Abstract
Although Dopamine and cAMP-regulated phosphoprotein, Mr 32000 (DARPP-32) is overexpressed in two-thirds of gastric cancers, it impact on molecular functions has not been fully characterized. In this study, we examined the role of DARPP-32 in gastric cancer cell invasion. Using matrigel coated Boyden chamber invasion assay, DARPP-32-overexpressing AGS cells showed 3-fold increase in invasion relative to the vector control (p<0.01). We also tested the trans-endothelial cell invasion as a measure of cell aggressiveness using the impedance-based HUVEC invasion assay and obtained similar results (p<0.001). Western blot analysis indicated that overexpression of DARPP-32 mediated an increase in the MT1-MMPand CXCR4 protein levels. Consistent with the role of MT1-MMP in cleaving extracellular matrix proteins initiating the activation of soluble MMPs, we detected a robust increase in MMP-2 activity in DARPP-32-overexpressing cells. The knockdown of endogenous DARPP-32 in the MKN-45 cells reversed these signaling events and decreased cell invasive activity. We tested whether the invasive activity mediated by DARPP-32 might involve sustained signaling via CXCR4-dependent activation of the MT1-MMP/MMP-2 pathway. The small-molecule CXCR4 antagonist (AMD3100) and CXCR4-siRNA blocked DARPP-32-induced cell invasion. We further examined our hypothesis that DARPP-32 could interact with CXCR4 and stabilize its levels following stimulation with its ligand, CXCL12. Using reciprocal co-immunoprecipitation and immunofluorescence experiments, we found that DARPP-32 and CXCR4 co-exist in the same protein complex. DARPP-32 prolonged the CXCR4 protein half-life and reduced ubiquitination of the CXCR4 protein, following treatment with its ligand, CXCL12. In conclusion, these findings demonstrate a novel mechanism by which DARPP-32 promotes cell invasion by regulating CXCR4-mediated activation of the MT1-MMP/MMP-2 pathway.
Keywords: MMPs, CXCL-12, MT1-MMP, invasion, DARPP-32
Introduction
Gastric cancer is one of the most common cancers worldwide (1). The prognosis for gastric cancer patients remains poor, especially in more advanced stages(2). Dopamine and cAMP-regulated phosphoprotein, Mr 32000 (DARPP-32), is abundantly expressed in striatal neurons and in striatonigral fibers (3). We have previously shown that DARPP-32 is overexpressed in approximately two-thirds of gastric cancers (4, 5). The expression of DARPP-32 occurs in the early stages of the gastric cancer progression cascade (4). Overexpression of DARPP-32 is associated with potent anti-apoptotic advantage and drug resistance in gastric cancer cells by promoting interaction between EGFR and ERBB3 and activating PI3K-AKT signaling (6, 7).
The invasion capacity of cancer cells determines their penetration power into surrounding tissues, a crucial early step in the metastatic cascade (8, 9). Metastasis, the major cause of morbidity and mortality in most cancers, is a complex pathophysiological process(10). Recently, it was suggested that chemokine stromal cell-derived factor-1α (SDF-1α, also known as CXC-chemokine ligand 12, CXCL-12) and its receptor, CXC-chemokine receptor 4 (CXCR4), are involved in gastric cancer invasion and metastasis (11–13). CXCR4 expression is associated with gastric cancer cell migration in vitro, and strong expression of CXCR4 by gastric cancer cells is significantly associated with lymphatic metastasis in patients with gastric cancer, suggesting that CXCR4 plays an important role during gastric cancer progression(14). Members of the MMP family are multifunctional zinc-dependent endopeptidases that can degrade a variety of ECM components. Almost all MMPs are secreted into the extracellular milieu, except six Membrane-Type Matrix Metalloproteinases (MT MMPs) (MT1-MMP to MT6-MMP, also called MT1-MMP, MMP-15, MMP-16, MMP-17, MMP-24 and MMP-25, respectively) (15, 16). MMPs share a conserved structure that consists of a signal peptide, a propeptide and a catalytic domain. Overexpression of MT1-MMP has been observed in gastric cancers, which is associated with the invasiveness of the cancer cells (17). It has reported that the expression of MT1-MMP may influence prognosis via tumor invasion of the gastric wall and lymph node metastasis, and activation of MMP-2 correlates with local invasion and lymphatic permeation in gastric cancers(18).
Although several molecules have been implicated in cancer metastasis, the detailed mechanism of gastric cancer invasion is not completely understood. In the present study, we have uncovered a novel mechanism by which DARPP-32 regulates CXCR4 and gastric cancer cell invasion. We have demonstrated that DARPP-32 interacts with CXCR4 promoting its stability and enhancing the MT1-MMP-regulated activation of MMP-2.
Materials and Methods
Cell Culture and Reagents
Human gastric cancer cell lines (AGS and MKN-45) and the immortalized human embryonic kidney epithelial cell line (HEK-293) were maintained in culture using either F12 medium or Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, CA). CXCL-12 and AMD3100 were purchased from BioVision (Mountain View, CA) and Tocris Bioscience (Ellisville, MO), respectively. Anti-Mouse and anti-Rabbit DARPP-32 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Abcam (Cambridge, MA), respectively. Anti-MT1-MMP and anti-CXCR4 antibodies were purchased from Abgent, and Sigma-Aldrich, respectively. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies; anti-MMP-2; and anti-β-actin antibodies were obtained from Cell Signaling Technology (Danvers, MA).
DARPP-32 expression and shRNA vectors
The flag-tagged coding sequence of DARPP-32 was cloned in pcDNA3.1 mammalian expression construct (Invitrogen). AGS cells were transfected with the expression construct and stable cell lines expressing DARPP-32 were generated (DP01 and DP02) (5). Lentivirus particles expressing DARPP-32, shRNA-DARPP-32or control shRNA were produced by GeneCopoeia (Rockville, MD).
Invasion Assay and HUVEC Invasion Assay
The effect of DARPP-32 on the invasion ability of AGS cells or MKN-45 cells was determined using matrigel invasion chambers (BD Biosciences). AGS cells stably expressing DARPP-32 were seeded into inserts at 1 × 104 cells per insert in serum-free medium and then transferred to wells filled with the culture medium containing 10% fetal bovine serum. After 24h incubation, non-invading cells on the top of the membrane were removed by cotton swabs. Invaded cells on the bottom of the membrane were fixed with 4% paraformaldehyde for 10 min, followed by staining with 0.05% crystal violet for 3 hr. Photographs were taken and all the cells on the entire membrane were counted. The relative invasion activity was calculated after normalization to cell migration.
We also used the transendothelial cell invasion assay as a measure for cell aggressiveness. The interaction of tumor cells with endothelial cells is a key event in tumor metastasis (19). The various components of tumor cell-endothelial cell interaction can be replicated in vitro by challenging a monolayer of human umbilical vein endothelial cells (HUVEC) with cancer cells. We modified an established in vitro invasion assay system in which the invasion of tumor cells after interaction with endothelial cells can be examined (20). The xCELLigence system (Roche Diagnostics) was used to monitor the change in the cell index. It measures the effect of any perturbations in a label-free real-time setting and measures as change in electrical impedance (change in resistance at cell-electrode interphase), which is recorded as cell index, and data point is collected every 5 min. The relative rate of invasion in transendothelial tumor cell invasion can be defined as: A reduction in the cell index of the HUVEC cells monolayer after the addition of the invasive cell line as a function of time, compared with the drop in the cell index with the addition of a non-invasive cell line, normalized with the cell index of HUVEC cell monolayer at a given time, as measured by the xCELLigence system. For transendothelial invasion assays, Roche E-plates (Cat# 05469813001) were treated with 100μl of 0.1% sterile gelatin (Sigma, St. Louis, MO) overnight at 4°C. Plates were washed once with sterile PBS before the addition of early passage HUVEC cells, obtained from Lonza Biosciences (Basil, Switzerland). HUVEC cells were grown in EBM-2 basal media (Cat# CC3156, Lonza Biosciences) supplemented with EGM-2 growth factors (Cat# CC4176, Lonza Biosciences). The E-plates were seeded with 25,000 HUVEC cells/100μl and incubated for 18h at 37°C. The cell index was monitored on the xCELLigence system while the monolayer was formed. Following the formation of the HUVEC monolayer, which is indicated by the plateau in the cell index, the EGM-2 media was removed and 100μl of RPMI + 5% serum containing media was added. The cell index was monitored for 4h and allowed to stabilize. After the cell index stabilized, the invading cells were added to each well at a density of 5000 cells/100μl. The cell index was normalized to the HUVEC monolayer and invasion was monitored over time. The experiments were performed in 6 wells per cell line. Rate of invasion of the cell lines were calculated according to the RTCA software version 1.2, within particular time intervals.
Gelatin Zymography
Gelatin zymography was performed in 12% SDS-PAGE that had been casted in the presence of 0.1% gelatin. Samples were prepared in non-reducing loading buffer. After electrophoresis, SDS was removed by 2.5% Triton X-100 to renature gelatinases. Gels were then incubated at 37°C for 72hr in an incubation buffer [50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 5 mM CaCl2, and 0.7 mg ZnCl2], and were then stained with 0.125% Coomassie Brilliant Blue R-250. TPA (12-O-Tetradecanoylphorbol 13-acetate) was used as positive control.
Immunofluorescence
Following cell fixation and permeabilization, immunofluorescence was performed with both anti-DARPP-32 (1:800) and anti-CXCR4 (1:200) antibodies. The cells were then washed with cold PBS three times for 3 min each, and incubated with both Alexa-Fluor 488 goat anti-rabbit secondary antibody (green, 1:400) and Alexa-Fluor 568 goat anti-moue secondary antibody (red, 1:800) (Invitrogen) at room temperature for 1hr. The cells were examined by fluorescence microscopy (Olympus America Inc., Center Valley, PA).
Immunoprecipitation and Western blotting
Cells were lysed with lysis buffer containing 0.5% Triton X-100, 150 mM NaCl, 5 mM EDTA, and 50 mM Tris supplemented with protease and phosphatase inhibitors. Immunoprecipitations of equivalent total protein amounts were performed at room temperature for 1h by using a primary antibody previously bound to 50 μl of Protein A Dynabeads per sample. The beads were washed four times with wash buffer. The beads in each tube were heated to 100°C for 5 min in 20 μl of sample buffer, and then clarified by magnet. Proteins were separated on 12.5% SDS-PAGE and transferred to Immobilon PVDF membrane (Millipore). Membranes were probed with specific antibodies. Proteins were then visualized by using horseradish peroxidase (HRP)-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP Substrate detection reagent (Millipore). β-actin was used as loading control.
Quantitative Real-time RT-PCR analysis(qRT-PCR)
Twenty pairs of de-identified gastric adenocarcinomas and their adjacent histological normal specimens were obtained from the NCI Human Cooperative Human Tissue Network in accordance with approved protocols. Total RNA was isolated from cell lines and human tissue samples by using the RNeasy Mini Kit (Qiagen, Valencia, CA). Total RNA (1 μg) was reverse transcribed by an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The qRT-PCR was performed using an iCycler and iCycler software version 3.0 (Bio-Rad). The primers for CXCR4 were forward: 5′-AATCTTCCTGCCCACCATCT-3′; reverse: 5′-GACGCCAACATAGACCACCT-3′; the primers for CXCL-12 were forward: 5′-GTGGTCGTGCTGGTCCTC-3′; reverse: 5′ AGATGCTTGACGTTGGCTCT-3′; and primers for HPRT1 were forward: RT-F: 5′-TTGGAAAGGGTGTTTATTCCTCA-3′; reverse: RT-R: 5′-TCCAGCAGGTCAGCAAAGAA-3′. The results of three independent experiments were subjected to statistical analysis. Fold change was calculated using the ΔΔC(t) method (21). HPRT1 was used as normalization control.
Ubiquitination Assays for CXCR4
HEK293 cells were transfected with HA-CXCR4, His-ubiquitin and pcDNA or DARPP-32. The next day, cells were transferred onto 6 cm dishes and allowed to grow for an additional 24h. The cells were then washed by aspirating medium and then replacing it with 4 mL of warm DMEM supplemented with 20 mM HEPES. We then incubated the cells in the same medium in the presence of 30 nM CXCL-12 for 30 min, rapidly wash cells on ice with cold PBS, and collected samples in 1 ml of lysis buffer. Samples were transferred into microcentrifuge tubes and placed at 4°C for 20 min, followed by sonification and centrifugation to pellet cellular debris. Cell lysates were incubated with an anti-HA polyclonal antibody and the immunoprecipitates were analyzed by SDS-PAGE followed by Western blotting using an anti-His antibody conjugated to HRP.
Statistical analyses
Data were expressed as mean ± SD of three independent experiments. Statistical significance of the in vitro studies was analyzed by a Student’s t test. Differences with p values ≤0.05 are considered significant.
Results
DARPP-32 enhances gastric cancer cells invasive activity
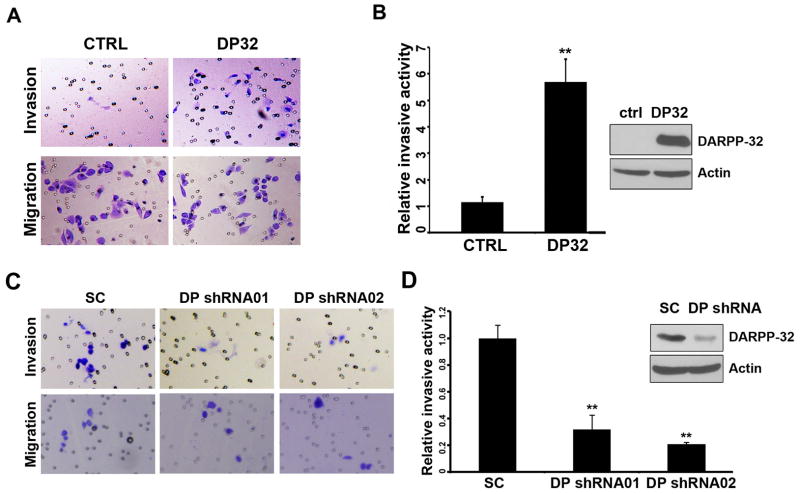
We detected a 5-fold increase in the invasive activity in DARPP-32-expressing cells, as compared to control AGS cells (Figure 1A–B). The knockdown of endogenous DARPP-32 using DARPP-32 specific shRNAs in MKN-45 cells (DARPP-32-shRNA01 and DARPP-32-shRNA02) inhibited the invasive activity (Figure 1C–D). We also performed invasion assay using CXCL-12/SDF-1 (100ng/ml) with low serum (1% FBS medium), a minimum amount to maintain cell survival. The results showed that DARPP-32 enhances CXCL-12-mediated cells invasion as compared to ctrl cells (Supplementary Figure S1).
Figure 1. DARPP-32 increased invasive activity in gastric cancer cells.
A) Invasion assays were performed using the matrigel-boyden chamber assay. AGS cells lentiviral expressing DARPP-32 (DP32) are more invasive than control (ctrl). B) The quantification demonstrates a significant increase in the number of invasive cells in AGS cells stably expressing DARPP-32 (p<0.01). Western blot demonstrates the levels of DARPP-32. C–D) The knockdown of DARPP-32 in the MKN-45 cells (shRNA01 and 02) led to a significant reduction in cell invasion (p<0.01). The levels of DARPP-32 are shown by Western blot analysis.
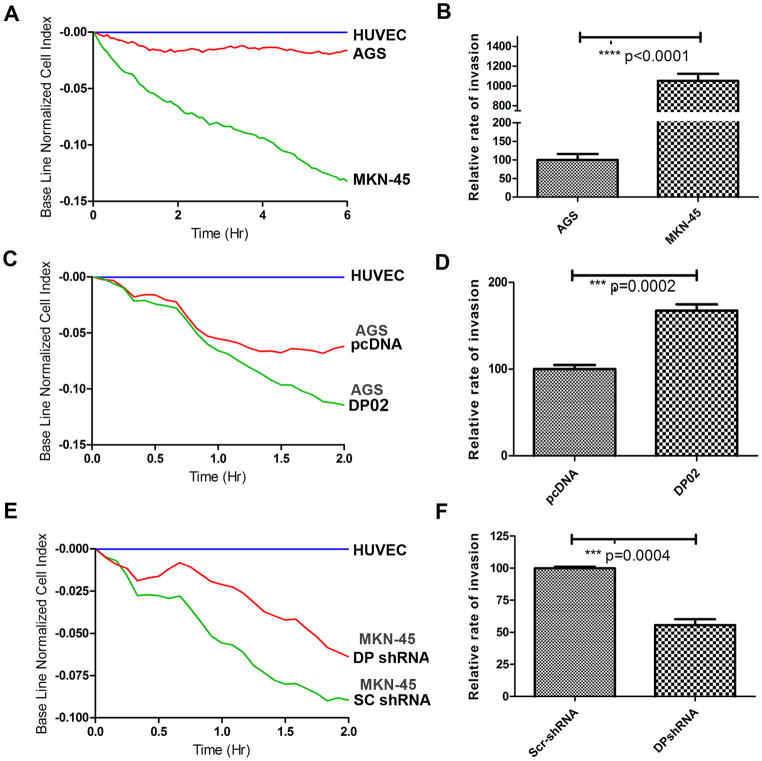
To address the question of whether DARPP-32 is able to mediate invasion, we utilized an impedance based endothelial cell invasion assay. This technique involves challenging a confluent monolayer of Human Umbilical Vein Endothelial Cells (HUVECs) with a second layer of metastatic cells that attach to and invade the HUVEC monolayer. First, we measured the invasive activity of wild-type AGS and MKN-45 cells; MKN-45 cells have a higher expression level of DARPP-32 protein as well as higher invasive activity compared with AGS cells (Figure 2A–B). The AGS cells stably overexpressing DARPP-32 resulted in significantly increased invasive activity (Figure 2C–D, p<0.001). In contrast, the knockdown of endogenous DARPP-32 in MKN-45 cells decreased cell invasion (Figure 2E–F, p<0.05). Taken together, these results have established an important, previously unknown, role for DARPP-32 in enhancing the invasive activity in gastric cancer cells.
Figure 2. DARPP-32 enhances gastric cancer cells invasion.
A–B) 5000 cells each of AGS or MKN-45 were used to challenge the HUVEC monolayer. The cell index was measured every 5 min by the xCELLigence system. The rate of change of cell index as a function of time was calculated as a measure of invasive activity. MKN45 cells have a higher change in cell index than AGS cells. C–D) The same as in A–B, using AGS cells stably expressing DARPP-32 or pcDNA demonstrates a significant increase in the rate of change of cell index in DARPP-32 expressing AGS cells (DP02) as compared to control (pcDNA) (p=0.0002). E–F) The same as in A–B, following knockdown of DARPP-32 (DP shRNA) or control (SC shRNA) in MKN45 cells, demonstrates a significant decrease in the rate of change of cell index (P=0.0004).
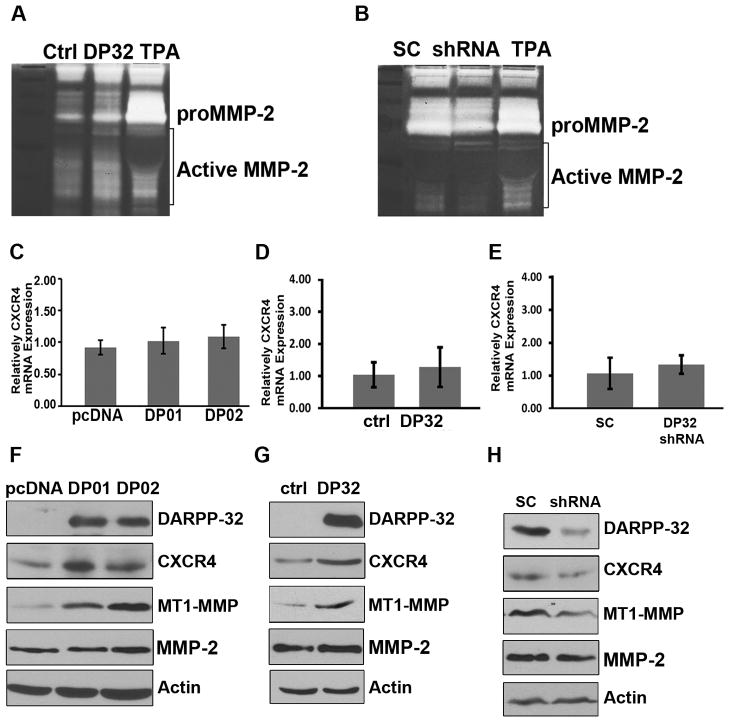
DARPP-32 induces CXCR4 and MT1-MMP protein expression and enhances MMP-2 activity The expression and activity levels of MMPs are known to play an in important role in cell invasion. To detect possible effects of DARPP-32 on the expression of MMPs, we utilized a RayBio® Human MMP Antibody Array, which comprises spotted antibodies specific for MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, TIMP-1, TIMP-2, and TIMP-4. We did not detect any significant changes in the MMPs protein levels (Supplementary Figure S2). Therefore, we though to examine the activity of MMP-9 and MMP-2, important mediators of cell invasion, using a zymography assay. Although we did not observe changes in the MMP-9 activity (data no shown), we detected a robust increase in MMP-2 activity in DARPP-32 overexpressing cells (Figure 3A) which was reversed upon he knockdown of endogenous DARPP-32 in MKN-45 cells (Figure 3B). Our next step was to examine the expression of CXCR4, a major protein that mediates regulation of MMPs and cell invasion in cancer. Using qRT-PCR, we could not detect significant changes in CXCR4 mRNA levels following overexpression of knockdown of DARPP-32 (Figure 3C–E). On the other hand, we detected changes in the protein level of CXCR4 and MT1-MMP in these cells, whereas MMP2 levels were unchanged (similar to our array results) (Figure 3F–G). Conversely, knockdown of endogenous DARPP-32 resulted in decreased CXCR4 and MT1-MMP protein levels in MKN-45 cells (Figure 3H). The MT1-MMP protein plays a major role in cleavage of extracellular matrix proteins initiating the activation of soluble MMPs (22). Therefore, our findings explain the noted changes in activity of MMP2. Taken together, these results suggest that the increased invasive activity of DARPP-32-expressing cells could be attributed to DARPP-32-mediated up-regulation of CXCR4 and MT1-MMP with a subsequent increase in the activity of MMP-2.
Figure 3. DARPP-32 enhances the chemokine receptor CXCR4 expression.
A) Gelatin zymography of AGS cells expressing lentiviral particles of DARPP-32 (DP32) demonstrates activation of MMP2 as compared to control particles (ctrl), TPA was used as a positive control. B) The lentiviral shRNA knockdown of endogenous DARPP-32 in MKN45 cells reversed these findings. The qRT-PCR analysis demonstrates non-significant differences in the level of CXCR4 in AGS cells stably overexpressing pcDNA-DARPP-32 (DP01 and DP02) (C) or lentivirus particles of DARPP-32 (D), as compared to controls. E) Similarly, no differences were observed following knockdown of endogenous DARPP-32 in MKN45 cells. Western blot analysis of AGS cells stably expressing DARPP-32 (F) or lentiviral DARPP-32particles (G) demonstrates up-regulation of CXCR4 and MT1-MMP, but not MMP-2. H) The lentiviral shRNA knockdown of endogenous DARPP-32 in MKN45 cells reversed these findings.
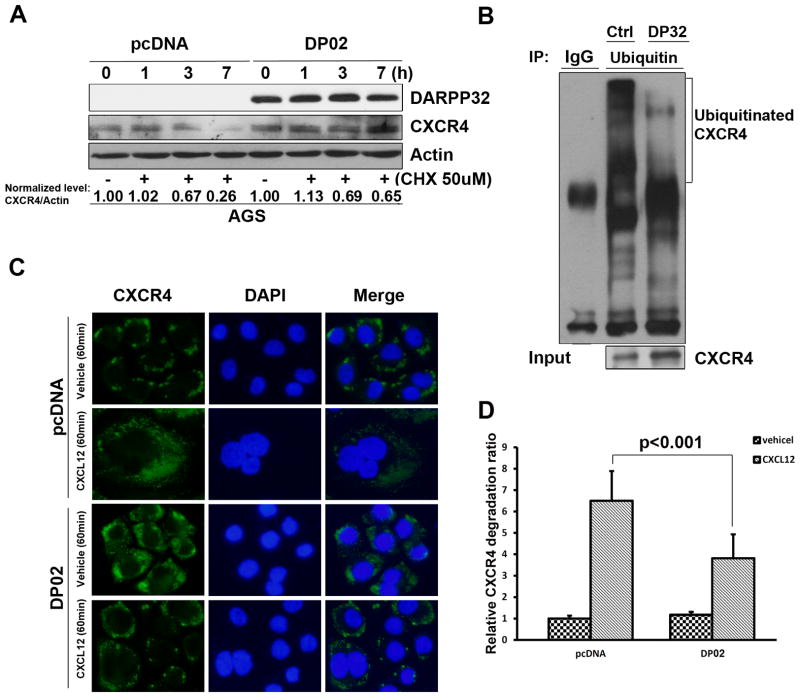
DARPP-32 interacts with CXCR4 and blocks CXCR4 degradation
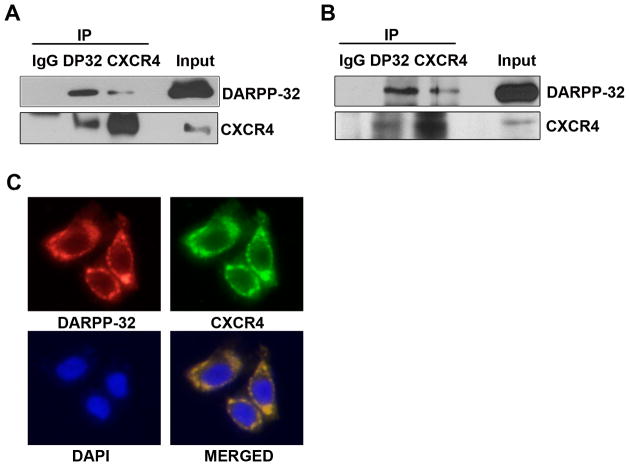
Because our data suggested that the DARPP-32-mediated up-regulation of CXCR4 was not transcriptional, we tested the hypothesis that DARPP-32 protein may bind to CXCR4, thereby affecting CXCR4 protein stability. Indeed, we found that CXCR4 co-immunoprecipitated with the exogenous DARPP-32 protein in HEK-293 cells (Figure 4A) and confirmed that endogenous DARPP-32 and CXCR4 proteins are also present in the same complex in MKN45 cells (Figure 4B). In line with these results, the immunofluorescence assay demonstrated the co-localization of DARPP-32 and CXCR4 on the cell membrane (Figure 4C). We also measured the changes in the CXCR4 protein half-life, following treatment with the protein synthesis inhibitor cyclohexamide (CHX) and found that DARPP-32 prolonged the CXCR4 protein half-life, as compared to control(Figure 5A). Next, we examined whether the stabilization of DARPP-32-induced CXCR4 was mediated through the ubiquitin-proteasome pathway. IP-Western blot showed reduced ubiquitination of CXCR4 protein after overexpression of DARPP-32 in HEK-293 cells (Figure 5B). To analyze the effects of DARPP-32 on CXCL-12-induced CXCR4 degradation, DARPP-32-expressing AGS cell were treated with CXCL-12 (30 nM) for 1h. As shown in Figure 5C–D, CXCR4 remained mainly localized to the plasma membrane after vehicle or CXCL-12 treatment of DARPP-32-expressing AGS cells. On the other hand, CXCR4 was mainly localized to punctate vesicles distributed throughout the cytoplasm in AGS control cells (Figure 5C–D). These data indicated that DARPP-32 significantly delayed the CXCL-12-induced degradation of CXCR4 protein.
Figure 4. DARPP-32 interacts with CXCR4.
A) HEK-293 cells were transiently transfected with DARPP-32 and CXCR4. Two-way co-immunoprecipitation and Western blot analyses demonstrates the interaction of DARPP-32 and CXCR4. B) Similar to (A), the protein interaction of endogenous DARPP-32 and CXCR4 was evaluated by co-immunoprecipitation in MKN-45 cells. C) Immunofluorescence analysis using AGS cells, following overexpression of DARPP-32, demonstrates co-localization of CXCR4 and DARPP-32 proteins on the cell membrane.
Figure 5. DARPP-32 promotes CXCR4 protein stability.
A) Western blot analysis, following the treatment with CHX (50 uM) for the indicated time points, demonstrated increased levels of CXCR4 protein in AGS cells stably expressing DARPP-32, as compared to control (pcDNA). B) HEK-293 cells co-transfected with HA-CXCR4, His-ubiquitin and pcDNA or DARPP-32 were treated with 30 nM CXCL-12 for 30 min. Cell lysates were incubated with an anti-HA polyclonal antibody and the immunoprecipitates were analyzed by SDS-PAGE followed by Western blotting using an anti-His antibody conjugated to HRP. Cell lysates were analyzed for the presence of HA-CXCR4 and demonstrated less CXCR4 ubiquitination in DARPP-32-expressing cells, as compared to control. C) AGS cells stably expressing DARPP-32 (DP02) or empty vector were treated with 30 nM CXCL-12 or vehicle for 60 min, followed by immunofluorescence staining with anti-CXCR4 (green) and DAPI (blue). CXCR4 remained mainly localized to the plasma membrane after vehicle or CXCL-12 treatment of DARPP-32-expressing AGS cells. On the contrary, CXCR4 was localized to diffuse punctate cytosolic vesicles in control cells D) Quantification of relative CXCR4 degradation shows a significant decrease in the relative CXCR4 degradation in the AGS cells stably-expressing DARPP-32 (p<0.001).
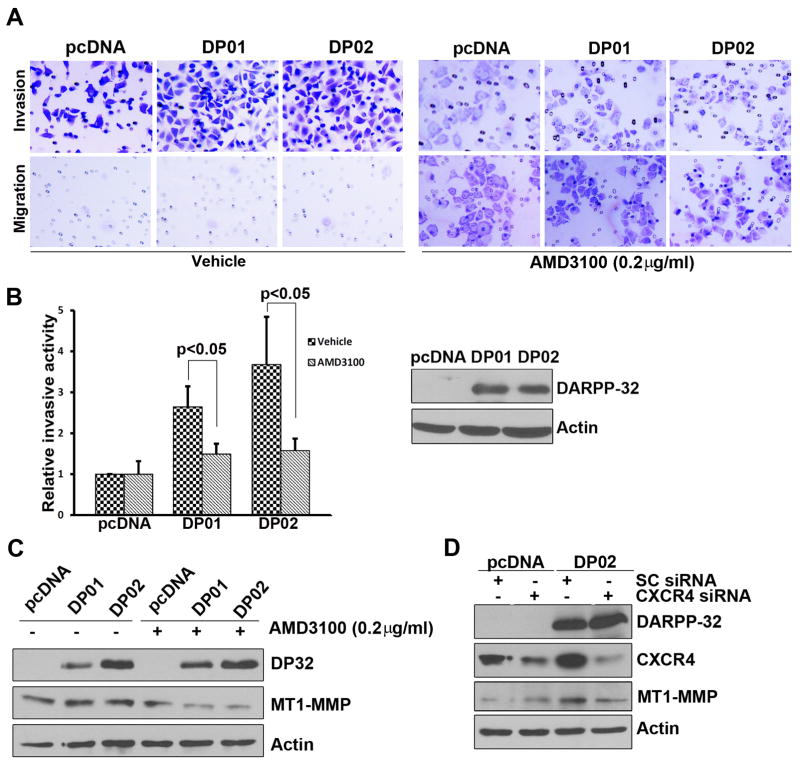
Blocking CXCR4 attenuates DARPP-32-mediated invasive properties in gastric cancer cells
To determine if CXCR4 is indeed the major determinant of the DARPP-32-mediated increased invasion potential, we utilized the small-molecule CXCR4 antagonist (AMD3100) and CXCR4 siRNA approaches. AMD3100 is a symmetric bicyclam, prototype non-peptide antagonist of the CXCR4 chemokine receptor. Using the migration and invasion assay as described above, an overnight treatment with AMD3100 (0.2 ug/ml) was sufficient to significantly diminish cell invasion in DARPP-32-expressing cells (Figure 6A–B). We next investigated if the changes in MT1-MMP expression in DARPP-32 expressing cells are also mediated by CXCR4. Indeed, the inhibition of CXCR4 using an overnight treatment with AMD3100, led to a remarkable reduction in MT1-MMP (Figure 6C). Similar results were obtained following the use of CXCR4 siRNA (Figure 6D). Taken together, the results indicate that CXCR4 is a major player of DARPP-32-mediated invasion in gastric cancer cells.
Figure 6. Blocking of CXCR4 disrupts DARPP-32-mediated cancer cells invasion.
A) Invasive assays were performed in the AGS cells-stably expressing DARPP-32 after treatment with AMD3100 (0.2 μg/ml) or vehicle. B) Quantification of invasion assay shows a significant decrease in relative cell invasion after AMD3100 treatment (p<0.05), the levels of DARPP-32 are shown in the Western blots. C) Western blot analysis following the above conditions demonstrates a decrease in the levels of MT1-MMP in DARPP-32 expressing cells (DP01 and 02). D) The knockdown of CXCR4 (CXCR4 siRNA) in AGS cells stably expressing DARPP-32 led to a decrease in the levels of CXCR4 and MT1-MMP, as compared to control (Sc siRNA).
CXCL-12 and CXCR4 mRNA level positively correlate with DARPP-32expression
We detected high levels of DARPP-32, CXCR4 and CXCL-12 in tumors, as compared to adjacent normal tissues (p<0.01) (Supplementary Figure S3). Statistical analysis using the Pearson’s method confirmed the positive correlation between CXCR4 and DARPP-32 or CXCL-12 and DARPP-32 in these tissue samples, with a correlation coefficient of 0.636 or 0.397, respectively (Figure 7A–B). These results suggest that that the expression of these components of cell invasion is intact in gastric tumors.
Figure 7. Positive correlation between expression levels of DARPP-32, CXCL-12 and CXCR-4.
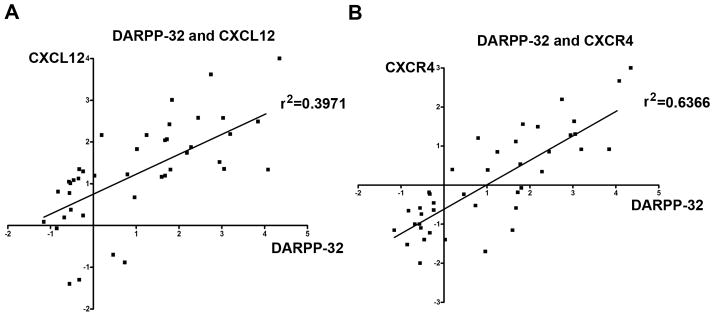
The qRT-PCR analysis of the expression levels of CXCL-12, CXCR4, and DARPP-32 in gastric tumors and their adjacent histologically normal non-tumor tissue samples demonstrated a statistically significant positive correlation between the levels of CXCL-12 and DARPP-32 (A) and between CXCR4 and DARPP-32 (B), suggesting that these components of invasion activity co-exist in gastric cancer cells.
Discussion
Although overexpression of DARPP-32 has been identified in several malignancies such as gastric, esophageal, colon, and breast cancers (4, 23, 24), the full spectrum of its biological functions in cancer remains uncharacterized. The ability of cancer cells to invade the nearby tissues and blood vessels is one of the crucial steps that determine the metastatic potential of a tumor (8, 9, 25, 26). In this study, we demonstrate a novel molecular mechanism by which DARPP-32 regulates CXCR4 and mediates cancer cell invasion.
The human chemokine superfamily includes at least 46 ligands, which bind to 18 functionally signaling G-protein-coupled receptors and two decoy or scavenger receptors (27). The chemokine CXCL-12/SDF-1 binds to the receptors CXCR4 and CXCR7 (28, 29). The chemokine receptor CXCR4 and its ligand CXCL-12/SDF-1 are known to play an important role in the development of invasion and metastases in several malignancies including gastric cancer {Smith, 2004 #259; Yasumoto, 2006 #227; Bohn, 2009 #283}. The current study examined a hypothesis that cancer cell invasion mediated by DARPP-32 in gastric cancer cells might involve a CXCR4-dependent pathway. We have demonstrated that DARPP-32 promotes invasion in gastric cancer cells by increasing CXCR4 protein levels. CXCR4 activated by CXCL-12 is rapidly internalized and targeted into the degradative pathway by an ubiquitin-dependent mechanism (30). Activation by CXCL-12 induces phosphorylation of CXCR4, then promotes binding to the E3 ubiquitin ligase, AIP4 (31). This is followed by internalization of CXCR4 onto early endosomes where the ubiquitin moiety serves as a sorting signal to direct the receptor to lysosomes for proteolysis (30). Because of our finding that exogenous and endogenous DARPP-32 interacted with CXCR4, we examined the protein stability levels of CXCR4. The results uncovered a novel mechanism whereby DARPP-32-CXCR4 interaction prolongs the CXCR4 protein half-life and blocks the CXCL-12-induced CXCR4 protein ubiquitination and degradation. Taken together, our findings suggest that DARPP-32 and CXCR4 binding could block CXCR4 internalization, thereby preventing its ubiquitination.
We did not identify a significant role for DARPP-32 in regulating the protein levels of several MMPs that included MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, TIMP-1, TIMP-2, and TIMP-4. However, we observed an increase in the activity of MMP2 and demonstrated an increase in the MT1-MMP protein level in DARPP-32 expressing cells. High expression levels of MT1-MMP were reported in gastric cancer metastasis (18). Various cell types employ MT1-MMP to alter their surrounding environment where MT1-MMP-mediated activation of MMP2 plays a key role during tumor invasion and metastasis (32, 33). It was also previously reported that CXCR4 and the MT1-MMP are mutually required during melanoma metastasis to lungs (34) and CXCL-12/SDF-1 is a key signaling event for MT1-MMP/MMP-2-dependent melanoma and breast cancer cell invasion (11, 33). These observations support our findings that DARPP-32 up-regulated MT1-MMP expression and promoted MMP-2 activity. Using small molecule inhibitors and siRNA knockdown approaches, we have confirmed the requirement of CXCR4 in promoting DARPP-32-dependent signaling events and cell invasion. Therefore, the sustained signaling via CXCR4-dependent activation of the MT1-MMP/MMP-2 pathway is a critical step in the DARPP-32-mediated cancer cell invasion. We also detected a positive correlation between high expression levels of DARPP-32, CXCR4 and CXCL-12 in primary gastric cancers, supporting the integrity of this axis in tumors.
In conclusion, our findings suggest that DARPP-32 plays an important role in invasion of gastric cancer cells. The ability of DARPP-32 to bind to CXCR4 and activate MT1-MMP/MMP-2 signaling provides a novel mechanism in mediating CXCR4 overexpression and metastasis in cancer.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by R01CA93999 (WER) from the National Institute of Health; Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, Department of Veterans Affairs, or Vanderbilt University.
Abbreviations
- DARPP-32
Dopamine and cAMP-regulated phosphoprotein, Mr 32000
- PVDF
Polyvinylidene Fluoride
- HRP
Horseradish peroxidase
- CHX
Cycloheximide
- CXCL-12
CXCchemokine ligand 12
- SDF-1α
Stromal cell-derived factor-1α
- CXCR4
CXC-chemokine receptor 4
- MT MMPs
Membrane-Type Matrix Metalloproteinases
- HEK-293
Human embryonic kidney epithelial cell line
- HUVEC
Human Umbilical Vein Endothelial Cells
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Shoumin Zhu, Email: shoumin.zhu@vanderbilt.edu.
Jun Hong, Email: jun.hong@vanderbilt.edu.
Manish K Tripathi, Email: manish.K.ripathi@vanderbilt.edu.
Vikas Sehdev, Email: vikas.sehdev@vanderbilt.edu.
Abbes Belkhiri, Email: abbes.belkhiri@vanderbilt.edu.
Wael El-Rifai, Email: wael.el-rifa@vanderbilt.edu.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer Journal international du cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–64. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Nairn AC, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32 and phosphatase inhibitor-1, two structurally related inhibitors of protein phosphatase-1, are both present in striatonigral neurons. J Neurochem. 1988;50:257–62. doi: 10.1111/j.1471-4159.1988.tb13258.x. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee K, Peng D, Brifkani Z, Belkhiri A, Pera M, Koyama T, et al. Dopamine and cAMP regulated phosphoprotein MW 32 kDa is overexpressed in early stages of gastric tumorigenesis. Surgery. 2010;148:354–63. doi: 10.1016/j.surg.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Rifai W, Smith MF, Jr, Li G, Beckler A, Carl VS, Montgomery E, et al. Gastric cancers overexpress DARPP-32 and a novel isoform, t-DARPP. Cancer Res. 2002;62:4061–4. [PubMed] [Google Scholar]
- 6.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 7.Zhu S, Belkhiri A, El-Rifai W. DARPP-32 Increases Interactions Between Epidermal Growth Factor Receptor and ERBB3 to Promote Tumor Resistance to Gefitinib. Gastroenterology. 2011;141:1738–48. e2. doi: 10.1053/j.gastro.2011.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung CM, Hsu CM, Hsu JT, Yeh TS, Lin CJ, Chen TC, et al. Predictive factors for lymph node metastasis in early gastric cancer. World J Gastroenterol. 2010;16:5252–6. doi: 10.3748/wjg.v16.i41.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namikawa T, Kobayashi M, Kitagawa H, Okabayashi T, Dabanaka K, Okamoto K, et al. Early gastric cancer with widespread duodenal invasion within the mucosa. Dig Endosc. 2010;22:223–7. doi: 10.1111/j.1443-1661.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 10.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 11.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo ZY, et al. HER2 Interacts With CD44 to Up-Regulate CXCR4 via Epigenetic Silencing of microRNA-139 in Gastric Cancer Cells. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Ying J, Xu Q, Zhang G, Liu B, Zhu L. The expression of CXCL12 and CXCR4 in gastric cancer and their correlation to lymph node metastasis. Med Oncol. 2011 doi: 10.1007/s12032-011-9990-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Kim SW, Kim HY, Li S, Yun HJ, Song KS, et al. Chemokine receptor CXCR4 expression, function, and clinical implications in gastric cancer. Int J Oncol. 2009;34:473–80. [PubMed] [Google Scholar]
- 15.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–51. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 16.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, et al. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–28. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimori K, Fukagawa T, Kosaka Y, Ishikawa K, Iwatsuki M, Yokobori T, et al. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bone marrow in gastric cancer cases. Ann Surg Oncol. 2008;15:2934–42. doi: 10.1245/s10434-008-9916-z. [DOI] [PubMed] [Google Scholar]
- 18.Mori M, Mimori K, Shiraishi T, Fujie T, Baba K, Kusumoto H, et al. Analysis of MT1-MMP and MMP2 expression in human gastric cancers. International journal of cancer Journal international du cancer. 1997;74:316–21. doi: 10.1002/(sici)1097-0215(19970620)74:3<316::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Okada T, Okuno H, Mitsui Y. A novel in vitro assay system for transendothelial tumor cell invasion: significance of E-selectin and alpha 3 integrin in the transendothelial invasion by HT1080 fibrosarcoma cells. Clin Exp Metastasis. 1994;12:305–14. doi: 10.1007/BF01753837. [DOI] [PubMed] [Google Scholar]
- 20.Rahim S, Beauchamp EM, Kong Y, Brown ML, Toretsky JA, Uren A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PLoS One. 2011;6:e19343. doi: 10.1371/journal.pone.0019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009;21:645–53. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Beckler A, Moskaluk CA, Zaika A, Hampton GM, Powell SM, Frierson HF, Jr, et al. Overexpression of the 32-kilodalton dopamine and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein in common adenocarcinomas. Cancer. 2003;98:1547–51. doi: 10.1002/cncr.11654. [DOI] [PubMed] [Google Scholar]
- 24.Wang MS, Pan Y, Liu N, Guo C, Hong L, Fan D. Overexpression of DARPP-32 in colorectal adenocarcinoma. Int J Clin Pract. 2005;59:58–61. doi: 10.1111/j.1742-1241.2004.00305.x. [DOI] [PubMed] [Google Scholar]
- 25.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10 (Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huskens D, Princen K, Schreiber M, Schols D. The role of N-glycosylation sites on the CXCR4 receptor for CXCL-12 binding and signaling and X4 HIV-1 viral infectivity. Virology. 2007;363:280–7. doi: 10.1016/j.virol.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 30.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–12. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 31.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–22. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 32.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 33.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer research. 2006;66:248–58. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartolome RA, Ferreiro S, Miquilena-Colina ME, Martinez-Prats L, Soto-Montenegro ML, Garcia-Bernal D, et al. The chemokine receptor CXCR4 and the metalloproteinase MT1-MMP are mutually required during melanoma metastasis to lungs. Am J Pathol. 2009;174:602–12. doi: 10.2353/ajpath.2009.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.