Abstract
Estrogen Therapy and Hormone Therapy are effective options for the prevention and treatment of osteoporosis, although because of their significant side effect profile, long term use for these applications is not recommended. Whereas SERMs (Selective Estrogen Receptor Modulators) exhibit a more favorable side effect profile, the currently available medicines in this class are substantially less effective in bone than classical estrogens. However, the results of substantial efforts that have gone into defining the mechanisms that underlie the pharmacology of estrogens, antiestrogens and SERMs have informed the development of the next generation of SERMs and have led to the development of TSECs (Tissue Selective Estrogen Complexes), a new class of ER-modulator. Further, the recent determination that the oxysterol 27-hydroxycholesterol functions as an endogenous SERM has highlighted an unexpected link between hypercholesterolemia and bone biology and must be considered in any discussions of ER-pharmacology. This review considers the most recent progress in our understanding of ER pharmacology and how this has and will be translated into new medicines for the treatment and prevention of osteoporosis.
Keywords: Bone Physiology, Selective Estrogen Receptor Modulator, Estrogen Receptor, Cholesterol, 27-Hydroxycholesterol, Liver X Receptor
Introduction
Whereas the primary roles of 17-β estradiol and other estrogens are as regulators of reproductive function, it is now appreciated that these steroid hormones have important activities in other processes unrelated to reproduction. Notable is the increased risk of osteoporosis, cardiovascular disease and vasomotor instability observed in women subsequent to the cessation of ovarian estrogen production that occurs at menopause [1, 2]. Dysregulated estrogen signaling has also been associated with an increased risk of breast and uterine cancers, and with other morbidities such as deep vein thrombosis and stroke. Thus, it has become clear that the same hormone can be recognized in different ways in different cells and under different physiological conditions. Together, these findings highlight the need to understand the molecular mechanisms underlying the pharmacological actions of estrogens and its receptors in physiology and disease as a means to optimize the delivery of currently available drugs and to inform the development of the next generation of drugs that target the estrogen receptor (ER).
The association between idiopathic osteoporosis in women and menopause was first described in 1940 and soon thereafter a specific cause and effect relationship between the loss of 17-β estradiol and bone pathology was established [3, 4]. Interestingly, not all women exhibit bone loss following cessation of ovarian function; a finding that (a) highlights the existence of redundant pathways that impact bone biology and/or (b) suggests that alternate, non ovarian sources of estrogens, may compensate for ovarian failure. There is substantial evidence in support of both hypotheses, although most compelling are the data showing that post-menopausal women who are taking aromatase inhibitors demonstrate an increased rate of bone loss and a significantly elevated risk of osteoporotic fracture; data which highlights the importance of non-ovarian estrogen production [5–11]. It is not surprising, therefore, that Estrogen Therapy (ET, estrogen alone) or Hormone Therapy (HT, estrogen combined with a progestin) are among the most effective therapies for the treatment and prevention of osteoporosis [12]. Both regimens normalize bone remodeling and unlike other antiresorptive therapies, such as bisphosphonates, their use is not associated with increased risk of atypical femoral fractures or osteonecrosis of the jaw [13–15]. However, while the beneficial effects of ET/HT in bone are unquestioned, the increased risk of breast cancer, cardiovascular disease and dementia observed in elderly women participating in the Womens’ Health Initiative (WHI) study, have resulted in a dramatic decrease in the use of estrogen containing medicines in the climacteric patient and in post-menopausal women at high risk for osteoporosis [16, 17]. Regardless, it is clear that ER and its signaling pathways are extremely useful targets in bone, and it is anticipated that compounds that modulate this axis, and exhibit a more favorable therapeutic index, will have considerable clinical utility. The Selective Estrogen Receptor Modulators (SERMs), a class of compounds whose relative ER-agonist/antagonist activity can differ between target organs, are a first step in this direction. Most SERMs do exhibit favorable selectivity towards bone but lack the efficacy of classical estrogens. However, the existing drugs of this class were discovered over 25 years ago as breast cancer therapeutics, or are follow-on drugs with similar mechanisms of action, whose bone sparing activities were discovered by accident. It is anticipated, therefore, that dissection of the molecular mechanisms underlying ER-pharmacology will facilitate a move from empirical to mechanism-based drug discovery, enabling the development of drugs optimized for their actions in bone. The primary objective of this review, therefore, is to provide an overview of what is known about estrogen/ER pharmacology in bone and how this information has and will be translated into new therapeutics for the prevention and treatment of osteoporosis.
Cellular targets of estrogen action in bone
Despite the dramatic effects of estrogens on the skeleton, an integrated model explaining how this hormone impacts bone formation and resorption has yet to emerge. This likely reflects the complexity of the cell biology of bone and the fact that estrogens regulate both bone cell intrinsic processes as well as the production of paracrine factors by other cells in the bone microenvironment that regulate bone cell function. Several excellent reviews have been published recently which discuss estrogen action in bone in detail; thus, only the essential concepts that are relevant to a discussion of ER-pharmacology will be discussed here.
Estrogens modulate peripheral calcium homeostasis in the intestine and kidney [18–20], however, their relatively modest effects on this process are unlikely to contribute significantly to their actions in bone. Thus, post-menopausal osteoporosis is almost definitely the result of an increase in the number and activity of both osteoblasts and osteoclasts and an imbalance in the tight control of osteoclastogenesis afforded by osteoblasts/preosteoblasts [21]. A similar imbalance is observed in mice and rats upon ovariectomy validating the use of these rodent models to study osteoporosis. It was initially surprising, therefore, that a significant bone phenotype was not observed in mice in which a global knockout of ERα or both ERα and β, had been accomplished. However, this unexpected finding has now been attributed to a significant increase in circulating androgens; hormones which exhibit both antiresorptive and anabolic actions in rodent bone, that results from impaired hypothalamic feedback by estrogens [21, 22]. This latter confounder encouraged others to generate conditional knockouts of ERα in involved cells in bone; a strategy that has proven to be extremely informative. Using a cre-recombinase driven by the lysozyme M promoter, and a floxed allele of ERα for instance, ERα expression was ablated in the neutrophils and monocyte/macrophage lineage (from which osteoclasts are derived) in mice. This was associated with an increase in osteoclast number and a corresponding decrease in trabecular bone mass [23]. Similar results were obtained by others who accomplished a specific knockdown of ERα in osteoclasts using a cre-recombinase driven by the cathepsin K promoter [24]. It was also shown that 17β-estradiol (E2) induces the expression of Fas ligand in osteoclasts which in turn acts in an autocrine manner to initiate apoptosis [24]. Thus, there is a strong body of evidence to suggest that osteoclasts are direct target of estrogens in bone.
One of the most interesting potential cellular targets of estrogen action in bone are TNFα (tumor necrosis factor alpha) producing T-cells. It has been observed that the number of such T-cells rise significantly in response to ovariectomy in mice and this results in an increase in the production of the major osteoclast differentiating factor, RANKL (receptor activator for NFκB ligand) and a corresponding increase in osteoclastogenesis [25]. The relevance of this finding was highlighted in a study performed in humans which demonstrated an increase in the number of TNFα and RANKL producing T-cells in women with post-menopausal osteoporosis [26]. Further, bone marrow lymphocytes and stromal cells from postmenopausal women have been shown to express higher levels of RANKL on their surfaces [27, 28]. It is unclear if TNFα is a direct target of ERα action or if its production is suppressed by an estrogen regulated pathway within the T-cells themselves or via actions in another cell that influences T-cell function. Defining the importance of T-cells as targets of estrogens in women with osteoporosis and the value of targeting these cells is a topic that is receiving considerable attention.
There are numerous, somewhat conflicting, reports that describe roles for 17-β estradiol in osteoblasts. Indeed, there are as many studies that indicate that 17-β estradiol increases ‘pre-osteoblast’ proliferation [29, 30], as those that conclude that this hormone is without effect or even inhibits proliferation of these cells in vitro [31]. This may reflect differences in the cell models used by different investigators (different primary and immortalized cell types) or the conditions under which many of the in vitro studies are performed. In mice, however, a significant increase in the number of osteoblast progenitors has been observed following ovariectomy; a result that mirrors that observed in post-menopausal women [32]. There are also a significant number of studies which indicate that 17-β estradiol induces the expression and activity of alkaline phosphatase, a marker of differentiated osteoblasts, and also modulates the expression of other genes associated with osteoblast activity including growth factors/cytokines (increased IGF-I, IGF-BP-4, TGFβ1, BMP-6, decreased IL1-α, IL-1β, IL-6), and hormone receptors (increased progesterone receptor, vitamin D receptor, decreased parathyroid hormone receptor) [reviewed by 33]. Recently, it has been shown in differentiated osteoblasts that nuclear factor-κB (NFκB) functions as an inhibitor of bone formation. This, is interesting in light of the observation that estrogen activated ERα is a robust inhibitor of NFκB [34]. Also of note is that 17-β estradiol induces the expression and secretion of osteoprotegrin (OPG) by osteoblasts, a protein which binds to and inactivates the critical osteoclast differentiation factor, RANKL, thus inhibiting osteoclastogenesis [35]. Estrogens also inhibit the production of the pro-resorbtive factor sclerostin [36]. Finally, there is evidence that that 17-β estradiol suppresses both osteoblast and osteocyte apoptosis [37–39]. This is significant in view of the recent work suggesting that osteocytes, and not osteoblasts, are the major source of the RANKL that controls osteoclastogenesis [40, 41]. Whereas there is abundant evidence for a role of ERα/estrogens in osteoblast biology, recent genetic studies have suggested that its function in osteoblast progenitors, differentiated osteoblasts and osteocytes are quite different. Notably, ablation of ERα expression in early mesenchymal progenitors resulted in a significant loss of cortical bone absent effect in trabecular/cancellous bone [42]. Paradoxically, however, knockdown of ERα expression in differentiated osteoblasts does not result in a bone defect per se but decreases osteoblast lifespan [42]. This is in contrast to the dramatic loss of BMD that occurs in mice in which ERα expression is ablated in late osteoblasts/osteocytes where a significant reduction in bone mineral density due to unloading is observed [43].
When taken together it is clear that ERα is a validated therapeutic target in bone, and that its activation by 17-β estradiol and other agonists has a positive effect on bone homeostasis. The complexity that is evident has made it difficult to identify a specific aspect of estrogen action, the targeting of which, would allow the development of more clinically useful ERα modulators. Thus, it is not surprising that none of the SERMs developed thus far, are as effective as classical ERα agonists in protecting against bone loss in postmenopausal women. Undoubtedly, a more detailed understanding of the mechanism of action of ERα in different bone cells and the incorporation of this information into mechanism based drug screens is likely to be a useful way to achieve optimal bone activity.
Estrogen receptors and their mechanism of action
Most of the biological activity that has been ascribed to estrogens can be explained by their actions on ERα and/or ERβ. These genetically distinct receptors are members of the nuclear receptor superfamily of ligand-inducible transcription factors [44–46]. ERα is more widely expressed than ERβ, with the latter being the major form expressed in the ovary, lung and prostate [47]. Whereas both ERs are expressed in bone, genetic and pharmacological studies have indicated that ERα is likely to be the most important mediator of estrogen action in this organ.
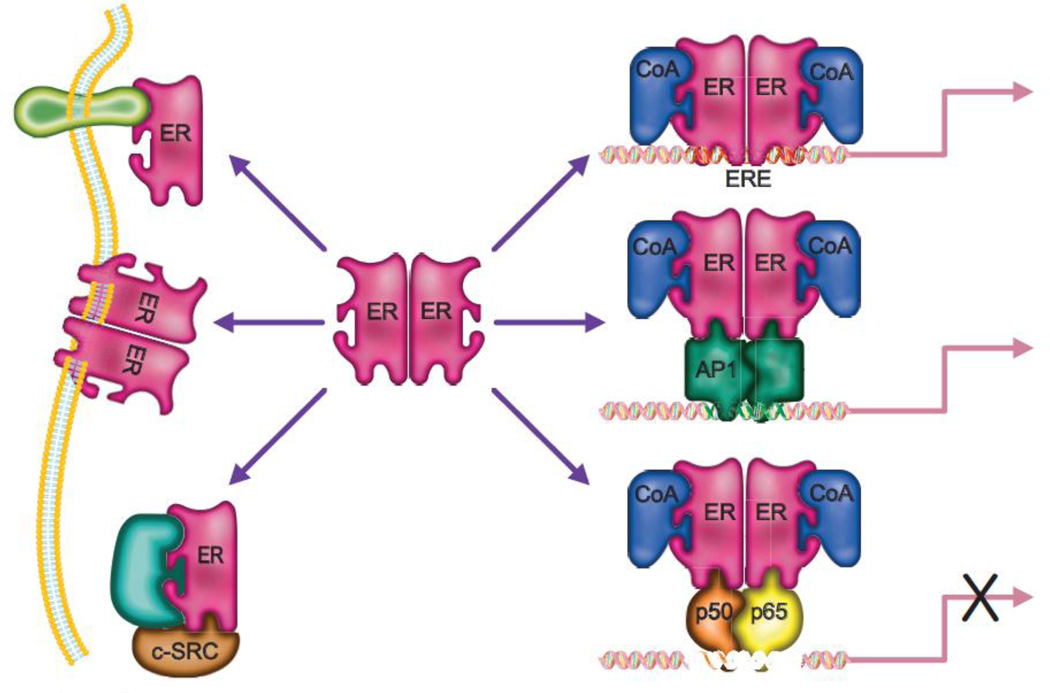
In the absence of hormone, ER is held in an inactive state through its association with an inhibitory heat-shock protein complex within the cytosol of target cells. Ligand binding induces an activating conformational change in the receptor allowing for the dissociation of the heat-shock protein complex and subsequent homo- or hetero-dimerization of ERα and ERβ [48, 49]. The dimers then translocate to the nucleus where they interact with the regulatory region of target genes, either directly via two zinc fingers that ‘recognize’ specific estrogen response elements (EREs), or indirectly by interacting with other transcription factors such as AP1, NFκB and Sp1 [50] that are located on DNA (Figure 1). The DNA bound receptor then nucleates the assembly of large protein complexes comprised of coregulators (coactivators or corepressors), the composition of which dictates whether the receptor activates or represses target gene expression and which determines the magnitude of these responses. Given that over 300 proteins have been identified that can interact with the ERs and which exhibit “coregulator” function it becomes apparent why even within a single cell the same receptor-ligand complex can exhibit different transcriptional activities [51].
Figure 1. Mechanisms of estrogen receptor (ER) action.
Upon binding an agonist the ERs (ERα and ERβ) can form homo or heterodimers and translocate to the nucleus where they associate with coactivator proteins (CoA) and impact transcription by either binding DNA directly at estrogen response elements (EREs), or by tethering to DNA-bound transcription factors such as AP1, nuclear factor-κB and Sp1. Alternatively ERs can act in a non-genomic manner by interacting with c-Src protein kinase complex, the regulatory subunit of phosphoinositide-3 kinase (p85), caveolins or the adaptor protein Shc. See text for details.
In addition to functioning as a transcription factor there are a significant number of studies which demonstrate that ER can function in a non-genomic manner to regulate the activity of several different signaling cascades. For instance, it has been shown in vitro that ERα (and to a lesser extent ERβ) can interact with the c-Src protein kinase complex, the regulatory subunit of phosphoinositide-3 kinase (p85), MAPK, caveolins or the adaptor protein Shc, and modulate the activity of the pathways in which these proteins participate [52, 53]. The relevance of these non-genomic actions of ERα/estradiol to bone function was highlighted by results of two separate in vitro studies, in which (a) a polymeric form of estradiol which cannot enter cells was shown to induce osteoclast apoptosis and (b) E2 prevented apoptosis of etoposide treated osteocytes via nitric oxide/cGMP [23, 54]. The same polymeric form of estradiol was able to prevent ovariectomy-associated loss of cortical bone but not trabecular bone in mice, indicating a potential role for non-genomic signaling of E2 in this compartment [55]. However, studies in mice transgenic for a mutant ERα that fails to bind DNA but should retain its non-genomic actions (nonclassical ER knock-in, NERKI), had an osteoporotic phenotype [56]. Further, the gene expression profiles between traditional ERα knockout mice and the NERKI mice were very similar, most likely due to the failure to bind EREs and modulate transcription [56]. Therefore, it is clear that further studies are required to clarify the precise roles of non-genomic actions of ER in bone.
Finally, although controversial, there are several studies which indicate that the G-protein coupled receptor, GPR30 can bind 17-β estradiol and that it may be a physiological membrane receptor for this hormone [57]. Determination of whether or not GPR30 is a bona fide membrane ER will require significant additional experimentation. Regardless, it is of interest to note that GPR30 is expressed in osteoblasts, osteocytes and osteoclasts, and appears to be upregulated by Runx2, a primary regulator of osteoblastogenesis [58–60]. Defining specific roles for GPR30 in mediating estrogen action in bone is complicated by the observation that its genetic knockout in mice results in complex bone phenotypes that are not the same in males and females. [61–63]
The molecular pharmacology of ER
Until relatively recently the simple models of nuclear receptor pharmacology posited that the binding of an agonist induced a conformational changes in its cognate receptor that was compatible with activation and that antagonists functioned solely by competitively inhibiting agonist binding. It was inferred therefore, that when corrected for affinity, all agonists would exhibit similar activity and antagonists would likewise be indistinguishable. However, this oversimplified explanation of ER-pharmacology was thrown into question by the results of an adjuvant study in breast cancer patients which showed that the “antiestrogen” tamoxifen actually exhibited significant estrogenic activity in bone. Specifically, rather than having a negative effect on bone, as expected, it was determined that tamoxifen actually protected against bone loss, increasing spinal BMD in post-menopausal women [64]. When coupled with other established activities of tamoxifen (ER-antagonist in the breast and ER-partial agonist in the uterus), it became clear that the descriptor “antiestrogen” was not appropriate for this drug and led to it been reclassified a Selective Estrogen Receptor Modulator (SERM). The SERM concept was reinforced by preclinical and clinical data showing that subtle changes in the chemical structures of ER-ligands could have a profound effect on their biological activity and suggested that ER-activity was influenced by the nature of the ligand to which it was bound. Notable in this regard was the demonstration that like tamoxifen, raloxifene exhibited robust bone protective activity and reduced breast cancer incidence but was remarkable in that it did not exhibit significant activity in the uterus. Thus, although identified in an empirical manner, raloxifene emerged as a very useful agent with which to treat and prevent osteoporosis [65]. Additional SERMs, such as idoxifene and levormeloxifene also emerged, but their clinical development was terminated when it became apparent that they exhibited significantly more agonist activity in the uterus when compared to raloxifene. Regardless, these compounds constituted a group of functionally distinct SERMs providing the tools needed to define the mechanism by which cells could distinguish between the different ER-ligand complexes.
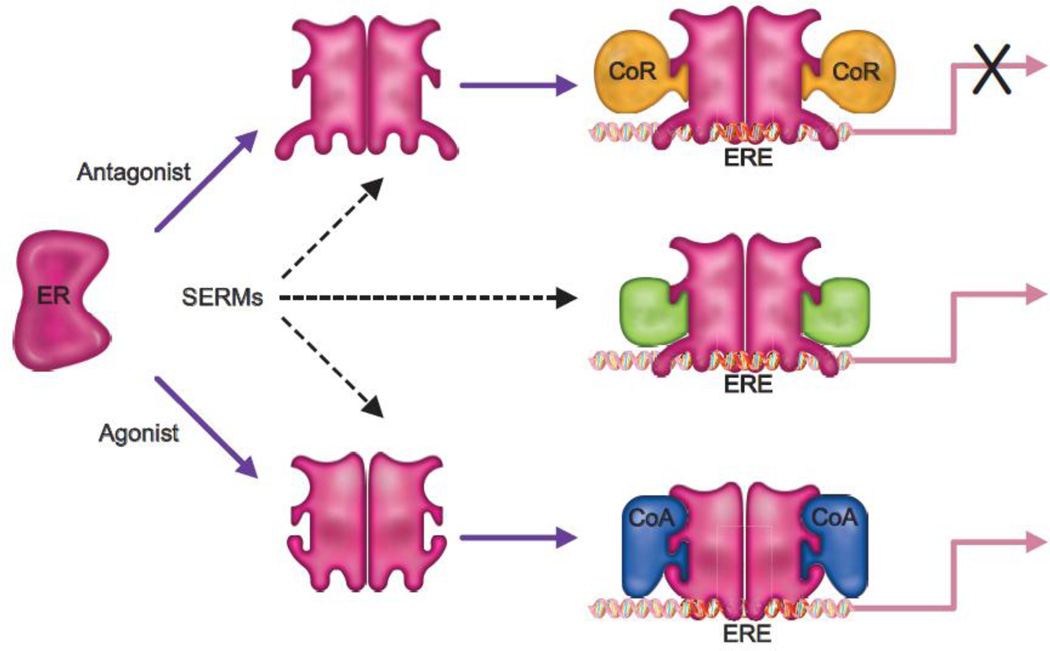
There has been a significant amount of interest in defining the mechanisms underlying the molecular pharmacology of SERMs. Resulting from this work has emerged the realization that the pharmacology of a given ER-modulator is influenced heavily by (a) its binding affinity for ERα and ERβ and the relative and absolute expression of these receptors in target cells, (b) the impact of the bound ligand on ER structure and how this influences the engagement of functionally distinct coregulators, (c) the impact of signaling pathways on the activity of different ER-coregulator complexes and, (d) the sequence of the ERE within the target gene and the transcription factor landscape close to the DNA bound receptor (Figure 2). There are of course many other factors that will impact ER-pharmacology (i.e. potential contributions of non-genomic activities) but those outlined above have endured as the most important [49]. As these rules evolved they were exploited in the development of mechanism-based screens for SERMs with unique activities, studies that were largely driven by efforts to develop compounds that mimicked the activity of tamoxifen in bone but which exhibited a more favorable profile in the uterus.
Figure 2. Updated model of ER pharmacology.
The overall conformation of ER is influenced by the nature of the ligand to which it is bound; an activity which enables the differential interaction of the receptor with functionally distinct coregulators. Pure agonists facilitate the interaction of the receptor with coactivators (CoA) and thus the cellular response to an ER-agonist complex will be determined by the relative expression level of functionally distinct coregulators in different target tissues. Antagonists, on the other hand allow the receptor to interact with only corepressors (CoR). Selective estrogen receptor modulators (SERMs) permit the bound ERs to interact with different subsets of coactivators and corepressors, permitting these drugs to elicit different activities in different tissues.
The first mechanism based screens for SERMs were established with the goal of identifying compounds whose pharmacological activities were similar to raloxifene but which had improved pharmaceutical properties. The first SERMs identified in this manner were lasofoxifene and bazedoxifene whose discovery utilized a series of in vitro molecular assays designed to report on ER-conformation [66]. Interestingly, in the Postmenopausal Evaluation and Risk-Reduction with Lasofoxifene (PEARL) trial, lasofoxifene was found to significantly reduce the risk of both vertebral and non-vertebral fractures, breast cancer, coronary events and stroke, although it was associated with an increased risk of thromboembolic events, hot flushes and benign endometrial changes [67, 68]. The second new SERM, bazedoxifene, has been approved for the treatment of postmenopausal osteoporosis in Europe and Japan, but is not registered in the United States. Like lasofoxifene, it was shown to significantly increase BMD and reduce the risk of vertebral fractures [69]. Furthermore, it is not associated with uterine or breast stimulation, [70]. However, it remains associated with thromboembolic events and hot flushes. Thus as expected, given the approaches used to guide their development, these compounds, when corrected for affinity, have similar properties to raloxifene. Importantly however, their discovery highlighted the utility of mechanism based screens for SERM identification and firmly established a relationship between the structure of an ER-ligand complex and its functional activity.
It has now become clear that differential recruitment by ER of functionally distinct coregulators is a key determinant of the pharmacology of its bound ligands, and that exploitation of this activity is likely to lead to the development of SERMs with distinct clinical activities. The first evidence for a role for coregulators in NR-pharmacology came from genetic studies in which disruption of a transcriptional corepressor switched tamoxifen from an antagonist to an agonist when assessed using a reconstituted ER responsive transcription system in yeast [71]. Soon thereafter, Onate et al identified the first mammalian coregulator SRC-1; a protein which interacted directly with ER (and other receptors) and increased its transcriptional activity [72]. It was subsequently shown that the relative agonist/antagonist activity of tamoxifen could be manipulated by increasing or decreasing the expression of SRC-1 in target cells [73]. From these data emerged the idea that it should be possible to identify compounds which facilitated the interaction of ER with specific coregulators and in this manner drugs with improved therapeutic profiles could be identified. Key to these types of approaches is the ability to define the coregulator “interactome” of ER and the results of follow-up studies that link specific coregulators to different biological processes i.e. inhibition of osteoclast differentiation. Interestingly, although a great number of ER-coregulators have been identified, a rigorous analysis of their individual roles in ER-biology/pharmacology has not been undertaken. However, we have reported the results of a proof of concept study for this general approach using the androgen receptor (AR) as the target. To this end, a comprehensive series of screens were used to identify over 300 proteins whose ability to interact with AR was influenced in a differential manner by different ligands. Using this approach it was determined that ligands that facilitated similar protein-protein interactions had remarkably similar activities when assessed for their ability to induce gene transcription and in cellular assays of relevance to AR action. These relationships were conserved across different cell lines affirming the importance of these activities in determining nuclear receptor pharmacology [74]. It is likely that a similar approach will result in the identification of comparable relationships between ER-ligand complexes, coregulator recruitment and pharmacology.
Enter an endogenous SERM: 27-hydroxycholesterol
The complexity of the pharmacological actions of SERMs has led to the suggestion that there may exist endogenous compounds that manifest “SERM-like” activities. Some initial data in support of this hypothesis came from studies in which the biological activities of individual components of the estrogen containing medicine, Premarin, were evaluated. This medicine, used for ET/HT, is a complex mixture of steroids which have been resolved and shown to exhibit estrogenic, progestagenic and androgenic activities. However, analysis of the pharmacology of the most abundant estrogens contained within Premarin revealed that (a) the affinity of individual estrogens for ERα was a poor predictor of biological activity, and (b) there existed steroidal estrogens whose relative agonist/antagonist activities varied between cells [75]. However, of more relevance to humans was the discovery by Umetani et al that the oxysterol, 27-hydroxycholesterol (27HC), can bind to and modulate the activity of ERα and ERβ both in vitro and in vivo [76, 77]. In mouse models of the cardiovascular system, 27HC behaves as an ER antagonist, and reduces the protective effects of 17-β estradiol [77], while in cellular models of ER positive breast cancer, 27HC behaves as an ER partial agonist [76]. More recently, we have demonstrated that 27HC has a detrimental effect on bone physiology; an activity that relates in part to its ability to disrupt ER action (discussed below) [78]. The independent work of other groups, suggesting that 27HC may be a key ancestral ER ligand, has heightened interest in the physiological/pathophysiological activities of 27HC in humans [79].
27HC, the most abundant circulating oxysterol, is a product of the “shunt” or secondary pathway of cholesterol metabolism. This conversion occurs primarily in macrophages where cholesterol is hydroxylated by CYP27A1. This polar oxysterol (27HC) can then be released into the plasma or further converted to cholestenoic acid by the same enzyme (CYP27A1), or 7α-hydroxylated by CYP7B1 expressed also within macrophages [80, 81]. In addition to macrophages, CYP27A1 expression has been detected in several other cell-types [82 and unpublished data], suggesting that both circulating and locally produced levels of 27HC may impact ER-action in target tissues. Importantly, circulating levels of 27HC are stoichiometrically correlated with those of cholesterol in both humans and in mice [83, 84], a result that has provided a potential mechanistic explanation for the bone phenotypes associated with hypercholesterolemia.
Hypercholesterolemia, 27-hydroxycholesterol, and bone physiology
Hypercholesterolemia is an established risk factor for osteoporosis in post-menopausal women [85–88]. Furthermore, patients with cardiovascular disease are at significantly higher risk of hip fracture; a result that suggests common etiologies for these pathological conditions [89]. Not surprisingly therefore, the use of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins), is associated with decreased fracture risk and with increased BMD [90–93]. Initially, based on some elegant studies from the Mundy laboratory, it was believed that the bone protective actions of statins related to their direct effects on osteoblast differentiation, secondary to their ability to inhibit protein prenylation [94, 95]. However, as a consequence of first pass metabolism, the extrahepatic levels of the currently available statins are unlikely to be high enough to exhibit significant direct actions on cholesterol metabolism in bone [96–98]. It is more likely, therefore, that it is the ability of statins to lower circulating cholesterol per se that is the primary mechanism by which this class of drugs manifest their bone protective actions. Supporting this hypothesis are studies which show that mice fed a high fat, high cholesterol (Western) diet develop an osteoporotic phenotype [99] and that this response can be observed also in mice in which dietary cholesterol alone is increased [29]. Furthermore, elevation of 27HC pharmacologically (by injection) or by genetically disrupting the CYP7B1−/− locus (the enzyme responsible for the catabolism of 27HC), results in significantly decreased trabecular and cortical bone [78]. Importantly, in the CYP7B1−/− mouse model the effects of 27HC on bone were partially reversed by administering pharmacological doses of 17-β estradiol, a result which implicates ER as one target of the pathological actions of 27HC. In these 28-day studies it was also observed that while ovariectomy alone had minimal effects on cortical bone in wild type mice, a dramatic loss of bone was observed in ovariectomized mice in which 27HC levels were elevated. By extrapolation, this latter finding suggests that post-menopausal women with elevated cholesterol may be at increased risk for cortical fractures.
Evaluation of the molecular pharmacology of 27-hydroxycholesterol identifies a new role for estrogens in bone biology
The inability of 17-β estradiol to completely reverse the effects of 27HC on bone suggested that the activity of this oxysterol on bone may involve more than its actions on ER. Moreover, whereas estrogen withdrawal leads to increased osteoblast numbers and elevated osteocalcin expression, decreased calvarial expression of osteocalcin was observed in 27HC treated mice. This result is the opposite of what would be expected if 27HC was functioning only as an ER antagonist or as a partial agonist. These results were interesting in light of other studies, that while controversial, indicated that 27HC could interact with and activate the transcriptional activity of LXRα and LXRβ, two genetically distinct members of the nuclear receptor superfamily implicated in cholesterol, fatty acid and glucose homeostasis [100, 101]. Importantly, using gene expression profiling we confirmed that this oxysterol does activate LXR in both osteoblasts and osteoclasts [29]. Furthermore, it was determined that treatment of female (ovary intact) mice with a synthetic LXR agonist (GW3095) for 28 days, resulted in decreased trabecular and cortical bone similar to what was observed in mice treated with 27HC. A systematic examination of the targets of LXR in bone revealed that 27HC or synthetic LXR agonists, result in the inhibition of osteoblast proliferation, down-regulation of genes associated with osteoblast differentiation and activity (RUNX2, osteocalcin, collagen 1A1 etc.), and inhibition of terminal differentiation and mineral deposition. Importantly, mice treated with GW3965 also showed decreased osteoblast numbers and decreased serum osteocalcin. When taken together these data revealed a very significant role for both LXR and ER in mediating the actions of 27HC in bone, although initially it was unclear if the pathways regulated by these receptors operated in parallel or if they functioned in a convergent manner.
In a search for potential points of convergence between ER and LXR signaling in bone we determined that 27HC mediated activation of LXR resulted in (a) the induction of both TNFα and RANKL mRNA and protein expression in primary osteoblasts in culture, and (b) that this led to increased osteoclastogenesis in an in vitro coculture model of primary osteoblasts and monocytes. These results were consistent with previous work demonstrating that TNFα is a direct target gene of LXR [102]. Importantly however, these LXR dependent effects of 27HC were completely inhibited by coadministration of 17-β estradiol. These data were consistent with the idea that 27HC impacts bone by binding to and (a) activating LXR and (b) inhibiting ER and disrupting the ability of this receptor to inhibit LXR activation.
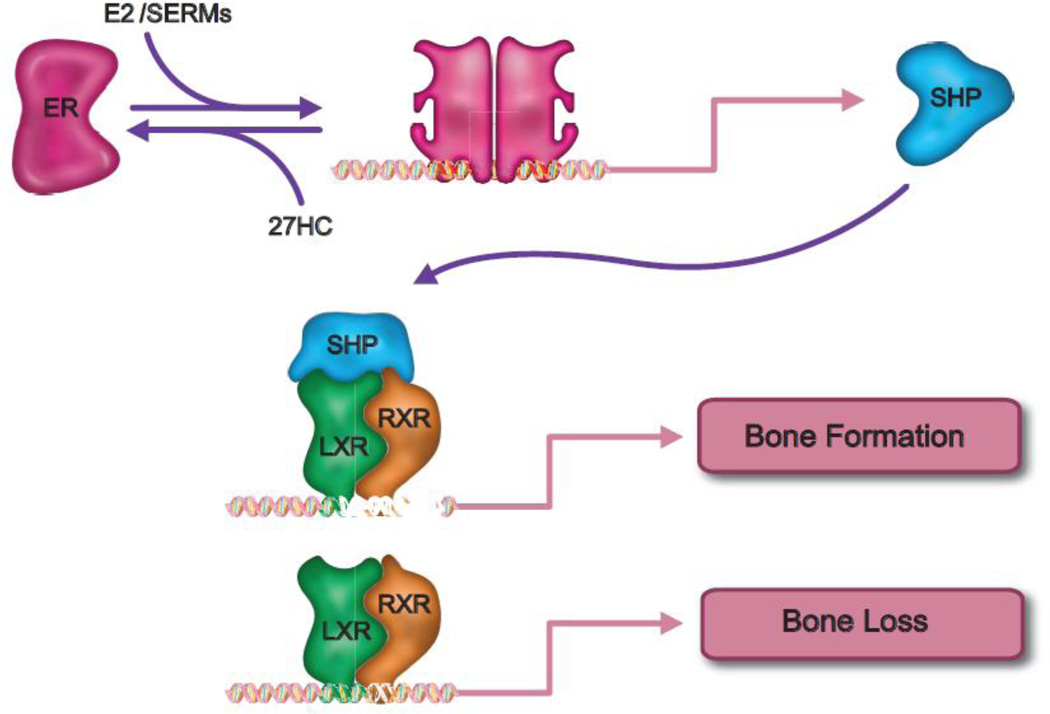
It has been demonstrated by several groups that the expression of the orphan nuclear receptor SHP (Small Heterodimeric Partner) is induced by 17-βestradiol. Similarly, we demonstrated in preosteoblasts that SHP expression is induced by 17-β estradiol and that this activity is attenuated by 27HC. These data are significant given the established roles of SHP as a repressor of LXR transcriptional activity. Importantly, we demonstrated that siRNA mediated knockdown of SHP expression completely inhibits the ability of estrogens to block LXR dependent induction of TNFα and RANKL. Interestingly, a significantly reduced BMD was reported in SHP−/− mice [103]. Considering these data it now appears that the negative bone pathology associated with increased 27HC is due in large part to its ability to (a) activate LXR and induce the expression of the proosteoclastic actions of TNFα and RANKL, (b) attenuate the expression of SHP, and (c) inhibit ER-dependent processes associated with osteoblast differentiation. The net effect of these activities is reduced osteoblast function and increased osteoclastogenesis (Figure 3).
Figure 3. A novel mechanism describing ER action in bone.
Some of the protective actions of estrogens in bone can be attributed to its ability to upregulate the expression of Small Heterodimeric Partner (SHP) in preosteoblasts. This protein functions as a negative regulator of the LXR/RXR heterodimer, which itself is a positive regulation of TNFα expression. Interestingly, by functioning as both an inhibitor of ER and an activator of LXR the cholesterol metabolite 27-hydroxycholesterol (27HC) can positively upregulate the expression of genes involved in osteoclastogenesis. See text for details.
Practical implications of the finding that 27HC is a negative regulator of bone homeostasis
Hyperocholesterolemia is a risk factor for osteoporosis, although in premenopausal women estrogens are protective as they increase HDL and suppress LDL production. Furthermore, as highlighted in our studies, estrogens can attenuate the impact of cholesterol (via 27HC) on bone. However, in postmenopausal women the situation is very much different as, in general, LDL rises and HDL is decreased. By inference, therefore, the resulting increase in 27HC will exhibit a negative impact on bone. However, cholesterol is a modifiable risk factor that can be controlled by statins and/or by dietary means. Thus, it is expected that statins in and of themselves would improve bone health by reducing the production of 27HC. The effects are likely to be somewhat reduced in women on ET/HT as it is expected that these treatment regimens will restore SHP expression in bone. However, statins are likely to be associated with a very positive effect on bone health in women with elevated cholesterol who elect not to take estrogens. It is likely also that the negative impact of aromatase inhibitors on bone health could be mitigated somewhat by lowering cholesterol (and by default 27HC) in breast cancer patients. Indeed, our data suggest that it may be informative to examine the impact of aromatase inhibitors on bone as a function of LDL cholesterol.
Whereas estrogens, in the context of ET/HT, exhibit robust anti-resorptive activity in the majority of postmenopausal women, the effectiveness of SERMs and the number of patients that actually respond to this class of drugs are significantly less. It will be important in the future to determine whether SERM response in bone is influenced by cholesterol status in patients and/or with statin usage. Regardless, it appears to us that there may be an opportunity to screen for “SERMs” that enable a robust induction of SHP in bone. It will be of interest also to assess the impact of specific CYP27A1 inhibitors on bone biology and define the potential utility of this approach to mitigate the impact of hypercholesterolemia on bone
Looking forward: from mechanistic insights to new drugs
The insights that have emerged from studies of the molecular pharmacology of ER have helped to inform the steps that should be taken in the development of the next generation of “SERMs”. It seems unlikely that it will be possible to develop a single molecule that will phenocopy the beneficial effects of classical estrogens and which will not increase the risk of breast and uterine cancers, DVTs and stroke. Efforts to do so thus far have resulted in the development of drugs that are much less efficacious in the desired organs. It now seems prudent to search for new classes of SERMs that are optimized for a particular biological response (i.e. antiresorptive activity) and screen against negative sequelae manifest in other target tissues. Until recently, it was unclear if this type of approach would be feasible. However, the ability to link coregulators to specific biological processes and the realization that it is possible to develop compounds that engender different coregulator interactions suggests that it will be possible to identify “process selective” SERMs. It is anticipated that in this manner the efficacy of ER-modulators in a given target tissue can be improved. This approach has been used to develop compounds which retain substantial activity on classical AR-responsive genes but which do not induce prostate cancer cell proliferation [74, 104]. Whereas it is generally held that exploitation of coregulator biology will yield new classes of ER modulators, it is likely that the availability of such compounds for clinical use is at least 10–15 years away.
One of the most interesting developments of late in the field of ER-biology is the development of a new class of medicines, the TSECs (Tissue Selective Estrogen Receptor Complexes). These medicines combine a SERM and an estrogen(s), and the blended activities of the constituents results in a clinically useful profile. Two TSECs have thus far been evaluated (raloxifene/17-β estradiol and bazedoxifene/conjugated equine estrogens). The clinical evaluation of the latter complex (Duavive) revealed that it effectively treats the climacteric symptoms associated with menopause and prevents bone loss without any negative impact on breast or in the uterus [105]. The inhibitory activity of the constituent SERM on the uterus obviates the need for concomitant use of a progestin. The mechanism of action of TSECs are not clear and resolution of a specific mechanism to describe their activities is difficult as both components of the TSEC compete for the same ligand binding pocket on the ERs. However, given our current understanding of the pharmacology of the individual components within the TSEC preparations it is likely that the major factors that contribute to their beneficial clinical activity are differences in the (a) pharmacodynamics and pharmacokinetics of the constituents, (b) penetration of the blood-brain barrier and (c) response of target tissues to estrogens. There is also evidence that heterodimerization of ER monomers occupied by different ligands may induce different gene expression profiles or that exposure of a cell to a SERM may influence the environment in which the estrogen acts and visa versa [106]. To that end, we have recently observed that ER stability is impacted by the SERM with which it is bound. This could influence the activity of an estrogen by reducing (bazedoxifene) or increasing (tamoxifen) the level of the target in involved tissues. It is likely, that no one mechanism will explain TSEC pharmacology and that each SERM/estrogen preparation will have distinct activities. Regardless, these new drugs represent the first fundamentally new concept in ET/HT in 20 years and should have a significant impact on the pharmacotherapy of the climacteric should be a useful approach to treat and prevent osteoporosis.
Highlights.
Estrogen Therapy and Hormone Therapy effectively prevent and treat osteoporosis but have significant side effect profiles.
SERMs (Selective Estrogen Receptor Modulators) exhibit a more favorable side effect profile
However currently available SERMs are substantially less effective in bone than classical estrogens
Discussion of ER pharmacology and ligands including endogenous SERMs
Translation of ER pharmacology into new medicines (such as TSECs) for the treatment and prevention of osteoporosis.
Acknowledgements
We would like to thank Sunghee Park for her help in the design of the figures.
Grant Support: NIH R37DK048807 (DPM), and a DOD postdoctoral award W81XWH-09-1-0613 (ERN), and by research grants from Novartis, Radius Health and Pfizer (DPM).
Abbreviations
- 27HC
27-hydroxycholesterol
- AR
androgen receptor
- ER
estrogen receptor
- LXR
Liver X Receptor
- RXR
Retinoid X Receptor
- SHP
Small Heterodimeric Partner
- SERM
Selective Estrogen Receptor Modulator
- TSEC
Tissue Selective Estrogen Receptor Complexes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Wardell and McDonnell have served as Ad-hoc consultants for Pfizer.
References
- 1.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 2.Vagenakis AG. Endocrine aspects of menopause. Clin Rheumatol. 1989;8(Suppl 2):48–51. doi: 10.1007/BF02207233. [DOI] [PubMed] [Google Scholar]
- 3.Albright F, Bloomberg E, Smith P. Postmenopausal osteoporosis. Transactions of the Association of American Physicians. 1940;55:298–305. [Google Scholar]
- 4.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 5.Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton LJ, 3rd, Riggs BL. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res. 2002;17:172–178. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, Shenkier TN, Tozer RG, Palmer MJ, Shepherd LE, Liu S, Tu D, Goss PE. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 9.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 10.Lonning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Paolini J, Polli A, Massimini G. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 11.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1995;122:9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95:3569–3577. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 15.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 16.Parente L, Uyehara C, Larsen W, Whitcomb B, Farley J. Long-term impact of the women's health initiative on HRT. Arch Gynecol Obstet. 2008;277:219–224. doi: 10.1007/s00404-007-0442-1. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.McKane WR, Khosla S, Burritt MF, Kao PC, Wilson DM, Ory SJ, Riggs BL. Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause--a clinical research center study. J Clin Endocrinol Metab. 1995;80:3458–3464. doi: 10.1210/jcem.80.12.8530583. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979;64:729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gennari C, Agnusdei D, Nardi P, Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71:1288–1293. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- 21.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 22.Windahl SH, Andersson G, Gustafsson JA. Elucidation of estrogen receptor function in bone with the use of mouse models. Trends Endocrinol Metab. 2002;13:195–200. doi: 10.1016/s1043-2760(02)00594-5. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell NH. RANK ligand and the regulation of skeletal remodeling. J Clin Invest. 2003;111:1120–1122. doi: 10.1172/JCI18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson ER, Dusell CD, Wang X, Howe MK, Evans G, Michalek RD, Umetani M, Rathmell JC, Khosla S, Gesty-Palmer D, McDonnell DP. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver x receptors. Endocrinology. 2011;152:4691–4705. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheven BA, Damen CA, Hamilton NJ, Verhaar HJ, Duursma SA. Stimulatory effects of estrogen and progesterone on proliferation and differentiation of normal human osteoblast-like cells in vitro. Biochem Biophys Res Commun. 1992;186:54–60. doi: 10.1016/s0006-291x(05)80774-0. [DOI] [PubMed] [Google Scholar]
- 31.Keeting PE, Scott RE, Colvard DS, Han IK, Spelsberg TC, Riggs BL. Lack of a direct effect of estrogen on proliferation and differentiation of normal human osteoblast-like cells. J Bone Miner Res. 1991;6:297–304. doi: 10.1002/jbmr.5650060312. [DOI] [PubMed] [Google Scholar]
- 32.Jilka RL, Takahashi K, Munshi M, Williams DC, Roberson PK, Manolagas SC. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101:1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spelsberg TC, Subramaniam M, Riggs BL, Khosla S. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol. 1999;13:819–828. doi: 10.1210/mend.13.6.0299. [DOI] [PubMed] [Google Scholar]
- 34.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 36.Modder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone. 2011;49:202–207. doi: 10.1016/j.bone.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC. Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus proapoptotic effects of estrogens. J Biol Chem. 2005;280:4632–4638. doi: 10.1074/jbc.M411530200. [DOI] [PubMed] [Google Scholar]
- 38.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 39.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 41.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer S, Bartell S, Warren A, Han L, Martin-Millan M, Ambrogini E, Xiong J, Weinstein R, Jilka R, O'Brien C, Almeida M, Manolagas S. ERα-deletion from Osteoblast Progenitors Abolishes the Protective Effect of Estrogens on Cortical Bone Mass in Both Female and Male Mice. American Society for Bone and Mineral Research Annual Meeting. 2011 Oral Presentation 1110. [Google Scholar]
- 43.Imai Y, Bonewald L, Kondoh S, Kato S. Estrogen Receptor α (ERα) Expression in Osteocytes is Necessary to Maintain Normal Bone Mineral Density (BMD) and Reduce Bone Loss Due to Unloading. American Society for Bone and Mineral Research Annual Meeting. 2011 Poster Presentation Number SA0310. [Google Scholar]
- 44.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 46.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 48.Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 49.Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 50.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 51.Lonard DM, O'Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 53.Improta-Brears T, Whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci U S A. 1999;96:4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17beta-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287:978–988. doi: 10.1074/jbc.M111.294959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartell SM, Warren A, Han l, Iyer S, Kim SH, Katzenellenbogen BS, Chambliss KL, Shaul PW, Katzenellenbogen JA, Roberson PK, Weinstein RS, O'Brien CA, Jika RL, Almeida M, Manolagas SC. An estrogen dendrimer conjugate incapable of stimulating the nuclear-initiated actions of estrogen receptors prevents the loss of cortical bone mass in estrogen deficient mice. JBMR. 2012;27:21. [Google Scholar]
- 56.Chokalingam K, Roforth MM, Nicks KM, McGregor U, Fraser D, Khosla S, Monroe DG. Examination of ERalpha Signaling Pathways in Bone of Mutant Mouse Models Reveals the Importance of ERE-Dependent Signaling. Endocrinology. 2012 doi: 10.1210/en.2012-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Chagin AS, Savendahl L. GPR30 estrogen receptor expression in the growth plate declines as puberty progresses. J Clin Endocrinol Metab. 2007;92:4873–4877. doi: 10.1210/jc.2007-0814. [DOI] [PubMed] [Google Scholar]
- 59.Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197:R1–R6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 60.Teplyuk NM, Galindo M, Teplyuk VI, Pratap J, Young DW, Lapointe D, Javed A, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Runx2 regulates G protein-coupled signaling pathways to control growth of osteoblast progenitors. J Biol Chem. 2008;283:27585–27597. doi: 10.1074/jbc.M802453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 62.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296:E490–E496. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 63.Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh JT, Clegg D, Zerwekh J, Oz OK. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J Bone Miner Res. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 65.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 66.McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol. 1995;9:659–669. doi: 10.1210/mend.9.6.8592512. [DOI] [PubMed] [Google Scholar]
- 67.Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 68.Goldstein SR, Neven P, Cummings S, Colgan T, Runowicz CD, Krpan D, Proulx J, Johnson M, Thompson D, Thompson J, Sriram U. Postmenopausal Evaluation and Risk Reduction With Lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18:17–22. doi: 10.1097/gme.0b013e3181e84bb4. [DOI] [PubMed] [Google Scholar]
- 69.Kawate H, Takayanagi R. Efficacy and safety of bazedoxifene for postmenopausal osteoporosis. Clin Interv Aging. 2011;6:151–160. doi: 10.2147/CIA.S15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulak J, Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology. 2011;152:3226–3232. doi: 10.1210/en.2010-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonnell DP, Vegeto E, O'Malley BW. Identification of a negative regulatory function for steroid receptors. Proc Natl Acad Sci U S A. 1992;89:10563–10567. doi: 10.1073/pnas.89.22.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 73.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen: 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 74.Norris JD, Joseph JD, Sherk AB, Juzumiene D, Turnbull PS, Rafferty SW, Cui H, Anderson E, Fan D, Dye DA, Deng X, Kazmin D, Chang CY, Willson TM, McDonnell DP. Differential presentation of protein interaction surfaces on the androgen receptor defines the pharmacological actions of bound ligands. Chem Biol. 2009;16:452–460. doi: 10.1016/j.chembiol.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dey M, Lyttle CR, Pickar JH. Recent insights into the varying activity of estrogens. Maturitas. 2000;34(Suppl 2):S25–S33. doi: 10.1016/s0378-5122(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 76.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 78.DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675–3685. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker ME. Insights from the structure of estrogen receptor into the evolution of estrogens: implications for endocrine disruption. Biochem Pharmacol. 2011;82:1–8. doi: 10.1016/j.bcp.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Babiker A, Andersson O, Lund E, Xiu RJ, Deeb S, Reshef A, Leitersdorf E, Diczfalusy U, Bjorkhem I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem. 1997;272:26253–26261. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- 81.Hansson M, Ellis E, Hunt MC, Schmitz G, Babiker A. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim Biophys Acta. 2003;1593:283–289. doi: 10.1016/s0167-4889(02)00398-1. [DOI] [PubMed] [Google Scholar]
- 82.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dusell CD, McDonnell DP. 27-Hydroxycholesterol: a potential endogenous regulator of estrogen receptor signaling. Trends Pharmacol Sci. 2008 doi: 10.1016/j.tips.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karuna R, Holleboom AG, Motazacker MM, Kuivenhoven JA, Frikke-Schmidt R, Tybjaerg-Hansen A, Georgopoulos S, van Eck M, van Berkel TJ, von Eckardstein A, Rentsch KM. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448–455. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 85.Orozco P. Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur J Epidemiol. 2004;19:1105–1112. doi: 10.1007/s10654-004-1706-8. [DOI] [PubMed] [Google Scholar]
- 86.Tanko LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32:8–14. doi: 10.1016/s8756-3282(02)00918-3. [DOI] [PubMed] [Google Scholar]
- 87.Tarakida A, Iino K, Abe K, Taniguchi R, Higuchi T, Mizunuma H, Nakaji S. Hypercholesterolemia accelerates bone loss in postmenopausal women. Climacteric. 2011;14:105–111. doi: 10.3109/13697137.2010.507888. [DOI] [PubMed] [Google Scholar]
- 88.Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 89.Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaelsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 90.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HA. Use of statins and fracture: results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 91.Gotoh M, Mizuno K, Ono Y, Takahashi M. Fluvastatin increases bone mineral density in postmenopausal women. Fukushima J Med Sci. 2011;57:19–27. doi: 10.5387/fms.57.19. [DOI] [PubMed] [Google Scholar]
- 92.Lupattelli G, Scarponi AM, Vaudo G, Siepi D, Roscini AR, Gemelli F, Pirro M, Latini RA, Sinzinger H, Marchesi S, Mannarino E. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism. 2004;53:744–748. doi: 10.1016/j.metabol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Rosenson RS, Tangney CC, Langman CB, Parker TS, Levine DM, Gordon BR. Short-term reduction in bone markers with high-dose simvastatin. Osteoporos Int. 2005;16:1272–1276. doi: 10.1007/s00198-005-1897-1. [DOI] [PubMed] [Google Scholar]
- 94.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 95.Weivoda MM, Hohl RJ. Effects of Farnesyl Pyrophosphate Accumulation on Calvarial Osteoblast Differentiation. Endocrinology. 2011 doi: 10.1210/en.2011-0016. [DOI] [PubMed] [Google Scholar]
- 96.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 97.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, Vega JM, Yang A, Alston BL, Brobst SW, Segal Y, Aberg JA. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 98.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 99.Demigne C, Bloch-Faure M, Picard N, Sabboh H, Besson C, Remesy C, Geoffroy V, Gaston AT, Nicoletti A, Hagege A, Menard J, Meneton P. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr. 2006;45:298–306. doi: 10.1007/s00394-006-0599-6. [DOI] [PubMed] [Google Scholar]
- 100.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 101.Song C, Liao S. Cholestenoic acid is a naturally occurring ligand for liver X receptor alpha. Endocrinology. 2000;141:4180–4184. doi: 10.1210/endo.141.11.7772. [DOI] [PubMed] [Google Scholar]
- 102.Landis MS, Patel HV, Capone JP. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J Biol Chem. 2002;277:4713–4721. doi: 10.1074/jbc.M108807200. [DOI] [PubMed] [Google Scholar]
- 103.Jeong BC, Lee YS, Bae IH, Lee CH, Shin HI, Ha HJ, Franceschi RT, Choi HS, Koh JT. The orphan nuclear receptor SHP is a positive regulator of osteoblastic bone formation. J Bone Miner Res. 2010;25:262–274. doi: 10.1359/jbmr.090718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joseph JD, Wittmann BM, Dwyer MA, Cui H, Dye DA, McDonnell DP, Norris JD. Inhibition of prostate cancer cell growth by second-site androgen receptor antagonists. Proc Natl Acad Sci U S A. 2009;106:12178–12183. doi: 10.1073/pnas.0900185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levine J. Treating menopausal symptoms with a tissue-selective estrogen complex. Gender medicine. 2011;8:57–68. doi: 10.1016/j.genm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Wardell SE, Kazmin D, McDonnell DP. Research resource: Transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol. 2012;26:1235–1248. doi: 10.1210/me.2012-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]