Abstract
Measles virus plays an important role as an environmental factor in the pathogenesis of Paget’s disease (PD). Previous studies have shown that IL-6 is increased in the bone marrow of Paget’s patients and that measles virus nucleocapsid protein (MVNP) induces IL-6 secretion by pagetic osteoclasts. Further, IL-6 plays a critical role in the development of pagetic osteoclasts and bone lesions induced by PD, but the mechanisms regulating IL-6 production by MVNP remain unclear. Our current studies revealed that MVNP expression in osteoclast precursors down-regulated Sirt1 mRNA and protein, a negative regulator of NF-κB activity, which is a key factor for IL-6 expression. MVNP expression in NIH3T3 cells also elevated Il-6 transcription and impaired the expression of Sirt1 mRNA both under basal conditions and upon activation of the Sirt1 upstream regulator FoxO3 by LY294002 (a PI3K/AKT inhibitor). Luciferase activity assays showed that constitutively active FoxO3 abolished the repressive effect of MVNP on reporters driven by either FoxO3 response elements or the Sirt1 promoter. Further, protein stability assays revealed that FoxO3 was degraded more rapidly in MVNP-expressing cells than in control cells following the addition of cycloheximide. Similarly, co-transfection of MVNP and FoxO3 into HEK293 cells demonstrated that MVNP decreased the protein levels of over-expressed FoxO3 in a dose-dependent manner. Treatment with the proteasome inhibitor, MG132, blocked the MVNP-triggered decrease of FoxO3, and the treatment with the serine/threonine phosphatase inhibitor, Calyculin A, revealed that MVNP increased phosphorylation of FoxO3. Further, over-expression of Sirt1 or treatment with the Sirt1 activator resveratrol blocked the increase in Il-6 transcription by MVNP. Finally, resveratrol reduced the numbers of TRAP positive multi-nuclear cells in bone marrow cultures from TRAP-MVNP transgenic mice to wild type levels. These results indicate that MVNP decreases FoxO3/Sirt1 signaling to enhance the levels of IL-6, which in part mediate MVNP’s contribution to the development of Paget’s disease.
Keywords: MVNP, FoxO3, Sirt1, Il-6, Paget’s disease, NF-κB
1. Introduction
Paget’s disease (PD) is characterized by abnormal osteoclasts (OCL) that produce high levels of IL-6, which is a critical inducer in the development of characteristic abnormalities of pagetic OCLs and bone lesions in vivo [1–3]. Environmental factors, such as measles virus, have been implicated in the pathogenesis of PD, and measles virus infection of host cells results in the induction of IL-6 [4–6]. We showed that mice expressing the measles virus nucleocapsid protein (MVNP) gene in OCL display a pagetic phenotype, develop pagetic bone lesions and their OCLs produce increased levels of IL-6 [7]. Importantly, loss of the IL-6 production in MVNP expressing mice resulted in loss of formation of pagetic OCL and bone lesions [1]. However, the mechanisms responsible for MVNP induction of IL-6 have yet to be fully elucidated.
The Forkhead-box class O (FoxO) transcription factors play evolutionarily conserved roles in a number of cell processes such as metabolism, differentiation, and cellular stress responses. The indispensable role of the FoxOs was recently demonstrated in maintaining skeletal homeostasis [8, 9]. Deletion of FoxO1, 3, and 4 increased TRAP-positive OCLs generated from mouse bone marrow cells, and FoxO3 was identified as the predominant FoxO in OCLs [8]. Targeted overexpression of FoxO3 in cells of the monocyte/macrophage lineage significantly decreased the numbers of osteoclast progenitors and mature osteoclast formation [10]. Interestingly, FoxO3 silencing [11] or genetic loss of FoxO3 [12] enhanced IL-6 secretion in dendritic cells. However, there is no FoxO3-binding site apparent in the Il-6 promoter [12], which indicates that FoxO3 regulates Il-6 transcription indirectly. Sirt1, a class III protein deacetylase, is one of the FoxO3 target genes [13, 14] and knockdown of FoxO3 expression severely suppressed starvation-induced gene expression of Sirt1 [14]. Genetic overexpression of Sirt1 can down-regulate the production of Il-6 through the down-modulation of NF-κB signaling [15]. Consistent with FoxO3 up-regulation of Sirt1 expression, FoxO3 expression antagonizes NF-κB signaling [16]. Thus, FoxO3/Sirt1 signaling appears to play an important role in controlling the transcription of IL-6.
In the current study, we tested the hypothesis that MVNP suppresses FoxO3/Sirt1 signaling, resulting in increased IL-6 production. To address this, we evaluated the effect of MVNP expression on FoxO3 and Sirt1 and their role in MVNP up-regulation of IL-6 in multiple model systems: transiently transfected HEK293 cells, HEK293R cells stably transfected with a human IL-1 type I receptor chain, NIH3T3 cells and human colony forming unit-granulocyte/macrophage (CFU-GM) stably transfected with MVNP cDNA, and bone marrow cultures from transgenic (TRAP-MVNP) mice expressing MVNP under regulation of the TRAP gene promoter [7].
2. Materials and Methods
2.1 Reagents and antibodies
Cycloheximide, MG132, anti-Flag M2 (F1804), anti-β-actin (A5316), and anti-α-tubulin (T9026) antibodies were from Sigma-Aldrich (St. Louis, MO). Anti-MVNP antibody [3E1] (ab9397) was from Abcam (Cambridge, MA). Anti-PARP (#9542) antibody and serine/threonine phosphatase inhibitor calyculin A (#9902) were from Cell Signaling Technology (Beverly, MA). Anti-FoxO3 (sc-11351), anti-Sirt1 (sc-15404), anti-phosphorylated Stat3Y705 (sc-7993), and anti-Stat3 (sc-483) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated IgG secondary antibodies were from GE Healthcare Life Sciences (Little Chalfont, UK). Polyvinylidene difluoride (PVDF) membranes were from Millipore (Bedford, MA). Recombinant tumor necrosis factor-α (TNF-α), mouse receptor activator of NF-κB ligand (RANKL) and monocyte colony-stimulating factor (M-CSF) were purchased from R&D Systems (Minneapolis, MN). IL-1β was from Upstate Biotechnology (Lake Placid, NY). The PI3K/AKT inhibitor LY294002 was from Calbiochem (La Jolla, CA). The Sirt1 activator resveratrol was from Enzo Life Sciences (Plymouth Meeting, PA).
2.2 Cell Culture
Empty vector (EV)- and MVNP-transduced NIH3T3 cells were cultured as previously described [17]. HEK293 cells were cultured in DMEM containing 10% FBS and 1 × pen/strep antibiotics (complete medium). HEK293R cells (a gift from Dr. Philip E. Auron [18]) are HEK293 cells stably transfected with a human IL-1 type I receptor chain. HEK293R cells were maintained in EMEM supplemented with 10% heat-inactivated FBS and 2 mg/ml L-glutamine. For primary cell culture from human, bone marrow cells were aspirated under 2% xylocaine anesthesia from the iliac crest of healthy normal volunteers into heparinized α-Minimal Essential Medium (α-MEM, Gibco BRL Invitrogen, Carlsbad, CA, USA) containing 5% FBS and bone marrow mononuclear (BMM) cells were then isolated as previously described [19, 20]. Stably transduced CFU-GM cells expressing EV or MVNP were prepared as described previously [21]. For primary cell culture from mice, whole bone marrow cells were flushed from long bones of 12- to 16-week-old wild-type or TRAP-MVNP transgenic mice [7] and plated on 100-mm tissue culture plates in α-MEM containing 10% fetal bovine serum (FBS, Invitrogen). Cells were incubated at 37°C in 5% CO2 overnight. Nonadherent cells were harvested and seeded into either 60-mm tissue culture dishes or 96-well plates. Cells then were cultured in α-MEM containing 10% FBS containing 100 ng/mL of M-CSF for 3 days. The cells were further cultured in α-MEM containing 10% FBS in the presence of 50 ng/mL of M-CSF and 50 ng/ml RANKL for 3 to 4 days to generate OCLs, and the level of OCL formation was determined by TRAP staining using a TRAP kit (Sigma No. 386A). The Institutional Review Board of the University of Pittsburgh approved these studies. All animal studies were approved by the Institutional Animal Care and Use Committees at the University of Pittsburgh School of Medicine, the VA Pittsburgh Healthcare System, and Virginia Commonwealth University.
2.3 Protein extraction and Western blotting
After washing with cold PBS, cells were lysed in cell lysis buffer as previously described [22]. Protein was extracted after centrifugation at 14,000×g for 15 min at 4°C. The CelLytic™ NuCLEAR™ extraction kit (Sigma-Aldrich) was used for the extraction of proteins from the cytosolic and nuclear fractions. Cell lysates were immunoprecipitated using anti-FLAG M2 antibody followed by Western blotting with various antibodies as indicated. Equal amounts of boiled protein samples were run on an 8% SDS polyacrylamide gel. The separated proteins were transferred to PVDF membranes. The following primary antibodies were used: anti-MVNP (1:3000), anti-FoxO3 (1:1000), anti-Sirt1 (1:1000), anti-Flag (1:3000), anti-Stat3 (1:1000), anti-phos-Stat3 (1:1000), anti-α-tubulin (1:20000), and β-actin (1:20000). Bands were detected using HRP-conjugated secondary antibodies (1:2000) and ECL™ reagents (GE Healthcare Life Sciences). Band densitometric analysis was performed using the TotalLab100 software (Nonlinear Dynamics).
2.4 RNA extraction and reverse transcription polymerase chain reactions (RT-PCR)
Total RNA was isolated using Trizol® reagent according to the manufacturer’s instructions. Reverse transcription was performed using a reverse transcription system kit (Promega, Cat.A3500). An aliquot of the product cDNA was used for real-time PCR with iQ™ SYBR Green Supermix and iCycler iQ PCR Detection System (Bio-Rad Laboratories). 18S rRNA was used as an internal control for data analysis. The nucleotide sequences of primers used for PCR were as follow: 18S rRNA 5′-CGCTTCCTTACCTGGTTGAT-3′ and 5′-GAGCGACCAAAGGAACCATA-3′; Il-6 5′-GAGTCCTTCAGAGAGATACAG-3′ and 5′-TGGTCTTGGTCCTTAGCC-3′; Sirt1 5′-ATCGGCTACCGAGACAAC-3′ and 5′-GTCACTAGAGCTGGCGTGT-3′.
2.5 Plasmids and luciferase reporter assays
The MVNP expression construct was generated by inserting E-MVNP cDNA [7] into the EcoRI site of the pCMV-Tag2C vector (Stratagene). Constructs expressing FHRE-Luc (Addgene plasmid 1789) [23], pTA-Luc SIRT1 promoter (−202) (Addgene plasmid 10971) [14], Flag-SIRT1 (Addgene plasmid 1791) [24], pcDNA3 Flag FKHRL1 AAA (FoxO3™) (Addgene plasmid 10709) and its wild-type form (Addgene plasmid 10708) [25] were obtained from Addgene, a non-profit plasmid distribution service. The IL-6 promoter (-225) luciferase reporter was kindly provided by Dr. Jian Zhang [26]. The NF-κB-responsive luciferase reporter (MHCκB NF-κB-pGL2 reporter) was a gift from Dr. Philip E. Auron [18].Transfection was done in HEK293 using Lipofectamine (Invitrogen), in HEK293R and CFU-GM cells using the FuGENE6 Reagent (Roche Molecular Biochemicals, Indianapolis, IN), or in NIH3T3 cells using Lipofectamine 2000 (Invitrogen).
2.6 Statistical analysis
Representative data were shown from at least 2 independent experiments with similar patterns. Statistical significance was analyzed by Student’s t-test or two-way analysis of variance (ANOVA). Values of P < 0.05 were considered significant.
3. Results
3.1 Expression of MVNP decreased Sirt1 gene expression
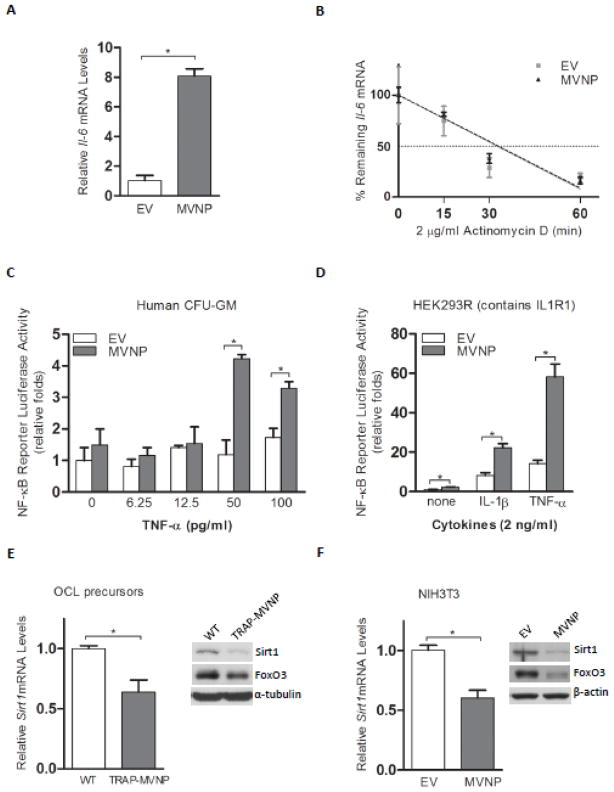
Real-time PCR demonstrated that MVNP expression in NIH3T3 cells elevates Il-6 mRNA (Figure 1A) as has been reported for OCL precursors expressing MVNP and Pagetic OCL [20]. Studies with Actinomycin D inhibition of transcription demonstrated that MVNP does not affect Il-6 mRNA stability (Figure 1B). Therefore, MVNP is modulating signaling that regulates Il-6 gene transcription. Il-6 expression is known to be regulated by a number of transcription factors under different conditions [27], but NF-κB is required for basal and some stimulated IL-6 gene regulation [28]. We have previously shown that MVNP increases NF-κB activity in human OCL precursors by EMSA [20]. Therefore, we determined if the presence of MVNP in human CFU-GM resulted in enhanced activation of a transfected NF-κB reporter (Figure 1C). TNF-α at concentrations of 50 or 100 pg/ml activated a transfected NF-κB reporter higher in MVNP-transduced CFU-GM cells as compared to EV-transduced cells. However, transfection of CFU-GM cells is very inefficient. Therefore, we also co-transfected the NF-κB reporter with MVNP/EV-expression plasmids into HEK293R (stably transfected with the IL-1R1) cells (Figure 1D). The results revealed that MVNP activated NF-κB by itself (~3-fold). Further, MVNP can synergize with both IL-1 and TNF-α to enhance their activation of NF-κB by ~3-fold (averaged over multiple independent experiments) beyond their large independent inductions of NF-κB activity (Figure 1D). This is similar to the 2~3-fold effect of MVNP on TNF-α-induced NF-κB activity observed with CFU-GM. Interestingly, these results form the novel observation that MVNP can synergize with IL-1β signaling as well as with TNF-α signaling to activate NF-κB, indicating that MVNP synergizes with both TRAF6 and TRAF2 signaling.
Figure 1. Expression of Sirt1 in MVNP expressing cells.
(A) Detection of Il-6 mRNA levels in EV- and MVNP-NIH3T3 cells by real-time PCR. *P < 0.01 as determined by student’s t-test. (B) Cells were treated with 2 μg/ml Actinomycin D for indicated times before cells were harvested for the analysis of Il-6 mRNA levels. (C) EV-CFU-GM and MVNP-CFU-GM co-transfected with a NF-κB reporter plasmid and a β-gal expression vector were treated with TNF-α for 24 hr. Cell lysate firefly luciferase values were divided by β-gal activity values for normalization in each sample for quadruplicate determinations. The means and standard deviations of the fold increases for each treatment set were plotted. *P < 0.01 as determined by as determined by student’s t-test. (D) HEK293R cells co-transfected with a NF-κB reporter and either an empty (EV) or MVNP-expression plasmid were stimulated with either vehicle (none), or 2 ng/ml IL-1β, or TNF-α for 24 hr. The relative luciferase units/μg protein for each of the triplicate samples were divided by the mean of the EV-transfected un-stimulated triplicates to derive a fold increase for each sample. The means and standard deviations of the fold increases for each treatment set (EV/MVNP) were plotted (None: 1.0 ± 0.1/2.4 ± 0.2; IL-1β: 8.3 ± 1.3/22.4 ± 1.9; TNF-α: 14.2 ± 1.8/58.3 ± 6.5). *P < 0.05 as determined by student’s t-test. (E) Bone marrow cells were derived from wild-type and TRAP-MVNP transgenic mice. After treatment with M-CSF for 3 days, osteoclast (OCL) precursors were harvested for total RNA and protein. Real-time PCR or Western blotting was performed to analyze the mRNA (left) or protein (right) levels of Sirt1, respectively. *P < 0.05 as determined by student’s t-test. (F) Expression of Sirt1 mRNA (left) and protein (right) was detected in EV- and MVNP-NIH3T3 cells. *P < 0.01 as determined by student’s t-test.
In light of the regulatory role of FoxO3 and Sirt1 on NF-κB activity and Il-6 gene regulation in other cell systems, we hypothesized that they may be involved in MVNP induction of NF-κB activity and Il-6 gene expression. To evaluate if MVNP affects FoxO3/Sirt1 signaling, we determined the levels of Sirt1 mRNA and protein in MVNP expressing and wild-type mouse OCL precursors. As shown in Figure 1E, both mRNA and protein levels of Sirt1 in cells from TRAP-MVNP transgenic mice were significantly lower than in cells from wild-type mice. We also analyzed Sirt1 mRNA and protein levels in a MVNP-stably transduced NIH3T3 cell line that has served as a model to study cellular effects of MVNP expression [17]. These data demonstrated that MVNP expression in NIH3T3 cells also repressed Sirt1 mRNA and protein (Figure 1F). The protein levels of FoxO3, a transcription factor that induces Sirt1 gene expression, were also found to be down-regulated by the expression of MVNP in both OCL precursors and NIH3T3 cells (Figure 1E, F).
3.2 MVNP reduced the transcriptional activity of FoxO3
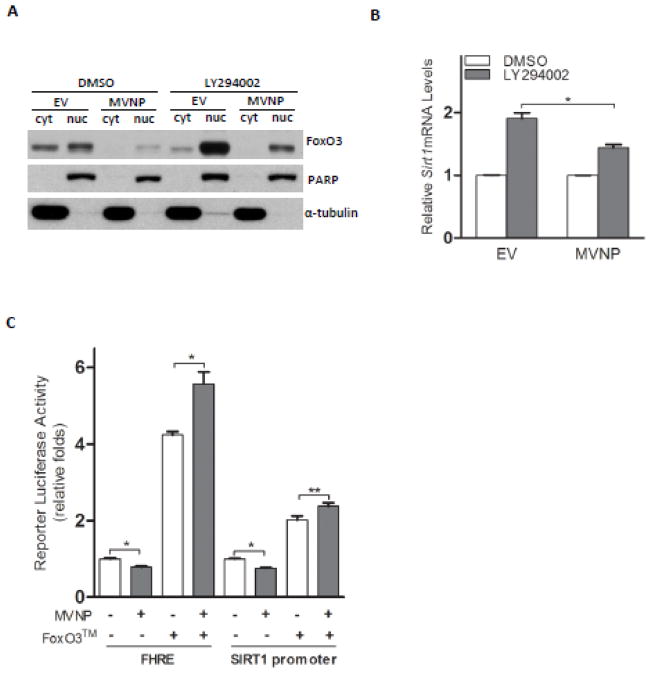
FoxO3 is known to be negatively regulated by the PI3K/AKT pathway [23]. Therefore, we treated both EV- and MVNP-NIH3T3 cells with LY294002, a classic PI3K inhibitor (Figure 2). Immunoblotting of proteins from the cytosolic and nuclear compartments revealed that in the absence of LY294002, the FoxO3 level in both cellular compartments in MVNP-NIH3T3 cells were clearly diminished compared to EV-NIH3T3 cells. Treatment with LY294002 stimulated increased FoxO3 nuclear localization in both cells, but didn’t abolish the difference between the nuclear FoxO3 in EV- and MVNP-NIH3T3 cells (Figure 2A). In concert with this result, MVNP strongly prevented the induction of Sirt1 mRNA expression by treatment with LY294002 (Figure 2B). Co-transfection of HEK293 cells with a MVNP-expression vector and luciferase reporters driven by either a FoxO3 response element (FHRE-Luc) or a SIRT1 promoter which contains the binding motifs for FoxO3 [14] revealed that the presence of MVNP suppressed the activity of both reporters by approximately 30% (Figure 2C). Co-overexpression of a constitutively active FoxO3 due to AKT phosphosite mutations T32A, S253A, and S315A (FoxO3™) eliminated the repressive effects of MVNP on both luciferase reporters. Interestingly, we observed that MVNP even enhanced the transcriptional activity of FoxO3™ slightly (Figure 2C).
Figure 2. FoxO3 regulation of Sirt1 mRNA was down-regulated by MVNP.
(A) Both EV-and MVNP-NIH3T3 cells were treated with 20 μM LY294002 for 2.5 hours. Cells were harvested and proteins in the cytosolic (cyt) and nuclear (nuc) compartments were isolated. FoxO3 protein levels were analyzed by Western blotting. PARP and-α-tubulin were used as controls for proteins from cytosolic and nuclear compartments, respectively. (B) After the treatment of LY294002 for 5 hours, cells were harvested for RNA extraction. Real-time PCR was performed to analyze the level of Sirt1 mRNA. The level of Sirt1 mRNA in EV or MVNP cells with DMSO treatment was set as 1.*P < 0.05 as determined by student’s t-test. (C) HEK293 cells were co-transfected with either FHRE-luciferase or Sirt1 promoter (−202) luciferase reporter together with the renilla luciferase construct (pRL-CMV, Promega). At 48 hours post-transfection, cells were harvested for dual-luciferase assays. Firefly luciferase values were divided by renilla luciferase values for normalization in each sample. Empty vector pCMV-Tag2C or pCDNA3.1 was used as control construct for MVNP (500 ng) or FoxO3™ (300 ng), respectively. A representative experiment is shown. Similar results were observed in 2 independent experiments. *P < 0.01 or **P < 0.05 as determined by student’s t-test.
3.3 MVNP induced proteasome-mediated degradation of FoxO3 protein
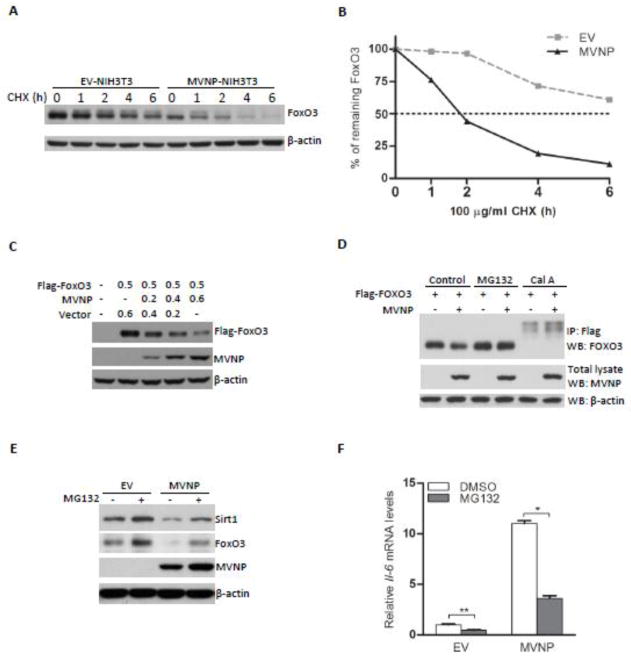
Expression studies demonstrated that MVNP doesn’t inhibit the gene expression of FoxO3 in NIH3T3 cells (Supplemental Figure 1). Therefore, we assessed if MVNP influenced the degradation of FoxO3 protein. We compared the stability of FoxO3 protein in EV- and MVNP-NIH3T3 cells. Expression of MVNP caused a more rapid decrease in FoxO3 protein levels after cycloheximide treatment (Figures 3A and B). FoxO3 has a half-life greater than 6 hours in EV-NIH3T3 cells; however, MVNP shortened the half-life of FoxO3 to less than 2 hours. Further, co-transfection of a FoxO3 expression vector with increasing amounts of the MVNP expression vector into HEK293 cells showed that increasing MVNP decreased the levels of the over-expressed FoxO3 in a dose-dependent manner (Figure 3C). These results indicate that MVNP decreases the stability of FoxO3 protein.
Figure 3. MVNP induced the degradation of FoxO3.
(A and B) The stability of FoxO3 protein was assayed by cycloheximide (CHX) chase in EV- and MVNP-NIH3T3 cells. Cells were harvested and protein lysates prepared at 0.5, 1, 2, 4, 6 hours (h) after addition of CHX. (A) Western blotting was performed to detect the FoxO3 levels at each time point. (B) Data represent densitometric analysis of the results in (A). (C) HEK293 cells were transfected with a Flag-FoxO3 plasmid and increasing amount of MVNP plasmid. At 36 hours post-transfection, cells were harvested and analyzed for the expression of Flag-FoxO3 and MVNP with their respective antibodies. (D) HEK293 cells were transfected with a Flag-FoxO3 plasmid and MVNP plasmid (500 ng of each). At 36 hours post-transfection, cells were harvested after 10 μM MG132 treatment for 2 hours or 100 nM calyculin A treatment for 40 minutes. Cell lysates were analyzed by immunoprecipitation and immunoblotting. A representative experiment is shown. Similar results were observed in 2 independent experiments. (E and F) Both EV- and MVNP-NIH3T3 cells were treated with 10 μM MG132 for 5 hours. Cells were then harvested for RNA and protein extraction. (E) Western blotting was performed to analyze the protein levels of FoxO3. (F) Real-time PCR was performed to analyze the levels of Il-6 mRNA. *P < 0.01 or **P < 0.05 as determined by student’s t-test.
Therefore, we investigated whether MVNP-induced down-regulation of FoxO3 was proteasome mediated. As shown in Figure 3D, treatment with the 26S proteasome inhibitor MG132 decreased proteasomal degradation of the transfected FoxO3, regardless of the presence of MVNP, and thereby prevented MVNP-induced alteration of FoxO3 levels in HEK 293 cells (Figure 3D). We also examined the effect of MG132 on the expression of FoxO3 and Sirt1in the NIH3T3 cells. Data showed that MG132 increased the protein levels of FoxO3 and Sirt1 (Figure 3E), which is correlated with a significant decrease in Il-6 mRNA expression in MVNP-NIH3T3 cells (Figure 3F). Since degradation of FoxO3 has been reported to be triggered by its serine/threonine (S/T) phosphorylation [29, 30], we investigated if a potent S/T phosphatase inhibitor, Calyculin A, would reveal that MVNP altered the S/T phosphorylation of FoxO3. Treatment with Calyculin A significantly increased the amount of phosphorylated FoxO3 detected, and thereby decreased the total FoxO3 level in the absence of MVNP (Figure 3D). However, the presence of MVNP further increased the amount of phosphorylated FoxO3 observed (Figure 3D). These data together indicate that MVNP activates a kinase to phosphorylate FoxO3 at target sites that increase its proteasomal degradation.
3.4 MVNP increased Il-6 transcription by down-regulation of Sirt1
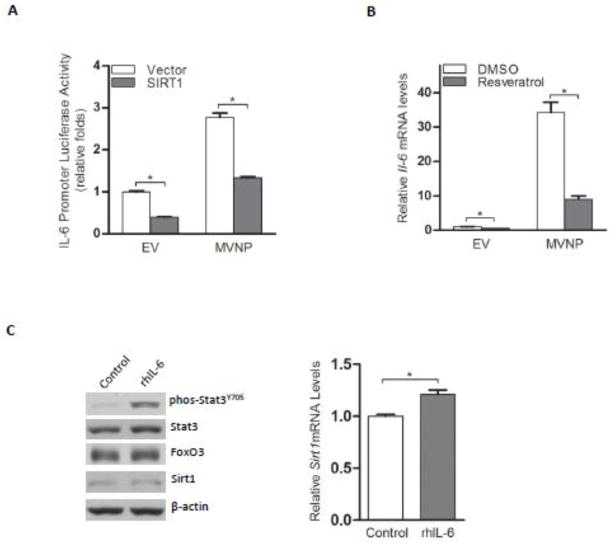
Overexpression of Sirt1 has been reported to reduce the production of Il-6 mRNA [15]. Similarly, we found that MVNP-NIH3T3 cells demonstrated higher IL-6 promoter luciferase reporter activity and ectopic expression of SIRT1 significantly decreased both the basal and MVNP-stimulated activity of the IL-6 reporter (Figure 4A). We then tested whether resveratrol, a Sirt1 activator, could suppress the high level of Il-6 mRNA in MVNP-NIH3T3 cells. As we expected, resveratrol treatment reversed the MVNP-stimulated increase of Il-6 mRNA (Figure 4B). Further, to determine if down-regulation of Sirt1 might be due to the high levels of IL-6, we treated EV-NIH3T3 cells with recombinant IL-6. Addition of IL-6 induced phosphorylation of Stat3, but didn’t suppress either FoxO3 protein levels or Sirt1 gene expression (Figure 4C). These results indicate that down-regulation of Sirt1 mRNA has a significant role in the high level of Il-6 transcription in MVNP expressing cells.
Figure 4. MVNP increased Il-6 through down-regulation of Sirt1.
(A) An IL-6 promoter (−225) luciferase reporter was transfected into EV- or MVNP-NIH3T3 cells together with a Sirt1 construct or an empty vector. *P < 0.01 as determined by student’s t-test. (B) Both EV- and MVNP-NIH3T3 cells were treated with resveratrol or the vehicle (DMSO) for 24 hours and harvested for Il-6 mRNA determination by real-time PCR. *P < 0.01 as determined by student’s t-test. (C) EV-NIH3T3 cells were treated with 10 ng/mL recombinant human Il-6 or left untreated for 4 hours. Western blotting or real-time PCR was performed to analyze the protein levels of Stat3, FoxO3, and Sirt1 (left) or mRNA levels of Sirt1 (right), respectively. *P < 0.05 as determined by student’s t-test.
3.5 Sirt1 activator resveratrol inhibited abnormal OCL differentiation in the presence of MVNP
Having found that the Sirt1 activator resveratrol inhibited the gene expression of Il-6, we investigated whether resveratrol could prevent the abnormal OCL differentiation induced by the expression of MVNP. As shown in Figure 5, the number of TRAP positive multinuclear cells was higher in the TRAP-MVNP cultures compared to wild-type cultures (P < 0.05, two-way ANOVA with Bonferroni post-tests). Resveratrol inhibited OCL differentiation of bone marrow cells within both wild-type and TRAP-MVNP mice (P < 0.001, two-way ANOVA). However, there was no significant difference in pairs of WT and TRAP-MVNP cultures at each of the resveratrol concentrations (10–50 μM). These results indicate that resveratrol blocks the enhancement of OCL formation induced by MVNP.
Figure 5. Resveratrol inhibited abnormal OCL differentiation in the presence of MVNP.
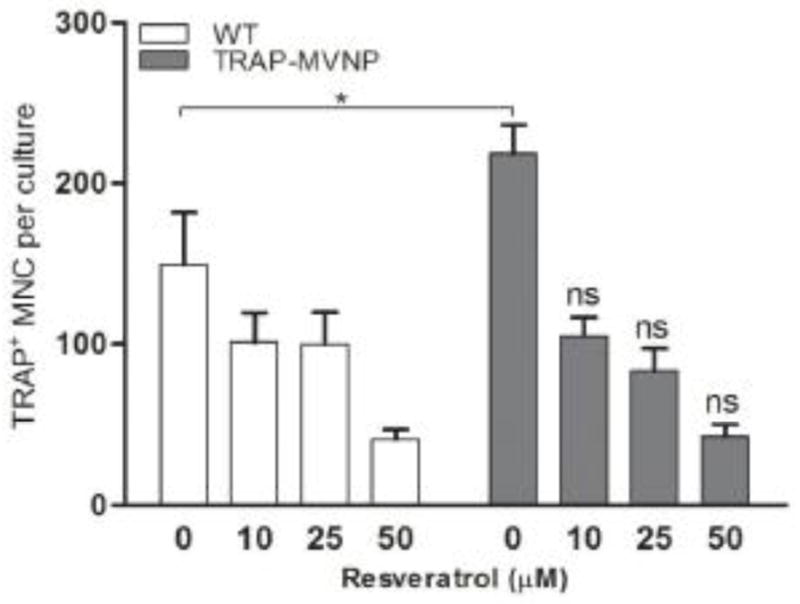
Nonadherent bone marrow cells were derived from wild-type and TRAP-MVNP mice long bone. After 3 days of pretreatment of M-CSF, the cells were treated with RANKL and different doses of resveratrol (0, 10 μM, 25 μM, and 50 μM). After 3 days of further culture, cells were fixed and processed for TRAP staining. TRAP positive multinuclear cells (MNC) were counted under a microscope. *P < 0.05 as determined by two-way ANOVA with Bonferroni post-tests; ns, not significant.
4. Discussion
Paget’s disease provided the first evidence of the importance of the role that IL-6 can play in OCL formation [2]. Pagetic OCLs express MVNP and produce IL-6 as an autocrine factor that stimulates their own activity [3, 31]. Using transgenic mice in which MVNP is targeted to the OCL lineage, Kurihara et. al. [7] reported that targeted expression of the MVNP gene in cells of the OCL lineage can induce pagetic-like bone lesions in vivo. Significantly, higher levels of IL-6 were detected in the marrow cultures from MVNP than from wild-type mice [7]. Importantly, MVNP mice lacking IL-6 did not develop pagetic OCL [1]. These results revealed that MVNP’s induction of IL-6 is a crucial mediator in the development of PD. We have shown that MVNP increases Il-6 mRNA via regulation of gene transcription in NIH3T3 cells. Further we demonstrated that MVNP can facilitate NF-κB signaling, a crucial mediator of IL-6 regulation, in osteoclasts, HEK293 cells, and NIH3T3 cells. Our results reveal that MVNP may exert its regulatory effect on IL-6 through mechanisms involving destabilization of FoxO3 and suppression of Sirt1, thereby decreasing the negative regulation of NF-κB activity and increasing IL-6 transcription.
We found that MVNP suppressed the expression of Sirt1 by down-regulating FoxO3 protein levels. Consistent with a previous study [8], we also observed that FoxO3 was expressed at the highest levels among all 3 FoxOs (FoxO1, FoxO3, and FoxO4) in differentiated OCLs (Supplemental Figure 2A). Interestingly, along with Sirt1, several other FoxOs target genes, such as Bim/Bcl2l11, Gadd45a, and Sod2, were also found to be suppressed in OCLs derived from TRAP-MVNP transgenic mice (Supplemental Figure 2B). Given the critical roles of these FoxO target genes in cellular responses to stress stimuli [24], further studies are warranted to elucidate whether the decrease of Bim/Bcl2l11, Gadd45a, and Sod2 contributes to the MVNP-induced pagetic osteoclast phenotype. Since gene expression of Sirt1 is regulated by FoxO3 [14], we examined the changes in the levels of FoxO3 mRNA and protein in MVNP-NIH3T3 cells. We observed slightly higher levels of FoxO3 mRNA in MVNP expressing cells (Supplemental Figure 1). However, the protein level of FoxO3 was dramatically reduced in whole cell lysates and either cytosol or nuclear compartments from these cells. Impairment of LY294002-promoted FoxO3 nuclear accumulation occurred in parallel with the suppression of Sirt1 induction. MVNP impairment of LY294002 induction of FoxO3-driven Sirt1 mRNA expression is likely due to the decreased FoxO3 protein levels observed in MVNP-expressing NIH3T3 cells, and therefore the LY294002-induced nuclear accumulation of FoxO3 was attenuated. These data strongly support the role of decreased functional FoxO3 protein in mediating the effects of MVNP on Sirt1 gene expression.
Protein stability assays showed that MVNP induced FoxO3 degradation, which indicates that MVNP acts on the upstream regulatory molecules targeting FoxO3 to enhance its degradation. When cells were treated with MG132, the protein levels of FoxO3 were increased and the effect of MVNP on FoxO3 protein disappeared. One would predict that blocking degradation of FoxO3 increased its protein level and would decrease the high levels of Il-6 mRNA caused by the insufficient FoxO3/Sirt1 signaling in MVNP-expressing cells. We have found that the levels of Il-6 mRNA in both MVNP-NIH3T3 and EV-NIH3T3 cells were reduced, which was coincident with the increased FoxO3 protein caused by MG132 treatment. Interestingly, our experiment using serine/threonine phosphatase inhibitor Calyculin A revealed that MVNP increased the phosphorylation of FoxO3. These results suggest that MVNP could affect the activity of FoxO3 by regulating the phosphorylation of FoxO3 sites that signal proteasomal degradation. To date, protein kinases AKT, ERK1/2, and IκB Kinase β (IKKβ) have been shown to down-regulate FoxO3 stability through phosphorylation [30] leading to FoxO3 ubiquitination and degradation [32]. AKT phosphorylation of FoxO3 at sites T32, S253, and S315 results in nuclear exclusion of FoxO3 [23]. In our experiments, we did not find any difference between EV- and MVNP-NIH3T3 cells in the ability of AKT inhibition by the PI3K inhibitor LY294002 to induce FoxO3 nuclear translocation, although the total FoxO3 in both cytoplasm and nucleus was lower in MVNP-containing cells. Thus, MVNP is unlikely to induce FoxO3 degradation via the PI3K/AKT pathway. On the other hand, the FoxO3™ activity was resistant to down-regulation by MVNP, suggesting that perhaps another kinase that targets the same or overlapping sites on FoxO3 as AKT is activated by MVNP. Phosphorylation of FoxO3 by either ERK1/2 (at S295, S354, S426) or IKKβ (at S644) also leads to nuclear exclusion and degradation of FoxO3 via the proteasome pathway [24, 33]. MVNP has been reported to associate with and activates virus-activated kinases including IKK family members TBK1 and IKKε [34, 35]. Recently, FoxO3 was demonstrated to be phosphorylated by IKK and the phosphorylation site for IKKε was not the same as the site for IKKβ [29]. Therefore, it is tempting to speculate that IKKε (and possibly TBK1) mediates the effect of MVNP on the degradation of FoxO3. In order to determine this, further experiments to identify the IKKε-targeted phosphorylation sites on FoxO3 are required. Furthermore, a regulatory feedback loop exists in FoxO3/Sirt1 signaling pathway, where FoxO3 can be deacetylated by Sirt1 and then subjected to ubiquitination [36] or methylation [37] which modulates FoxO3 stability. Taken together, the mechanisms fine-tuning FoxO3 expression and activity in the presence of MVNP warrant future study.
Consistent with our finding that MVNP decreased FoxO3/Sirt1 signaling and increased Il-6 mRNA expression, activation of Sirt1 by resveratrol strongly reversed the stimulatory effect of MVNP on Il-6 mRNA expression. Fischer-Posovszky et. al. reported that resveratrol suppression of Il-6 expression was partially rescued with Sirt1 knockdown [38]. Resveratrol and its analogues can inhibit osteoclastogenesis, although part of this is Sirt1 independent, and relies instead on the capacity of resveratrol to act as a free radical scavenger and decrease ROS generation [39–41]. The inhibitory effect of resveratrol on Il-6 expression in the presence of MVNP along with our previous report that Il-6 expression is required for the MVNP-induced pagetic phenotype in mice [1] prompted us to test whether resveratrol could suppress the effects of MVNP on the formation of pagetic OCL in vitro. In agreement with our hypothesis, resveratrol dramatically inhibited the enhanced osteoclastogenesis in bone marrow cultures from MVNP transgenic mice down to wild-type levels. This result, in combination with a recent finding that Sirt1mediated resveratrol-inhibition of normal osteoclastogenesis through regulation of NF-κB signaling [42], supports the concept that resveratrol activation of Sirt1 counteracts the Sirt1 suppression by MVNP in the TRAP-MVNP cultures leading to decreased NF-κB activity and reduced osteoclastogenesis. However, the exact mechanisms involved in resveratrol’s effect require further study.
In summary, these results demonstrate a novel mechanism underlying the effect of MVNP on IL-6 expression through the down-regulation of FoxO3/Sirt1 signaling (Figure 6). Our results indicate that increased Sirt1expression or activation could reverse the up-regulation of IL-6 caused by MVNP, which is found in 70% of PD patients [1]. It is noteworthy that, since high levels of IL-6 can also be detected in patients with PD not carrying MVNP transcripts, other environmental or genetic mechanisms might contribute to the increase of IL-6 as well. Whether these also act via modulation of FoxO3 and/or Sirt1 is unknown. Nevertheless, approaches that activate Sirt1, such as using its activator resveratrol, may prevent the development or progression of PD on the basis of the critical environmental factor MVNP.
Figure 6. Schematic representation of IL-6 gene regulation by MVNP modulation of FoxO3 and Sirt1.
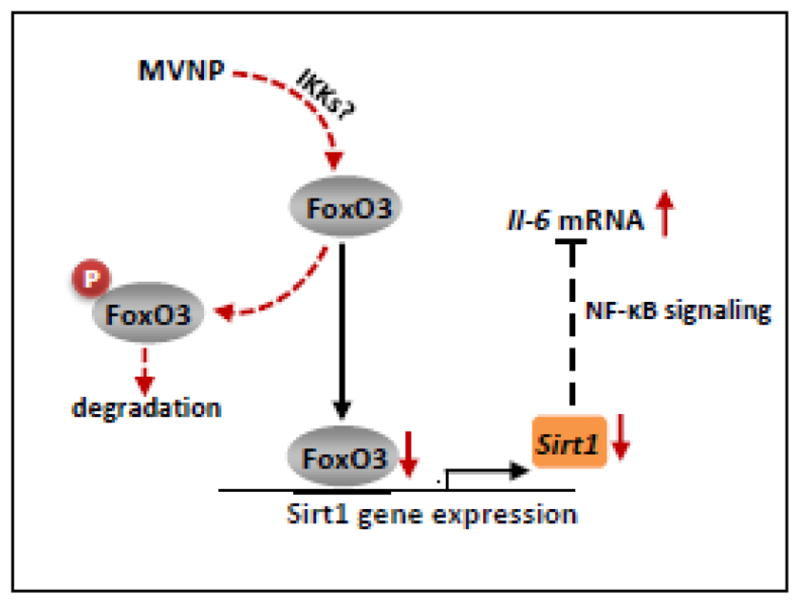
MVNP-increased phosphorylation of FoxO3, perhaps by members of the IKK family, leads to degradation of FoxO3 and down-regulation of Sirt1 expression. Decreased Sirt1 leads to more active NF-κB and increased IL6 gene expression.
Supplementary Material
Highlights.
Measles virus nucleocapsid protein (MVNP) induces IL-6 gene transcription.
MVNP decreases FoxO3 protein stability and thereby decreases expression of a repressor of NF-κB activity, Sirt1.
Activation of Sirt1 by resveratrol decreased the impact of MVNP on IL-6 expression.
Resveratrol blocked the MVNP-induced pagetic osteoclast phenotype of increased OCL formation.
Acknowledgments
The authors gratefully thank Dr. Philip Auron for the HEK293R cells and the MHCBκ NF-κB-pGL2 reporter plasmid, Dr. William Sellers for the wildtype and mutant FoxO3-pcDNA3 plasmids, Dr. Michael Greenberg for the FHRE-luc and Flag-Sirt1 plasmids, Dr. Toren Finkel for the pTA-Luc Sirt1 promoter plasmid (all supplied through Addgene), Dr. Jian Zhang for the IL6 promoter reporters, and the Veterans Administration Pittsburgh Healthcare System, Research and Development for use of the facilities. This work was supported by the National Institutes of Health (NIAMS grants R01 AR057308 to G.D.R. and R01 AR057310 to D.L.G.).
Abbreviations
- PD
Paget’s disease
- MVNP
measles virus nucleocapsid protein
- OCL
osteoclast
- FoxO
forkhead-box class O
- TRAP
tartrate resistant acid phosphatase
- TRAP-MVNP
MVNP expression under regulation of the TRAP gene promoter
- WT
wild-type
- PVDF
polyvinylidene difluoride
- TNF-α
tumor necrosis factor-α
- RANKL
receptor activator of NF-κB ligand
- M-CSF
monocyte colony-stimulating factor
- EV
empty vector
- DMEM
Dulbecco’s Modified Eagle Medium
- EMEM
Eagle’s Minimum Essential Medium
- α-MEM
α-Minimal Essential Medium
- BMM
bone marrow mononuclear
- RT-PCR
reverse transcription polymerase chain reactions
- CFU-GM
colony forming unit-granulocyte/macrophage
- TRAF
tumor necrosis factor receptor-associated factor
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Feng-Ming Wang, Email: fw2@iupui.edu.
Aliye Sarmasik, Email: asarmasik@hotmail.com.
Yuko Hiruma, Email: yukohiruma@hotmail.com.
Quanhong Sun, Email: sunq2@upmc.edu.
Benedicte Sammut, Email: sammutb@upmc.edu.
Jolene J. Windle, Email: jjwindle@vcu.edu.
G. David Roodman, Email: groodman@iupui.edu.
Deborah L. Galson, Email: dlgalson@gmail.com.
References
- 1.Kurihara N, Hiruma Y, Yamana K, Michou L, Rousseau C, Morissette J, Galson DL, Teramachi J, Zhou H, Dempster DW, Windle JJ, Brown JP, Roodman GD. Contributions of the measles virus nucleocapsid gene and the SQSTM1/p62(P392L) mutation to Paget’s disease. Cell Metab. 2011;13:23–34. doi: 10.1016/j.cmet.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–66. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 3.Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A, Smith JF, Singer FR. Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest. 1992;89:46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helin E, Vainionpaa R, Hyypia T, Julkunen I, Matikainen S. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology. 2001;290:1–10. doi: 10.1006/viro.2001.1174. [DOI] [PubMed] [Google Scholar]
- 5.Manchester M, Eto DS, Oldstone MB. Characterization of the inflammatory response during acute measles encephalitis in NSE-CD46 transgenic mice. J Neuroimmunol. 1999;96:207–17. doi: 10.1016/s0165-5728(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 6.Schneider-Schaulies J, Schneider-Schaulies S, Ter Meulen V. Differential induction of cytokines by primary and persistent measles virus infections in human glial cells. Virology. 1993;195:219–28. doi: 10.1006/viro.1993.1363. [DOI] [PubMed] [Google Scholar]
- 7.Kurihara N, Zhou H, Reddy SV, Garcia Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in mice. J Bone Miner Res. 2006;21:446–55. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- 8.Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–46. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida M. Unraveling the role of FoxOs in bone--insights from mouse models. Bone. 2011;49:319–27. doi: 10.1016/j.bone.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartell SM, Ambrogini E, Han L, Warren A, Shelton RS, Zhao H, Qiu X, Goellner J, O’Brien CA, Almeida MJS, Manolagas SC. Targeted FoxO3 overexpression in osteoclast progenitors and mature osteoclasts inhibits NF-Kb signaling and osteoclastogenesis and increases bone mass. J Bone Miner Res. 2011;26(Suppl 1) (Available at http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007101-127&terms=) [Google Scholar]
- 11.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–72. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–13. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A. 2012;109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 15.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–13. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Kurihara N, Reddy SV, Araki N, Ishizuka S, Ozono K, Cornish J, Cundy T, Singer FR, Roodman GD. Role of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget’s Disease. J Bone Miner Res. 2004;19:1154–64. doi: 10.1359/JBMR.040312. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y, Kumar A, Koyama Y, Peng H, Arman A, Boch JA, Auron PE. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004;279:1768–76. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]
- 19.Kukita A, Chenu C, McManus LM, Mundy GR, Roodman GD. Atypical multinucleated cells form in long-term marrow cultures from patients with Paget’s disease. J Clin Invest. 1990;85:1280–6. doi: 10.1172/JCI114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurihara N, Reddy SV, Menaa C, Anderson D, Roodman GD. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000;105:607–14. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka S, Kurihara N, Reddy SV, Cornish J, Cundy T, Roodman GD. (23S)-25-Dehydro-1{alpha}-hydroxyvitamin D3–26,23-lactone, a vitamin D receptor antagonist that inhibits osteoclast formation and bone resorption in bone marrow cultures from patients with Paget’s disease. Endocrinology. 2005;146:2023–30. doi: 10.1210/en.2004-1140. [DOI] [PubMed] [Google Scholar]
- 22.Wang FM, Galson DL, Roodman GD, Ouyang H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp Hematol. 2011;39:999–1006. doi: 10.1016/j.exphem.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 24.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Johnston G, Stebler B, Keller ET. Hydrogen peroxide activates NFkappaB and the interleukin-6 promoter through NFkappaB-inducing kinase. Antioxid Redox Signal. 2001;3:493–504. doi: 10.1089/15230860152409121. [DOI] [PubMed] [Google Scholar]
- 27.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 28.Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci. 1996;1:d340–57. doi: 10.2741/a136. [DOI] [PubMed] [Google Scholar]
- 29.Luron L, Saliba D, Blazek K, Lanfrancotti A, Udalova IA. FOXO3 as a new IKK-epsilon-controlled check-point of regulation of IFN-beta expression. European journal of immunology. 2012;42:1030–7. doi: 10.1002/eji.201141969. [DOI] [PubMed] [Google Scholar]
- 30.Yang JY, Hung MC. Deciphering the role of forkhead transcription factors in cancer therapy. Curr Drug Targets. 2011;12:1284–90. doi: 10.2174/138945011796150299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roodman GD. Regulation of osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:100–9. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- 32.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813:1938–45. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servant MJ, ten Oever B, LePage C, Conti L, Gessani S, Julkunen I, Lin R, Hiscott J. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J Biol Chem. 2001;276:355–63. doi: 10.1074/jbc.M007790200. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–51. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Chan CH, Chen K, Guan X, Lin HK, Tong Q. Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene. 2012;31:1546–57. doi: 10.1038/onc.2011.347. [DOI] [PubMed] [Google Scholar]
- 37.Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, Espejo A, Bedford MT, Gozani O, Gygi SP, Brunet A. Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging. 2012;4:462–79. doi: 10.18632/aging.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, Wabitsch M. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr. 2010;92:5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- 39.Kupisiewicz K, Boissy P, Abdallah BM, Hansen FD, Erben RG, Savouret JF, Soe K, Andersen TL, Plesner T, Delaisse JM. Potential of resveratrol analogues as antagonists of osteoclasts and promoters of osteoblasts. Calcif Tissue Int. 2010;87:437–49. doi: 10.1007/s00223-010-9399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ, Lee SH, Lee KU, Kim GS, Kim SW, Koh JM. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–37. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaisse JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–52. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- 42.Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286:11492–505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.