Abstract
We determined the first nationwide inventories of alkylphenol surfactants in U.S. sewage sludges (SS) using samples from the U.S. Environmental Protection Agency's 2001 national SS survey. Additionally, analysis of archived 3-year outdoor mesocosm samples served to determine chemical fates in SS-amended soil. Nonylphenol (NP) was the most abundant analyte (534±192 mg/kg) in SS composites, followed by its mono- and di-ethoxylates (62.1±28 and 59.5±52 mg/kg, respectively). The mean annual load of NP and its ethoxylates in SS was estimated at 2408–7149 metric tonnes, of which 1204–4289 is applied on U.S. land. NP compounds showed observable loss from SS/soil mixtures (1:2), with mean half-lives ranging from 301 to 495 days. Surfactant levels in U.S. SS ten-times in excess of European regulations, substantial releases to U.S. soils, and prolonged half-lives found under field conditions, all argue for the U.S. to follow Europe's move from 20 years ago to regulate these chemicals.
Keywords: Surfactants, Alkylphenol ethoxylates, Biosolids, National inventory, Soil half-life, Emerging contaminants
1. Introduction
Alkylphenol ethoxylates (APEOs) are extensively used as surfactants in commercial and industrial products. These chemicals are produced in excess of one million pounds per year in the U.S. and thus are classified as high production volume (HPV) chemicals. Nonylphenol ethoxylates (NPEOs) represent about 80-85% of all APEOs, with an annual consumption estimated at 123,000 to 168,000 metric tonnes in the U.S. (U.S. Environmental Protection Agency, 2010). Due to their widespread use, significant amounts of APEOs enter wastewater treatment plants (WWTPs), where they readily undergo biotransformation to form alkylphenols (primarily nonylphenol) and their short chained ethoxylates (mono- and di-ethoxylates) (Ahel et al., 1994a; Johnson et al., 2005; Koh et al., 2005; Nakada et al., 2006; Shao et al., 2003). Studies have shown WWTP effluents are one of the major sources of these degradates in the environment (Ahel et al., 1994b; Fries and Püttmann, 2003; Langford et al., 2005; Petrovic et al., 2002a; Petrovic et al., 2002b; Sabik et al., 2003). About 60% of the APEO mass entering WWTPs is released to aquatic environments (Naylor et al., 1992). AP and APEOs have been detected in surface waters, groundwater, WWTP sludge, sediments, soil, air, and even in drinking water (Ahel et al., 1994a; Naylor et al., 1992; La Guardia et al., 2001; Ahel et al., 1996; Falkenberg et al., 2003; Vikelsøe et al., 2002; Barber et al., 1988; Dachs et al., 1999; Petrovic et al., 2003). The degradates are shown to be more persistent, lipophilic, and bioaccumulative than their long-chained parent APEOs (Ahel et al., 1994a; Ekelund et al., 1993). One of the major concerns associated with these metabolites is their ability to mimic natural hormones and induce endocrine disruption in aquatic and terrestrial organisms (Soto et al., 1991; Jobling and Sumpter, 1993; Jobling et al., 1996; Renner, 1997). Substantial literature exists that defines elevated aquatic toxicity of these metabolites (European Commission, 2002a; Staples et al., 2009). Nonylphenol (NP), octylphenol (OP) and their ethoxylates have also been detected in human milk, thereby providing a pathway for exposure of newborns to these endocrine disruptors (Ademollo et al., 2008). NP is also associated with respiratory toxicity, inhibition of growth of neural stem cells, increased proliferation of mammary gland cells, and chromosomal aberrations (Argese et al., 1994; Kudo et al., 2004; Kudo et al., 2004; Colerangle and Roy, 1996; Roy et al., 1998). Due to these concerns, several European countries initiated a voluntary phase-out in mid 1990s to reduce the production and use of NP derivatives (OSPAR Commission, 2006). Recently, the U.S. Environmental Protection Agency (U.S. EPA) initiated both voluntary and regulatory actions on NP and NPEOs in the U.S. (U.S. EPA, 2012).
The fate and occurrence of APEOs and degradates in the environment has been studied in detail over the past two decades and has been critically reviewed (La Guardia et al., 2001; Ying et al., 2002; Soares et al., 2008; Giger et al., 2009; Sharma et al., 2009; Dubroca et al., 2003; González et al., 2010; Shang et al., 1999; Bradley et al., 2008; Hesselsøe et al., 2001; Langdon et al., 2011; González et al., 2012). One potential exposure pathway for humans is from land application of processed sewage sludge (biosolids) on agricultural soil. Levels of 1 g/kg or more have been reported in dry sludge (Giger et al., 1984; Ahel et al., 1994a; Ahel et al., 1994b; Ahel and Giger, 1985). The U.S. EPA has performed several national sewage sludge surveys, but AP and APEOs have not been included in these surveys. Though other studies have reported concentrations of NP and NPEOs in U.S. sludge at levels varying between 4.8 and 1,380 mg/kg, these concentrations are limited to specific study locations and to a maximum of 11 U.S. WWTPs (La Guardia et al., 2001; Xia and Jeong, 2004; Xia et al., 2010).
The present study is intended to provide a national baseline level for these compounds in U.S. sewage sludges to enable risk assessment. In a research collaboration, unused samples from EPA's 2001 survey were acquired and are being archived in the Biodesign Institute at Arizona State University as part of the U.S. National Biosolids Repository (Chari and Halden, 2012). The sewage sludge composites analyzed in this study constitute a representative sample (94 WWTP facilities) of the more than 16,000 WWTPs in the U.S. The purpose of national surveys is to determine levels of trace organics in sewage sludges for future monitoring and regulation needs. The present study was performed to extend this effort to other emerging contaminants that were excluded from past U.S. EPA studies. Additionally, archived samples of outdoor mesocosms were analyzed to investigate the fate over three years of AP and APEOs in agricultural soil amended with sewage sludge. Mesocosm samples originated from a study conducted from 2005 to 2008 in Baltimore, Maryland. The approach of analyzing archived sewage sludge composites and mesocosm samples has been validated previously in studies of pharmaceuticals and personal care products (PPCPs) performed to evaluate their nationwide occurrence in sewage sludges and fate in sewage sludge-amended soils (Chari and Halden, 2012; McClellan and Halden, 2010; Walters et al., 2010). The present work employed a similar methodology to analyze for AP and short chain APEOs with the goal of providing: (i) the first national inventory of these compounds in U.S. sewage sludges; (ii) estimates of national loading of AP and APEOs to soils as a result of sewage sludge land application; and (iii) half-lives of AP and APEOs in soils amended with sewage sludges.
2. Materials and methods
2.1. Sample description
2.1.1. Sewage sludge mega composites
Sewage sludge samples for the 2001 national sewage sludge survey (NSSS) were collected by U.S. EPA between February and March 2001 according to an established protocol (U.S. EPA, 2001). After completion of the 2001 NSSS, samples were transferred to Arizona State University and stored in amber glass jars (500 mL) at -20°C for further analysis. Sewage sludge samples were randomly grouped into five composite samples, each containing solids from between 21 and 24 individual treatment plants. Detailed sampling procedure and preparation of composites are provided as supplementary information. A duplicate of composite sample #1 was prepared to serve as a blind duplicate. The composites were prepared to establish mean national baseline levels for study analytes by examining a small and more manageable number of samples. The validity, benefits and limitation of this mega composite approach have been discussed in detail previously (Chari and Halden, 2012; McClellan and Halden, 2010).
2.1.2. Soil/sludge time-series samples
Sewage sludge for the mesocosm study were originally obtained from a full-scale activated sludge treatment plant (Heidler et al. 2006) located in the mid-Atlantic region of the U.S (see supplementary information for additional information). Agricultural soil was obtained from the United States Department of Agriculture – Agricultural Research Service (USDA-ARS) Beltsville Agricultural Research Center (BARC). Sewage sludge and soil were mixed at a ratio of 1:2, which is high compared to the typical land application rate of sewage sludge (e.g., 1:10 after mixing), but lower than the application rate of pure sewage sludge in forestry (1:1). This substantial application rate was chosen to enable the potential observation of multiple half-lives of sewage sludge-borne compounds in soils and to facilitate the detection of degradates. The sludge/soil mixtures were seeded with plants and were exposed to ambient outdoor weather condition of the greater Baltimore region in Maryland. Detailed information on sampling locations, sampling techniques, and experimental setup are provided as supplementary information and in peer-reviewed literature (Walters et al., 2010). Samples were collected on day 57, 115, 520, 859, and 995 and were archived in the Biodesign Institute laboratory at -20°C until the chemical analysis was performed.
2.2. Sample analysis
Both sewage sludge composites and sludge/soil mixtures were shipped to a commercial lab (AXYS Analytical Services Ltd., Sydney, British Columbia, Canada) for analysis of C8-C9 APs and C9 APEOs [octylphenol (OP), 4-nonylphenols (NP), 4-nonylphenol monoethoxylates (NP1EO) and 4-nonylphenol diethoxylates (NP2EO)]. The commercial lab specializes in the analysis of traditional and emerging contaminants and also developed EPA Method 1694 for pharmaceuticals and personal care products. The samples (2 g-dry) underwent digestion with methanolic potassium hydroxide and liquid-liquid extracted with hexane followed by non-aqueous acetylation (AXYS Analytical Services Ltd., 2004). Acetylated extracts were then cleaned up using a 5% silica chromatography column. A recovery standard was added prior to analysis. Instrumental analysis was performed on a RTX-5 capillary gas chromatography column coupled to a low-resolution mass spectrometer (LRMS) (Finnigan MAT Incos 50 Agilent GC-MSD, Agilent Technologies, Palo Alto, CA). The LRMS was operated in electron impact (EI) mode using multiple ion detection (MID) acquiring at least 2 characteristic ions for each target analyte and surrogate standard (Table S1). Quality assurance and quality control procedures included method blanks and matrix spikes to evaluate recovery rates in percent as described in detail elsewhere (McClellan and Halden, 2010; Walters et al., 2010). All concentrations are reported on a dry weight (dw) basis. Precision between samples and duplicates was expressed as relative percent difference (RPD). Method performance and RPD calculations are shown in supplementary information.
3. Results and discussion
3.1. National baseline levels and inventories of AP and APEOs in U.S. sewage sludges
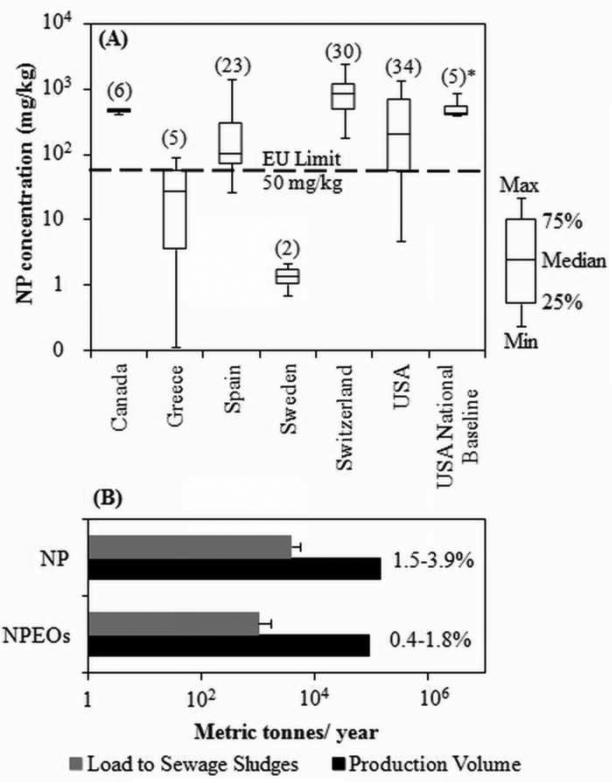
NP, NP1EO and NP2EO were consistently detected in all composite sewage sludge samples, whereas OP was below the detection limit (<1.1 μg/kg) in all the samples. This finding is consistent with known surfactant usage patterns that heavily favor NP derivatives (80-85%) over OP derivatives (U.S. EPA, 2010). NP was the most abundant compound detected at a mean concentration of 534±192 mg/kg, followed by NP1EO and NP2EO at 62.1±28 and 59.5±52 mg/kg, respectively (Table 1). This relative abundance in sewage sludge is in agreement with previously reported concentrations of these compounds (La Guardia et al., 2001; González et al., 2010). It is suggested that NP polyethoxylate surfactants undergo degradation by losing their ethoxy groups, thus leading to the initial formation of NP1EO and NP2EO (Minamiyama et al., 2006; Nunez et al., 2007), and ultimately NP (Ahel et al., 1994a). Also, the stability of these compounds increases with decreasing ethoxylate chains, and as a result NP is present at the highest concentration in sewage sludge. The national baseline concentrations reported in the present study are within the range of concentrations reported in other U.S. studies (Figure 1A). The median concentration was comparable to sludge concentrations reported in countries like Canada and Switzerland, but significantly higher than concentrations reported in sludge samples from Greece, Spain, and Sweden (Figure 1A). The lower concentration observed in Europe likely are linked to the voluntary phase out of NP in these countries (OSPAR Commission, 2006). Currently there are no regulations or limits for NP and NPEOs in sewage sludge in the U.S. The European Union has set a limit of 50 mg/kg for NP in sludge for use on land (European Commission, 2000). The mean baseline level of NP detected in the present study is more than ten times this limit suggesting the immediate need for regulations in the U.S.
Table 1.
Concentrations detected in U.S. sewage sludges collected in 2001 and calculated annual loading of AP and APEOs to different environmental compartments.
| Compound | Sewage Sludge Concentration Avg. (Min, Max) (mg/kg) | Frequency Detected (%) | Estimated Annual AP and APEOs Load Min-Max (Metric Tonnes/yr)2 |
|||
|---|---|---|---|---|---|---|
| To Sewage Sludge | To Land Application | To Landfills | To Incineration | |||
| 4-Nonylphenol | 534 (405, 861) | 100 | 2066-5510 | 1033-3306 | 351-937 | 413-1102 |
| 4-Nonylphenol monoethoxylate | 62.1 (34.3, 103) | 100 | 175-659 | 87-396 | 30-112 | 35-132 |
| 4-Nonylphenol diethoxylate | 59.5 (32.8, 153) | 100 | 167-979 | 84-588 | 28-166 | 33-196 |
| Octylphenol | <MDL1 | 0 | - | - | - | - |
MDL- Method detection limit
These values were calculated based on the estimated percentage of total sewage sludges use and disposal (50-60% to land application;17% to landfills; 20% to incineration) (NEBRA, 2007; Jones-Lepp and Stevens, 2007; National Research Council, 2002)
Figure 1.
(A) Box and whisker plot of nonylphenol concentrations in sewage sludge detected in different countries. The number within parenthesis represents the number of sludge samples analyzed. *five composite samples prepared from 110 sewage sludge samples from 94 U.S. WWTPs were analyzed in the present study. The dashed line represent NP limit in sludge for land use set by European Union (EU). (B) Annual estimated sewage sludge load of AP and APEOs compared with their production volume in the U.S. in year 2001. The percentage next to the bars represents the amount of produced mass that becomes sequestered in sewage sludges. Whisker bars represent estimated maxima and minima.
Based on the estimated sewage sludge production of 5.1 – 6.4 million metric tonnes (5.6 – 7 million U.S. short tons) in the year 2001 (NEBRA, 2007; Jones-Lepp and Stevens, 2007; National Research Council, 2002), the nationwide annual loading rates to sewage sludge for NP and NPEOs were calculated (Table 1). The annual mean loading rate of total NP and NPEOs was 2408 – 7149 metric tonnes, with the most abundant compound being NP with a rate of 2066 – 5510 metric tonnes in U.S. sewage sludges. Based on the estimate of the percentage of total sewage sludges applied on land (50-60%) (NEBRA, 2007; Jones-Lepp and Stevens, 2007; National Research Council, 2002), the mean loading rate of total NP and NPEOs to agricultural soil was found to be 1204-4,289 metric tonnes per year. A significant amount of NP and NPEOs (409-1215 metric tonnes/year) was also estimated to go to landfills as an alternative disposal route for unwanted sewage sludge (Table 1). In order to grasp the true abundance of these compounds in sewage sludge, their load in sewage sludge was compared to the respective U.S. production volume (Figure 1B). Accordingly, it was estimated that up to 1.8 and 3.9% of the total produced volume of NPEOs and NP in 2001 was sequestered in sewage sludges, respectively. The estimated percent for NPEOs is conservative, since the production volume of NPEOs includes a wide range of NP polyethoxylates (NPnEOs) while the present study analyzed for only mono- and di-ethoxylates.
3.2. Fate of AP and APEOs in sludge/soil mesocosms
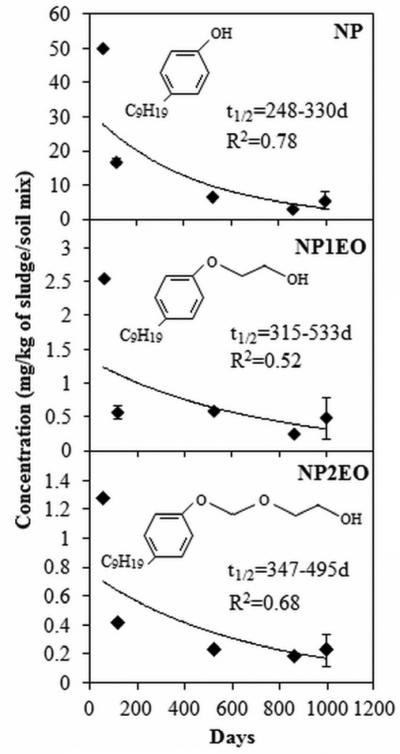
NP, NP1EO and NP2EO were consistently detected in sludge/soil mixtures incubated in ambient outdoor conditions for 3 years, whereas OP was not observed (< 1.1 μg/kg). Control samples of soil that did not receive sewage sludge showed background levels of NP ranging between 1.6 and 1.9 mg/kg dw, while the rest of the analytes were below the respective detection limit (< 12.2, 33.9, and 1.1 μg/kg for NP1EO, NP2EO, and OP, respectively). NP was the most abundant compound found early in the sludge/soil mixture experiment at 50.3 mg/kg dw, followed by NP1EO and NP2EO at 2.6 and 1.3 mg/kg, respectively. These levels indicate that the majority of the mass of NP and NPEOs detected originated from sewage sludge. All three detected analytes showed appreciable losses during the mesocosm weathering study (Figure 2). The first-order degradation curves for these three compounds suggest mean half-lives of 301, 495, and 462 days for NP, NP1EO, and NP2EO, respectively. Since no effort was taken to capture the leachate from these soils, these half-lives represent the net loss of compounds through a combination of abiotic and biotic processes including leaching. About 60-70% of the compounds were lost from sludge/soil mixtures within 120 days; but residual concentrations persisted some 995 days after sewage sludge application. This is in agreement with a previous field study that showed residual concentrations of NP and NPEOs in soil 320 days after the last sludge application (Marcomini et al., 1989).
Figure 2.
Concentrations and half-life (t1/2) of NP and NPEOs in soil amended with sewage sludge over time. Error bars represent minimum and maximum concentration.
Previous studies have shown a shorter half-life for NP in soil. One study showed a half-life of 23 and 16 days, respectively, for NP in sewage sludge-amended soil and sewage sludge-amended soil with plants (Brown et al., 2009). The experiment was performed under laboratory conditions by mixing 0.5 g of freeze-dried sewage sludge with 16 g of air-dry soil (1:32 ratio). Half-lives as short as 3-6 days have been determined for NP in sludge-soil mixtures under aerobic conditions (Hesselsøe et al., 2001). One study showed that application of low amounts of sewage sludge did not inhibit the degradation of NP in soil, but higher application rates (1:1) showed slower degradation kinetics. The slow mineralization of NP in heavily amended soil was related to oxygen limitation from high biological oxygen demand of sewage sludge and restricted gaseous diffusion due to sludge aggregates (Hesselsøe et al., 2001; Topp and Starratt, 2000). The longer half-life observed in this study for these surfactants could have resulted from the higher application rate of sewage sludge to soil (1:2), temporary oxygen-limited conditions after rainfall events and intrinsic properties of the soil and microbial community. The half-lives reported in this study should be considered conservative when applied to agricultural soils that typically see lower sewage sludge application rates, and best-case scenarios for forests, range land and reclamation sites, where sewage sludge are surface applied without mixing (U.S. EPA, 2000).
4. Conclusions
Though alkylphenolic surfactants have been studied in depth and critically reviewed in the past two decades, land application of sewage sludge is one underappreciated route of exposure to terrestrial biota. Though previous studies suggest short half-lives for these endocrine disruptors in soil, the extended half-lives shown in this study may prevail in regions with limited oxygen conditions and higher land application rates of sewage sludge. This nationwide study of the U.S. arrives some 20 years after a series of reports on the occurrence of NP and NPEOs in European sewage sludges that triggered a ban of these compounds in several products due to toxicity concerns. Surfactant levels reported in the present work exceed limits set for European countries by over an order of magnitude, suggesting that the U.S. may have to follow suit and take regulatory action to protect human health and the environment.
Supplementary Material
Highlights.
First national survey of alkylphenol surfactants in U.S. sewage sludges
Nonylphenol (NP) and its ethoxylates were consistently detected in all samples
Levels of NP in U.S. biosolids exceed regulatory limit set by European Union
Significant surfactant releases to U.S. soils via biosolids land application
Half-lives >300 days for NP and its ethoxylates observed in outdoor soil mesocosms
Acknowledgement
We thank Rick Stevens, Harry B. McCarty, and the U.S. EPA for providing the sewage sludge samples from the 2001 National Sewage Sludge Survey. We thank Yakov Pachepsky from the United States Department of Agriculture Agricultural Research Service (USDA-ARS) Beltsville Agricultural Research Center for providing the soil samples. We also thank Barbara Halden for her help with sampling and maintenance of the soil mesocosms. We would like to acknowledge the laboratory staff of AXYS Analytical Services Ltd. for performing chemical analysis. This study was supported in part by the Johns Hopkins Center for a Livable Future and by National Institute of Environmental Health Sciences grant 1R01ES015445 and its supplements. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule. First study providing national inventories of alkylphenol surfactants in U.S. sewage sludges (SS), shows significant release of chemicals to U.S. soils through SS land application.
References
- Ademollo N, Ferrara F, Delise M, Fabietti F, Funari E. Nonylphenol and octylphenol in human breast milk. Environment International. 2008;34:984–987. doi: 10.1016/j.envint.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ahel M, Schaffner C, Giger W. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment--III. Occurrence and elimination of their persistent metabolites during infiltration of river water to groundwater. Water Research. 1996;30:37–46. [Google Scholar]
- Ahel M, Giger W, Koch M. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment--I. Occurrence and transformation in sewage treatment. Water Research. 1994a;28:1131–1142. [Google Scholar]
- Ahel M, Giger W, Schaffner C. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment—II. Occurrence and transformation in rivers. Water Research. 1994b;28:1143–1152. [Google Scholar]
- Ahel M, Giger W. Determination of alkylphenols and alkylphenol mono-and diethoxylates in environmental samples by high-performance liquid chromatography. Analytical Chemistry. 1985;57:1577–1583. [Google Scholar]
- Argese E, Marcomini A, Bettiol C, Perin G, Miana P. Submitochondrial particle response to linear alkylbenzene sulfonates, nonylphenol polyethoxylates and their biodegradation derivatives. Environmental Toxicology and Chemistry. 1994;13:737–742. [Google Scholar]
- AXYS Analytical Services Ltd. Method MLA-004 Determination of Nonylphenols and nonylphenol ethoxylates by GC/MS. Sidney, B.C.: 2004. [Google Scholar]
- Barber LB, Thurman EM, Schroeder MP, LeBlanc DR. Long-term fate of organic micropollutants in sewage-contaminated groundwater. Environmental Science & Technology. 1988;22:205–211. doi: 10.1021/es00167a012. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Barber LB, Kolpin DW, McMahon PB, Chapelle FH. Potential for 4-n-nonylphenol biodegradation in stream sediments. Environmental Toxicology and Chemistry. 2008;27:260–265. doi: 10.1897/07-333R.1. [DOI] [PubMed] [Google Scholar]
- Brown S, Devin-Clarke D, Doubrava M, O'Connor G. Fate of 4-nonylphenol in a biosolids amended soil. Chemosphere. 2009;75:549–554. doi: 10.1016/j.chemosphere.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Chari BP, Halden RU. Validation of mega composite sampling and nationwide mass inventories for 26 previously unmonitored contaminants in archived biosolids from the US National Biosolids Repository. Water Research. 2012;46:4814–4824. doi: 10.1016/j.watres.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colerangle JB, Roy D. Exposure of environmental estrogenic compound nonlyphenol to noble rats alters cell-cycle kinetics in the mammary gland. Endocrine. 1996;4:115–122. doi: 10.1007/BF02782756. [DOI] [PubMed] [Google Scholar]
- Dachs J, Van Ry DA, Eisenreich SJ. Occurrence of estrogenic nonylphenols in the urban and coastal atmosphere of the lower Hudson River estuary. Environmental Science & Technology. 1999;33:2676–2679. [Google Scholar]
- Dubroca J, Kollmann A, Mougin C, Chaplain V, Touton I, Brault A. Effect of nonylphenol surfactants on fungi following the application of sewage sludge on agricultural soils. Journal of Environmental Quality. 2003;32:1269–1276. doi: 10.2134/jeq2003.1269. [DOI] [PubMed] [Google Scholar]
- Ekelund R, Granmo Å, Magnusson K, Berggren M, Bergman Å. Biodegradation of 4-nonylphenol in seawater and sediment. Environmental pollution. 1993;79:59–61. doi: 10.1016/0269-7491(93)90178-q. [DOI] [PubMed] [Google Scholar]
- European Commission . Working document on sludge, third draft, 27 April 2000. Brussels; Belgium: 2000. [July 24, 2012]. http://ec.europa.eu/environment/waste/sludge/pdf/sludge_en.pdf. [Google Scholar]
- European Commission . Risk Assessment Report and Summary. Vol. 10. European Commission; Luxembourg: 2002a. 4-Nonylphenol (Branched) and Nonylphenol. http://esis.jrc.ec.europa.eu/doc/risk_assessment/REPORT/4-nonylphenol_nonylphenolreport017.pdf. [Google Scholar]
- Falkenberg J, Persson B, Hojsholt U, Rokkjaer A, Wahid M. NIRAS, Allerod. Report to the Danish Environmental Protection Agency; Denmark: 2003. Typical values for diffuse soil pollution in Danish urban soil. [Google Scholar]
- Fries E, Püttmann W. Occurrence and behaviour of 4-nonylphenol in river water of Germany. Journal of Environmental Monitoring. 2003;5:598–603. doi: 10.1039/b302229n. [DOI] [PubMed] [Google Scholar]
- Giger W, Gabriel FLP, Jonkers N, Wettstein FE, Kohler HPE. Environmental fate of phenolic endocrine disruptors: field and laboratory studies. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2009;367:3941–3963. doi: 10.1098/rsta.2009.0148. [DOI] [PubMed] [Google Scholar]
- Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225:623–625. doi: 10.1126/science.6740328. [DOI] [PubMed] [Google Scholar]
- González M, Martín J, Camacho-Muñoz D, Santos J, Aparicio I, Alonso E. Degradation and environmental risk of surfactants after the application of compost sludge to the soil. Waste Management. 2012;32:1324–1331. doi: 10.1016/j.wasman.2012.02.023. [DOI] [PubMed] [Google Scholar]
- González M, Martin J, Santos J, Aparicio I, Alonso E. Occurrence and risk assessment of nonylphenol and nonylphenol ethoxylates in sewage sludge from different conventional treatment processes. Science of the Total Environment. 2010;408:563–570. doi: 10.1016/j.scitotenv.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Heidler J, Sapkota A, Halden RU. Persistence, Partitioning, and Accumulation in Digested Sludge of the Topical Antiseptic Triclocarban During Wastewater Treatment. Environmental Science & Technology. 2006;40:3634–3639. doi: 10.1021/es052245n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselsøe M, Jensen D, Skals K, Olesen T, Moldrup P, Roslev P, Mortensen GK, Henriksen K. Degradation of 4-nonylphenol in homogeneous and nonhomogeneous mixtures of soil and sewage sludge. Environmental Science & Technology. 2001;35:3695–3700. doi: 10.1021/es010024l. [DOI] [PubMed] [Google Scholar]
- Jobling S, Sumpter JP, Sheahan D, Osborne JA, Matthiessen P. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environmental Toxicology and Chemistry. 1996;15:194–202. [Google Scholar]
- Jobling S, Sumpter J. Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic toxicology. 1993;27:361–372. [Google Scholar]
- Johnson A, Aerni HR, Gerritsen A, Gibert M, Giger W, Hylland K, Jürgens M, Nakari T, Pickering A, Suter MJF. Comparing steroid estrogen, and nonylphenol content across a range of European sewage plants with different treatment and management practices. Water Research. 2005;39:47–58. doi: 10.1016/j.watres.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Jones-Lepp T, Stevens R. Pharmaceuticals and personal care products in biosolids/sewage sludge: the interface between analytical chemistry and regulation. Analytical and Bioanalytical Chemistry. 2007;387:1173–1183. doi: 10.1007/s00216-006-0942-z. [DOI] [PubMed] [Google Scholar]
- Koh Y, Lester J, Scrimshaw M. Fate and behavior of alkylphenols and their polyethoxylates in an activated sludge plant. Bulletin of Environmental Contamination and Toxicology. 2005;75:1098–1106. doi: 10.1007/s00128-005-0862-1. [DOI] [PubMed] [Google Scholar]
- Kudo C, Wada K, Masuda T, Yonemura T, Shibuya A, Fujimoto Y, Nakajima A, Niwa H, Kamisaki Y. Nonylphenol induces the death of neural stem cells due to activation of the caspase cascade and regulation of the cell cycle. Journal of Neurochemistry. 2004;88:1416–1423. doi: 10.1046/j.1471-4159.2003.02270.x. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E, Mainor TM. Alkylphenol ethoxylate degradation products in land-applied sewage sludge (biosolids). Environmental Science & Technology. 2001;35:4798–4804. doi: 10.1021/es0109040. [DOI] [PubMed] [Google Scholar]
- Langdon KA, Warne MSJ, Smernik RJ, Shareef A, Kookana R. Field dissipation of 4-nonylphenol, 4-t-octylphenol, triclosan and bisphenol A following land application of biosolids. Chemosphere. 2011;86:1050–1058. doi: 10.1016/j.chemosphere.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Langford KH, Scrimshaw MD, Birkett JW, Lester JN. Degradation of nonylphenolic surfactants in activated sludge batch tests. Water Research. 2005;39:870–876. doi: 10.1016/j.watres.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Marcomini A, Giger W, Capel P, Lichtensteiger T, Brunner P. Behavior of aromatic surfactants and PCBs in sludge-treated soil and landfills. Journal of Environmental Quality. 1989;18:523–528. [Google Scholar]
- McClellan K, Halden RU. Pharmaceuticals and personal care products in archived US biosolids from the 2001 EPA national sewage sludge survey. Water Research. 2010;44:658–668. doi: 10.1016/j.watres.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamiyama M, Ochi S, Suzuki Y. Fate of nonylphenol polyethoxylates and nonylphenoxy acetic acids in an anaerobic digestion process for sewage sludge treatment. Water Science & Technology. 2006;53:221–226. doi: 10.2166/wst.2006.356. [DOI] [PubMed] [Google Scholar]
- Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Research. 2006;40:3297–3303. doi: 10.1016/j.watres.2006.06.039. [DOI] [PubMed] [Google Scholar]
- National Research Council (US). Committee on Toxicants, Pathogens in Biosolids Applied to Land . Biosolids Applied to Land: Advancing Standards and Practices. National Academy Press; 2002. [Google Scholar]
- Naylor CG, Mieure JP, Adams WJ, Weeks JA, Castaldi FJ, Ogle LD, Romano RR. Alkylphenol ethoxylates in the environment. Journal of the American Oil Chemists’ Society. 1992;69:695–703. [Google Scholar]
- North East Biosolids Residuals Association (NEBRA) A national biosolids regulation, quality, end use & disposal survey. 2007 http://www.nebiosolids.org/uploads/pdf/NtlBiosolidsReport-20July07.pdf.
- Nunez L, Turiel E, Tadeo J. Determination of nonylphenol and nonylphenol ethoxylates in environmental solid samples by ultrasonic-assisted extraction and high performance liquid chromatography-fluorescence detection. Journal of Chromatography A. 2007;1146:157–163. doi: 10.1016/j.chroma.2007.01.101. [DOI] [PubMed] [Google Scholar]
- OSPAR Commission Implementation of PARCOM Recommendation 92/8 on Nonylphenol/Nonylphenol-Ethoxylates (NP/NPE) 2006.
- Petrovic M, Barceló D, Diaz A, Ventura F. Low nanogram per liter determination of halogenated nonylphenols, nonylphenol carboxylates, and their non-halogenated precursors in water and sludge by liquid chromatography electrospray tandem mass spectrometry. Journal of the American Society for Mass Spectrometry. 2003;14:516–527. doi: 10.1016/S1044-0305(03)00139-9. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Fernández-Alba AR, Borrull F, Marce RM, Mazo EG, Barceló D. Occurrence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Environmental Toxicology and Chemistry. 2002a;21:37–46. [PubMed] [Google Scholar]
- Petrovic M, Solé M, López De Alda MJ, Barceló D. Endocrine disruptors in sewage treatment plants, receiving river waters, and sediments: integration of chemical analysis and biological effects on feral carp. Environmental Toxicology and Chemistry. 2002b;21:2146–2156. [PubMed] [Google Scholar]
- Renner R. European bans on surfactant trigger transatlantic debate. Environmental Science & Technology. 1997;31:316–320. doi: 10.1021/es972366q. [DOI] [PubMed] [Google Scholar]
- Roy D, Colerangle JB, Singh KP. Is exposure to environmental or industrial endocrine disrupting estrogen-like chemicals able to cause genomic instability? Frontiers in Bioscience. 1998;3:913–921. doi: 10.2741/a332. [DOI] [PubMed] [Google Scholar]
- Sabik H, Gagne F, Blaise C, Marcogliese D, Jeannot R. Occurrence of alkylphenol polyethoxylates in the St. Lawrence River and their bioconcentration by mussels (Elliptio complanata). Chemosphere. 2003;51:349–356. doi: 10.1016/S0045-6535(02)00862-7. [DOI] [PubMed] [Google Scholar]
- Shang DY, Macdonald RW, Ikonomou MG. Persistence of nonylphenol ethoxylate surfactants and their primary degradation products in sediments from near a municipal outfall in the Strait of Georgia, British Columbia, Canada. Environmental Science & Technology. 1999;33:1366–1372. [Google Scholar]
- Shao B, Hu J, Yang M. Nonylphenol ethoxylates and their biodegradation intermediates in water and sludge of a sewage treatment plant. Bulletin of Environmental Contamination and Toxicology. 2003;70:527–532. doi: 10.1007/s00128-003-0018-0. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Anquandah GAK, Yngard RA, Kim H, Fekete J, Bouzek K, Ray AK, Golovko D. Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: A review on occurrence, fate, and treatment. Journal of Environmental Science and Health Part A. 2009;44:423–442. doi: 10.1080/10934520902719704. [DOI] [PubMed] [Google Scholar]
- Soares A, Guieysse B, Jefferson B, Cartmell E, Lester J. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environment International. 2008;34:1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environmental Health Perspectives. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples CA, Weeks J, Hall JF, Naylor CG. Evaluation of aquatic toxicity and bioaccumulation of C8-and C9-alkylphenol ethoxylates. Environmental toxicology and chemistry. 2009;17:2470–2480. [Google Scholar]
- Topp E, Starratt A. Rapid mineralization of the endocrine-disrupting chemical 4-nonylphenol in soil. Environmental Toxicology and Chemistry. 2000;19:313–318. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Biosolids Technology Fact Sheet: Land Application of Biosolids. 2000 EPA 832-F-00-064: www.epa.gov/region8/water/biosolids/pdf/BiosolidsTechSheetLA.pdf.
- U.S. Environmental Protection Agency (U.S. EPA) 2001 National Sewage Sludge Survey Report. 2001 EPA-822-R-07-006: http://water.epa.gov/scitech/wastetech/biosolids/upload/sludgesurvey9-2007.pdf.
- U.S. Environmental Protection Agency (U.S. EPA) Nonylphenol (NP) and Nonylphenol Ethoxylates (NPEs) Action Plan. 2010 RIN 2070-ZA09. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/RIN2070-ZA09_NPNPEs%20Action%20Plan_Final_2010-08-09.pdf.
- U.S. Environmental Protection Agency (U.S. EPA) [July 26, 2012];Nonylphenol and Nonylphenol Ethoxylates Action Plan Summary. 2012 2012: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/np-npe.html.
- Vikelsøe J, Thomsen M, Carlsen L. Phthalates and nonylphenols in profiles of differently dressed soils. Science of the Total Environment. 2002;296:105–116. doi: 10.1016/s0048-9697(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Walters E, McClellan K, Halden RU. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Research. 2010;44:6011–6020. doi: 10.1016/j.watres.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, Hundal LS, Kumar K, Armbrust K, Cox AE, Granato TC. Triclocarban, triclosan, polybrominated diphenyl ethers, and 4-nonylphenol in biosolids and in soil receiving 33-year biosolids application. Environmental Toxicology and Chemistry. 2010;29:597–605. doi: 10.1002/etc.66. [DOI] [PubMed] [Google Scholar]
- Xia K, Jeong CY. Photodegradation of the endocrine-disrupting chemical 4-nonylphenol in biosolids applied to soil. Journal of Environmental Quality. 2004;33:1568–1574. doi: 10.2134/jeq2004.1568. [DOI] [PubMed] [Google Scholar]
- Ying GG, Williams B, Kookana R. Environmental fate of alkylphenols and alkylphenol ethoxylates--a review. Environment International. 2002;28:215–226. doi: 10.1016/s0160-4120(02)00017-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.