Summary
The heat shock transcription factor (HSF) is a conserved regulator of heat shock-inducible gene expression. Organismal roles for HSF in physiological processes such as development, aging, and immunity have been defined largely through studies of the single C. elegans HSF homolog, hsf-1. However, the molecular and cell biological properties of hsf-1 in C. elegans are incompletely understood. We generated animals expressing physiological levels of an HSF-1::GFP fusion protein and examined its function, localization, and regulation in vivo. HSF-1::GFP was functional as measured by its ability to rescue phenotypes associated with two hsf-1 mutant alleles. Rescue of hsf-1 stress, aging, and development phenotypes was abolished in a DNA-binding-deficient mutant, demonstrating that the transcriptional targets of hsf-1 are critical to its function even in the absence of stress. Under non-stress conditions, HSF-1::GFP was found primarily in the nucleus. Following heat shock, HSF-1::GFP rapidly and reversibly redistributed into dynamic, sub-nuclear structures that share many properties with human nuclear stress granules, including colocalization with markers of active transcription. Rapid formation of HSF-1 stress granules required HSF-1 DNA binding activity and the threshold for stress granule formation was altered by growth temperature. HSF-1 stress granule formation was not induced by inhibition of IGF signaling, a pathway previously suggested to function upstream of hsf-1. Our findings suggest that development, stress, and aging pathways may regulate HSF-1 function in distinct ways, and that HSF-1 nuclear stress granule formation is an evolutionarily conserved aspect of HSF-1 regulation in vivo.
Keywords: Heat shock factor, aging, longevity
Introduction
The heat shock transcription factor (HSF) plays essential and evolutionarily conserved roles in the activation of heat shock-inducible gene expression. While HSFs are best recognized as regulators of stress-induced gene expression, they also contribute to more complex organismal physiological processes such as development, growth, aging, immunity, and reproduction. HSFs are also central to many pathophysiological processes and contribute to the tumorogenic phenotype as well as to the pathology underlying diseases of protein misfolding, such as Huntington’s and Alzheimer’s diseases (Hsu et al. 2003; Morley & Morimoto 2004; Cohen et al. 2006; Dai et al. 2007). Given the importance of HSFs to these physiological and pathophysiological states, understanding the mechanisms that regulate HSF function could provide new insights and therapeutic strategies for a variety of diseases (Westerheide & Morimoto 2005).
In mammals, HSF1 encodes the master regulator of the heat shock response(Akerfelt et al. 2010). Regulation of HSF1 is thought to occur at several levels. In the absence of heat shock, mammalian HSF1 exists primarily as a monomeric protein that is bound and repressed by heat shock protein 90 (HSP90) (Voellmy & Boellmann 2007). Following an acute heat shock, HSP90 binds to misfolded client proteins, releasing HSF1 so that it can trimerize, bind sequence-specific heat shock elements (HSEs), and transactivate gene expression. In human (and other primate but not rodent) cells, HSF1 also responds to stress by localizing to subnuclear structures termed nuclear stress granules (or nuclear stress bodies; (Biamonti & Vourc'h 2010)). HSF1 stress granules represent the binding of HSF1 to heterochromatic, pericentromeric repeat regions of DNA, leading to transcription of non-coding RNAs (ncRNAs) (Jolly et al. 1997; Jolly et al. 1999; Denegri et al. 2002; Jolly et al. 2004; Eymery et al. 2010). Stress granule formation seems to be determined by specific DNA sequences, since rodent HSF1 can be induced to form stress granules when it is in the presence of human chromosomes (Denegri et al. 2002). Despite intensive study, the functional role of HSF1 stress granules and their associated ncRNAs remains one of the most mysterious aspects of HSF-1 regulation. Since HSF1 stress granule formation has only been observed in primate cells, their study has been hampered by the lack of a suitable system in which to manipulate stress granule formation and ncRNA synthesis. Moreover, while the biochemical and cell biological properties of HSF1 are well described in isolated cells, studies investigating whether similar regulation occurs in a multicellular organism are more limited.
Recent studies in Caenorhabditis elegans have played a major role in understanding how HSF contributes to organismal physiology and pathophysiology. While vertebrates express four major HSFs, worms express a single HSF homolog, hsf-1. By sequence homology, C. elegans HSF-1 contains N-terminal DNA binding and trimerization domains, with a putative transactivation domain at the C-terminus (Hajdu-Cronin et al. 2004). RNAi-mediated knockdown of hsf-1 gives rise to a progeric phenotype (Garigan et al. 2002), while hsf-1 overexpression promotes longevity and delays age-related protein misfolding and proteotoxicity (Hsu et al. 2003; Morley & Morimoto 2004; Cohen et al. 2006). hsf-1 also plays important functional roles in C. elegans innate immunity (Singh & Aballay 2006), where it functions in the intestine to inhibit pathogen-induced protein aggregation (Mohri-Shiomi & Garsin 2008). Despite these many roles, the localization pattern and dynamic regulation of the C. elegans HSF-1 protein expressed at physiological levels have not been reported.
Here, we generated C. elegans expressing a functional single-copy HSF-1::GFP transgene driven by the native hsf-1 promoter. We find that in vivo, HSF-1::GFP is a ubiquitously-expressed, predominantly nuclear protein. Following heat shock, HSF-1::GFP does not exhibit further nuclear enrichment but does undergo rapid and reversible reorganization into sub-nuclear structures that share many characteristics with human HSF1 stress granules. The rapid formation of these structures in C. elegans required DNA binding activity but was not induced by inactivation of the insulin/IGF signaling. These studies are among the first to demonstrate the dynamic nuclear behavior of HSF in native tissues in a live animal setting, suggesting that nuclear stress granule formation may be an evolutionarily ancient mechanism for regulating HSF in vivo.
Results
HSF-1 constitutively localizes to the nucleus and forms nuclear granules upon heat shock
Many HSF-1-dependent processes have been studied in C. elegans and the properties of overexpressed C. elegans HSF-1 have recently been investigated (Chiang et al. 2012). However, overexpressed C. elegans HSF-1 produces gain-of-function phenotypes (Hsu et al. 2003; Morley & Morimoto 2004; Singh & Aballay 2006; Chiang et al. 2012) and may not accurately reflect the physiological properties of endogenous HSF1. To characterize these properties, we generated a fluorescent reporter that fused GFP to the C-terminus of the HSF-1 protein and integrated this transgene at single-copy level into the C. elegans genome (Figure 1A) (Frokjaer-Jensen et al. 2008). Quantitative real-time PCR confirmed that this transgenic line expressed hsf-1 at near physiological levels (Figure 1B). In contrast with previous studies of overexpressed HSF-1::GFP, single copy HSF-1::GFP localized predominantly to the nucleus under basal conditions (20°C) (Figure 1C). Following heat shock (35°C for 1 min or 20 min), HSF-1::GFP did not exhibit further accumulation of fluorescence intensity within the nucleus (Figure S1), suggesting that during the earliest phases of heat shock, nuclear translocation of HSF-1::GFP is a minor component of its regulation in C. elegans. However, heat shock did result in the redistribution of HSF-1::GFP into distinct sub-nuclear structures (Figure 1C,D), a property reminiscent of human HSF1, which forms structures termed HSF1 nuclear stress granules upon heat shock (Cotto et al. 1997; Jolly et al. 1997). HSF-1::GFP granule formation also occurred in the hsf-1(ok600) deletion mutant, indicating that stress granule formation does not require the endogenous HSF-1 protein (data not shown). A PCR fusion-derived HSF-1::YFP protein lacking Gateway adaptor sequences also showed nuclear enrichment and stress granule formation (Figure S2). Quantification of the size of C. elegans HSF1 stress granules revealed an average diameter of 0.6±0.2 μm, slightly smaller than human HSF1 stress granules, which distribute among two populations of structures with diameters of 0.5–1.6 µm and 1.6–3.0 µm (Figure 1E) (Cotto et al. 1997). The C. elegans HSF-1 structures were also similar in number to human HSF1 granules (7.1±2.7 granules per nucleus in C. elegans (Figure 1F) versus 6.8±2.4 granules per nucleus in HeLa cells (Cotto et al. 1997)). Stress-induced HSF-1::GFP granules were observed in all examined tissue types (Figure S3). Polyploid intestinal nuclei appeared to contain more granules per nucleus than other cell types, but intestinal autofluorescence hindered precise quantification (data not shown). Granules formed within one minute during heat shock at 35°C and dispersed following an hour of recovery at 20°C (Figure S3). After recovery, HSF-1::GFP could re-form nuclear granules with subsequent heat shocks, although reformation required a longer heat shock suggesting that stress granule formation exhibits adaptation (Figure S4). These granules formed in locations similar to the initial granules, suggesting that another existing nuclear structure, such as DNA or RNA, acts as a scaffold for granule formation. When animals were grown at 16°C, HSF-1::GFP formed granules at heat shock temperatures ≥ 28 °C (Figure S5). Animals grown at 20°C or 25°C formed few or no granules at this temperature, but did form stress granules when exposed to ≥ 33°C (Figure S5). This suggests that the induction of HSF-1::GFP granules by heat shock is adaptable and regulated by growth temperature. Together these data suggest that stress-induced HSF-1::GFP nuclear granules in C. elegans resemble human HSF-1 stress granules in their number, kinetics, reformation, and reversibility.
Figure 1. HSF-1::GFP is broadly expressed and condenses into nuclear granules following heat shock.
(A) Diagram of the hsf-1p::hsf-1(cDNA)::GFP::unc-54 3’UTR expression construct (drSi13) used in this study. hsf-1p is 4 kb of sequence upstream of the hsf-1 start ATG. Notations above the construct diagram indicate changes made to the transgene (R to A at residue 145 and HA insertion after residue 370); notations below indicate relative positions of mutations in the endogenous gene (diagram not to scale.) (B) qRT-PCR comparing wild type (N2) and HSF-1::GFP hsf-1 mRNA levels relative to actin mRNA, normalized to N2. Shown is the mean relative expression ± SEM in drSi13 for three independent experiments. hsf-1 mRNA in a wild-type background is less than double control wild type, suggesting compensatory mechanisms acting on hsf-1 expression. (C) HSF-1::GFP localizes primarily to the nucleus at 20°C. (D) After 1 min of 35°C heat shock, HSF-1::GFP collects into nuclear puncta (arrows). Shown are four merged (Z-dimension) deconvolved slices depicting hypodermal nuclei. Scale bar = 5μm. (E) HSF-1::GFP granule size in hypodermal cells after 1 min 35°C heat shock (N = 349 granules). (F) Number of HSF-1::GFP granules per cell in hypodermal cells after 1 min 35°C heat shock. (N = 100 nuclei). (G-I) drSi41, a single copy line expressing hsf-1p::hsf-1::HA::unc-54 3’UTR, in which the HA tag is inserted into the region between the putative trimerization and transactivation domains of the hsf-1 cDNA. drSi41 worms were either heat shocked (G, 30 min at 35°C) or not (F, 30 min at 25°C), dissected, fixed, probed for HA (red, G-I), and stained with Hoechst dye (blue, G’-I’). Heat shocked N2 worms are shown as a control (I). Shown are nine merged (Z-dimension) deconvolved slices. Dotted line indicates outline of nuclei as determined by Hoechst. Scale bar = 5 µm.
We considered the possibility that the behavior of HSF-1::GFP may represent an artifact of the GFP tag. We therefore attempted to immunolocalize endogenous worm HSF-1 using a human antibody previously reported to recognize worm HSF-1 by immunoblot.(Alavez et al. 2011; Volovik et al. 2012) Although we could detect human HSF1 expressed in worms, we were unable to immunolocalize C. elegans HSF-1::GFP with this antibody following heat shock (Figure S6). Furthermore, we were also unable to detect a bacterially expressed C. elegans 6XHis-HSF-1 fusion protein by Western blot with either this antibody or another reported cross-reactive anti-human HSF-1 antibody (Chiang et al. 2012), although both antibodies robustly detected human 6XHis-HSF1 (Figure S7). As an alternative approach, we generated single-copy transgenic animals expressing an HA-tagged form of HSF-1. We inserted the HA tag into the HSF-1 protein between the predicted DNA binding and transactivation domains (Hajdu-Cronin et al. 2004). Like HSF-1::GFP, HSF-1::HA localized to the nucleus and re-distributed into granule-like structures following heat shock (Figure 1F-H). HSF-1::HA retained function, as measured by its ability to rescue an hsf-1 mutant (Figure 2A). These data show that HSF-1 is a predominantly nuclear protein that undergoes rapid and reversible changes in sub-nuclear distribution in response to acute heat shock in C. elegans.
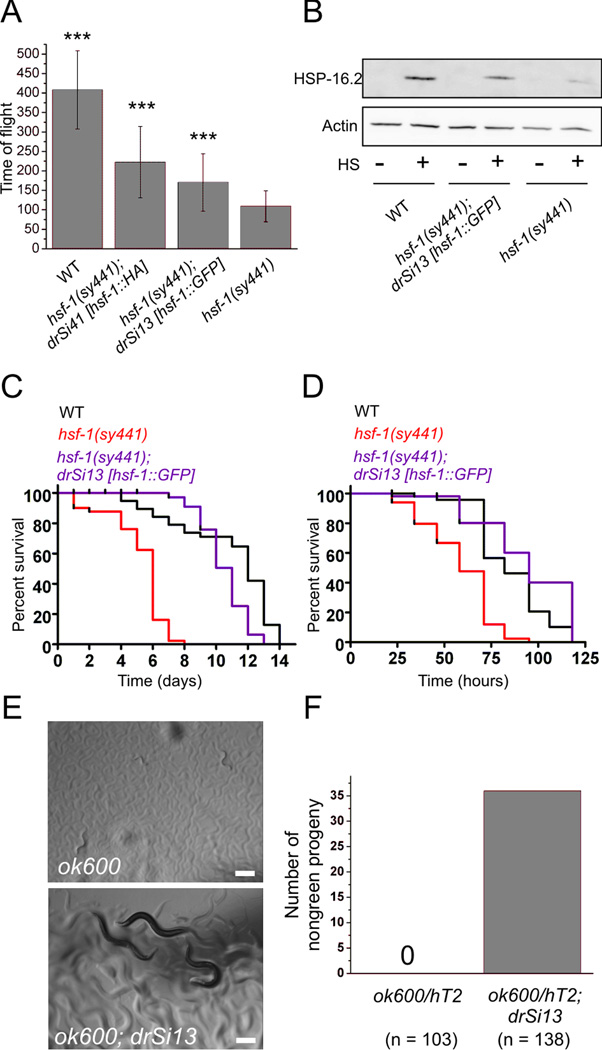
Figure 2. HSF-1::GFP is functional.
(A) Wild type (WT), hsf-(sy441);drSi41[hsf-1p::hsf-1::HA::unc-54 3’UTR], hsf-(sy441);drSi13[hsf-1p::hsf-1::GFP::unc-54 3’UTR], and hsf-1(sy441) eggs were placed at 25°C and allowed to grow until wild type was L4/young adult. Worms were analyzed for size (time of flight) in a COPAS Biosort (N ≥ 89 animals. Mean ± SD, *** - p < 0.001 as compared to hsf-1(sy441)). (B) Representative Western against HSP-16.2 (top panel) and β-actin (bottom panel) on WT, hsf-1(sy441);drSi13, and hsf-1(sy441) worms ± a 35°C 3h heat shock followed by 3 hr recovery at 16°C. Relative HSP-16.2:actin ratio for WT : hsf-1(sy441);drSi13 : hsf-1(sy441) is 1.0:0.67:0.22. (C) Lifespan of WT, hsf-1(sy441), and hsf-1(sy441);drSi13 animals at 25°C (N = 50 for all). (p < 0.0001 between hsf-1(sy441) and hsf-1(sy441);drSi13; p = 0.0036 between WT and hsf-1(sy441);drSi13) (D) Survival of WT, hsf-1(sy441), and hsf-1(sy441); drSi13 animals on P. aeruginosa PA14 at 25°C (N = 50 for all) (p < 0.0001 between hsf-1(sy441) and hsf-1(sy441);drSi13; p = 0.498 between WT and hsf-1(sy441);drSi13). (E) Images of ok600 homozygous animals with (lower) or without (upper) the drSi13 HSF-1::GFP transgene. Scale bar = 100μm. (F) Quantification of the number of ok600 homozygous animals that reach L4 stage or later within 3 days at 20°C with or without the drSi13 HSF-1::GFP transgene.
HSF-1::GFP is functional
We considered the possibility that the GFP fusion might disrupt HSF-1 protein function. We tested this by assessing the ability of HSF-1::GFP to rescue hsf-1 mutant phenotypes. We also investigated the ability of a human HSF1::GFP protein to functionally substitute for C. elegans HSF-1. The hsf-1(sy441) allele introduces a stop codon at position 585 preceeding the predicted C-terminal transactivation domain, and mutant animals exhibit temperature-sensitive growth arrest, reduced expression of HSP-16.2 after heat shock, shortened lifespan, and sensitivity to pathogens (Hajdu-Cronin et al. 2004; Singh & Aballay 2006). A second hsf-1 allele, ok600, encodes a frameshifting deletion that eliminates potential regulatory and transactivation domains at the C-terminus of the HSF-1 protein (Figure 1A) and causes a 100% penetrant larval arrest phenotype at all growth temperatures, suggesting that ok600 is a more severe allele than sy441. Whether the lethality of ok600 is due to loss of hsf-1 or another linked mutation has not been described. Single-copy worm hsf-1p::hsf-1::GFP rescued the temperature-dependent growth defects, temperature-dependent induction of HSP-16.2, lifespan reduction, and enhanced pathogen sensitivity of hsf-1(sy441) mutants (Figure 2A-D). Unlike hsf-1 overexpression transgenes, single-copy HSF-1::GFP did not cause increased longevity or enhanced pathogen resistance, suggesting that this transgene does not produce gain-of-function phenotypes like that observed for hsf-1 overexpression (Figure 2C,D). HSF-1::GFP was also able to rescue the larval lethality associated with the hsf-1(ok600) allele, indicating that the ok600 lethal phenotype is indeed due to loss of hsf-1 function (Figure 2E,F). Human HSF1::GFP under the control of the C. elegans hsf-1 promoter was expressed, properly localized to the nucleus, and capable of forming granules following heat shock (Figure S6, S8A), albeit with substantially slower kinetics than those observed with worm HSF-1::GFP (1 hr of heat shock required for human HSF1::GFP versus 1 min for C. elegans HSF-1::GFP). Despite this, human HSF1::GFP was unable to rescue hsf-1(sy441) mutant phenotypes (Figure S8B-F), suggesting that worm and human HSF1 are not functionally interchangeable. These data show that, in addition to its roles in aging, C. elegans hsf-1 is an essential gene required for larval development and that worm HSF-1::GFP, but not human HSF1::GFP, is functional and capable of rescuing hsf-1-associated mutant phenotypes in C. elegans.
HSF-1::GFP granules are dynamic and induced by specific environmental stressors
We next considered the possibility that HSF-1::GFP granules may simply be the result of protein aggregation caused by heat shock. We used fluorescence recovery after photobleaching (FRAP) to examine the mobility of the HSF-1::GFP protein within stress-induced nuclear granules. In contrast to protein aggregates induced by either stress or aging (Moronetti Mazzeo et al. 2012), puncta of HSF-1::GFP exhibited rapid recovery after photobleaching (Video S1). Similar results have been shown for human HSF1 stress granules (Jolly et al. 1999). These data demonstrate that the HSF-1::GFP granules are not aggregates but rather are composed of dynamic HSF-1::GFP molecules.In addition to heat shock, other environmental stressors, including cadmium and azetidine, induce the formation of HSF1 granules in human cells (Jolly et al. 1999). We asked if C. elegans HSF-1::GFP also forms nuclear granules in response to various environmental stressors. Exposure of HSF-1::GFP worms to osmotic stress (219 mM NaCl), heavy metals (1µm cadmium), or ethanol (100mM) did not induce granule formation (Figure 3D-F). However, exposure to sodium azide, a well known inhibitor of cytochrome oxidase and cellular ATP production and a commonly used anesthetic in the C. elegans field (Sulston & Hodgkin 1988), induced robust HSF-1::GFP granule formation similar to that observed with heat shock (Figure 3B,C). Conditions that induced granule formation also activated hsf-1-dependent gene expression, as measured using a reporter transgene (hsp-16.2p::GFP) (Figure 3H–M). HSF-1::GFP granule formation can therefore be induced by elevated temperature or through azide-dependent inhibition of ATP production, demonstrating that heat shock per se is not required for granule formation.
Figure 3. Nuclear granules form in response to heat shock and sodium azide.
Worms expressing drSi13 HSF-1::GFP were anesthetized 30 min in 1 mM levamisole at either room temperature (A), 35°C (HS) (B), 5 mM sodium azide (C), 219 mM NaCl (D), 100 μM CdCl2 (E), or 100 mM ethanol (F). Scale bar = 5μm. (G) Percent of hypodermal nuclei with ≥ one visible granule were quantified for each condition (N ≥ 10 worms per condition, representing ≥ 85 nuclei. Mean ± SEM, *** - p < 0.001 vs. control, n.s. - not significant). (H-M) TJ375 (hsp-16.2p::GFP) worms were subjected to 30 min of the same conditions as in A-F, followed by recovery at 20°C for 4 hr before imaging. Scale bar = 100μm.
Post-translational modification of HSF-1 temporally lags granule formation
Previous data from several species demonstrate that upon heat shock, HSF-1 is subject to a number of post-translational modifications (Akerfelt et al. 2010). We asked if C. elegans HSF-1::GFP was similarly modified after heat shock. Consistent with other recent observations of HSF-1 in C. elegans (Chiang et al. 2012), HSF-1::GFP shifted towards a higher molecular weight in response to heat shock (Figure S9A), suggesting it is the target of stress-inducible post-translational modification (PTM). While previous studies showed that this PTM is due to phosphorylation (Chiang et al. 2012), we were unable to confirm this by phosphatase assay due to the extremely low abundance of the single-copy HSF-1::GFP protein (data not shown). The molecular weight shift was not apparent after one minute of heat shock but was detectable after 10 minutes of heat shock, thus temporally lagging the observed kinetics of HSF-1 stress granule formation (Figure S9B).
DNA binding promotes HSF-1 stress granule formation
Studies in human cells have established that human HSF1 nuclear stress granules do not represent the binding of HSF1 to HSEs in canonical HSF1 targets like HSP70 (Jolly et al. 1997), but rather HSF1 binding to and transcription of non-coding satellite II and III repeats, elements specific to primate genomes (Jolly et al. 2002; Eymery et al. 2010). Supporting this model, the DNA binding domain of HSF1 is required for stress granule formation in human cells (Jolly et al. 2002). To ask if DNA binding was also required for C. elegans HSF-1::GFP stress granules formation, we generated a point mutation (R145A, equivalent to human R71A, Figure 4A) in a completely conserved amino acid within the DNA binding domain that has been previously shown to be required for HSF-1-HSE DNA binding (Inouye et al. 2003; Morley & Morimoto 2004). We expressed this HSF-1(R145A) as a fusion with GFP under the native hsf-1 promoter at single copy level. Like wild-type HSF-1::GFP, HSF-1(R145A)::GFP was expressed and localized to the nucleus; however, the ability of HSF-1(R145A)::GFP to form nuclear granules in response to acute heat shock was significantly reduced (Figure 4B–D). This suggests that DNA binding promotes HSF-1 granule formation in C. elegans.
Figure 4. HSF-1 DNA binding promotes stress granule formation and developmental rescue of hsf-1(sy441).
(A) Alignment of the region of the DNA binding domain containing R145 (red) from the indicated species. (B,C) Images of drSi13 HSF-1::GFP (WT) and drSi28 HSF-1(R145A)::GFP (R145A) taken after a 1 min 35°C heat shock, showing granule formation (arrow). Scale bar = 5μm. Shown are four merged (Z-dimension) deconvolved slices. (D) Number of granules per nucleus was quantified for WT and R145A. (N ≥ 18 worms, representing ≥ 140 nuclei for each line. Mean ± SD, *** - p < 0.001). (E) N2 wild type (WT), hsf-1(sy441), and hsf-1(sy441);drSi28 eggs were placed at 25°C and allowed to grow until wild type was L4/young adult. Worms were analyzed for size (time of flight) in a COPAS Biosort (N ≥ 30 animals. Mean ± SD, *** - p < 0.001, n.s. - not significant). (F) Representative Western against HSP-16.2 (top panel) and β-actin (bottom panel) on young adult wild type, hsf-1(sy441);drSi28, and hsf-1(sy441) worms ± a 3 hr 35°C heat shock followed by 3 hr recovery at 16°C.
As HSF-1 mutant phenotypes are probably due to an inability to transactivate target gene expression, we examined the ability of HSF-1(R145A)::GFP to rescue the hsf-1 mutant alleles ok600 and sy441. Because the sy441 point mutation is viable and the ok600 deletion allele lethal, sy441 likely results in the expression of a partially functional protein, which is predicted to contain intact DNA binding and oligomerization domains but lack the putative C-terminal transactivation domain. Given that the active form of HSF-1 is thought to be a trimer (Baler et al. 1993), we hypothesized that the DNA binding-deficient HSF-1(R145A), which contains a functional transactivation domain, might be able to interact with the truncated hsf-1(sy441) protein and provide the missing transactivation function. Consistent with this model, hsf-1(sy441) animals expressing the HSF-1(R145A)::GFP transgene produced a marked increase in stress-inducible gene expression as compared to the hsf-1(sy441) background alone (Figure 4F), though quantification of this was difficult due to the very low levels of HSP-16.2 expression in hsf-1(sy441). However, HSF-1(R145A)::GFP was unable to rescue the developmental functions of HSF-1 in either the sy441 or ok600 backgrounds (Figure 4E and data not shown). The ability of HSF-1(R145A)::GFP to partially rescue the stress-inducible phenotype of hsf-1(sy441) but not the developmental phenotype suggests that the functional requirements for hsf-1 in stress responses and development may be genetically separable.
HSF-1 granules in C. elegans colocalize with markers of active transcription
In human cells, HSF1 granules represent sites of HSF1 binding at satellite II and III repeats, colocalization with markers of active transcription like phosphorylated RNA polymerase II and acetylated histones, and transcription of ncRNAs (Denegri et al. 2002; Jolly et al. 2002; Jolly et al. 2004; Metz et al. 2004). Because C. elegans contains little if any satellite repeat DNA sequences (Sulston & Brenner 1974), the putative site(s) of HSF-1 stress granule binding are unknown. Therefore, we asked whether worm HSF-1 stress granules also colocalized with general markers of active transcription. We stained control and heat shocked animals for either the phosphorylated Serine 2 form of RNA polymerase II (RNA polII Ser2p) or the acetylated Lysine 5 form of histone H2A (H2Aac), both of which have been used as markers of active transcription in human HSF1 stress granules (Jolly et al. 2004). Both of these markers showed colocalization with some (but not all) stress-induced HSF-1::GFP granules (Figure 5A–C, I–K, arrows). The overall number of H2Aac foci was significantly increased by heat shock, consistent with transcriptionally active HSF-1 granules being induced by stress (Figure S10). Although these data do not directly demonstrate transcriptional activity of HSF-1 within nuclear stress granules, they strongly suggest that active transcription is occurring at some sites of HSF-1 granule formation.
Figure 5. HSF-1::GFP granules colocalize with markers of active transcription.
drSi13 HSF-1::GFP worms grown at 25°C were heat shocked for 1.5 hr at 35°C (HS) or put at 25°C 1.5 hr (no HS) and intestinal nuclei were probed for GFP (green, B,F,J,N) histone H2A acetylated on Lysine 5 (red, A,E), or RNA polymerase II phosphorylated on Serine 2 (red, I,M. Exposure times in I and M were different because we observed substantially reduced RNA polII Ser2p staining post-heat shock). Nuclei (intestinal) were detected by Hoechst staining (D,H,L,P). Nuclear staining showed puncta of fluorescence, some of which show colocalization of GFP and an active transcription marker (arrows). Other puncta exhibit GFP-only (carrot) or active transcription marker-only (open arrowhead) staining. Scale bar = 5μm.
IGF and neuronal genetic pathways influence HSF-1 granule formation
Previous studies have shown that several genetic and physiological pathways require HSF-1 function. For example, extension of lifespan via activation of insulin signaling requires HSF-1 activity (Hsu et al. 2003; Morley & Morimoto 2004). Likewise, a recently described neuronal pathway regulates HSF-1-dependent gene expression (Prahlad et al. 2008; Prahlad & Morimoto). To determine if these pathways regulate HSF-1 in a way similar to that of heat shock, we examined the expression, localization, and stress-inducible behavior of HSF-1::GFP in the IGF mutant daf-2(e1370) and in the AIY neuron mutant ttx-3(ks5). In daf-2(e1370), in the absence of stress, HSF-1::GFP localized to and was evenly distributed within the nucleus (of hypodermis, intestine and other cell types), as was previously observed in non-stressed wild-type animals (Figure S11E,F, and data not shown). Granule formation could still be induced, but quantification of granule formation revealed a statistically significant decrease in the number of granules induced by heat shock in a daf-2(e1370) background (Figure S11H) in hypodermal nuclei. The effect of daf-2(e1370) on granule formation in other tissues was more difficult to quantify due to the small size of the nuclei and autofluorescence, although stress-induced granule formation was observed (data not shown). Likewise, in the AIY interneuron mutant ttx-3(ks5), (previously shown to be required for non-neuronal heat shock-inducible gene expression) HSF-1::GFP remained localized to the nucleus in the absence of stress. Following heat shock of ttx-3, HSF-1::GFP continued to form stress granules in hypodermal nuclei (Figure S11D) and other cell types (data not shown). However, as with daf-2, the number of granules induced by heat shock in ttx-3 animals was reduced (Figure S11G). These findings show that IGF and AFD signaling promote proper stress-inducible HSF-1::GFP granule formation but do not alter HSF-1::GFP nuclear localization in non-stressful environments, suggesting that the mechanism(s) by which IGF signaling regulates HSF-1 is distinct from the mechanisms that regulate HSF-1 in response to heat shock.
Discussion
While most of the work demonstrating a functional role for HSF1 in the regulation of aging and diseases of protein aggregation has been carried out in C. elegans, there is still much to learn regarding the molecular properties of HSF-1 in this system or any organismal context. Our findings fill important gaps in our knowledge of HSF biology, provide new resources with which to study this conserved transcription factor, and describe insights into HSF-1 regulation in C. elegans that both concur with and contradict other recent studies (Chiang et al. 2012).
The major model for HSF1 regulation predicts it to be predominantly cytoplasmic under control conditions, constrained by interactions with cytoplasmic heat shock proteins (HSPs) (Voellmy & Boellmann 2007). Following heat stress, HSPs are competed away by interactions with misfolded client proteins, allowing HSF1 to trimerize and translocate to the nucleus. While all studies agree that HSF1 is localized to the nucleus following stress, the localization of HSF1 prior to stress has been controversial. For example, Drosophila HSF1 is considered a constitutively nuclear protein (Westwood et al. 1991; Yao et al. 2006), but some studies have suggested that it is predominantly cytoplasmic and undergoes nuclear translocation with heat shock (Zandi et al. 1997). Likewise, human HSF1 has been reported both as a nuclear protein under all conditions (Martinez-Balbas et al. 1995; Mercier et al. 1999) and as a predominantly cytoplasmic protein that undergoes stress-induced nuclear translocation (Baler et al. 1993; Sarge et al. 1993). The reasons for these inconsistencies are unclear but may be due to artifacts of overexpression or biochemical preparations that artificially place inactive HSF1 in the cytoplasm (Mercier et al. 1999). Recently, Chiang et al. reported that an HSF-1::GFP protein in C. elegans exhibited diffuse nucleo-cytoplasmic fluorescence under control conditions, converting to weak nuclear localization after heat shock (Chiang et al. 2012). This finding contrasts with our observations that HSF-1::GFP is a nuclear protein before and after stress. How can this discrepancy be explained? One possibility is that the HSF-1::GFP fusion protein differs between the two studies and these differing sequences alter localization. Both fusion proteins are derived from an HSF-1 cDNA fused with C-terminal GFP via different linker sequences. The presence of the GFP tag does not affect localization, since an HSF-1::HA protein exhibits similar nuclear localization to our HSF-1::GFP protein. Likewise, our linker sequences also do not affect localization since an HSF-1::YFP protein lacking linkers also localized to the nucleus and formed granules with stress. It is possible that overexpression of HSF-1::GFP in the Chiang et al. study alters localization. High-level expression may overwhelm HSF-1 regulatory mechanisms, driving dysregulated HSF-1 into the cytoplasm. Such dysregulation would be unlikely to affect our HSF-1 reporter due to its physiological level of expression. This discrepancy could be best resolved by examining the localization of the endogenous HSF-1 protein, but such studies will require the generation of new antibodies that are compatible with immunofluorescence techniques in C. elegans.
One important new finding of our work is that heat shock induces C. elegans HSF-1 to form discrete subnuclear foci that are similar to structures previously thought to occur only in primate cells. While C. elegans and human HSF-1 foci share many characteristics (Table S1), we have not shown that all properties are shared between the two structures. For example, human HSF1 granules do not colocalize with standard HSF1 targets (Jolly et al. 1997) but rather with heterochromatic pericentromeric satII and satIII repeats (Jolly et al. 2004; Metz et al. 2004; Eymery et al. 2010), where they promote the transcription of ncRNAs that remain associated with the stress granule. While we have shown the C. elegans HSF-1 granules involve DNA binding and are (in a subset of granules) associated with transcription, we have not shown that such binding is distinct from the binding of HSF-1 to target promoters or that these binding sites are associated with the transcription of ncRNAs. Additionally, worms do not have centromeric sequences or satellite repeat DNA, so it remains possible that the specific properties of stress granule binding sites in C. elegans may be distinct from those in human cells. Despite these potential differences, it is also possible that the C. elegans stress granules are sites of centromere-independent ncRNA transcription. If so, C. elegans could provide important insights regarding the role of such ncRNAs in hsf-1-dependent processes. This is currently an important but unanswered question in the HSF1 field to which studies in C. elegans could make an important contribution.
Our data also suggest that the mechanisms regulating HSF-1 may not be the same in all contexts. In C. elegans, hsf-1 has been primarily studied at the phenotypic level where it has roles in aging, immunity, and development. Prior to our work, it was not clear if these processes acted through mechanisms similar to those by which temperature regulates HSF-1 activity. We found that activation of HSF-1 via heat shock induces granule formation, but inactivation of insulin signaling via mutation of daf-2 does not. It bears noting that we were able to induce granule formation with sodium azide, demonstrating that heat shock itself is not required for granule formation. Like inhibition of insulin signaling, inhibition of AFD signaling via ttx-3 mutation also does not constitutively induce or prevent granule formation, though it does reduce number of granules visible per nucleus. This observation could be due to a requirement of neuronal signaling for stress-induced granule formation, or possibly due to a decrease in total HSF-1, as HSF-1 levels were not quantified in this line. Altogether, our findings provide an alternative model to that proposed by Chiang et al. (Chiang et al. 2012) and suggest that insulin signaling and temperature might employ distinct mechanisms to control HSF-1 activity.
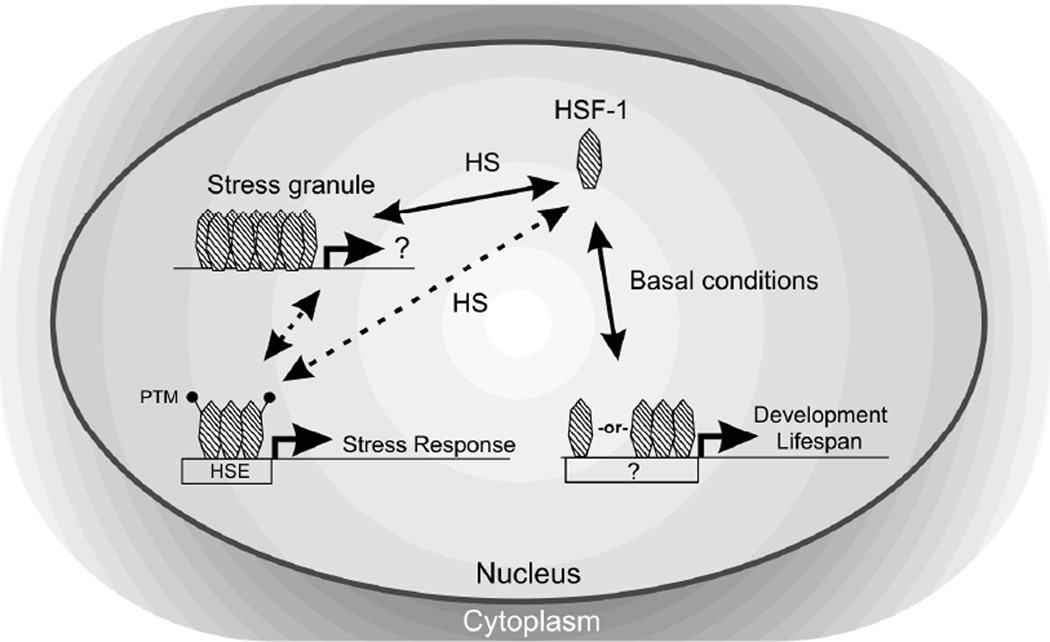
Consistent with the idea that multiple mechanisms may regulate HSF-1, we also found evidence that the activity of HSF-1 in development and heat shock are not two outcomes of a single activation pathway, but rather two mechanistically different activation pathways (Figure 6). Similar observations have been noted for Drosophila hsf-1 (Jedlicka et al. 1997) and C. elegans hsf-1 (Walker et al. 2003). We have built on these observations by showing that the DNA binding-deficient HSF-1(R145A) molecule could partially complement the transactivation-deficient hsf-1(sy441) mutant for stress-inducible gene expression but not development. This could be explained through stress-specific oligomerization of HSF-1(R145A) and HSF-1(sy441) molecules, both of which possess intact putative trimerization domains, leading to an oligomer containing functional domains for both transactivation and DNA binding. An alternative possibility is that the R145A mutant may retain low levels of DNA binding activity that are sufficient to rescue hsf-1 stress functions but not developmental functions. Regardless, the fact that HSF-1(R145A) is incapable of rescuing development strongly suggests that HSF-1 has transcriptional targets even in the absence of stress. Such targets may even be transcribed by monomeric HSF-1, likely the predominant form in the absence of stress (Figure 6). Further testing of this model, using more precise deletion alleles that eliminate specific HSF-1 functional domains, is called for.
Figure 6. Model for HSF-1 regulation in C. elegans.
HSF-1 is a predominately nuclear protein in C. elegans, and its modes of activity under basal conditions and stress conditions (HS) differ. Stress-inducible activity is distinguished by stress granule formation, oligomerization, and post-translational modification of HSF-1. Due to its oligomeric nature, we hypothesize that physiological levels of the DNA binding-deficient HSF-1(R145A) can still associate with the active HSF-1 complex and contribute transactivation function to stress-inducible targets in trans. The observation that HSF-1(R145A) cannot rescue developmental defects in the sy441 transactivation-deficient background suggests that HSF-1 activity in the context of development may not operate in trans. Basal targets of HSF-1, including genes involved in development and possibly lifespan, require DNA binding activity, but may not involve stress granule formation or oligomerization.
In conclusion, we have provided new in vivo insights into the regulation of HSF-1 in C. elegans and its mechanism of regulation by heat shock, including a potentially evolutionarily conserved sub-nuclear behavior that was previously thought to be present only in primates. Expression of epitope-tagged, physiological levels of HSF-1 in vivo offers new experimental opportunities to understand how this protein integrates development, stress, aging, and metabolic pathways in a live organism setting to determine condition-specific gene expression.
Experimental Procedures
C. elegans strains and culture
Strains were cultured on standard NGM media with OP50 bacteria. For a list of all strains and alleles used in this study, see ‘Supplemental Methods’.
Molecular Biology and Transgenics
Detailed description of the cloning methods and expression constructs used in this study can be found in the ‘Supplemental Methods’. Single-copy-compatible constructs were integrated into the chromosome II locus (ttTi5605) using the direct insertion MosSCI technique (Frokjaer-Jensen et al. 2008). The single copy nature of the insertion was verified by PCR using primers flanking the recombination interval (OG967 and OG970). The HSF-1(R145A)::GFP construct (pOG124) w) )as created by site-directed mutagenesis (QuikChange II Kit, Cat. #200523) of pOG34. The sequences of all primers used in this study can be found in Table S1.
Statistical Analysis
Survival studies were analyzed using the Kaplan-Meier log-rank function (GraphPad Software). Comparisons of means were analyzed with either a two-tailed Students t-test (2 groups) or ANOVA (3 or more groups) using Bonferroni post-test analysis. A paired t-test was used in Figure S1, others were unpaired. p-values of <0.05 were considered significant.
Supplementary Material
Acknowledgements
We thank Aaron Gitler for the human hsf-1 clone, Chris Link for the HSP-16.2 antibody, Peter Klein and John Murray for immunofluorescence antibodies, Meera Sundaram and the CGC for strains. This work was supported by NIH training grant T32 GM07229 (E.A.M.) and by NIH grant R01AA017580 (T.L.). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R, Dahl G, Voellmy R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Molecular and cellular biology. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biamonti G, Vourc'h C. Nuclear stress bodies. Cold Spring Harbor perspectives in biology. 2010;2:a000695. doi: 10.1101/cshperspect.a000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cotto J, Fox S, Morimoto R. HSF1 granules: a novel stress-induced nuclear compartment of human cells. Journal of cell science. 1997;110(Pt 23):2925–2934. doi: 10.1242/jcs.110.23.2925. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denegri M, Moralli D, Rocchi M, Biggiogera M, Raimondi E, Cobianchi F, De Carli L, Riva S, Biamonti G. Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies. Molecular biology of the cell. 2002;13:2069–2079. doi: 10.1091/mbc.01-12-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymery A, Souchier C, Vourc'h C, Jolly C. Heat shock factor 1 binds to and transcribes satellite II and III sequences at several pericentromeric regions in heat-shocked cells. Experimental cell research. 2010;316:1845–1855. doi: 10.1016/j.yexcr.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu-Cronin YM, Chen WJ, Sternberg PW. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics. 2004;168:1937–1949. doi: 10.1534/genetics.104.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Inouye S, Katsuki K, Izu H, Fujimoto M, Sugahara K, Yamada S, Shinkai Y, Oka Y, Katoh Y, Nakai A. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Molecular and cellular biology. 2003;23:5882–5895. doi: 10.1128/MCB.23.16.5882-5895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Mortin MA, Wu C. Multiple functions of Drosophila heat shock transcription factor in vivo. The EMBO journal. 1997;16:2452–2462. doi: 10.1093/emboj/16.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Konecny L, Grady DL, Kutskova YA, Cotto JJ, Morimoto RI, Vourc'h C. In vivo binding of active heat shock transcription factor 1 to human chromosome 9 heterochromatin during stress. The Journal of cell biology. 2002;156:775–781. doi: 10.1083/jcb.200109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Metz A, Govin J, Vigneron M, Turner BM, Khochbin S, Vourc'h C. Stress-induced transcription of satellite III repeats. The Journal of cell biology. 2004;164:25–33. doi: 10.1083/jcb.200306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Morimoto R, Robert-Nicoud M, Vourc'h C. HSF1 transcription factor concentrates in nuclear foci during heat shock: relationship with transcription sites. Journal of cell science. 1997;110(Pt 23):2935–2941. doi: 10.1242/jcs.110.23.2935. [DOI] [PubMed] [Google Scholar]
- Jolly C, Usson Y, Morimoto RI. Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6769–6774. doi: 10.1073/pnas.96.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Balbas MA, Dey A, Rabindran SK, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- Mercier PA, Winegarden NA, Westwood JT. Human heat shock factor 1 is predominantly a nuclear protein before and after heat stress. Journal of cell science. 1999;112(Pt 16):2765–2774. doi: 10.1242/jcs.112.16.2765. [DOI] [PubMed] [Google Scholar]
- Metz A, Soret J, Vourc'h C, Tazi J, Jolly C. A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. Journal of cell science. 2004;117:4551–4558. doi: 10.1242/jcs.01329. [DOI] [PubMed] [Google Scholar]
- Mohri-Shiomi A, Garsin DA. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. The Journal of biological chemistry. 2008;283:194–201. doi: 10.1074/jbc.M707956200. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Molecular biology of the cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moronetti Mazzeo LE, Dersh D, Boccitto M, Kalb RG, Lamitina T. Stress and aging induce distinct polyQ protein aggregation states. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10587–10592. doi: 10.1073/pnas.1108766109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V, Morimoto RI. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1106557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Molecular and cellular biology. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Aballay A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13092–13097. doi: 10.1073/pnas.0604050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 587–606. [Google Scholar]
- Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Advances in experimental medicine and biology. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- Volovik Y, Maman M, Dubnikov T, Bejerano-Sagie M, Joyce D, Kapernick EA, Cohen E, Dillin A. Temporal requirements of heat shock factor-1 for longevity assurance. Aging cell. 2012;11:491–499. doi: 10.1111/j.1474-9726.2012.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. Faseb J. 2003;17:1960–1962. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. The Journal of biological chemistry. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Westwood JT, Clos J, Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991;353:822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Yao J, Munson KM, Webb WW, Lis JT. Dynamics of heat shock factor association with native gene loci in living cells. Nature. 2006;442:1050–1053. doi: 10.1038/nature05025. [DOI] [PubMed] [Google Scholar]
- Zandi E, Tran TN, Chamberlain W, Parker CS. Nuclear entry, oligomerization, and DNA binding of the Drosophila heat shock transcription factor are regulated by a unique nuclear localization sequence. Genes & development. 1997;11:1299–1314. doi: 10.1101/gad.11.10.1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.