LE, Sun H, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral contact analysis using 3D MRI-based knee models. Journal of biomechanics. 2004;37:1499–1504. doi: 10.1016/j.jbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Dillon CF, Rasch EK, Gu QP, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. Journal of Rheumatology. 2006;33:2271–2279. [PubMed] [Google Scholar]
- Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, Wirth W, Evelhoch JL. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis and rheumatism. 2005a;52:3132–3136. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208:491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Lemberger B, Gratzke C, Hudelmaier M, Glaser C, Englmeier KH, Reiser M. In vivo cartilage deformation after different types of activity and its dependence on physical training status. Annals of the rheumatic diseases. 2005b;64:291–295. doi: 10.1136/ard.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Reiser M, Englmeier KH, Putz R. In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging--from image to data, from data to theory. Anat Embryol (Berl) 2001;203:147–173. doi: 10.1007/s004290000154. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Tieschky M, Faber S, Englmeier KH, Reiser M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat Embryol (Berl) 1999;200:419–424. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. Journal of biomechanics. 2001;34:1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:187–197. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 259–300. [Google Scholar]
- Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- Guterl CC, Gardner TR, Rajan V, Ahmad CS, Hung CT, Ateshian GA. Two-dimensional strain fields on the cross-section of the human patellofemoral joint under physiological loading. Journal of biomechanics. 2009;42:1275–1281. doi: 10.1016/j.jbiomech.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran JP, Sibole S, van Donkelaar CC, van Turnhout MC, Oomens CW, Weiss JA, Guilak F, Erdemir A. Multiscale Mechanics of Articular Cartilage: Potentials and Challenges of Coupling Musculoskeletal, Joint, and Microscale Computational Models. Annals of biomedical engineering. 2012 doi: 10.1007/s10439-012-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudelmaier M, Glaser C, Hohe J, Englmeier KH, Reiser M, Putz R, Eckstein F. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis and rheumatism. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. Journal of biomechanics. 1998;31:423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. Journal of biomechanics. 2010;43:2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Liedtke WB, Guilak F. Effect of Initial Osmolarity on Hypo-osmotically Induced Calcium Signaling in Chondrocytes. Trans Orthop Res Soc. 2010;35:876. [Google Scholar]
- Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, Rubash HE. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon) 2005;20:736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Liu F, Kozanek M, Hosseini A, Van de Velde SK, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral cartilage deformation during the stance phase of gait. Journal of biomechanics. 2010;43:658–665. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Annals of the rheumatic diseases. 1992;51:844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis and rheumatism. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- O'Connell GD, Jacobs NT, Sen S, Vresilovic EJ, Elliott DM. Axial creep loading and unloaded recovery of the human intervertebral disc and the effect of degeneration. J Mech Behav Biomed Mater. 2011;4:933–942. doi: 10.1016/j.jmbbm.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paajanen H, Lehto I, Alanen A, Erkintalo M, Komu M. Diurnal fluid changes of lumbar discs measured indirectly by magnetic resonance imaging. J Orthop Res. 1994;12:509–514. doi: 10.1002/jor.1100120407. [DOI] [PubMed] [Google Scholar]
- Peffers MJ, Milner PI, Tew SR, Clegg PD. Regulation of SOX9 in normal and osteoarthritic equine articular chondrocytes by hyperosmotic loading. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1502–1508. doi: 10.1016/j.joca.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis and rheumatism. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld JP, Kauffmann C, Beaudoin G, Berthiaume MJ, de Guise JA, Bloch DA, Camacho F, Godbout B, Altman RD, Hochberg M, Meyer JM, Cline G, Pelletier JP, Martel-Pelletier J. Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:351–360. doi: 10.1016/s1063-4584(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Roberts N, Hogg D, Whitehouse GH, Dangerfield P. Quantitative analysis of diurnal variation in volume and water content of lumbar intervertebral discs. Clin Anat. 1998;11:1–8. doi: 10.1002/(SICI)1098-2353(1998)11:1<1::AID-CA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- Setton LA, Zhu W, Mow VC. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. Journal of biomechanics. 1993;26:581–592. doi: 10.1016/0021-9290(93)90019-b. [DOI] [PubMed] [Google Scholar]
- Sitoci KH, Hudelmaier M, Eckstein F. Nocturnal changes in knee cartilage thickness in young healthy adults. Cells, tissues, organs. 2012;196:189–194. doi: 10.1159/000333456. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Terry ME, Utturkar GM, Spritzer CE, Queen RM, Irribarra LA, Garrett WE, DeFrate LE. Measurement of in vivo anterior cruciate ligament strain during dynamic jump landing. Journal of biomechanics. 2011;44:365–371. doi: 10.1016/j.jbiomech.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieschky M, Faber S, Haubner M, Kolem H, Schulte E, Englmeier KH, Reiser M, Eckstein F. Repeatability of patellar cartilage thickness patterns in the living, using a fat-suppressed magnetic resonance imaging sequence with short acquisition time and three-dimensional data processing. J Orthop Res. 1997;15:808–813. doi: 10.1002/jor.1100150604. [DOI] [PubMed] [Google Scholar]
- Torzilli PA, Dethmers DA, Rose DE, Schryuer HF. Movement of interstitial water through loaded articular cartilage. Journal of biomechanics. 1983;16:169–179. doi: 10.1016/0021-9290(83)90124-0. [DOI] [PubMed] [Google Scholar]
- Van de Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis and rheumatism. 2009;60:3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginckel A, Almqvist F, Verstraete K, Roosen P, Witvrouw E. Human ankle cartilage deformation after different in vivo impact conditions. Knee Surg Sports Traumatol Arthrosc. 2011a;19:137–143. doi: 10.1007/s00167-010-1159-4. [DOI] [PubMed] [Google Scholar]
- Van Ginckel A, Roosen P, Almqvist KF, Verstraete K, Witvrouw E. Effects of in vivo exercise on ankle cartilage deformation and recovery in healthy volunteers: an experimental study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011b;19:1123–1131. doi: 10.1016/j.joca.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Vincent KR, Conrad BP, Fregly BJ, Vincent HK. The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. PM R. 2012;4:S3–S9. doi: 10.1016/j.pmrj.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Guo XE, Sun D, Mow VC, Ateshian GA, Hung CT. The functional environment of chondrocytes within cartilage subjected to compressive loading: a theoretical and experimental approach. Biorheology. 2002;39:11–25. [PubMed] [Google Scholar]
- Waterton JC, Solloway S, Foster JE, Keen MC, Gandy S, Middleton BJ, Maciewicz RA, Watt I, Dieppe PA, Taylor CJ. Diurnal variation in the femoral articular cartilage of the knee in young adult humans. Magn Reson Med. 2000;43:126–132. doi: 10.1002/(sici)1522-2594(200001)43:1<126::aid-mrm15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wise BL, Niu J, Yang M, Lane NE, Harvey W, Felson DT, Hietpas J, Nevitt M, Sharma L, Torner J, Lewis CE, Zhang Y. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken) 2012;64:847–852. doi: 10.1002/acr.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BL, Sah RL. Mechanical asymmetry during articulation of tibial and femoral cartilages: local and overall compressive and shear deformation and properties. Journal of biomechanics. 2010;43:1689–1695. doi: 10.1016/j.jbiomech.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuertz K, Urban JP, Klasen J, Ignatius A, Wilke HJ, Claes L, Neidlinger-Wilke C. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–1522. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]